Abstract
Significance: Tumor microenvironment (TME) is a complex term that includes extracellular matrix, blood vessels, endothelial, stromal, and inflammatory cells, and other supporting structures of the particular organ; and physiological components such as oxygen, pH, nutrients, waste products, signaling molecules, reducing/oxidizing species, growth factors, protumorigenic factors, etc. TME is now widely recognized as a major contributor to cancer aggression and treatment resistance and as a potential target for therapeutic intervention. Recent Advances: Among important physiological parameters of the TME, tissue hypoxia is considered to be a consequence of imbalanced angiogenesis and is associated with changes in metabolic pathways, including a higher dependence on glycolysis resulting in tissue acidosis. Both hypoxia and acidosis affect the tissue redox status and its key intracellular component, glutathione (GSH). Numerous publications support that these local TME conditions select for outgrowth of cells with appropriate phenotypes, which can reflect underlying genetics. Critical Issues: Here, we hypothesize that specific patterns of local TME, namely, tumor oxygenation, extracellular pH, redox, and GSH homeostasis, acting in orchestrated mechanism, can promote cancer cell survival, while at the same time being highly toxic and mutagenic for normal cells, thus contributing to the growth of cancers at the expense of the normal tissues they are invading. This review summarizes the experimental observations that support the hypothesized Janus-faced character of the redox axis. Future Directions: Normalizing the TME redox parameters may decrease the selection pressure for malignant phenotypes, therefore providing a tool for TME-targeted anticancer therapy. Antioxid. Redox Signal. 21, 723–729.
Introduction
In recent years, cancer research has experienced a paradigm shift from a seemingly obvious target, tumor cells, toward a key support system of cancer, tumor microenvironment (TME) (64). Carcinogenesis is a phenomenon that occurs in tissues, not in individual cancer cells. Malignant cells generated by causal genetic insults behave differently depending on their specific microenvironment. This explains why numerous therapeutic effects and treatment strategies elaborated in vitro are not reproduced in further studies in animals and humans. In general, TME is a complex term that includes two interrelated and interacting principal components: the physical TME (extracellular matrix, blood vessels, endothelial, stromal, and inflammatory cells, and other supporting structures of the particular organ); and physiological TME (oxygen, pH, nutrients, waste products, signaling molecules, reducing/oxidizing species, growth factors, protumorigenic factors, etc.). TME is now widely recognized as an integral, essential part of cancer, a major contributor to cancer aggression and treatment resistance and as a potential target for therapeutic intervention. Among important physiological parameters of the TME, tissue hypoxia (66) is considered to be a consequence of imbalanced angiogenesis and is associated with changes in metabolic pathways, including a higher dependence on glycolysis (70) resulting in tissue acidosis (5, 32). Both hypoxia and acidosis affect the tissue redox status (51) and its key intracellular component, glutathione (GSH) (20, 69). In this study, we hypothesize that specific patterns of tumor redox and GSH homeostasis can promote cancer cell survival, while at the same time being highly toxic and mutagenic for nontransformed cells. This review summarizes the experimental observations that support the hypothesized Janus-faced character of the redox axis of the TME.
TME Redox Is Characterized by Low Redox Potential and High Reducing Capacity
The important role of tissue redox microenvironment and redox signaling in physiology and pathophysiology is widely speculated, but not widely accepted. This discrepancy is due to the difficulty in deriving a quantitative description of the redox microenvironment, which requires accounting for the simultaneous status of the numerous redox couples. Among other redox couples, the glutathione disulfide–glutathione couple (GSSG/2GSH) is of particular importance due to the very high millimolar concentration range of the intracellular GSH, which is considered as an intracellular redox buffer (63). Therefore, the redox state of the GSSG/2GSH is accepted as an important indicator of the intracellular redox environment.
