Abstract
CD34+ cells are nonpermissive to infection by HIV strains X4 and R5, despite the fact that many CD34+ cells express high levels of the viral receptor protein CD4 and the coreceptor CXCR4 on their surface. In these cells, the co-receptor CCR5 protein, which, like CXCR4, is a chemokine receptor, is detected mainly intracellularly. We hypothesized that CD34+ cells secrete CCR5-binding chemokines and that these factors interfere with HIV R5 interactions with these cells, possibly by binding CCR5 or by inducing its internalization. We found that human CD34+ cells and CD34+KIT+ cells, which are enriched in myeloid progenitor cells, expressed and secreted the CCR5 ligands RANTES, MIP-1α, and MIP-1β and that IFN-γ stimulated expression of these chemokines. In contrast, SDF-1, a CXCR4 ligand, was not detectable in the CD34+KIT+ cells, even by RT-PCR. Conditioned media from CD34+ cell culture significantly protected the T lymphocyte cell line PB-1 from infection by R5 but not X4 strains of HIV. Interestingly, the secretion of endogenous chemokines decreased with the maturation of CD34+ cells, although ex vivo, expanded megakaryoblasts still secreted a significant amount of RANTES. Synthesis of CCR5-binding chemokines by human CD34+ cells and megakaryoblasts therefore largely determines the susceptibility of these cells to infection by R5 HIV strains. We postulate that therapeutic agents that induce the endogenous synthesis of chemokines in human hematopoietic cells may protect these cells from HIV infection.
J. Clin. Invest. 104:1739–1749 (1999).
Introduction
HIV-infected subjects frequently exhibit a variety of hematologic abnormalities, including anemia, neutropenia, and thrombocytopenia, in addition to the invariable loss of CD4+ lymphocytes (1, 2). The pathogenesis of bone marrow dysfunction is probably multifactoral in origin (3, 4). Hematopoietic cells may be damaged directly by HIV in addition to being inhibited by HIV-related proteins, proinflammatory cytokines, and chemokines, which are upregulated in response to infection (1, 2).
HIV entry is dependent on binding to both the CD4 antigen and to chemokine receptors present on the cell surface (1, 4, 5). The macrophagotropic R5 HIV strains use CCR5 as a coreceptor, whereas lymphotropic X4 HIV strains use CXCR4. The natural ligands for CCR5 (MIP-1α, MIP-1β, and RANTES) and CXCR4 (SDF-1) may interfere with R5 and X4 HIV entry into the cells (5). In support of this theory, natural killer (NK) cells, which secrete several β-chemokines, have been reported to be highly resistant to HIV infection (6–8).
Most researchers agree that human CD34+ hematopoietic progenitor cells are highly resistant to HIV infection (3, 9, 10). We recently confirmed this observation in a set of experiments in which human CD34+ cells were exposed to R5 and X4 HIV envelope-pseudotyped viruses that contained a sensitive luciferase reporter gene (9). Because CD34+ cells express CD4 (2, 3, 11) in addition to CXCR4 and CCR5 (10), the explanation of this phenomenon is puzzling. We hypothesized that CD34+ cells may produce HIV-related chemokines and thus remain resistant to HIV infection in a manner analogous to that of NK cells (6, 7). CD34+ cells have previously been shown to produce a variety of cytokines including growth factors (IL-1β, IL-8, TGF-β1, KL, and STK-1L) (12–15), and it may be that these cells also secrete important protective chemokines. We also looked for the expression and production of chemokines in megakaryocytic, myelocytic, and erythroid precursors derived from CD34+ progenitor cells ex vivo.
We report that R5 HIV–binding chemokines (MIP-1α, MIP-1β, and RANTES) are secreted by normal human CD34+ cells. The secretion of endogenous chemokines decreased with the maturation of CD34+ cells, with the exception of ex vivo expanded cultures of megakaryoblasts, which still secreted a significant amount of RANTES. The endogenous secretion of CCR5-binding chemokines by various subsets of normal human hematopoietic cells may influence their infectability with R5 HIV strains.
Methods
Normal human hematopoietic cells.
Light-density marrow mononuclear cells (MNC) were obtained from 10 consenting, healthy donors, and were depleted of adherent cells and T lymphocytes to obtain A–T– MNC as described (9). Cord blood was isolated from 5 term deliveries after obtaining consent from patients; blood was depleted of erythroblasts.
Selection of CD34+ cells.
A–T– MNC were enriched for CD34+ cells by immunoaffinity selection with MiniMACS paramagnetic beads (Miltenyi Biotec, Auburn, California, USA) according to the manufacturer’s protocol. The purity of isolated CD34+ cells was greater than 95% by FACS® analysis.
Selection of CD34+KIT+ A–T– MNC by FACS®.
The KIT receptor–positive subset of CD34+ cells was isolated by FACS® as described (13, 16). Briefly, A–T– MNC were enriched for CD34+ cells by immunoaffinity selection with MiniMACS paramagnetic beads, and subsequently labeled for 30 minutes at 4°C with anti–KIT receptor mAb SR-1 directly conjugated with Cy5 (20 μL/106 cells; Becton Dickinson Immunocytometry Systems, San Jose, California, USA). After the final incubation, cells were washed 3 times in ice-cold PBS supplemented with 5% bovine calf serum (BCS) and then sorted with a FACStar Plus II cell sorter (Becton Dickinson Immunocytometry Systems). The purity of isolated CD34+KIT+ cells was greater than 98%.
Ex vivo expansion of human erythroid, megakaryocytic, and myeloid cells.
CD34+ cells were expanded in a serum-free liquid system as reported previously (17–19). Briefly, CD34+ A–T– MNC (104 cells/mL) were resuspended in Iscove’s DMEM (GIBCO BRL, Grand Island, New York, USA) supplemented with 25% artificial serum containing 1% delipidated, deionized, and charcoal-treated BSA; 270 μg/mL iron-saturated transferrin; 20 μg/mL insulin; and 2 mmol/L L-glutamine (all from Sigma Chemical Co., St. Louis, Missouri, USA). Erythrocyte burst-forming unit (BFU-E) growth was stimulated with recombinant human (rH) erythropoietin (2 U/mL) and rH KIT ligand (10 ng/mL). Megakaryocyte colony-forming unit (CFU-Meg) growth was stimulated with rH thrombopoietin (50 ng/mL) and rH IL-3 (10 ng/mL). Granulocyte–macrophage colony-forming unit (CFU-GM) growth was stimulated with rH IL-3 (10 ng/mL) and rH KIT ligand (10 ng/mL). Cytokines were obtained from R&D Systems Inc. (Minneapolis, Minnesota, USA). Cultures were incubated at 37°C in a fully humidified atmosphere supplemented with 5% CO2. Under these conditions, approximately 100% of BFU-E–derived cells were positive for glycophorin A, 100% of CFU-GM–derived cells were negative for glycophorin A and αIIb/β3 and expressed CD33, and 85% of CFU-Meg–derived cells were positive for αIIb/β3. In some experiments, CFU-Meg–derived cells were further enriched for αIIb/β3+ cells (to > 97% of total cells) by additional selection with immunomagnetic beads (Miltenyi Biotec).
