Abstract
Standard integration-proficient lentiviral vectors (IPLVs) are effective at much lower doses than other vector systems and have shown promise for gene therapy of Parkinson's disease (PD). Their main drawback is the risk of insertional mutagenesis. The novel biosafety-enhanced integration-deficient lentiviral vectors (IDLVs) may offer a significant enhancement in biosafety, but have not been previously tested in a model of a major disease. We have assessed biosafety and transduction efficiency of IDLVs in a rat model of PD, using IPLVs as a reference. Genomic insertion of lentivectors injected into the lesioned striatum was studied by linear amplification-mediated polymerase chain reaction (PCR), followed by deep sequencing and insertion site analysis, demonstrating lack of significant IDLV integration. Reporter gene expression studies showed efficient, long-lived, and transcriptionally targeted expression from IDLVs injected ahead of lesioning in the rat striatum, although at somewhat lower expression levels than from IPLVs. Transgenic human glial cell line-derived neurotrophic factor (hGDNF) expression from IDLVs was used for a long-term investigation of lentivector-mediated, transcriptionally targeted neuroprotection in this PD rat model. Vectors were injected before striatal lesioning, and the results showed improvements in nigral dopaminergic neuron survival and behavioral tests regardless of lentiviral integration proficiency, although they confirmed lower expression levels of hGDNF from IDLVs. These data demonstrate the effectiveness of IDLVs in a model of a major disease and indicate that these vectors could provide long-term PD treatment at low dose, combining efficacy and biosafety for targeted central nervous system applications.
Introduction
Lentiviral vectors and adeno-associated viral vectors (AAVs) have been widely used for preclinical and some clinical gene therapies in the central nervous system (CNS) (Bjorklund et al., 2000; Abordo-Adesida et al., 2005). Retroviral vector integration has mediated some serious adverse events in clinical trials (Hacein-Bey-Abina et al., 2003), and although lentiviral vectors have a safer profile (Biffi et al., 2011; Aiuti et al., 2013), some examples of feline lentiviral vector-mediated oncogenesis have been reported in fetal models (Themis et al., 2005; Condiotti et al., 2013; Nowrouzi et al., 2013). In contrast, integration-deficient lentiviral vectors (IDLVs) have provided similar transduction efficiencies to classical integration-proficient lentiviral vectors (IPLVs) in a range of cell types, while having a significantly reduced risk of insertional mutagenesis (Yáñez-Muñoz et al., 2006; Wanisch and Yáñez-Muñoz, 2009). IDLVs are commonly produced using class I integrase mutations (most frequently encoding a D64V change), whereby viral DNA fails to integrate into the host genome and forms episomal DNA circles instead. As these viral episomes lack replication signals, they are stable in quiescent cells but progressively diluted in proliferating cells. Hence, IDLVs are ideally suited for applications in the postmitotic CNS environment (Peluffo et al., 2013), but to our knowledge they have not been previously applied in the study of a major disease. Additionally, most experiments using IDLVs have focused on a relatively short time scale (weeks), with very few studies so far confirming efficient and long-lasting CNS gene expression in vivo (Philippe et al., 2006; Yáñez-Muñoz et al., 2006; Apolonia et al., 2007; Rahim et al., 2009; Hutson et al., 2012).