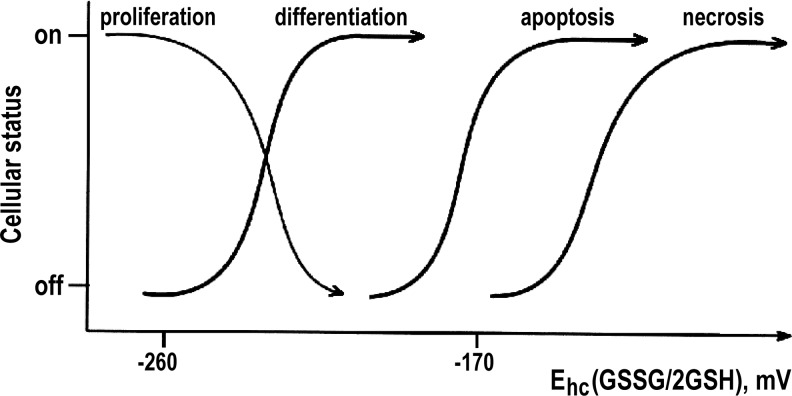
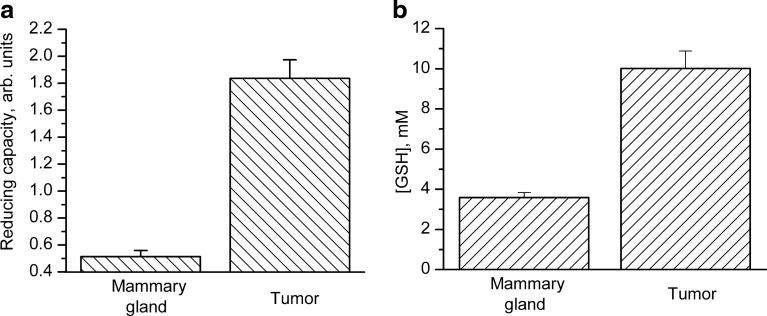
Figure 1 illustrates the relationship between the GSH redox potential and cellular state. The most negative GSH redox potential (from −260 to −220 mV) is characteristic for proliferating cells, while apoptotic and necrotic cells characteristically have the highest GSH redox potentials (≥−170 mV) (63). In proliferating cancer cells, low GSH redox potentials have been measured in vitro (34, 63). Furthermore, a high reducing capacity (4, 42) and high intracellular GSH (4, 42, 62) contents have been reported from in vivo measurements in animal cancer models, as illustrated in Figure 2. A negative redox potential reflects TME redox thermodynamics, and indicates a shift of redox balance between oxidizing and reducing capacities in favor of the latter one. A relevant question is whether a highly reducing TME can be interpreted as deriving from decreased oxidative stress or low rates of reactive oxygen species (ROS) production?
FIG. 1.
Simplified scheme of the relationship between the GSH redox potential and biological cellular status. Redox potential, Ehc, for the glutathione disulfide–glutathione couple (GSSG/2GSH), is an important indicator of the intracellular redox environment that reflects cellular status. Ehc (GSSG/2GSH) has the most negative value for proliferating cells, including cancer cells, and is more positive for differentiated cells. A number of oxidative stress-related pathologies, for example, inflammation, are characterized by further increase in GSH redox potential and are often associated with GSH depletion. If Ehc (GSSG/2GSH) becomes too positive, then apoptotic redox signals are initiated, resulting in the removal of cells that have lost an ability to control their redox environment. Very high values of the Ehc leave only necrosis or necroptosis as a path to cell death. Adapted from Schafer and Buettner (63) with permission of Elsevier, Inc.
FIG. 2.
Tumor reducing capacity and GSH content. The reducing capacity of extracellular tissue microenvironment and concentration of intracellular GSH assessed in the normal mammary glands and mammary tumors of female FVB/N mice using in vivo electron paramagnetic resonance technique. (a) The reduction rate of the nitroxide redox probe in extracellular media of normal mammary glands and tumors. (b) Intracellular GSH concentrations measured using paramagnetic GSH-sensitive probes. Adapted from Bobko et al. (4) with permission.
Role of TME in ROS Generation
In cases wherein there is a high level of oxidative stress, for example, due to elevated levels of ROS generation at low pH or in inflammation, the tissue microenvironment is characterized by high redox potential and high oxidizing capacity. Whereas TME is characterized by low redox potential and high reducing capacity, elevated levels of intra- and extracellular ROS generation (2, 46, 49, 55) and products of lipid peroxidation (19, 43) have been detected in almost all cancers. This is not entirely surprising since the higher reducing capacity of TME may promote electron flux from reducing equivalents to the corresponding substrates, such as oxygen or iron, followed by a cascade of free radical reactions and oxidative damage in spite of the very negative redox potential. Superoxide generation is widely considered to represent the initial step in ROS formation followed by its dismutation to hydrogen peroxide and further aggravated by the possible formation of highly reactive hydroxyl radicals in the Fenton-type reactions, catalyzed by reduced metals. The oxidative phosphorylation chain of mitochondria (9) and membrane-associated NADPH-dependent oxidases (NOX) (2, 38) are two major sources of intracellular and extracellular superoxide generation, respectively. We suspect that additional mechanisms of extracellular superoxide generation are facilitated by specific features of TME.