Isolation of human blood platelets and activation of platelets by thrombin.
Platelets were isolated by differential centrifugation of whole blood. Briefly, blood obtained by venipuncture from normal volunteers for studies (approved by the University of Pennsylvania Institutional Human Subjects Review Board) was anticoagulated with 3.8% sodium citrate. Platelets were isolated by centrifugation of the blood for 20 minutes at 150 g at room temperature, and were used for further studies requiring platelet-rich plasma. Subsequently, platelets were gel-filtered in calcium-free Tyrode’s buffer (5 mM HEPES at pH 6.5, 4 mM NaH2PO4, 137 mM NaCl, 2.6 mM KCl, 1 mM MgCl2, and 5 mM glucose) with a pH of 7.4, containing 1 mg/mL albumin. To obtain washed platelets, prostaglandin E1 was added to platelet-rich plasma to a final concentration of 1 μM. Platelets were then pelleted by centrifugation at room temperature for 20 minutes at 800 g. The pellet was resuspended and washed twice in a Tyrode’s buffer supplemented with 1 mg/mL albumin, 5 U/mL apyrase, and 1 mM EGTA (all from Sigma Chemical Co.). After each wash, platelets were centrifuged at room temperature for 15 minutes at 900 g. The final pellet was resuspended in HEPES buffer (pH 7.5) to a concentration of 3 × 108 platelets/mL. After each preparation, platelets were tested for their ability to aggregate with ADP in the absence or presence of 200 μg/mL fibrinogen and 1 mM CaCl2; platelets were used within 5 hours. For a release study, aliquots of washed platelets were stimulated for 5 minutes in an aggregometer cuvette with agonist (10 μM ADP or 2 nM thrombin), and then centrifuged for 3 minutes at 1,200 g. The supernatant was used for detecting the presence of RANTES by ELISA.
FACS® analysis of chemokine receptor expression.
Bone marrow MNC (BMMNC) were double stained with chemokine receptor–specific mAbs to CCR5 and CXCR4; primary mAbs were then detected with phycoerythrin (PE) conjugated sheep anti-mouse mAbs (Sigma Chemical Co., St. Louis, Missouri, USA). Subsequently, cells were stained with an anti-CD34 mAb directly conjugated with FITC anti–HPCA-1 (20 μL/106 cells; Becton Dickinson Immunocytometry Systems) as described (9). CD4 was detected as described previously (9). The mAbs used for chemokine receptor expression were purchased from R&D Systems Inc. (clone 12G5 for CXCR4 and clones 531 and 549 for CCR5). CCR5 was also detected with clone mAbs 2D7 (PharMingen, San Diego, California, USA) and CTC5 (a kind gift from Protein Design Laboratories Inc., Fremont, California, USA). Data analysis was performed using CellQuest software from Becton Dickinson Immunocytometry Systems.
Detection of intracellular chemokines by FACS®.
Cells were isolated using MiniMACS magnetic beads. Purity of the isolated cells was greater than 95% according to FACS® staining. After isolation, cells were resuspended in 1 mL of Iscove’s DMEM supplemented with 25% serum-free medium and cultured in a 24-well plate for 24 hours with or without IFN-γ (1,000 U/mL). Four to six hours before the staining procedure, GolgiStop (PharMingen) was added to cell cultures. Cells were stained with Cytofix/Cytoperm (PharMingen) according to the manufacturer’s protocol. Briefly, cells were washed twice with a staining buffer and then resuspended in 250 μL of Cytofix/Cytoperm and incubated on ice for 20 minutes. The cells were then washed twice with Perm/Wash solution (PharMingen) and stained with labeled anti-chemokine mAbs (anti-RANTES or anti–MIP-1α; PharMingen). In some cases the reaction was blocked with unlabeled Abs before staining. After staining, cells were washed twice with Perm/Wash and resuspended in a staining buffer. Cells were analyzed using a FACSCalibur flow cytometer.
Detection of RANTES in CD34+KIT+ BMMNC cells.
GolgiStop was added to the cell cultures 4–6 hours before the staining procedure. Subsequently, cells were washed twice and resuspended in 100 μL of staining buffer. Antibodies against CD34 and KIT were added, and cells were incubated for 30 minutes on ice. Cells were then washed and resuspended in 250 μL of Cytofix/Cytoperm (PharMingen) and incubated on ice for 20 minutes. Cells were washed twice with Perm/Wash solution and stained with labeled anti-chemokine Abs (anti-RANTES or anti–MIP-1α; PharMingen). In some cases, cells were blocked with unlabeled Abs before staining. Finally, the cells were washed twice with Perm/Wash, resuspended in staining buffer, and analyzed using FACStar.
ELISA assay for detection of chemokines.
Secretion of MIP-1α, MIP-1β, and RANTES by hematopoietic cells was detected by Quantikine human MIP-1α, MIP-1β, and RANTES immunoassay (all from R&D Systems Inc.) according to the manufacturer’s protocol. Equal numbers of CD34+ cells, CD34+KIT+ cells, and day 6 and day 11 normal human CFU-GM–, BFU-E–, and CFU-Meg–derived cells were cultured for 24 hours in serum-free media before collecting cell-conditioned media for ELISA. Media from each cell culture was analyzed by a quantitative sandwich enzyme immunoassay technique. Sensitivity of the ELISA assays for MIP-1α, MIP-1β, and RANTES was greater than 31 pg/mL, greater than 31 pg/mL, and greater than 15 pg/mL, respectively.
Isolation of mRNA from CD34+ cells.
CD34+ cells were analyzed for chemokine mRNA expression (MIP-1α, MIP-1β, RANTES, and SDF-1) by RT-PCR. Briefly, cells were lysed in 200 μL of RNAzol (Biotecx Laboratories Inc., Houston, Texas, USA) with 22 μL of chloroform as described (13, 19). The aqueous phase was collected and mixed with 1 volume of isopropanol (Sigma Chemical Co.). RNA was precipitated overnight at –20°C. The RNA pellet was washed in 75% ethanol and resuspended in water that had been autoclaved 3 times.
RT-PCR.
Briefly, mRNA (0.5 μg) was reverse transcribed with 500 U of Moloney murine leukemia virus reverse transcriptase and 50 pmol of an oligodeoxynucleotide primer complementary to the 3′ end of the reported chemokine sequences of MIP-1α (5′-CAC TCA GCT CCA GGT CGC TGA-3′), MIP-1β (5′-GTA CTC CTG GCC CAG GAT TC-3′), RANTES (5′-CTC ATC TCC AAA GAG TTG ATG-3′), and SDF-1 (5′-CAC ATG TTG AAC CTC TTG TTT AAA AGC-3′). The resulting cDNA fragments were amplified using 5 U of Taq polymerase and primers specific for the 5′ end of chemokine sequences of MIP-1α (5′-CCT TGC TGT CCT CCT CTG CAC-3′), MIP-1β (5′-TGT CCT CCT CAT GCT AGT AG-3′), RANTES (5′-TCA TTG CTA CTG CCC TCT GCG-3′), and SDF-1 (5′-AAC GCC AAG GTC GTG GTC GTG CTG-3′). Amplification of β-actin mRNA was performed simultaneously using specific primers as reported previously (9, 13, 19). Amplified products (10 μL) were electrophoresed on a 2% agarose gel and transferred to a nylon filter before photographing. Specificity of the amplified products was further confirmed by Southern blotting (data not shown).