Parkinson's disease (PD) affects over 6 million individuals worldwide and is considered the second most common neurodegenerative disease, after Alzheimer's disease (Lyons and Pahwa, 2011). PD is an idiopathic disorder caused by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and subsequent depletion of dopamine levels in the striatum (Chen et al., 2005). Protection of the scant remaining dopaminergic neurons has been suggested as a therapeutic stratagem since the discovery of neuroprotective effects of glial cell line-derived neurotrophic factor (GDNF) on dopaminergic neurons in vitro (Lin et al., 1993). GDNF exerts its effects by regulating cell survival and cell differentiation through PI3K/Akt and Ras/ERK pathways (Toth et al., 2002). The potential neuroprotection and neurorestoration effects of GDNF have been examined in both rodent and nonhuman primate models of PD with positive effects shown in a variety of them (Kirik et al., 2000; Eslamboli et al., 2003; Pascual et al., 2008). These encouraging results then prompted initial open-label clinical trials, which suggested that continuous delivery of GDNF (or its relative, neurturin) by protein infusion or viral vector-mediated delivery was efficacious in advanced PD patients (Gill et al., 2003; Marks et al., 2008). However, such results were not replicated in double-blind, phase II clinical trials (Lang et al., 2006; Marks et al., 2010). At this time, the mechanism underlying the uneven success of GDNF in these trials is unclear (Broadstock and Yáñez-Muñoz, 2012). Viral vector-mediated delivery offers stability of expression, but, once administered, expression of the neurotrophic factor is often constitutive. Therefore, it is crucial that such expression is both safe and well-tolerated. The use of viral vectors allows transcriptional targeting, which may also be beneficial (Jakobsson et al., 2003; Hioki et al., 2007; Li et al., 2010). Further trials are being considered to search for an effective, safe, and stable vector-based system to deliver GDNF (Bartus et al., 2011; Richardson et al., 2011).
Following on these investigations, we have assessed the efficacy of the novel IDLVs for therapy in a PD rodent model. We report that IDLVs can provide efficient, long-lived, and transcriptionally targeted transduction of brain cells when injected ahead of striatal lesioning with 6-hydroxydopamine (6-OHDA). While human glial cell line–derived neurotrophic factor (hGDNF) gene expression was higher from IPLVs, transduction with IDLVs resulted in equally efficient neuroprotection and long-term improvements of behavioral symptoms in lesioned rats. Lack of significant IDLV integration was confirmed in animals injected with vector after striatal lesioning. These results underscore the effectiveness of IDLVs in the CNS and the scope for low-dose, low-risk therapies based on these novel vectors.
Materials and Methods
Lentiviral vector production and titration
Concentrated stocks of the 2nd- and 3rd-generation lentiviral vectors, both IPLVs and IDLVs, were produced by calcium phosphate transfection into HEK-293T cells as previously described (Yáñez-Muñoz et al., 2006). Lentiviral transfer plasmids used in this study were pRRLsc-CMVp-eGFP-W, pRRLsc-hGFAPp-eGFP-W, and pHR′-hGFAPp-hGDNF-W. All vectors were self-inactivating, contained a central polypurine tract/central termination sequence (cPPT/cTS) and Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), and were pseudotyped with the vesicular stomatitis virus G protein (VSV-G) envelope. Viral titers were quantified by either flow cytometry using a FACSCanto II (BD Biosciences) or qPCR using a Rotogen Q (Qiagen) as described (Yáñez-Muñoz et al., 2006), or by p24 ELISA kit (SAIC) following the manufacturer's instructions.
Animals
All animal experiments were performed in accordance with the UK Animals (Scientific Procedures) Act, 1986. Forty-five male Sprague-Dawley rats (Charles River) weighing 250–300 g at the start of the study were housed 2–3 rats/cage. Animals were maintained in a standard 12 hr light/dark cycle with free access to food and water.
Stereotaxic injection
Animals were kept under isoflurane anesthesia (5% in 100% O2 for induction and 2.5% in 100% O2 for maintenance). The nose bar was set at −3.3 mm. All injections were made using a 20 μl Hamilton syringe held within an automated syringe pump (World Precision Instruments), and the injection rate was 0.5 μl/min.
6-OHDA lesioning and lentiviral vector injections for vector integration analysis
Thirty minutes before surgical procedures, animals were injected with a combined solution of pargyline (5 mg/kg, i.p.) and desipramine (25 mg/kg, i.p.). A single injection of 6-OHDA (12.5 μg in 2.5 μl of 0.9% saline and 0.02% ascorbic acid) was made into the median forebrain bundle (coordinates from Bregma: AP −2.8; ML +2.0; DV–9.0 mm). Animals were then allowed to recover for 2 weeks. To assess the effectiveness of 6-OHDA lesioning, animals were habituated in 40 cm flat-bottomed hemispherical bowls for 15 min. Animals were then injected with apomorphine hydrochloride (Tocris; 1 mg/kg, i.p., dissolved in sterile water). Rotational behavior was scored for 30 min postinjection. Animals displaying at least four net contraversive rotations per minute were deemed to have over 90% loss of striatal dopamine terminals (Hefti et al., 1980).