Highly reducing extracellular TME (4) favors electron flux from reductants to dissolved oxygen resulting in superoxide production. Metal ions in their reduced state, for example, Fe2+ or Cu+, are well-known reducing agents for oxygen that catalyze superoxide production. In respect to this, iron transport proteins, transferrin and ferritin, as well as copper-binding ceruloplasmin, can act as sources of metal ions. Furthermore, tumor-associated extracellular acidosis (15, 48, 72) may potentiate dissociation of protein-bound metals [e.g., Fe3+ from transferrin (13, 58)], while highly reducing TME favors metal ion reduction and, therefore, ion redox cycling. Among the mechanisms of metal reduction could be the reduction of ferric iron, Fe3+, as well as other metal cations (e.g., Cu2+) by reactive thiol, cysteinyl-glycine (12, 17) whose extraordinary low pKa=6.4 provides a high fraction of metal-binding thiolate anions. The synthesis of cysteinyl-glycine is catalyzed by plasma membrane-bound γ-glutamyltransferase (GGT) from extracellular GSH. The activity of GGT (12, 21, 27, 29, 30) as well as expression of GSH complex export transporters, GS-X pumps (1, 14, 45), are often significantly elevated in cancer cells, including human malignancies, supporting the potential importance of GGT/GSH-dependent mechanism in TME extracellular superoxide production (17).
Many redox reactions are pH dependent with redox potential being increased at lower pH (63). In regard to superoxide (E0’=940 mV), a decrease of extracellular pH (pHe) by about 0.5 units, often observed in solid tumors, results in a threefold increase in its protonated form, hydroperoxyl radical (E0’=1060 mV) capable of oxidation of unsaturated lipids. An enhanced lipid peroxidation during tissue acidosis has been previously reported (6, 31, 47). Therefore, extracellular TME acidosis may potentiate enhanced oxidative stress and oxidizing damage (73).
Numerous studies of ischemia/reperfusion phenomena demonstrate that tissue ischemia induces tissue acidosis (41) and accumulation of reducing equivalents (lower redox potential) (74), followed by a burst of ROS production upon onset of reperfusion (26). Interestingly, the spatial distribution of oxygen in TME is also not constant, but varies over time due to periodic microregional blood flow fluctuations with periodicities ranging from 20 to 60 min (waves) (7) to large-scale circadian changes (tides). These temporal oscillations create local cyclic hypoxia events in TME oxygenation resembling an ischemia/reperfusion model, and therefore may facilitate similar bursts in ROS generation.
Janus-Faced Tumor Redox Microenvironment: Oxidative Stress in Cancer Cells Versus Normal Cells
An imbalance between antioxidants and oxidants in favor of oxidants, potentially leading to damage, is termed “oxidative stress” (65). The presence of random oxidative alterations of DNA, proteins, and lipids are well documented in cancers and cancer cells (18, 43, 46) supporting the presence of some level of oxidative stress. As first suggested by Oberley et al. (55), oxidative stimuli may result in signaling pathways leading to proliferation, while an overall more reducing environment might be the result of adaptation in response to an oxidative stress. Cancer cells express increased levels of antioxidant proteins (2, 46) to detoxify ROS suggesting that a delicate balance of intracellular ROS levels is required for cancer cell function.
High levels of GSH, a major intracellular redox buffer and antioxidant (63), have been found in various tumor types, being up to several-fold greater than that in surrounding tissues (4, 20, 33, 67, 69). The overexpression of GSH in tumors has been related to enhanced cell proliferation (39), decreased levels of apoptosis (28), increased resistance to chemotherapeutic drugs (40, 67), and radiation therapy (53). Moreover, high GSH content in tumor cells is typically associated with higher activities of GSH-dependent antioxidant enzymes, including glutathione peroxidase, glutathione transferase (GSH-Tr), and glutathione reductase (GSSG-Rx) (16). Therefore, elevated levels of GSH and antioxidant GSH-dependent enzymes play a central role in protection of cancer cells against intracellular oxidative stress. On the other hand, as discussed in the previous section, overexpression of GGT in cancer cells is related to the GGT/GSH-dependent mechanism of superoxide production in extracellular TME. An extracellular superoxide generation in TME is aggravated by high reducing capacity, low pHe, and local cycling hypoxia. In the absence of high levels of GSH, the cells in the TME are subjected to large excursions in redox.
