Abstract
Study Objectives:
To test the hypotheses that brain oxygen partial pressure (PtO2) in response to obstructive apneas changes with age and that it might lead to different levels of cerebral tissue oxidative stress.
Design:
Prospective controlled animal study.
Setting:
University laboratory.
Participants:
Sixty-four male Wistar rats: 32 young (3 mo old) and 32 aged (18 mo).
Interventions:
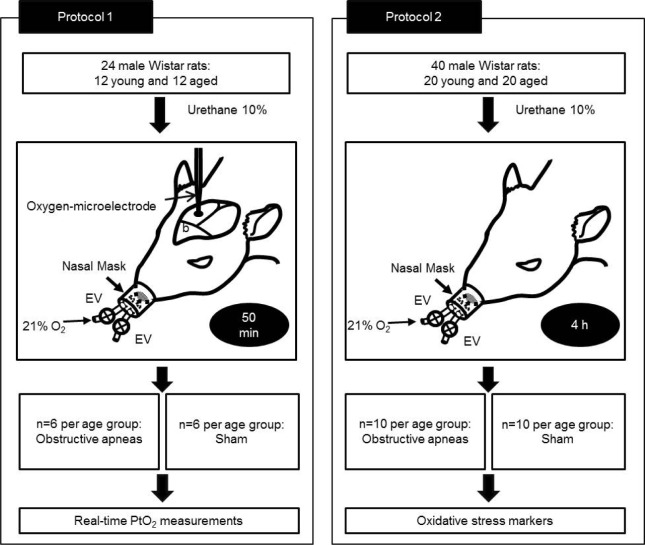
Protocol 1: Twenty-four animals were subjected to obstructive apneas (50 apneas/h, lasting 15 sec each) or to sham procedure for 50 min. Protocol 2: Forty rats were subjected to obstructive apneas or sham procedure for 4 h.
Measurements and Results:
Protocol 1: Real-time PtO2 measurements were performed using a fast-response oxygen microelectrode. During successive apneas cerebral cortex PtO2 presented a different pattern in the two age groups; there was a fast increase in young rats, whereas it remained without significant changes between the beginning and the end of the protocol in the aged group. Protocol 2: Brain oxidative stress assessed by lipid peroxidation increased after apneas in young rats (1.34 ± 0.17 nmol/mg of protein) compared to old ones (0.63 ± 0.03 nmol/mg), where a higher expression of antioxidant enzymes was observed.
Conclusions:
The results suggest that brain oxidative stress in aged rats is lower than in young rats in response to recurrent apneas, mimicking obstructive sleep apnea. This could be due to the different PtO2 response observed between age groups and the increased antioxidant expression in aged rats.
Citation:
Dalmases M, Torres M, Márquez-Kisinousky L, Almendros I, Planas AM, Embid C, Martínez-Garcia MA, Navajas D, Farré R, Montserrat JM. Brain tissue hypoxia and oxidative stress induced by obstructive apneas is different in young and aged rats. SLEEP 2014;37(7):1249-1256.
Keywords: aging, animal model, obstructive apnea, oxidative stress, tissue oxygenation
INTRODUCTION
Obstructive sleep apnea (OSA) is a highly prevalent respiratory disorder that affects 5% to 14% of the middle-aged population.1 It is characterized by recurrent episodes of partial or complete obstruction of the upper airway that result in intermittent hypoxia, hypercapnia, increased respiratory effort, sympathetic activation, and disruption of sleep architecture. OSA has been associated with a decrease in quality of life and an increase in morbidity and mortality from metabolic,2 neural, and cardiovascular3 alterations.
One of the well-known consequences of OSA in middle-aged patients is neurocognitive impairment,4 which has been related in animal models to oxidative stress and neuronal death (apoptosis and/or necrosis) induced by cyclical hypoxia.5–7 Recent data from a young rat model of OSA have reported an increase in average cerebral oxygen partial pressure (PtO2) and its cyclic oscillations in response to obstructive apneas as a mechanism responsible, at least in part, for reactive oxygen species production in brain tissue.8
There is little evidence, however, about the brain effects of OSA in elderly patients. The population pyramid is changing because of increasing longevity and it is expected that in the next decades 20% of the Western population will be elderly.9 Moreover, OSA and cognitive impairment10 both increase dramatically with aging, with published studies reporting OSA prevalence of 30% and as high as 50%.11,12 Although the effects of this chronic disease are expected to increase further in our aging society, few studies have focused on this topic. Consequently, better knowledge of the effects of OSA at an advanced age is required to evaluate its clinical significance, which is still unclear. It is noticeable that the available data from studies addressing the cognitive impairment in elderly patients with OSA have provided conflicting results. Whereas some authors have reported a relationship between cognitive impairment and sleep disordered breathing,13,14 others have not,15,16 possibly because of the presence of comorbidities and declining brain functions with age. To avoid these comorbidities, the use of animal models could enhance our understanding of how OSA consequences observed mainly in middle-aged patients interact with the functional decline associated with the natural history of aging and shed light on the mechanisms underlying cognitive impairment.