Four days after apomorphine injection, under isofluorane-induced anesthesia, animals received a single 2.5 μl injection of either IDLV-CMVp-eGFP (2×108 eGFP vector units/ml), IPLV-CMVp-eGFP (2×108 eGFP vector units/ml), or DMEM (PAA) into the left striatum (coordinates from Bregma: AP +1.0; DV −5.0; ML +2.8 mm). Groups of animals were as follows: 6-OHDA-lesioned IDLV (n=5), 6-OHDA-lesioned IPLV (n=4), nonlesioned IDLV (n=3), nonlesioned IPLV (n=3), and nonlesioned vehicle (n=2). One week postvector injection, animals were killed by exposure to a rising concentration of CO2 followed by cervical dislocation. The striata were rapidly dissected into RNAlater for subsequent linear amplification-mediated PCR (LAM-PCR).
Integration site analyses by 3′LTR-mediated LAM-PCR
3′LTR LAM-PCR was performed as previously described to determine the lentiviral vector integration sites (Schmidt et al., 2007). In brief, 400–1100 ng of DNA per sample was used for linear PCR to preamplify 3′ vector genome junctions. After double-strand synthesis, restriction digestion (Tsp509I and HpyCH4IV) and adapter ligation, two nested exponential PCRs were performed. LAM-PCR amplicons were prepared for sequencing by 454 technology (454/Roche System) as previously described (Paruzynski et al., 2010). A final PCR step was set up to include 454 sequencing adaptors and sample-specific barcodes. After sequencing, the obtained sequences were trimmed and aligned to the rat genome (assembly rn4) by a semiautomated bioinformatical data mining pipeline as previously described (Arens et al., 2012).
Lentiviral vector injections and 6-OHDA lesioning to test neuroprotective effects
Animals received a single injection of IPLV-GFAPp-hGDNF (n=10), IDLV-GFAPp-hGDNF (n=10), IPLV-GFAPp-eGFP (n=5), or IDLV-GFAPp-eGFP (n=5) into two sites of the right striatum (5 μl/site; 109 viral DNA copies/ml or 2×104 ng p24/ml). Stereotaxic coordinates from Bregma were (1) AP +1.8; ML −2.5; DV −5.0 mm; (2) AP 0.0; ML −3.5; DV −5.0 mm. Two weeks postvector transduction, animals were injected with a combined solution of pargyline (5 mg/kg, i.p.) and desipramine (25 mg/kg, i.p.). Thirty minutes later, 6-OHDA (2.5 μg/μl in 0.9% sterile saline and 0.02% ascorbic acid) was injected into the same locations as for vector administration, 2 μl/site. Reagents were obtained from Sigma Aldrich, unless stated otherwise.
Amphetamine-induced rotational behavior in hGDNF-treated animals
Rotational tests were performed from 3 weeks after 6-OHDA lesioning, once per month for a period of 4 months. Animals were acclimatized in 40-cm-diameter bowls for 30 min before injection with amphetamine (Sigma; 5 mg/kg, i.p., dissolved in sterile water). The rotations were assessed for 90 min postinjection as one whole head to tail revolution, with contralateral and ipsilateral rotations counted separately.
hGDNF detection by ELISA
Animals were sacrificed by cervical dislocation as described above. The striata were isolated and frozen immediately in liquid nitrogen. Tissues were homogenized in complete lysis buffer (Roche) followed by a centrifugation at 14,000×g at 4°C for 15 min. Levels of hGDNF were determined by hGDNF ELISA kit (Promega) following the manufacturer's instructions.