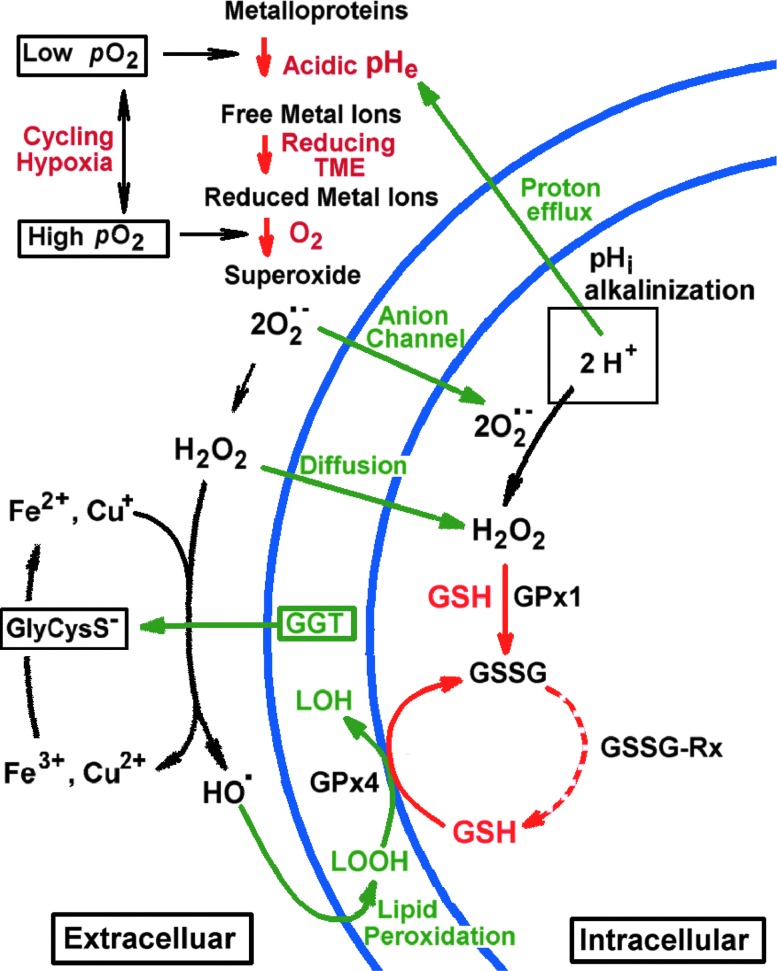
In summary, TME exposes both cancer and nontransformed cells to elevated ROS production. The corresponding oxidative stress is tightly controlled in cancer cells by overexpressed antioxidant defense at a level sufficient to provide a signaling pathway to proliferation, while avoiding a fatal oxidative damage. However, the same elevated level of extracellular ROS generation may be highly toxic and mutagenic for normal cells (50) due to significantly lower activities of antioxidant enzymes and GSH content. Adaptation of the cells in response to an oxidative stress may include an increase in intracellular GSH content and GSH redox potential contributing to signaling for proliferation and malignant transition. The proposed paradigm of Janus-faced TME is illustrated in Figure 3.
FIG. 3.
Janus-faced tumor redox microenvironment. The discussed key components of the TME, oxygen, pH, redox, and GSH, are shown in red. Hypoxia-induced acidosis potentiates accumulation of free metal ions such as Fe3+ and Cu2+. In its turn, a high reducing capacity of TME promotes metal ion reduction to the Fenton-active state, for example, via γ-glutamyltransferase (GGT)/GSH-dependent generation of reducing cysteinyl-glycine dipeptide, GlyCysS−. A cycling local hypoxia in TME facilitates further electron transfer to oxygen with formation of O2•− radical, triggering the radical reaction cascade of O2•− dismutation to H2O2 followed by OH-radical formation via the Fenton reaction. Low reactive oxygen species (ROS), H2O2, and O2•−, penetrate into tumor cells by diffusion or via anion channels, latter contributing to the increase of intracellular pH (pHi) (8, 37) with a corresponding decrease in oxidizing potential of ROS. In contrary, low acidic extracellular pH (pHe) in TME enhances ROS oxidizing potential toward surrounding cells that may result in oxidative damage and mutagenesis as well as adaptive response (e.g., increase of GSH) and changing cellular phenotypes. Increase in H2O2, O2•−, GSH, and pH has been shown contribute into the triggering cells in the proliferation stage (8). Compared with normal cells, cancer cells are well protected against oxidative stress, in part, by elevated GSH content and activities of GSH-dependent antioxidant enzymes, including glutathione peroxidases (GPx1 and GPx4 denote peroxidases that target hydrogen peroxide and lipid peroxide, correspondingly) and glutathione reductase (GSSG-Rx). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Selection Pressure of Specific TME Conditions
Genetic heterogeneity of tumors is recognized as a major cause of therapy resistance. This heterogeneity arises from genome instability in combination with a highly selective TME (25). Local TME conditions select for outgrowth of cells with appropriate phenotypes, which can reflect underlying genetics (24). Numerous publications support that among these specific TME conditions, hypoxia and acidosis lead to genomic instability (3, 23, 24, 59). We believe that one of the mechanisms of the TME influence on mutagenesis may be related to the corresponding alterations of TME redox characterized by both high reducing capacity and enhanced extracellular ROS generation, as illustrated in Figure 3. It is evolutionarily reasonable that, since tumors evolved within hypoxic and acidic environments and related oxidizing pressure, they would recapitulate these as they grow, as it provides them with a selective advantage. Cancer cells are well protected against oxidizing pressure, in part, by elevated GSH content and activities of GSH-dependent antioxidant enzymes. This allows successful cancers to avoid severe oxidative damage and to maintain a delicate balance of intracellular ROS levels required for cancer cell functions such as activation of redox signaling pathways leading to proliferation. On the other hand, exposing normal cells to cyclic oxidizing pressure may result in DNA damage, leading to cell death or malignant transition.