The current study was therefore designed to test the following hypotheses: first, that the dynamic changes in brain PtO2 in response to obstructive apneas might depend on age; and second, that the age-dependent PtO2 response would cause different levels of brain oxidative stress in young and aged rats.
We measured PtO2 in the cerebral cortex of young and old rats subjected to recurrent obstructive apneas. To assess the potential oxidative stress induced by apneas and the underlying mechanisms, we also analyzed lipid peroxidation in brain tissue and assessed the response in antioxidant systems in both age groups.
MATERIALS AND METHODS
Animals
The study, which was approved by the Ethics Committee of Animal Experimentation of the University of Barcelona, was carried out on 64 male Wistar rats. Thirty-two rats were 3 mo old and the other 32 rats were 18 mo old. As shown in Figure 1, there were two different protocols. In the first, 24 rats (12 young and 12 aged) were subjected to obstructive apneas or sham for 50 min to measure real-time PtO2. In the second, another set of 20 rats per age group were subjected to obstructive apneas (n = 10) or sham (n = 10) for 4 h and used to determine brain oxidative stress.
Figure 1.
Study design. Protocol 1: Animals underwent obstructive apneas or sham during 50 min and oxygen partial pressure (PtO2) in cerebral cortex was measured in real time. Protocol 2: Rats were subjected to obstructive apneas or sham for 4 h and brain oxidative stress markers were determined.
The animals were housed in standard cages, maintained on a 12-h light–dark cycle within a constant temperature range (22–24°C) and fed with standard rodent chow (Panlab, Barcelona, Spain) and tap water ad libitum. Before starting the corresponding protocol, the rats were anesthetized intraperitoneally with urethane 10% (1 g/kg) and at the end of the experiment they were sacrificed by exsanguination.
Application of Obstructive Apneas
A previously described noninvasive experimental setting was used for this study.17 The system mimicked the cyclical airway obstructions and the decrease in oxygen saturation observed in patients with OSA. This model was based on a nasal mask with one tube open to the atmosphere and another connected to an airflow source to prevent rebreathing. To allow the controlled application of airway obstructions or spontaneous breathing, two electronically synchronized electrovalves were closed or opened, respectively.
The animals were subjected to recurrent obstructive apneas with a pattern of 50 apneas/h lasting 15 sec each for 50 min or 4 h, depending on the protocol, as described previously. The sham group was identically instrumented, but the valve system of the nasal mask was always open and did not apply any airway obstructions.
Measurements of PtO2 in Brain Tissue
Real-time PtO2 measurements were made in brain tissue using a modified Clark's polarographic fast-response oxygen microelectode pipette (OX-50, Unisense A/S, Denmark; 50 μm diameter, 90% response time < 2 sec) connected to an amplified picoamperimeter (Unisense A/S). The oxygen microelectrode was calibrated before each experiment in water at 100% O2, 21% O2, and in an oxygen-free solution (sodium hydroxide 0.1 M, sodium ascorbate 0.1 M) (MicOX software, Unisense A/S).
To insert the microelectrode, a small orifice was carefully made in the dura mater to expose the cerebral cortex and then the oxygen sensor was inserted to a depth of 2 mm from the cortex surface by a micrometric positioner; it was then retracted 0.5 mm to a point with a stable PtO2 value between 30-50 mmHg.
Arterial oxygen saturation (SaO2) was measured by a pulse-oximeter (504; Critical Care Systems, Inc, Waukesha, WI, USA). PtO2 and SaO2 signals were sampled at 60 Hz and data were stored for subsequent analysis.
Determination of Oxidative Stress Markers
After 4 h of recurrent apneas or sham, the animals were sacrificed and cerebral cortex samples were excised and immediately stored at -80°C to determine lipid peroxidation, oxidized glutathione (GSSG) and antioxidant enzymes (Cu-Zn super-oxide dismutase-1 (SOD), catalase (CAT), glutathione peroxidase-1 (GPx), and glutathione reductase (GPr)).
1. Lipid Peroxidation Measurements
Lipid peroxidation in brain tissue was assessed by measuring the content of lipid hydroperoxide (LPO) using an assay kit (#705002, Cayman Chemical Company, Ann Arbor, Michigan, USA) that measures hydroperoxides using the redox reactions with ferrous ions after lipid hydroperoxide extraction into chloroform. The assay was carried out following the manufacturer's instructions.