Detection of chemokines in CD34+ cells, CFU-Meg–derived cells, and platelets by Western blot.
Purified CD34 cells (5 × 106), purified day 14 CFU-Meg–derived cells (5 × 106), gel-filtered platelets (108), or bone marrow stromal cells (5 × 106) were lysed in 0.5 mL of Mammalian Protein Extraction Reagent (M-PER) buffer (Pierce Chemical Co., Rockford, Illinois, USA) supplemented with protease and phosphatase inhibitors, for 15 minutes on ice. Cell lysates were precleared by centrifugation at 15,000 g for 15 minutes at 4°C. Protein concentration in supernatants was measured by Bradford assay using a kit from Bio-Rad Laboratories Inc. (Hercules, California, USA). Supernatants were mixed with tricine loading buffer and boiled for 5 minutes. Equal amounts of protein were resolved on 16.5% Tris-Tricine Ready Gels (Bio-Rad Laboratories Inc.) and transferred to 0.05-μm nitrocellulose membranes (Schleicher & Schuell Inc., Keene, New Hampshire, USA). Membranes were subsequently blocked in 5% BSA for 60 minutes at room temperature. The goat polyclonal primary Abs (α-RANTES, α−MIP-1α, and α−MIP-1β, all from R&D Systems Inc.) were added at a concentration of 1.5 μL/mL and membranes were incubated overnight at room temperature. Membranes were then washed and incubated with sheep anti-goat horseradish peroxidase–conjugated secondary Ab (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) for 1 hour. Finally, membranes were developed with ECL reagent from Amersham Pharmacia Biotech (Piscataway, New Jersey, USA) before being dried and exposed to film.
Infection of PB-1 cells with HIV.
The T lymphocyte cell line PB-1 (a kind gift from M. Wasik, Department of Pathology & Laboratory Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA) was maintained in RPMI (GIBCO BRL) supplemented with 10% BCS (HyClone Laboratories, Logan, Utah, USA). Cells were infected on day 0 with Ba-L (R5 HIV) or HXB.2 (X4 HIV). All of these HIV strains (kind gifts of F. Gonzalez-Scarano, Department of Neurology, University of Pennsylvania, Philadelphia, Pennsylvania, USA) are propagated in a P3 laboratory at the University of Pennsylvania. Initially, 105 PB-1 cells (20) were resuspended in 200 μL of medium and exposed to 2 ng of HIV protein. The multiplicity of infection for both viruses was 0.002–0.02, having been carefully established using U373-MAGI-CCR5 and U373-MAGI-CXCR4 tests as described (21). Subsequently, 300 μL of conditioned medium collected from serum-free cultures of CD34+ cells, megakaryoblasts, erythroblasts, or myeloblasts was added to the PB-1 cells. As negative and positive controls we used medium alone, conditioned medium containing human CD34+ cells that were heat inactivated before addition to the cultures, and conditioned medium from human CD34+ cell cultures, respectively. In our further infection studies, we used both viruses at a multiplicity of infection of 0.02, and used conditioned medium collected from CD34+ cell cultures, conditioned medium collected from human CD34+ cell cultures to which were added the neutralizing goat polyclonal Abs α-RANTES (10 μg/mL), α–MIP-1α (50 μg/mL), and α–MIP-1β (50 μg/mL) (R&D Systems Inc.), and medium alone that was mock cleared with an equal amount (110 μg/mL) of the nonreactive goat Ig (Sigma Chemical Co.). The PB-1 cells were subsequently incubated for 24 hours in 5% CO2 at 37°C. After incubation, PB-1 cells were washed and resuspended in DMEM with 10% BCS and placed into the tissue culture incubator. Aliquots of medium were aspirated on days 1, 5, and 10 of culture for p24 ELISA assays. Infection of the PB-1 cells was measured by using p24 ELISA (NEN Life Science Products Inc., Boston, Massachusetts, USA).
Statistical analysis.
Mean and SD were calculated on a MacIntosh computer using Instat 1.14 software (GraphPad Software for Science Inc., San Diego, California, USA). Data were analyzed using the Student’s t test for unpaired samples. Statistical significance was defined as P < 0.05.
Results
Expression of HIV-related chemokine receptors and CD4 antigen by human CD34+ cells.
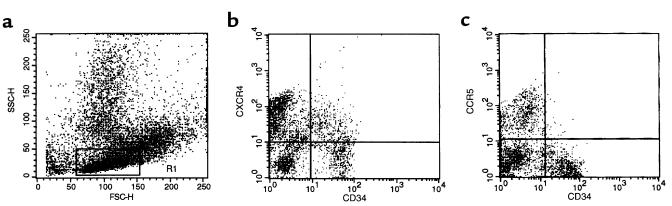
We studied the expression of CXCR4, CCR5, and CD4 on human bone marrow CD34+ cells by FACS® analysis. As reported by others (3, 22), we confirmed that 15–50% of CD34+ cells expressed CD4 (not shown). As shown in Figure 1, 50–75% of human CD34+ cells express CXCR4, but less than 5% of CD34+ cells were CCR5+. However, by mRNA analysis, we found high levels of both CXCR4 and CCR5 mRNA in CD34+ cells (9).
Figure 1.
Expression of chemokine receptors on bone marrow CD34+ BMMNC. BMMNC were isolated from bone marrow aspirates of healthy donors by Ficoll-gradient centrifugation and then stained with mAbs against CD34 antigen (FITC) and chemokine receptors (PE). (a) Forward (FSC) and side scatter (SSC) analysis of BMMNC. Lymphocyte region is defined by R1. (b) Analysis of cells dual-labeled with FITC anti-CD34 mAb and PE anti-CXCR4 mAb. (c) Analysis of cells dual-labeled with FITC anti-CD34 mAb and PE anti-CCR5 mAb. Data from at least 5 different donors were analyzed with similar results. Data from a representative donor is presented.
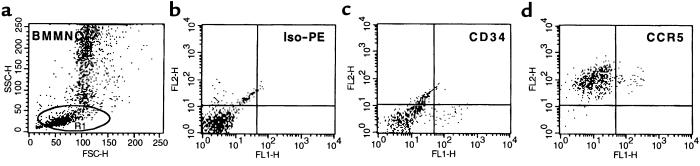
We then asked if this discrepancy between the results of RNA analysis and immunostaining might be explained by the fact that CCR5 protein was expressed intracellularly. To address this issue, we looked for intracytoplasmic expression of this molecule. Accordingly, the BMMNC were permeabilized and then stained for CD34 antigen and CCR5. Figure 2 shows the observed high intracellular expression of the CCR5 protein in all permeabilized CD34+ bone marrow cells.
Figure 2.