Conclusion
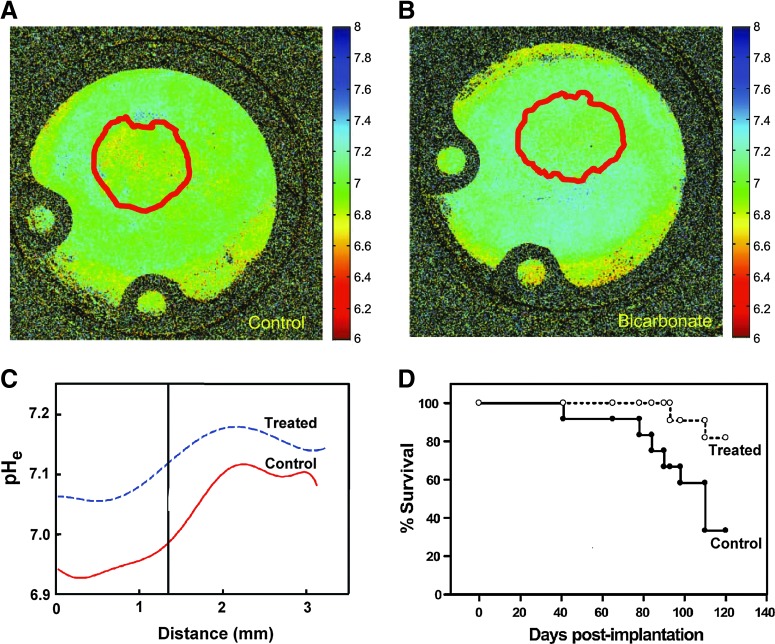
A new hypothesis of Janus-faced TME is proposed. High reducing capacity, hypoxia, and acidosis of extracellular TME expose both cancer and nontransformed cells to elevated ROS production, while providing cancer cells with a selective advantage. Normalizing TME may decrease the selection pressure for malignant phenotypes, therefore providing a tool for TME-targeted anticancer therapy. Indeed, there are data supporting the beneficial effects of targeting selected TME parameters, including tumor hypoxia (22, 52, 54, 71), acidosis (4, 35, 36, 60, 61, 68) (see Fig. 4), redox (10, 57), and GSH content (11, 44, 56). A combination therapy aimed to normalize several of these interrelated TME parameters may provide further therapeutic advantages.
FIG. 4.
Effect of bicarbonate on tumor microenvironment pHe and survival of tumor-bearing animals. Microscopic pHe gradients in tumors inoculated into the window chamber were measured by fluorescence ratio imaging of SNARF-1 pH probe. Representative pHe images are shown for untreated (A) and bicarbonate-treated (B) mice. Red lines, region of interest of tumor. (C) Distributions of pHe along radial lines for control and bicarbonate-treated tumors. “0” is centroid of tumor, and vertical line indicates tumor edge. (D) Effect of bicarbonate treatment on survival of female SCID mice bearing MDA-MB-231 breast tumors. Mice were started on drinking water (ad libitum) supplemented with 200 mM NaHCO3 at day 6 postinjection of MDA-MB-231 cells. Data are plotted as a Kaplan–Meier survival curve, the difference in the survival curve for the bicarbonate versus control animals was tested using the log-rank test (p=0.027). Adapted from Robey et al. (60) with permission. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Abbreviations Used
- GGT
γ-glutamyltransferase
- GlyCysSH
cysteinyl-glycine dipeptide
- GPx1
glutathione peroxidase 1
- GPx4
glutathione peroxidase 4
- GSH
glutathione
- GSH-Tr
glutathione transferase
- GSSG
glutathione disulfide
- GSSG-Rx
glutathione reductase
- NOX
NADPH-dependent oxidase
- pHe
extracellular pH
- pHi
intracellular pH
- ROS
reactive oxygen species
- TME
tumor microenvironment
Acknowledgments
This work was partly supported by NIH grants EB014542, CA077575-10, and U54 CA 143970-01.