2. Measurement of Glutathione
The cerebral cortex was homogenized, sonicated in 3.3% 5-sulfosalicyclic acid (1 mL per 200 mg of tissue) and centrifuged at 12,000 xg for 30 min at 4°C. The supernatant was used to determine GSSG and the pellet was used to determine total protein concentration (Brad ford Method, Bio-Rad, Hercules, CA, USA). The reaction is based on the oxidation of DTNB (5,5'-dithio-bis-(2-nitrobenzoic acid)) followed by measurement at 405-412 nm in a spectrophotometer. The formation of GSSG was determined in 100 μL of the supernatant after the addition of 2 μL of 2-vinylpyridine.
3. Measurement of Superoxide Dismutase-1, Catalase, Glutathione Peroxidase-1, and Glutathione Reductase
Total protein was extracted using radioimmunoassay buffer (RIPA) containing 0.01 M phosphate buffered saline, sodium dodecyl sulfate, sodium deoxycholate, the nonionic detergent Igepal, and the complete protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany). The protein concentration of samples was determined with the Bradford assay (Bio-Rad). Twenty-five μg of protein extract were resolved by sodium dodecyl sulfate polyacrylamide gel, and proteins were transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore, Bedford, MA, USA). Rabbit polyclonal and monoclonal antibodies were used against the following proteins: catalase (#ab1877, Abcam) diluted 1:1,000, glutathione peroxidase (#ab22604, Abcam) diluted 1:1,000, and glutathione reductase (#ab124995, Abcam) diluted 1:1,000, and a goat polyclonal antibody was used against superoxide dismutase-1 (#sc-8637, Santa Cruz) diluted 1:1,000. A mouse monoclonal antibody against β-actin (Clone AC-15, Sigma-Aldrich) diluted 1:500,000 was used as the loading control. Antibodies were diluted in Tris-buffered saline containing 0.5% Tween 20 and were incubated overnight at 4°C. The following day, the membranes were incubated with horseradish peroxidaseconjugated mouse antigoat immunoglobulin G (IgG) (Sigma-Aldrich) or goat antirabbit IgG (Amersham Biosciences) diluted 1:2,000 for 2 h at room temperature. The blots were developed with the use of a chemiluminescent substrate (ECL Western blotting Analysis System; Amersham Biosciences). The optical density of the bands was measured by densitometric analysis (Molecular Imager GS800, Bio-Rad). Band intensity was divided by the intensity of β-actin to correct for differences in the amount of protein loaded on each gel. Samples with rats from the different experimental groups were run in each gel and values were expressed as fold versus mean sham young.
Data Processing and Statistical Analysis
In rats subjected to obstructive apneas, SaO2 and cerebral PtO2 were measured at baseline and at the maximum and minimum values attained during the obstructive apnea period. The time and age dependence of SaO2 and brain PtO2 were carried out by two-way repeated-measures analysis of variance (ANOVA) with one repeated factor (time) by comparing the sample points at the first apnea and every 10 min with respect to either the baseline value in the maximum or the first apnea value in the minimum. Post hoc pairwise comparisons were undertaken by means of the Student-Newman-Keuls method. In rats subjected to sham treatment, SaO2 and cerebral PtO2 were recorded at the same time points as in the apneic rats and the same statistical analysis was performed. The differences in LPO, GSSG, and antioxidant enzymes were assessed by two-way ANOVA by age (young versus aged) and treatment (sham versus apnea), followed by the Bonferroni post hoc analysis to determine the statistical significance of group differences, which was set at a level of P < 0.05. All data are presented as mean ± standard error. Statistical analyses were performed with SigmaPlot (Systat Software, Inc).
RESULTS
Oxygen Partial Pressure in Brain Tissue
In rats subjected to sham procedure, young and aged groups presented similar SaO2 values at baseline (young: 95.2 ± 0.3%; aged: 94.9 ± 0.9%), which remained without significant differences during 50 min of spontaneous breathing (Figure 2). Moreover, both groups showed similar PtO2 values at baseline (young: 37.7 ± 4.4 mmHg; aged: 39.6 ± 4.2 mmHg) without significant differences during measurements.
Figure 2.
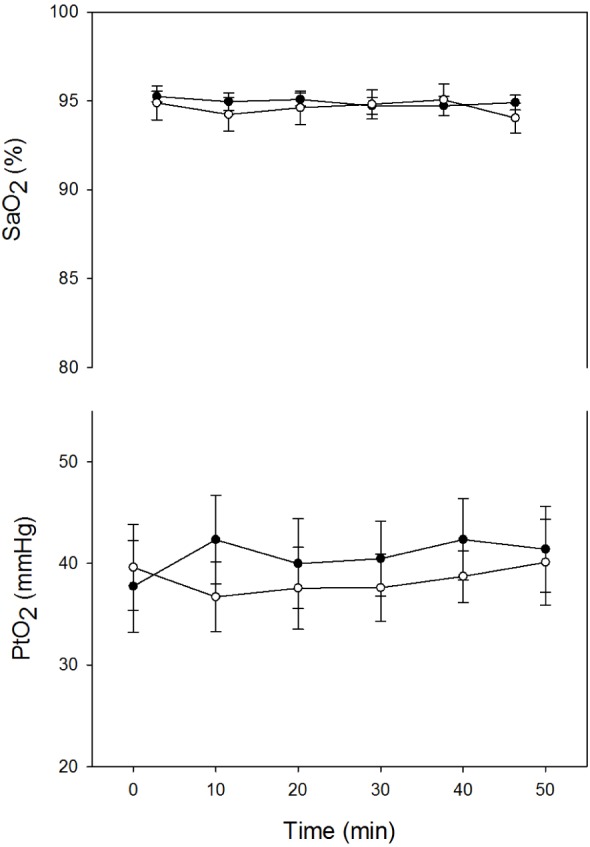
Time course of arterial oxygen saturation (SaO2) and oxygen partial pressure in cerebral cortex (PtO2) recorded in young (closed circles) and aged (open circles) sham rats. Results are shown as mean ± standard error.