Intracellular expression of CCR5 on bone marrow CD34+ BMMNC. BMMNC were isolated from bone marrow aspirates of healthy donors by Ficoll-gradient centrifugation. Cells were permeabilized before staining with mAbs against CD34 antigen (FITC) and CCR5 receptor (PE). (a) Forward (FSC) and side scatter (SSC) analysis of permeabilized BMMNC. Lymphocyte region is defined by R1. (b) Iso-PE staining. (c) FITC anti-CD34. (d) Analysis of cells dual-labeled with FITC anti-CD34 mAb and PE anti-CCR5 mAb. Data from at least 3 different donors were analyzed with similar results. Data from a representative donor is presented. We obtained similar data by using 3 other mAbs (clones 2D7 and 549 from R&D Systems Inc., and CTC5 from Protein Design Laboratories Inc.) for CCR5 detection.
There are 2 possible explanations for the observation that CCR5 is expressed intracellularly but not on the cell surface. CCR5 proteins may not mature beyond the Golgi apparatus in CD34+ cells. Alternatively (or in addition), CCR5 may be internalized rapidly from the cell membrane after binding to specific chemokines (MIP-1α, MIP-1β, or RANTES) that are secreted by CD34+ cells into the culture medium.
CD34+ cells express mRNA for several HIV-related chemokines.
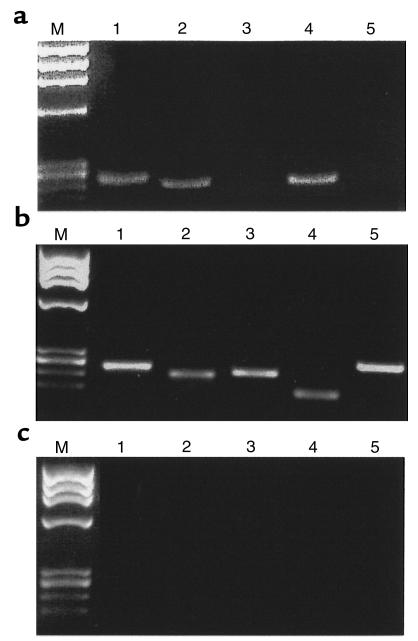
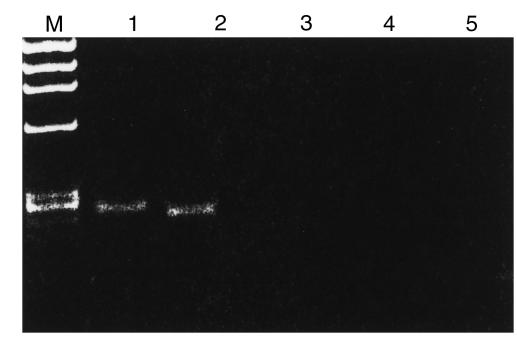
To test the second hypothesis, we performed analyses to discover whether CD34 cells synthesize or secrete MIP-1α, MIP-1β, or RANTES. We isolated mRNA from both total CD34+ cells and CD34+KIT+ BMMNC, and tested for mRNA expression of MIP-1α, MIP-1β, and RANTES. Simultaneously, we evaluated the expression of mRNA for SDF-1. We found that CD34+ cells express mRNA for MIP-1α, MIP-1β, and RANTES (Figure 3a, lanes 1, 4, and 2 respectively), but apparently little SDF-1 (Figure 3, lane 3). A similar pattern of MIP-1α, MIP-1β, and RANTES mRNA expression was observed by RT-PCR in CD34+KIT+ BMMNC (Figure 4, lanes 1 and 2). These cells, however, did not express mRNA for SDF-1 (Figure 4, lane 3).
Figure 3.
RT-PCR analysis of chemokine expression in human CD34+ cells. (a) MIP-1α (lane 1), RANTES (lane 2), SDF-1 (lane 3), MIP-1β (lane 4), and MCP-1 (lane 5). (b) Positive RT-PCR control reactions for MIP-1α (lane 1), MIP-1β (lane 2), RANTES (lane 3), MCP-1 (lane 4), and SDF-1 (lane 5). Expression of MIP-1α, MIP-1β, and RANTES were detected in mRNA isolated from BMMNC, whereas expression of MCP-1 and SDF-1 were detected in mRNA isolated from bone marrow–derived stroma fibroblasts. (c) Negative RT-PCR control reactions (using H2O instead of mRNA) for MIP-1α (lane 1), RANTES (lane 2), SDF-1 (lane 3), MIP-1β (lane 4), and MCP-1 (lane 5). Lane M, molecular weight marker (ΦX174 DNA/HaeIII).
Figure 4.
RT-PCR analysis of chemokine expression in human CD34+KIT+ cells sorted by FACS®. Lanes 1–3 show MIP-1α, RANTES, and SDF-1, respectively. Lanes 4–6 show negative RT-PCR control reactions for MIP-1α, RANTES, and SDF-1, respectively. Data from 3 different donors was analyzed with similar results. Data from a representative donor are presented. Lane M, molecular weight marker (ΦX174 DNA/HaeIII).
Presence of chemokine proteins in CD34+ cells by intracellular immunostaining and Western blot analysis.
We used both FACS® analysis and Western blot assays to determine whether the mRNAs encoding CCR5-related chemokines were translated in CD34+ cells. Human CD34+ cells were permeabilized and then stained with PE anti–MIP-1α or PE anti-RANTES mAbs. As shown in Figure 5, more than 95% of human CD34+ cells isolated by magnetic beads expressed MIP-1α. We obtained similar data when we analyzed CD34+ cells for intracellular expression of RANTES (not shown). We next looked for intracellular chemokine expression in CD34+KIT+ cells, which are enriched for human myeloid progenitor cells. A representative example of these studies is shown in Figure 6. These results confirmed our RT-PCR data showing that RANTES (Figure 6) and MIP-1α (data not shown) are highly expressed in purified human CD34+KIT+ BMMNC. Of note, we also found mRNA for MIP-1α, MIP-1β, and RANTES in purified (by FACS®) human CD34+CD4+ BMMNC (data not shown). Because all these cells express CCR5 intracellularly, we postulate that β-chemokines are expressed by a population of CD34+CD4+CCR5+ cells, which is a potential target for R5 HIV infection. Similarly, CFU-Meg–derived αIIb/β3+ cells (which highly express RANTES) are in fact CD4+CCR5+ (9), and therefore potential targets for R5 HIV infection.
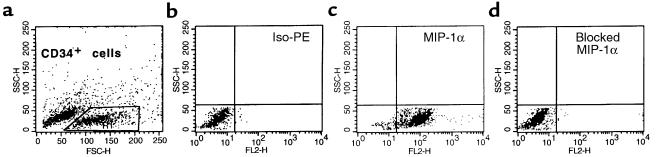
Figure 5.
Intracellular staining for MIP-1α in CD34+ cells isolated using MiniMACS beads and subsequently permeabilized. (a) Forward and side scatter analysis of CD34+ cells isolated by MiniMACS beads. (b) Iso-PE staining. (c) PE anti–MIP-1α mAb. (d) MIP-1α detection has been specifically blocked by addition of MIP-1α. Data from 3 different donors were analyzed with similar results. Data from a representative donor are presented.