References
- 1.Backos DS, Franklin CC, and Reigan P. The role of glutathione in brain tumor drug resistance. Biochem Pharmacol 83: 1005–1012, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Bauer G. Tumor cell-protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res 32: 2599–2624, 2012 [PubMed] [Google Scholar]
- 3.Bindra RS. and Glazer PM. Genetic instability and the tumor microenvironment: towards the concept of microenvironment-induced mutagenesis. Mutat Res 569: 75–85, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bobko AA, Eubank TD, Voorhees JL, Efimova OV, Kirilyuk IA, Petryakov S, Trofimiov DG, Marsh CB, Zweier JL, Grigor'ev IA, Samouilov A, and Khramtsov VV. In vivo monitoring of pH, redox status, and glutathione using L-band EPR for assessment of therapeutic effectiveness in solid tumors. Magn Reson Med 67: 1827–1836, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahimi-Horn MC, Chiche J, and Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol 19: 223–229, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bralet J, Schreiber L, and Bouvier C. Effect of acidosis and anoxia on iron delocalization from brain homogenates. Biochem Pharmacol 43: 979–983, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Brurberg KG, Graff BA, and Rofstad EK. Temporal heterogeneity in oxygen tension in human melanoma xenografts. Br J Cancer 89: 350–356, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18: 775–794, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Cadenas E. and Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29: 222–230, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Chaiswing L. and Oberley TD. Extracellular/microenvironmental redox state. Antioxid Redox Signal 13: 449–465, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Chen Z, Hu Y, and Huang P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by beta-phenethyl isothiocyanate: mechanisms for anti-leukemia activity. Antioxid Redox Signal 15: 2911–2921, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti A, Franzini M, Paolicchi A, and Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res 30: 1169–1181, 2010 [PubMed] [Google Scholar]
- 13.Dautry-Varsat A, Ciechanover A, and Lodish HF. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A 80: 2258–2262, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bittencourt Junior PI, Curi R, and Williams JF. Glutathione metabolism and glutathione S-conjugate export ATPase (MRP1/GS-X pump) activity in cancer. I. Differential expression in human cancer cell lines. Biochem Mol Biol Int 45: 1227–1241, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Dhimitruka I, Bobko AA, Eubank TD, Komarov DA, and Khramtsov VV. Phosphonated trityl probe for concurrent in vivo tissue oxygen and pH monitoring using EPR-based techniques. JACS 135: 5904–5910, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.di Ilio C, Sacchetta P, del Boccio G, la Rovere G, and Federici G. Glutathione peroxidase, glutathione S-transferase and glutathione reductase activities in normal and neoplastic human breast tissue. Cancer Lett 29: 37–42, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Dominici S, Paolicchi A, Corti A, Maellaro E, and Pompella A. Prooxidant reactions promoted by soluble and cell-bound gamma-glutamyltransferase activity. Methods Enzymol 401: 484–501, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Epe B. Role of endogenous oxidative DNA damage in carcinogenesis: what can we learn from repair-deficient mice? Biol Chem 383: 467–475, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Erejuwa OO, Sulaiman SA, and Ab Wahab MS. Evidence in support of potential applications of lipid peroxidation products in cancer treatment. Oxid Med Cell Longev 2013: 931251, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrela JM, Ortega A, and Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci 43: 143–181, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Franzini M, Corti A, Lorenzini E, Paolicchi A, Pompella A, De Cesare M, Perego P, Gatti L, Leone R, Apostoli P, and Zunino F. Modulation of cell growth and cisplatin sensitivity by membrane gamma-glutamyltransferase in melanoma cells. Eur J Cancer 42: 2623–2630, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Gee HE, Ivan C, Calin GA, and Ivan M. HypoxamiRs and cancer: from biology to targeted therapy. Antioxid Redox Signal 2013[Epub ahead of print]; DOI: 10.1089/ars.2013.5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillies RJ. and Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J Bioenerg Biomembr 39: 251–257, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Gillies RJ. and Gatenby RA. Hypoxia and adaptive landscapes in the evolution of carcinogenesis. Cancer Metastasis Rev 26: 311–317, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Gillies RJ, Verduzco D, and Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer 12: 487–493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grill