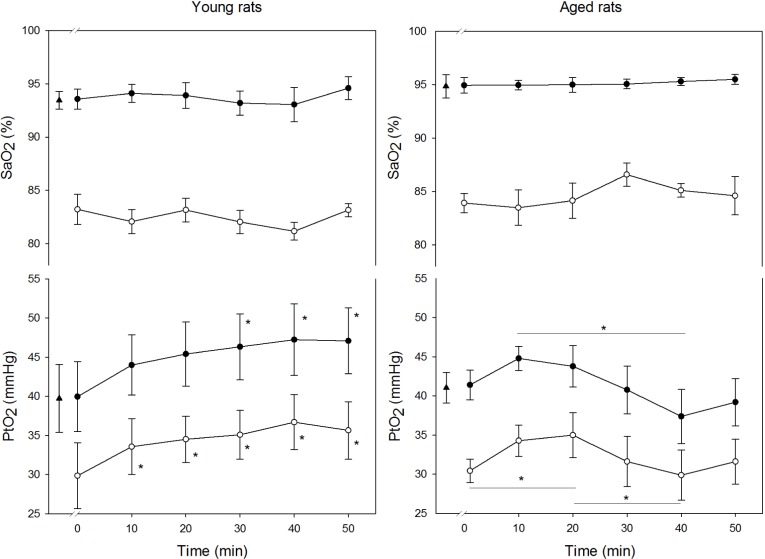
In the apnea groups, SaO2 showed a fast decrease in each apnea followed by a recovery, with maximum and minimum values typical of patients with OSA (values ranging from 93.0 ± 0.6% to 81.6 ± 0.5% in young rats and from 95.3 ± 0.4% to 84.6 ± 0.6% in aged rats). Remarkably, the SaO2 showed a stable cyclical pattern during the entire duration of the experiment and no significant differences were observed in the SaO2 values between age groups.
When comparing young and old rats, PtO2 in the cerebral cortex showed a different temporal response (interaction between age and time P < 0.05) (Figure 3). In young rats, according to the results we previously reported,8 the maximum and minimum PtO2 values experienced a fast increase during recurrent apneas. The maximum values showed a significant rise from 39.7 ± 4.3 mmHg at baseline to 47.1 ± 4.2 mmHg after 50 min of apneas (P < 0.05). The minimum values attained at the end of the protocol also increased from 29.8 ± 4.2 mmHg at the first apnea to 35.6 ± 3.7 mmHg at 50 min (P < 0.05).
Figure 3.
Time course of maximum (closed circles) and minimum (open circles) values of oxygen partial pressure (PtO2) in cerebral cortex and arterial oxygen saturation (SaO2) recorded in both age groups during the application of obstructive apneas. Baseline value is represented by a triangle. Results are shown as mean ± standard error. * P < 0.05 with respect to the baseline in maximum and respect to the first apneic event in minimum.
Interestingly, this type of increase did not occur in aged rats, where PtO2 in the cerebral cortex presented a stable pattern, without significant differences in the maximum and minimum values between the beginning and the end of the experiment (maximum: 41.0 ± 2.0 mmHg versus 39.2 ± 3.0 mmHg, minimum: 30.4 ± 1.5 mmHg versus 31.6 ± 2.9 mmHg). However, PtO2 showed significant fluctuations during the apnea period.
Oxidative Stress Markers
Lipid Peroxidation
LPO levels in cerebral cortex were significantly increased (P < 0.01) after obstructive apneas in young rats (1.34 ± 0.17 nmol/mg of protein) compared with both their controls (0.86 ± 0.08 nmol/mg of protein) and aged rats subjected to apneas (0.63 ± 0.03 nmol/mg) (Figure 4).
Figure 4.