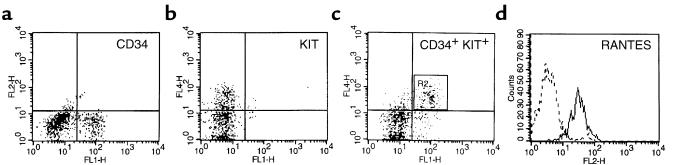
Figure 6.
Intracellular staining for RANTES in CD34+KIT+ MNC. (a) FITC anti-CD34 staining. (b) Cy5 anti-KIT staining. (c) R2 cells dual-labeled with FITC anti-CD34 mAb and Cy5 anti-KIT mAb. (d) Expression of intracellular RANTES in CD34+KIT+ cells from R2. Data from 3 different donors were analyzed with similar results. Data from a representative donor are presented. Cells in area R2 are dual-labeled CD34+KIT+ cells.
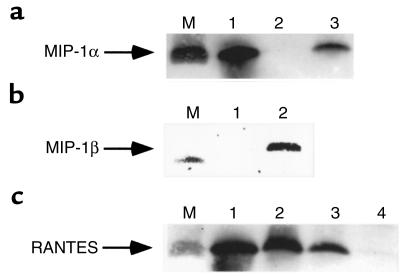
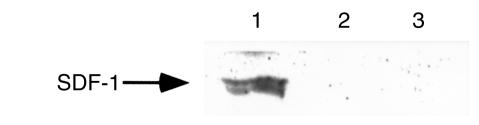
Finally, we looked for expression of CCR5-binding chemokines in lysates of CD34+ cells by Western blot analysis. We detected the presence of MIP-1α, MIP-1β, and RANTES in those cells (Figure 7). We also found the presence of RANTES protein in human CFU-Meg–derived αIIb/β3+ cells and platelets (Figure 7c, lanes 2 and 1, respectively). As expected, SDF-1 protein was not present in lysates from CD34+ cells or CFU-Meg–derived αIIb/β3+ cells (Figure 8, lanes 2 and 3), but it was easily detectable in lysates prepared from human bone marrow stroma fibroblasts (Figure 8, lane 1).
Figure 7.
Western blots showing expression of chemokines in cell lysates from human hematopoietic cells. (a) Expression of MIP-1α in CD34+ cells (lane 3) in CFU-Meg–derived αIIb/β3+ cells (lane 2). Lane 1: recombinant MIP-1α (positive control). Lane M: protein size marker (∼7 kDa). (b) Expression of MIP-1β in CD34+ cells (lane 2) and in CFU-Meg–derived αIIb/β3+ cells (lane 1). Lane M: protein size marker (∼7 kDa). (c) Expression of RANTES in human platelets (lane 1), CFU-Meg–derived cells (lane 2), and CD34+ cells (lane 3). RANTES is not expressed in CFU-GM–derived cells (lane 4). Lane M: protein size marker (∼7 kDa).
Figure 8.
Western blots showing expression of SDF-1 in lysates from human bone marrow stroma fibroblasts (lane 1), human CFU-Meg–derived αIIb/β3+ cells (lane 2), and human CD34+ cells (lane 3).
Secretion of chemokines by human CD34+ cells.
To examine whether CD34+ cells secreted chemokines, purified CD34+ cells were cultured for 24 hours in serum-free medium, and supernatants collected from those cells were evaluated for the presence of chemokine proteins by a very sensitive ELISA assay. We found that CCR5-binding chemokines (MIP-1α, MIP-1β, and RANTES) were secreted by human CD34+ cells (Table 1). The amount secreted by 106 purified CD34+ cells was up to 1–2 ng each of MIP-1α and MIP-1β during 24 hours of ex vivo culture.
Table 1.
Secretion of CCR5 chemokines by normal human hematopoietic cells
Expression of chemokines in differentiating hematopoietic cells.
We next assessed intracellular expression and secretion of chemokines in human hematopoietic cells grown in serum-free liquid cultures of myeloid, megakaryocytic, and erythroid lineages. We previously reported that both CXCR4 and CCR5 receptors are expressed by cells of myeloid and megakaryocytic lineage (9).
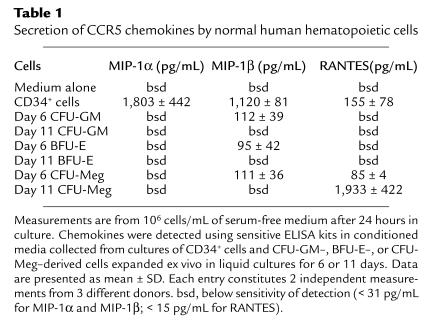
In this study, we found that CCR5-binding chemokines were downregulated in all hematopoietic lineages (erythroid, myeloid, and megakaryocytic) during differentiation (Table 1), except for RANTES, which showed high levels of expression in the megakaryocytic lineage (Table 1 and Figure 9b). Similarly, we detected a high level of RANTES by Western blot (Figure 7c, lane 1) in lysates prepared from peripheral blood platelets; RANTES was also detected by ELISA (data not shown) in the supernatant harvested from human platelets activated by thrombin.
Figure 9.
Intracellular staining for RANTES in megakaryoblasts and myeloblasts that were expanded ex vivo for 11 days under serum-free conditions. (a) Forward and side scatter analysis of day 11 CFU-Meg–derived cells. (b) Expression of RANTES in day 11 megakaryoblasts. (c) Forward and side scatter analysis of day 11 CFU-GM–derived cells. (d) Expression of RANTES in day 11 myeloblasts. Data from 4 different donors were analyzed with similar results. Data from a representative donor are presented.
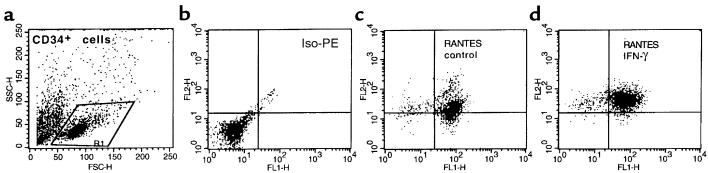
Expression of RANTES, MIP-1α, and MIP-1β in human CD34+ cells is upregulated after stimulation with IFN-γ.
For many cells, chemokine secretion is known to increase after inflammatory stimulation (8). To determine if this was also true for CD34+ cells, we exposed purified CD34+ cells to the inflammatory cytokines IFN-α, IFN-β, IFN-γ, TNF-α, and IL-1α. The intracellular expression of chemokines was then evaluated by FACS®. Of all the cytokines tested, only IFN-γ upregulated expression of CCR5-binding chemokines in CD34+ cells (Table 2 and Figure 10).
Table 2.
IFN-γ increases secretion of MIP-1α, MIP-1β, and RANTES from CD34+ cells
Figure 10.
Intracellular staining for RANTES in CD34+ cells that were isolated using MiniMACS beads and then permeabilized. (a) Forward and side scatter analysis of CD34+ cells isolated using MiniMACS beads. (b) Iso-PE staining. (c) PE anti-RANTES mAb. (d) PE anti-RANTES mAb in CD34+ cells stimulated for 24 hours with IFN-γ (1,000 U/mL). Data from 3 different donors were analyzed with similar results. Data from a representative donor are presented.