Immunohistochemistry
Rat brains were collected, fixed in ice-cold 4% PFA, and maintained at 4°C for 3 days. Coronal sections were sliced at 50 μm thickness on a vibratome (Campden Instruments) and then incubated in 1% BSA blocking buffer for an hour. Rabbit anti-TH antibody (Cat AB152, 1:1000; Millipore) was added to sections containing the SN, while mouse anti-NeuN (Cat MAB377, 1:500; Millipore), rabbit anti-GFAP (Cat Z0334, 1:500; Dako), or rabbit anti-Iba1 (Cat 019–19741, 1:500; Wako) antibody was added to striatal sections. Sections were maintained at 4°C overnight and then washed and incubated with either goat antimouse AlexaFluor555 (Cat A21424, 1:500; Invitrogen) or goat antirabbit AlexaFluor555 (Cat A21428, 1:500; Invitrogen) antibody for an hour, in the dark. After another three washes in 1× PBS, sections were incubated with 1 μg/ml DAPI for 15 min and subsequently washed in 1× PBS before being mounted onto SuperFrost slides.
Image capture
Brain sections were visualized under an inverted fluorescence Axio Observer D1 microscope. Images were captured with an AxioCam combined with AxioVision software. Equipment and software were from Carl Zeiss.
Cell counts
Overlapping parts from each section containing the SN were captured and stitched automatically by AxioVision software to create a mosaic image of the section. The number of TH+ cells within the SNpc (identified through Paxinos and Watson Rat Brain Map, 6th edition, 2007) on each section was counted manually using ImageJ software. Cell counts from approximately 30 SNpc-containing serial sections were averaged to obtain the total TH+ cell number per section of the SNpc in the corresponding brain hemisphere.
Measurement of eGFP intensity
After image capture, the area of the striatum on each section was subsequently identified according to the Paxinos and Watson Rat Brain Map (6th edition, 2007). The eGFP intensity within this area was quantified by AxioVision software and divided by the area to obtain the value of eGFP intensity, expressed as arbitrary units/μm2. The values for approximately 60 striatal sections from each brain were averaged to obtain the value for the corresponding brain.
Statistical analysis
Using Prism 5 software (GraphPad), data were analyzed and shown as means±S.E.M., with error bars representing the S.E.M.; “n” refers to the number of animals per group. Comparisons of statistical significance were assessed by one- or two-way ANOVA followed by Bonferroni's post hoc test or 2-tailed Student's t-test. Significant levels were set at *p<0.05, **p<0.01, ***p<0.001.
Results
Residual integration levels of IDLVs in intact and lesioned rat striata
We and others have demonstrated that IDLVs only integrate at residual levels in a variety of tissues, including the CNS (Yáñez-Muñoz et al., 2006; Mátrai et al., 2011; Chick et al., 2012). To confirm that this is also the case in the 6-OHDA-lesioned rat brain, we performed comparative large-scale lentiviral insertion site analysis by LAM-PCR followed by high-throughput sequencing (pyrosequencing). Animals were lesioned with 6-OHDA or mock-treated, and 18 days later injected in the right striata with IDLV or IPLV expressing eGFP. One week after vector injection, striata were harvested and analyzed for vector integration. As expected, there were considerably fewer integration sites for IDLVs than IPLVs (Table 1). Most interestingly, frequencies of integration events were broadly similar within vector types when comparing lesioned and nonlesioned striata. These data suggest that disease status had no effect on the overall integration frequency of lentiviral vectors. Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/hum) details the integration sites of IDLVs in the present study.
Table 1.
Residual Integration of Integration-Deficient Lentiviral Vectors Is Not Affected by Striatal Lesion Status
| Integration sites | ||
|---|---|---|
| Lesioned | Nonlesioned | |
| Exactly mappable | ||
| IDLV | 9 | 8 |
| IPLV | 1023 | 494 |
| Total | ||
| IDLV | 18 | 8 |
| IPLV | 1154 | 555 |
IDLV, integration-deficient lentiviral vector; IPLV, integration-proficient lentiviral vector; LAM-PCR, linear amplification-mediated polymerase chain reaction.
The following groups of animals were analyzed: lesioned IDLV (n=5), lesioned IPLV (n=4), nonlesioned IDLV (n=3), and nonlesioned IPLV (n=3). LAM-PCR samples were subjected to high-throughput pyrosequencing. The raw sequence data were processed to determine possible integration sites. These were mapped onto the rat genome, with the majority being exactly mappable and few multiple hits. A total of 104,601 sequence reads were obtained and distributed as follows: lesioned IDLV (38,917), lesioned IPLV (38,162), nonlesioned IDLV (15,080), and nonlesioned IPLV (12,442).