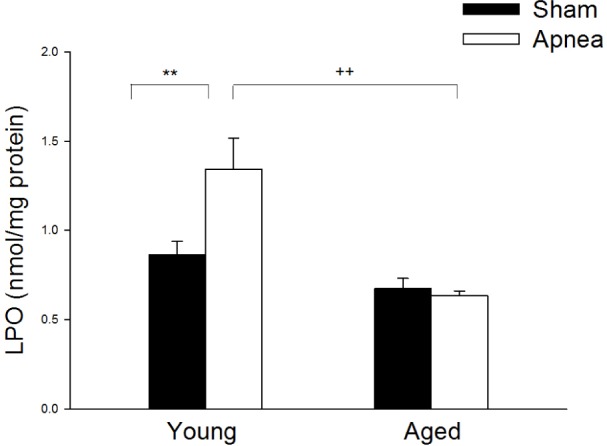
Lipid peroxidation levels in cerebral cortex in young and aged rats subjected to sham or obstructive apneas for 4 h. Results are shown as mean ± standard error. ** is the difference between treatment P < 0.01 and ++ is for age differences P < 0.01.
Oxidized Glutathione
GSSG significantly increased (P < 0.05) in brain tissue from young rats subjected to obstructive apneas (0.108 ± 0.009 nmol/ mg of protein) compared with their controls (0.084 ± 0.004 nmol/mg of protein). In contrast, brain GSSG levels after apneas in aged rats were not significantly different from those of aged sham rats (Figure 5).
Figure 5.
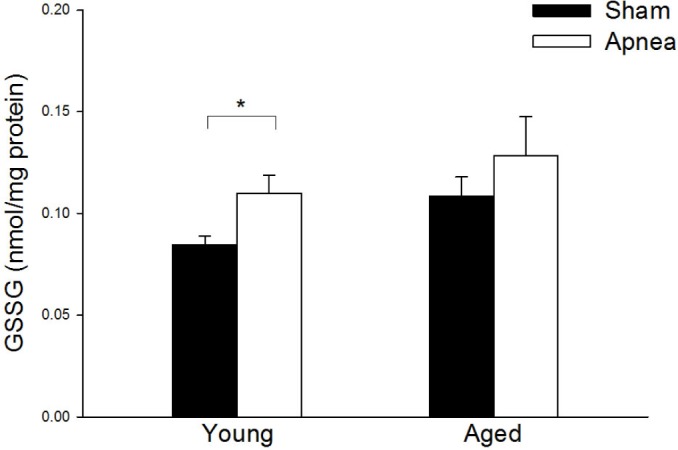
Oxidized glutathione (GSSG) levels in cerebral cortex for all groups. Results are shown as mean ± standard error. * is difference between treatment P < 0.05. There were no differences between age groups.
Antioxidant Enzymes
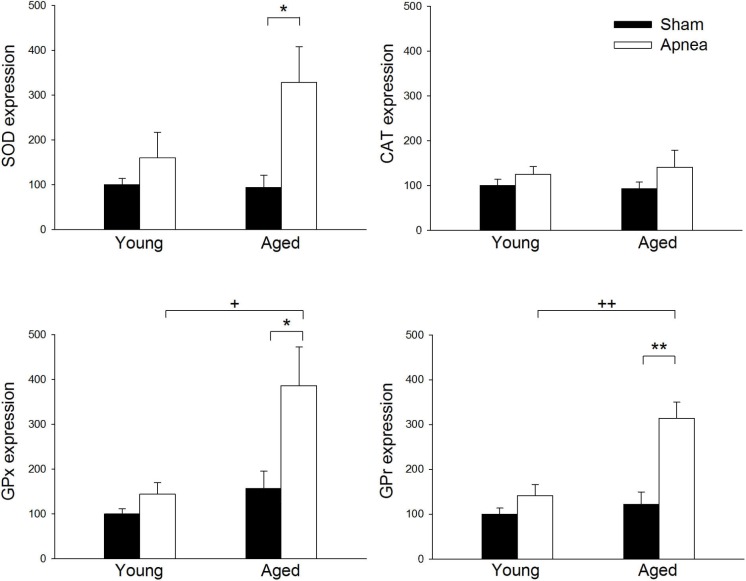
The expression of SOD, GPx, and GPr was significantly higher after apnea mainly in aged rats, showing percentages of increase over young sham rats of 227%, 286%, and 213%, respectively (Figure 6). No significant differences were observed in CAT expression in either young or aged rats after apneas.
Figure 6.
Cu-Zn superoxide dismutase-1 (SOD), catalase (CAT), glutathione peroxidase-1 (GPx), and glutathione reductase (GPr) expression in brain tissue in young and aged rats subjected to apneas or sham. Results are expressed as percentage of increase over young sham rats. Results are shown as mean ± standard error. * and ** are differences between treatment P < 0.05 and P < 0.01, respectively. + and ++ are differences between age P < 0.05 and P < 0.01, respectively.