Conditioned medium collected from CD34+ cells interfered with infection of PB-1 cells with R5 but not X4 HIV strains.
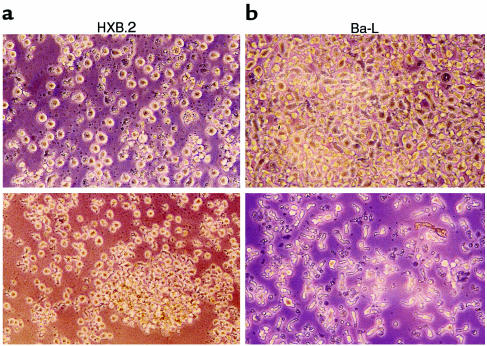
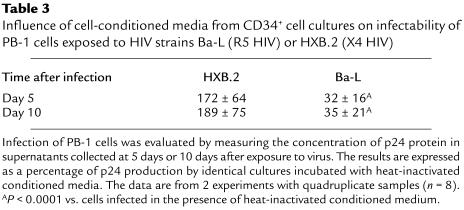
As a final test of our hypothesis, we investigated the question of whether endogenously secreted chemokines might prevent HIV entry into susceptible target cells. To address this issue, we attempted to infect the susceptible PB-1 T lymphocyte cell line (20) with X4 (HXB.2) or R5 (Ba-L) HIV strains in the presence of conditioned medium collected from CD34+ cell cultures. Infection of PB-1 cells was evaluated both morphologically (Figure 11) and by measuring the concentration of HIV-related p24 protein in supernatants collected from infected cells at day 5 or day 10 after exposure to virus (Table 3). We found that conditioned medium from cultures of CD34+ cells — which contain CCR5-binding chemokines — significantly decreased infection of PB-1 cells by R5 HIV (Ba-L) compared with both conditioned medium that was heat inactivated before addition to the cells (Figure 11) and normal medium that did not contain chemokines. The same conditioned medium, however, did not protect PB-1 cells from X4 (HXB.2) HIV infection.
Figure 11.
PB-1 cells infected with different strains of HIV in the presence of conditioned medium collected from CD34+ cell cultures, which was added fresh (top) or heat inactivated before addition (bottom). (a) Cells infected with HXB.2 (X4 HIV). (b) Cells infected with Ba-L (R5 HIV). Pictures were taken 5 days after infection.
Table 3.
Influence of cell-conditioned media from CD34+ cell cultures on infectability of PB-1 cells exposed to HIV strains Ba-L (R5 HIV) or HXB.2 (X4 HIV)
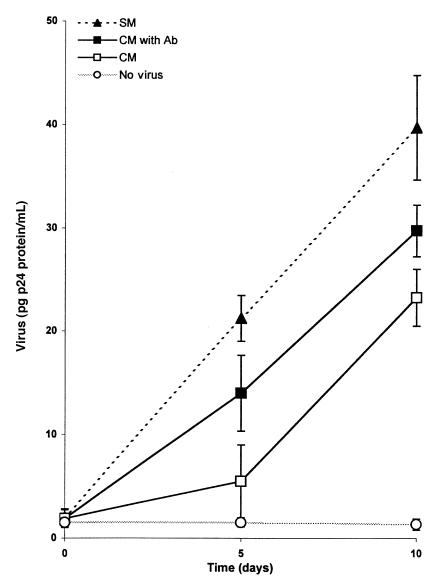
Finally, to assess whether CC chemokines were responsible for the observed suppression of the infectability of PB-1 cells, we used neutralizing mAbs to MIP-1α, MIP-1β, and RANTES to block the influence of those chemokines on R5 HIV infection. Figure 12 shows that the inhibition of p24 production by PB-1 cells infected with R5 HIV (Figure 12) in the presence of supernatants from CD34+ cell cultures could be only partially abrogated by preclearing conditioned medium with neutralizing Abs directed against MIP-1α, MIP-1β, and RANTES. Again, conditioned medium harvested from CD34+ cells did not protect PB-1 cells from infection by X4 HIV (data not shown).
Figure 12.
PB-1 cells infected with R5 HIV (Ba-L) in the presence of conditioned medium (CM) collected from CD34+ cell cultures, conditioned medium that contained a cocktail of neutralizing Abs (50 μg/mL α–MIP-1α, 50 μg/mL α–MIP-1β, and 10 μg/mL α-RANTES) (CM with Ab), and control culture medium (SM) that was mock cleared with an equal amount of nonreactive goat Ig (110 μg/mL). Results are representative of 3 separate experiments and are presented as mean p24 (ng/mL) of triplicate measurements. The multiplicity of infection for both viruses was 0.02. There was a significant decrease in Ba-L p24 production at day 5 and day 10 in the cultures when PB-1 cells were infected in the presence of supernatant from CD34+ cell cultures (P < 0.001). The decrease in Ba-L p24 production was only partially abrogated by preclearing conditioned medium from CD34+ cells with neutralizing mAbs.
Similar results were obtained using conditioned medium collected from megakaryoblast cells expanded in culture ex vivo (data not shown). Conditioned media collected from these cells contained RANTES (Table 1), and inhibited infection of PB-1 cells by R5 HIV. In contrast, conditioned media collected from cultures of myeloid and erythroid cells — which do not secrete CCR5-binding chemokines (Table 1) — generated no protection in our assay (data not shown).
Discussion
The resistance of human CD34+ cells to HIV infection is not completely understood from a molecular point of view (9). A significant population of CD34+ cells expresses the CD4 molecule (10). Although it is known that more than 50% of CD34+ cells express CXCR4 (9, 11, 23), surface expression of CCR5 on CD34+ cells is controversial (4, 9, 22, 24). In this study, we demonstrate the intracellular presence of CCR5, and therefore speculate that the ability to detect surface expression of CCR5 may depend on differences in cell preparation or staining procedures. To explain our observations, we hypothesized that human CD34+ cells might secrete CCR5-binding chemokines that could downregulate CCR5 from the surface and thus interfere with HIV entry into cells.
When we examined human CD34+ cells for expression of HIV-related chemokines, we found that CD34+KIT+ cells highly express mRNAs that encode the CCR5-binding chemokines MIP-1α, MIP-1β, and RANTES, but not the CXCR4-binding chemokine SDF-1. Moreover, these CCR5-binding chemokines were detected by intracellular staining in CD34+KIT+ cells and by Western blot in CD34+ cells. Finally, MIP-1α, MIP-1β, and RANTES were detected by sensitive ELISA assays performed on supernatants collected from purified CD34+ cell cultures.