HP, Zweier JL, Kuppusamy P, Weisfeldt ML, and Flaherty JT. Direct measurement of myocardial free radical generation in an in vivo model: effects of postischemic reperfusion and treatment with human recombinant superoxide dismutase. J Am Coll Cardiol 20: 1604–1611, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Grimm C, Hofstetter G, Aust S, Mutz-Dehbalaie I, Bruch M, Heinze G, Rahhal-Schupp J, Reinthaller A, Concin N, and Polterauer S. Association of gamma-glutamyltransferase with severity of disease at diagnosis and prognosis of ovarian cancer. Br J Cancer 109: 610–614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall AG. Review: The role of glutathione in the regulation of apoptosis. Eur J Clin Invest 29: 238–245, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Hanigan MH. Expression of gamma-glutamyl transpeptidase provides tumor cells with a selective growth advantage at physiologic concentrations of cyst (e)ine. Carcinogenesis 16: 181–185, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Hanigan MH, Gallagher BC, Townsend DM, and Gabarra V. Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis 20: 553–559, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassan W, Ibrahim M, Nogueira CW, Ahmed M, and Rocha JB. Effects of acidosis and Fe (II) on lipid peroxidation in phospholipid extract: comparative effect of diphenyl diselenide and ebselen. Environ Toxicol Pharmacol 28: 152–154, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Haulica A. and Ababei L. Comparative study of glycolytic activity in the erythrocytes of animals with chronic experimental hypoxia and with tumours. Neoplasma 21: 29–35, 1974 [PubMed] [Google Scholar]
- 33.Huang Z, Komninou D, Kleinman W, Pinto JT, Gilhooly EM, Calcagnotto A, and Richie JP, Jr., Enhanced levels of glutathione and protein glutathiolation in rat tongue epithelium during 4-NQO-induced carcinogenesis. Int J Cancer 120: 1396–1401, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Hutter DE, Till BG, and Greene JJ. Redox state changes in density-dependent regulation of proliferation. Exp Cell Res 232: 435–438, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim-Hashim A, Cornnell HH, Abrahams D, Lloyd M, Bui M, Gillies RJ, and Gatenby RA. Systemic buffers inhibit carcinogenesis in TRAMP mice. J Urol 188: 624–631, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim Hashim A, Cornnell HH, Coelho Ribeiro Mde L, Abrahams D, Cunningham J, Lloyd M, Martinez GV, Gatenby RA, and Gillies RJ. Reduction of metastasis using a non-volatile buffer. Clin Exp Metastasis 28: 841–849, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikebuchi Y, Masumoto N, Tasaka K, Koike K, Kasahara K, Miyake A, and Tanizawa O. Superoxide anion increases intracellular pH, intracellular free calcium, and arachidonate release in human amnion cells. J Biol Chem 266: 13233–13237, 1991 [PubMed] [Google Scholar]
- 38.Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci 100: 1382–1388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang YJ. and Enger MD. Glutathione content and growth in A549 human lung carcinoma cells. Exp Cell Res 187: 177–179, 1990 [DOI] [PubMed] [Google Scholar]
- 40.Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat Res 202: 343–361, 1988 [DOI] [PubMed] [Google Scholar]
- 41.Komarov DA, Dhimitruka I, Kirilyuk IA, Trofimiov DG, Grigor'ev IA, Zweier JL, and Khramtsov VV. Electron paramagnetic resonance monitoring of ischemia-induced myocardial oxygen depletion and acidosis in isolated rat hearts using soluble paramagnetic probes. Magn Reson Med 68: 649–655, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, and Mitchell JB. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res 62: 307–312, 2002 [PubMed] [Google Scholar]
- 43.Lauschke H, Tolba R, Burger B, Minor T, and Hirner A. Lipid peroxidation as additional marker in patients with colorectal cancer. Results of a preliminary study. Eur Surg Res 34: 346–350, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Lee SY, Kang TH, Knoff J, Huang Z, Soong RS, Alvarez RD, Hung CF, and Wu TC. Intratumoral injection of therapeutic HPV vaccinia vaccine following cisplatin enhances HPV-specific antitumor effects. Cancer Immunol Immunother 62: 1175–1185, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leier I, Jedlitschky G, Buchholz U, Center M, Cole SP, Deeley RG, and Keppler D. ATP-dependent glutathione disulphide transport mediated by the MRP gene-encoded conjugate export pump. Biochem J 314 (Pt 2): 433–437, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liou GY. and Storz P. Reactive oxygen species in cancer. Free Radic Res 44: 479–496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lipscomb DC, Gorman LG, Traystman RJ, and Hurn PD. Low molecular weight iron in cerebral ischemic acidosis in vivo. Stroke 29: 487–492; discussion 493, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Lora-Michiels M, Yu D, Sanders L, Poulson JM, Azuma C, Case B, Vujaskovic Z, Thrall DE, Charles HC, and Dewhirst MW. Extracellular pH and P-31 magnetic resonance spectroscopic variables are related to outcome in canine soft tissue sarcomas treated with thermoradiotherapy. Clin Cancer Res 12: 5733–5740, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Luanpitpong S, Talbott SJ, Rojanasakul Y, Nimmannit U, Pongrakhananon V, Wang L, and Chanvorachote P. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J Biol Chem 285: 38832–38840, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Knudsen ES, Sotgia F, and Lisanti MP. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 9: 3256–3276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto K, Hyodo F, Matsumoto A, Koretsky AP, Sowers AL, Mitchell JB, and Krishna MC. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res 12: 2455–2462, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Meijer TW, Kaanders JH, Span PN, and Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res 18: 5585–5594, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Mitchell JB, Biaglow JE, and Russo A. Role of glutathione and other endogenous thiols in radiation protection. Pharmacol Ther 39: 269–274, 1988 [DOI] [PubMed] [Google Scholar]
- 54.Moyer MW. Targeting hypoxia brings breath of fresh air to cancer therapy. Nat Med 18: 636–637, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Oberley LW, Oberley TD, and Buettner GR. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Med Hypotheses 7: 21–42, 1981 [DOI] [PubMed] [Google Scholar]
- 56.Ortega A, Mena S, and Estrela JM. Glutathione in cancer cell death. Cancers 3: 1285–1310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Policastro LL, Ibanez IL, Notcovich C, Duran HA, and Podhajcer OL. The tumor microenvironment: characterization, redox considerations, and novel approaches for reactive oxygen species-targeted gene therapy. Antioxid Redox Signal 19: 854–895, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Princiotto JV. and Zapolski EJ. Difference between the two iron-binding sites of transferrin. Nature 255: 87–88, 1975 [DOI] [PubMed] [Google Scholar]
- 59.Reynolds TY, Rockwell S, and Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res 56: 5754–5757, 1996 [PubMed] [Google Scholar]
- 60.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, and Gillies RJ. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 69: 2260–2268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robey IF. and Martin NK. Bicarbonate and dichloroacetate: evaluating pH altering therapies in a mouse model for metastatic breast cancer. BMC Cancer 11: 235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roshchupkina GI, Bobko AA, Bratasz A, Reznikov VA, Kuppusamy P, and Khramtsov VV. In vivo EPR measurement of glutathione in tumor-bearing mice using improved disulfide biradical probe. Free Radic Biol Med 45: 312–320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schafer FQ. and Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Siemann DW. (Ed). Tumor Microenvironment Chichester, UK; Hoboken, NJ: John Wiley & Sons, Ltd., 2011, p. 436 [Google Scholar]
- 65.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 82: 291–295, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padhani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D, Croft B, Hoffman J, Liu G, Stone H, and Sullivan D. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 82: 699–757, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, and Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013: 972913, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vishvakarma NK. and Singh SM. Mechanisms of tumor growth retardation by modulation of pH regulation in the tumor-microenvironment of a murine T cell lymphoma. Biomed Pharmacother 65: 27–39, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Voegtlin C. and Thompson JW. Glutathione content of tumor animals. J Biol Chem 70: 801–806, 1926 [Google Scholar]
- 70.Warburg O. On the origin of cancer cells. Science 123: 309–314, 1956 [DOI] [PubMed] [Google Scholar]
- 71.Wilson WR. and Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 11: 393–410, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Wojtkowiak JW, Rothberg JM, Kumar V, Schramm KJ, Haller E, Proemsey JB, Lloyd MC, Sloane BF, and Gillies RJ. Chronic autophagy is a cellular adaptation to tumor acidic pH microenvironments. Cancer Res 72: 3938–3947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang HY, Hormi-Carver K, Zhang X, Spechler SJ, and Souza RF. In benign Barrett's epithelial cells, acid exposure generates reactive oxygen species that cause DNA double-strand breaks. Cancer Res 69: 9083–9089, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu X, Zuo L, Cardounel AJ, Zweier JL, and He G. Characterization of in vivo tissue redox status, oxygenation, and formation of reactive oxygen species in postischemic myocardium. Antioxid Redox Signal 9: 447–455, 2007 [DOI] [PubMed] [Google Scholar]