DISCUSSION
Our study shows that the brain effects of obstructive apneas mimicking OSA change with aging, leading to substantially different responses in both oxygen partial pressure as well as in levels of oxidative stress markers in tissue. We found that brain PtO2 in young rats increased during apnea whereas, in contrast, it remained without significant changes between the beginning and the end of the protocol in aged rats. This increase in PtO2 values was accompanied by higher cerebral oxidative stress in young animals compared to old animals, in which we also observed increased expression of antioxidant enzymes. The lower oxidative stress in aged rats is therefore attributable to a better balance between the presence of oxidative stress generated by apneas (mainly induced by changing PtO2 values) and an adequate response of antioxidant systems.
To carry out this work we used a validated experimental setting to apply recurrent obstructive apneas in a noninvasive and controlled manner.17 This model was able to mimic the respiratory events of patients with OSA and so, in addition to intermittent hypoxia, rats also experienced recurrent hyper-capnia and inspiratory effort. For the PtO2 measurements we used a Clark's polarographic microelectrode pipette, which is especially suited to measuring PtO2 in tissues and is commonly used for this purpose.18,19 Its small size induces minimal tissue trauma and allows measurements at a microregional level, in contrast to imaging techniques that explore a greater volume of tissue and might include large blood vessels. However, because of differences in regional blood flow between brain areas, our results could not be representative of other parts of the brain.
Animals were anesthetized with urethane and this could have limitations in terms of effects on the cardiovascular system as well as in inducing a sleeplike state. Unlike other anesthetics, urethane promotes spontaneous and cyclical alternations of brain state that resemble sleep state alternations and promotes a condition of unconsciousness that closely mimics the full spectrum of natural sleep20; although is not fully equal and results should be cautiously extrapolated. Regarding cardiovascular effects, it is described that with urethane anesthesia (1 g/kg) blood gas values are very similar to those of nonanesthetized animals. Moreover, despite anesthesia, SaO2 values recorded in both age groups concur to a reasonable extent with the values observed in patients with OSA. Furthermore, we assessed whether anesthesia affected age groups in a different manner by measuring PtO2 and SaO2 in young and aged sham rats. The results showed that PtO2 and SaO2 remained stable over time and without significant differences among age groups, demonstrating that the results found after apnea were not attributable to age differences in anesthetic response.
Despite the fact that no differences in SaO2 values were observed, either between the apnea groups or during the experiment, cerebral PtO2 in response to apneas showed a markedly different behavior in each age group. In young rats, in line with the results previously published,8 the maximum and minimum PtO2 values gradually increase over the course of the 50 min, being significantly higher at the end of the experiment relative to the baseline and to the first apnea decrease, respectively. In contrast, this particular cerebral response was not observed in aged animals, where this rise did not occur and PtO2 only presented oscillations around the mean baseline value but without differences between the beginning and the end of the experiment. Considering that cerebral metabolism decreases with age,21 lower values of PtO2 do not appear to be caused by increased oxygen consumption but are instead attributable to reduced supply. These data suggest that compensatory mechanisms increasing the oxygen availability in brain tissue in response to apneas might be altered or less efficient with aging. The oxygen supply to the brain is mainly regulated by changes in cerebral blood flow, which in turn are regulated by multiple stimuli (hypoxia, blood pressure, and hypercapnia).
According to previous studies,22 the vasodilator response induced by hypoxia is preserved in aged animals (even older than those used in our study) except in severe hypoxia (PaO2 values approximately 25 mmHg), with lower values than those achieved in our apnea experiments. Another mechanism involved in maintaining blood flow is dynamic cerebral autoregulation (the capacity to maintain cerebral flow despite changes in blood pressure), and it has been reported that this mechanism is not affected by aging, even when negative pressures are applied to the circulatory system.23 Hypercapnia is another factor regulating the oxygen supply in two ways: increasing the release of oxygen in tissue by a right shift in the oxyhemoglobin dissociation curve24 and inducing vasodilatation (in addition to the hypoxia-induced vasodilatatory effect).25 In this respect, the vasodilator response to hypercapnia decreases with age both in animal studies26 and in healthy elderly subjects, as assessed by either functional magnetic resonance imaging27 or a frequency domain tissue oximeter.28 This deterioration could be related to arteriosclerosis, which is common in the elderly, and some authors have attributed this lack of response to a loss of prostaglandin-mediated vasodilatation.29 Taking into account previous studies by our group,30 we observed that the PtO2 pattern in aged rats is similar to that obtained in animals subjected to intermittent hypoxia alone, endorsing the hypothesis that the mechanisms involved could be related to hyper-capnia. Therefore, although hypercapnia was not the main cause of apnea-induced blood flow changes, the different results in PtO2 observed among age groups could be explained, at least in part, by age differences in the response to hypercapnia.