We propose, therefore, that CD34+ cells are in part protected from R5 HIV infection by the endogenous production of CCR5-binding chemokines. These chemokines may block CCR5 receptors on the cell surface, preventing HIV from using them as a coreceptor for entry into the cells. An additional possibility is that ligand-bound receptors may be rapidly internalized, leaving few surface receptors for HIV entry. It has been reported that endogenously secreted β-chemokines might be complexed with proteoglycans and might compete more effectively than do recombinant chemokines with HIV binding to CCR5 (25). During myeloid differentiation in vitro, the endogenous secretion of chemokines is not detectable after day 6. We hypothesize that surface CCR5 is no longer rapidly internalized or blocked on myeloid cells after day 6, and that these cells become more susceptible to R5 infection (1, 5). In contrast, megakaryocytic cells continue to secrete RANTES and to show little surface CCR5, and are resistant to R5 HIV entry (9). The erythroid lineage is similarly resistant to HIV entry (9). In this case, however, the cells not only stop secreting chemokines, but they also no longer express CXCR4 and CCR5 on the cell surface (26).
In agreement with our hypothesis, we demonstrated that the conditioned media containing CCR5-binding chemokines collected from CD34+ cells and megakaryoblasts inhibited R5 HIV entry into the T lymphocyte cell line PB-1. At the same time, conditioned media collected from cultures of myeloblast and erythroblast cells, which lack any CCR5-binding chemokines, were without effect. This protective effect could be only partially abrogated by preclearing conditioned medium collected from CD34+ cell cultures with neutralizing mAbs against MIP-1α, MIP-1β, and RANTES. This observation supports data that have been published by other investigators (27, 28), who argue that in supernatants collected from some human cell cultures (of NK cells or CD8+ lymphocytes), there are factors that in addition to β-chemokines could modulate infectability of cells by HIV. Therefore, we postulate that human CD34+ cells, similar to NK cells and CD8+ lymphocytes, may potentially secrete other factors that interfere with infectability by HIV.
The proposed protective effect of autosecretion of CCR5-binding chemokines may explain the resistance of CD34+ cells to R5 HIV infection, but a parallel model can not explain the resistance of CD34+KIT+ cells to X4 HIV infection. This situation may be analogous to that observed for human NK cells (6, 7), which secrete a similar panel of CCR5-related chemokines but not SDF-1, and like CD34+ cells remain highly resistant to both R5 HIV and X4 HIV infection (6, 7).
Other mechanisms may also contribute to HIV resistance. The resistance of the target cells may be caused by differences in sulfation of chemokine receptors (29), the presence of high molecular isoform of receptors on the cell surface (30), by cross-desensitization of the chemokine receptors (31, 32), or by a combination of these.
Our studies also demonstrate that IFN-γ is an upregulator of chemokine expression by human hematopoietic cells. Other proinflammatory cytokines tested in this study (IFN-α, IFN-β, TNF-α, TNF-β, and IL-1α) were ineffective in this role. Therefore, inflammatory cytokines that are released during HIV infection, particularly in response to HIV-related infectious complications, may modulate the infectability of hematopoietic cells (1). They can potentially alter the expression of chemokines and chemokine receptors. For example, we previously reported that IFN-γ upregulates expression of CXCR4, but not CCR5, on the surface of human CD34+ cells (9). It has also been reported that IFN-γ may upregulate the expression of CCR5 on human monocytes (33).
The biological role of chemokines endogenously secreted by human CD34+ cells is not clear. Some chemokines, such as MIP-1α, have been reported to inhibit hematopoiesis, particularly when present at very low levels, which prevents their dimerization to inactive molecules (8, 34–36). Therefore, the secretion of MIP-1α by CD34+ cells suggests the presence of an autocrine negative regulatory loop in those cells. A similar negative regulatory loop has been shown in CD34+ cells for human TGF-β1 (12). On the other hand, the fact that human CD34+ cells secrete MIP-1α may explain why, in some experimental systems, the addition of exogenous MIP-1α does not affect cell proliferation (37–40). Whether secreted MIP-1β and RANTES have an autocrine effect is even less clear. The continued expression of RANTES in the megakaryocytic lineage may be linked to storage of RANTES and other chemokines in platelet α-granules (8). Although the late expression of RANTES is probably not important for megakaryopoiesis, it is related to the proinflammatory process (8).
Finally, the secretion of CCR5 chemokines by human CD34+ cells explains, at least in part, why these cells do not show calcium flux, chemotaxis, or phosphorylation of MAPK p42/44 kinases after stimulation with MIP-1α, MIP-1β, or RANTES (26, 41). These cells do not make SDF-1, have detectable CXCR4 on their surface, and respond to SDF-1 exposure with calcium flux, chemotaxis, and phosphorylation of the kinases MAPK p42/44 and Akt (26, 41–43).
We conclude that human CD34+KIT+ cells secrete MIP-1α, MIP-1β, and RANTES, whereas SDF-1 is not expressed in these cells. The secretion of CCR5-binding chemokines decreases with the maturation of CD34+ cells. Megakaryoblasts, however, still secrete a significant amount of RANTES into the culture medium. We propose that the endogenous production of CCR5-binding chemokines by various subsets of human normal hematopoietic cells provides clues to their relative infectability with R5 HIV strains. Hence, CD34+ cells and megakaryoblasts that secrete CCR5-binding chemokines are naturally protected from R5 HIV infection. Finally, an autocrine role for chemokines in regulating the biology of human hematopoietic cells requires further study.
Acknowledgments
This work was supported by National Institutes of Health grants R01 HL-61796-01 and the Leukemia Society of America grant 6497-00 (to M.Z. Ratajczak), and R01 AI-4083 (to G.N. Gaulton). The authors are indebted to M. Poncz for critical comments. The technical support of D.O. Conover is appreciated.
References
- 1.Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 2.Hoxie, J.A. 1995. Hematologic manifestations of AIDS. In Hematology: basic principles and practice. R. Hoffman et al., editors. Churchill Livingstone, New York. 2171–2200.
- 3.Moses A, Nelson J, Bagby GC. The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91:1479–1495. [PubMed] [Google Scholar]
- 4.Lee B, Doranz BJ, Ratajczak MZ, Doms RW. An intricate web: chemokine receptors, HIV-1 and hematopoiesis. Stem Cells. 1998;16:79–88. doi: 10.1002/stem.160079. [DOI] [PubMed] [Google Scholar]
- 5.Cairns JS, D’Souza MP. Chemokines and HIV-1 second receptors: the therapeutic connection. Nat Med. 1998;4:563–568. doi: 10.1038/nm0598-563. [DOI] [PubMed] [Google Scholar]
- 6.Oliva A, et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest. 1998;102:223–231. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehniger TA, et al. Natural killer cells from HIV-1+ patients produce CC-chemokines and inhibit HIV-1 infection. Blood. 1998;92(Suppl. 1):544a. [PubMed] [Google Scholar]
- 8.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 9.Lee B, Ratajczak J, Doms RW, Gewirtz AM, Ratajczak MZ. Coreceptor/chemokine receptor expression on human hematopoietic cells: biological implications for HIV-1 infection. Blood. 1999;93:1145–1156. [PubMed] [Google Scholar]
- 10.Shen H, et al. Intrinsic human immunodeficiency virus type 1 resistance of hematopoietic stem cells despite coreceptor expression. J Virol. 1999;73:728–737. doi: 10.1128/jvi.73.1.728-737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1α and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- 12.Cardoso AA, et al. Release from quiescence of CD34+ CD38– human umbilical cord blood cells reveals their potentiality to engraft adults. Proc Natl Acad Sci USA. 1993;90:8707–8711. doi: 10.1073/pnas.90.18.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratajczak MZ, et al. Expression and physiologic significance of Kit ligand and stem cell tyrosine kinase-1 receptor ligand in normal human CD34+, c-Kit+ marrow cells. Blood. 1995;86:2161–2167. [PubMed] [Google Scholar]
- 14.Watari K, Lansdorp PM, Dragowska W, Mayani H, Schrader JW. Expression of interleukin-1β gene in candidate human hematopoietic stem cells. Blood. 1994;84:36–42. [PubMed] [Google Scholar]
- 15.Behringer D, Kresin V, Henschler R, Mertelsmann R, Lindermann A. Cytokine and chemokine production by CD34+ haematopoietic progenitor cells: detection in single cells. Br J Haematol. 1997;97:9–14. doi: 10.1046/j.1365-2141.1997.d01-2143.x. [DOI] [PubMed] [Google Scholar]
- 16.Ratajczak MZ, et al. CD34+, kit+, rhodamine123low phenotype identifies a marrow cell population highly enriched for human hematopoietic stem cells. Leukemia. 1998;12:942–950. doi: 10.1038/sj.leu.2401027. [DOI] [PubMed] [Google Scholar]
- 17.Ratajczak MZ, Ratajczak J, Machalinski B, Mick R, Gewirtz AM. In vitro and in vivo evidence that ex vivo cytokine priming of donor marrow cells may ameliorate post-transplant thrombocytopenia. Blood. 1998;91:353–359. [PubMed] [Google Scholar]
- 18.Ratajczak J, et al. An improved serum free system for cloning human “pure” erythroid colonies. The role of the different growth factors and cytokines on BFU-E formation by the bone marrow and cord blood CD34+ cells. Folia Histochem Cytobiol. 1998;36:55–60. [PubMed] [Google Scholar]
- 19.Ratajczak J, et al. The role of insulin (INS) and insulin-like growth factor-I (IGF-I) in regulating human erythropoiesis. Studies in vitro under serum-free conditions—comparison to other cytokines and growth factors. Leukemia. 1998;12:371–381. doi: 10.1038/sj.leu.2400927. [DOI] [PubMed] [Google Scholar]
- 20.Majka M, et al. Expression and function of HIV-1 co-receptor on human hematopoietic cell lines. Blood. 1998;92(Suppl. 1):166a. [Google Scholar]
- 21.Vodicka MA, et al. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology. 1997;233:193–198. doi: 10.1006/viro.1997.8606. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, et al. Intrinsic human immunodeficiency virus type 1 resistance of hematopoietic stem cells despite coreceptor expression. J Virol. 1999;73:728–737. doi: 10.1128/jvi.73.1.728-737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowalska MA, et al. Megakaryocyte precursors, megakaryocytes and platelets express the HIV co-receptor CXCR4 on their surface: determination of response to stromal-derived factor-1 by megakaryocytes and platelets. Br J Haematol. 1999;104:220–229. doi: 10.1046/j.1365-2141.1999.01169.x. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz ME, et al. Peripheral blood-derived CD34+ progenitor cells: CXC chemokine receptor 4 and CC chemokine receptor 5 expression and infection by HIV. J Immunol. 1998;161:4169–4176. [PubMed] [Google Scholar]
- 25.Wagner L, et al. β-chemokines are released from HIV-1 specific cytolytic T-cell granules complexed to proteoglycans. Nature. 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 26.Majka M, et al. The stromal derived factor-1 (SDF-1) receptor – CXCR4 is expressed on the human erythroid progenitors and SDF-1 is an important chemotactic factor for early human erythroid cells. Comparison to other chemokine receptor – chemokines axes. Blood. 1999;92(Suppl. 1):366a. [Google Scholar]
- 27.Fehinger TA, et al. Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. J Immunol. 1998;161:6433–6438. [PubMed] [Google Scholar]
- 28.Moriuchi H, Moriuchi M, Combadiere C, Murphy PM, Fauci AS. CD8+ T-cell-derived soluble factor(s), but not β-chemokines RANTES, MIP-1α, and MIP-1β, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farzan M, et al. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 30.Lapham CK, Zaitseva MB, Lee S, Romantseva T, Golding H. Fusion of monocytes and macrophages with HIV-1 correlates with biochemical properties of CXCR4 and CCR5. Nat Med. 1999;5:303–308. doi: 10.1038/6523. [DOI] [PubMed] [Google Scholar]
- 31.Lefkowitz PJ. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 32.Gutkind JS. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 33.Hariharan D, et al. Interferon-γ upregulates CCR5 expression in cord and adult blood mononuclear phagocytes. Blood. 1999;93:1137–1144. [PubMed] [Google Scholar]
- 34.Broxmeyer HE, et al. Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. J Immunol. 1993;150:3448–3458. [PubMed] [Google Scholar]
- 35.Mantel C, Kim YJ, Cooper S, Kwon B, Broxmeyer HE. Polymerization of murine macrophage inflammatory protein 1α inactivates its myelosuppressive effects in vitro: the active form is monomer. Proc Natl Acad Sci USA. 1993;90:2232–2236. doi: 10.1073/pnas.90.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su S, et al. Inhibition of immature erythroid progenitor cell proliferation by macrophage inflammatory protein-1α by interacting mainly with a C-C chemokine receptor, CCR1. Blood. 1997;90:605–611. [PubMed] [Google Scholar]
- 37.Cashman JD, Eaves CJ, Sarris AH, Eaves AC. MCP-1, not MIP-1α, is the endogenous chemokine that cooperates with TGF-β to inhibit the cycling of primitive normal but not leukemic (CML) progenitors in long-term human marrow cultures. Blood. 1998;92:2338–2344. [PubMed] [Google Scholar]
- 38.Cook DN, et al. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 39.Patel VP, et al. Molecular and functional characterization of two novel human C-C chemokines as inhibitors of two distinct classes of myeloid progenitors. J Exp Med. 1997;185:1163–1172. doi: 10.1084/jem.185.7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller JS, McCullar V, Verfaille CM. Ex vivo culture of CD34+/Lin–/DR– cells in stroma-derived soluble factors, interleukin-3, and macrophage inflammatory protein-1α maintains not only myeloid but also lymphoid progenitors in a novel switch culture assay. Blood. 1998;91:4516–4522. [PubMed] [Google Scholar]
- 41.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohle R, et al. The chemokine receptor CXCR4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- 43.Majka, M., Ratajczak, J., Kowalska, M.A., and Ratajczak, M.Z. 2000. Binding of stromal derived factor-1a (SDF-1a) to CXCR4 chemokine receptor in normal human megakaryoblasts but not in platelets induces phosphorylation of mitogen-activated protein kinase p42/44 (MAPK), ELK-1 transcription factor and serine/threonine kinase AKT. Eur. J. Haematol. In press. [DOI] [PubMed]