This study also shows differences in brain oxidative stress assessed by lipid peroxidation; this is higher in young rats subjected to obstructive apneas than in old ones, for which there were no changes in LPO levels after recurrent airway obstructions. In support of this notion, GSSG significantly increased after apneas in young rats, providing evidence of a higher degree of oxidative stress and higher consumption of GSH. We also assessed the antioxidant system by analyzing enzymes such as SOD, CAT, GPx, and GPr. All of these are antioxidant enzymes responsible for preventing the spread of oxidative damage by catalyzing reactions that convert free radicals and oxygen reactive species into less harmful substances. SOD scavenges superoxide, converting it to H2O2 which is catabolized to water mainly by catalase preventing the formation of hydroxyl radical. GPx might also convert H2O2 to water in the oxidative reaction of glutathione and GPr reduces GSSG to keep up intracellular levels of GSH. We found that the expression of antioxidant enzymes was higher after apnea mainly in aged rats. These results are in agreement with previous studies showing that enzymatic antioxidant response to hypoxia/reoxygenation in aging rats did not diminish or even increase31 and that a good level of inducibility is maintained in old animals.32 This finding is probably the result of the presence of a low degree of oxidative stress at baseline in aged rats, as suggested by the higher GSSG in the aged sham group, which could act as an adaptive mechanism preventing reactive oxygen species increase. Accordingly, this lower oxidative stress observed in old rats, compared to young rats, may be caused by increased expression of antioxidants as well as a decreased reactive oxygen species production in brain tissue during apneas. In a previous work30 on young rats, our group described lower oxidative stress, in an intermittent hypoxia model, when PtO2 did not increase during the experiment, as observed here in aged rats. Moreover, the pattern of a progressive increase in PtO2 values, as found in young rats subjected to apneas, was associated with high levels of oxidative stress. Our results agree with several studies that described an increase in brain oxidative stress in murine models of sleep apnea or intermittent hypoxia using young rats, which in turn has been associated with behavioral impairments and learning deficits,33 and even apoptosis in brain tissue.34
Despite the high prevalence of sleep apnea in the elderly population, we could locate only one study in the literature of the effects of this disease in aged brain substrate. In that study, an increased susceptibility to chronic intermittent hypoxia, evaluated as changes in proteasomal activity and neuronal apoptosis, was described in old rats.35 The difference in the results can be explained by the fact that during intermittent hypoxia the PtO2 pattern in young rats is different from that observed when applying apneas, as previously explained. Therefore, subjecting young and old rats to intermittent hypoxia probably reduces the actual differences between the age groups in terms of PtO2 in brain tissue and could result in a higher susceptibility in the old group. The obstructive apnea experimental setting there seems more suitable, because it closely mimics OSA events, than intermittent hypoxia for studying brain changes associated with obstructive apneas, especially when comparing different age groups.
To our knowledge there are no data describing either the changes in brain oxygenation or in oxidative stress after apnea in advanced age. Our findings are free from confounding factors caused by comorbidities and show for the first time how OSA interacts with aging. However, the current results were obtained in an acute model and therefore the chronic responses remain unknown. In a chronic phase, the observed vascular response as well as the brain oxidative stress could vary according to the balance between any damage induced by chronic apneas and the ability of the adaptive mechanisms to protect from or repair such damage.
In conclusion, our study, which shows a differential age-dependent effect of OSA on brain tissue, helps to shed light on the effect of OSA in the elderly population and the underlying mechanisms. The current study points the way to future experimental studies, adding some of the comorbidities typical of OSA to the aging paradigm.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported in part by SEPAR and Spanish Ministry of Science and Innovation (PI11/01892 and SAF2011-22576). Work was performed at Universitat de Barcelona, Barcelona, Spain. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Miguel A. Rodríguez for his help with the laboratory procedures.
ABBREVIATIONS
- CAT
catalase
- DTNB
5,5'-dithio-bis-(2-nitrobenzoic acid)
- GPr
glutathione reductase
- GPx
glutathione peroxidase-1
- GSSG
oxidized glutathione
- LPO
lipid peroxidation
- OSA
obstructive sleep apnea
- PtO2
oxygen partial pressure
- RIPA
radioimmunoassay buffer
- SaO2
arterial oxygen saturation
- SOD
Cu-Zn superoxide dismutase-1
Footnotes
A commentary on this article appears in this issue on page 1161.
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trzepizur W, Le Vaillant M, Meslier N, et al. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest. 2013;143:1584–9. doi: 10.1378/chest.12-1652. [DOI] [PubMed] [Google Scholar]
- 3.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 4.Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54:787–93. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veasey S. Insights from animal models into the cognitive consequences of adult sleep-disordered breathing. ILAR J. 2009;50:307–11. doi: 10.1093/ilar.50.3.307. [DOI] [PubMed] [Google Scholar]
- 6.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–50. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapino C, Bianchi G, Di Giulio C, et al. HIF-1alpha cytoplasmic accumulation is associated with cell death in old rat cerebral cortex exposed to intermittent hypoxia. Aging Cell. 2005;4:177–85. doi: 10.1111/j.1474-9726.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 8.Almendros I, Montserrat JM, Torres M, González C, Navajas D, Farré R. Changes in oxygen partial pressure of brain tissue in an animal model of obstructive apnea. Respir Res. 2010;11:3. doi: 10.1186/1465-9921-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–9. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Shahar E, Nieto FJ, et al. Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 12.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea- hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Zion M, Stepnowsky C, Johnson S, Marler M, Dimsdale JE, Ancoli-Israel S. Cognitive changes and sleep disordered breathing in elderly: differences in race. J Psychosom Res. 2004;56:549–53. doi: 10.1016/j.jpsychores.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Kim HC, Young T, Mathews CG, et al. Sleep-disordered breathing and neurophysiological deficits: a population-based study. Am J Crit Care Med. 1997;156:1813–9. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 15.Sforza E, Roche F, Thomas-Anterion C, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33:515–21. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips BA, Berry D.T.R, Schmitt FA, Magan LK, Gerhardstein D, Cook YR. Sleep-disordered breathing in the healthy elderly. Clinically significant? Chest. 1992;101:345–9. doi: 10.1378/chest.101.2.345. [DOI] [PubMed] [Google Scholar]
- 17.Carreras A, Almendros I, Acerbi I, Montserrat JM, Navajas D, Farré R. Obstructive apneas induce early release of mesenchymal stem cells into circulating blood. Sleep. 2009;32:117–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Offenhauser N, Thomsen K, Caesar K, Lauritzen M. Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J Physiol. 2005;565:279–94. doi: 10.1113/jphysiol.2005.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takano T, Tian GF, Peng W, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–62. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- 20.Clement EA, Richard A, Thwaites M, Ailon J, Peters S, Dickson CT. Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS One. 2008;3:e2004. doi: 10.1371/journal.pone.0002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman WE, Pelligrino D, Miletich DJ, Albrecht RF. Brain metabolic changes in young vs aged rats during hypoxia. Stroke. 1985;16:860–3. doi: 10.1161/01.str.16.5.860. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman WE, Albrecht RF, Miletich DJ. Cerebrovascular response to hypoxia in young vs aged rats. Stroke. 1984;15:129–33. doi: 10.1161/01.str.15.1.129. [DOI] [PubMed] [Google Scholar]
- 23.Carey BJ, Eames PJ, Blake MJ, Panerai RB, Potter JF. Dynamic cerebral autoregulation is unaffected by aging. Stroke. 2000;31:2895–900. doi: 10.1161/01.str.31.12.2895. [DOI] [PubMed] [Google Scholar]
- 24.Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J Exp Biol. 2009;212:3387–93. doi: 10.1242/jeb.023697. [DOI] [PubMed] [Google Scholar]
- 25.Hare GM, Kavanagh BP, Mazer CD, et al. Hypercapnia increases cerebral tissue oxygen tension in anesthetized rats. Can J Anaesth. 2003;50:1061–8. doi: 10.1007/BF03018375. [DOI] [PubMed] [Google Scholar]
- 26.Lartaud I, Bray-des-Boscs L, Chillon JM, Atkinson J, Capdeville-Atkinson C. In vivo cerebrovascular reactivity in Wistar and Fischer 344 rat strains during aging. Am J Physiol. 1993;264:H851–8. doi: 10.1152/ajpheart.1993.264.3.H851. [DOI] [PubMed] [Google Scholar]
- 27.Riecker A, Grodd W, Klose U, et al. Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J Cereb Blood Flow Metab. 2003;23:565–73. doi: 10.1097/01.WCB.0000056063.25434.04. [DOI] [PubMed] [Google Scholar]
- 28.Gatto R, Hoffman WE, Mueller M, Paisansathan C, Charbel F. Age effects on brain oxygenation during hypercapnia. J Biomed Op. 2007;12 doi: 10.1117/1.2804705. 062113. [DOI] [PubMed] [Google Scholar]
- 29.Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol. 2012;112:1884–90. doi: 10.1152/japplphysiol.01270.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almendros I, Farré R, Planas AM, et al. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34:1127–33. doi: 10.5665/SLEEP.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Romero R, Cañuelo A, Martínez-Lara E, et al. Aging affects but does not eliminate the enzymatic antioxidative response to hypoxia/reoxygenation in cerebral cortex. Exp Gerontol. 2006;41:25–31. doi: 10.1016/j.exger.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Amicarelli F, Ragnelli AM, Aimola P, et al. Age-dependent ultrastructural alterations and biochemical response of rat skeletal muscle after hypoxic or hyperoxic treatments. Biochim Biophys Acta. 1999;1453:105–14. doi: 10.1016/s0925-4439(98)00088-x. [DOI] [PubMed] [Google Scholar]
- 33.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–53. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- 34.Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 35.Gozal D, Row BW, Kheirandish L, et al. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J Neurochem. 2003;86:1545–52. doi: 10.1046/j.1471-4159.2003.01973.x. [DOI] [PubMed] [Google Scholar]