Lentiviral vector-mediated eGFP expression in the striatum of 6-OHDA-lesioned rats
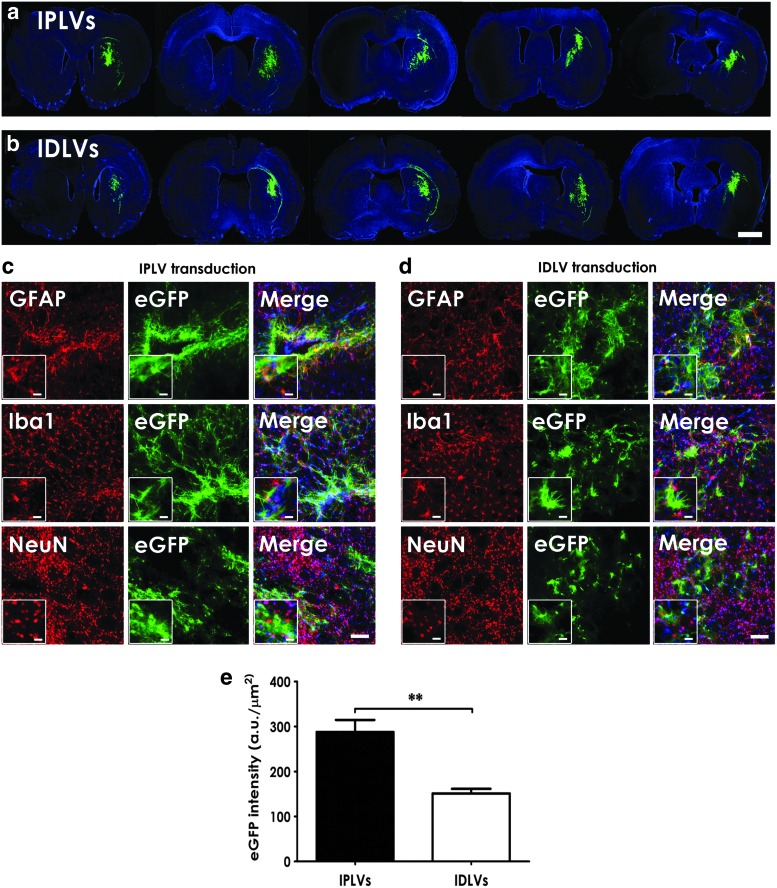
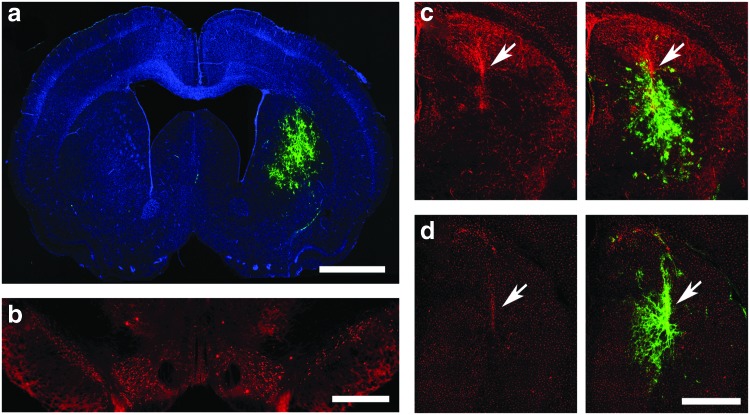
Animals were injected with IPLV- or IDLV-GFAPp-eGFP vectors, followed by 6-OHDA lesioning 2 weeks later. Brain tissue was harvested 5 months after vector injection. We first demonstrated successful transduction with widespread eGFP production within the striatum (Fig. 1a and b). Cell identification by morphology and marker expression indicated that specific cell types were targeted, with mainly astrocytes being transduced; no significant microglia or neuron transduction was noticed (Fig. 1c and d). Levels of eGFP in groups treated with IDLVs were about twofold lower than in those receiving IPLVs (Fig. 1e). No eGFP expression in the noninjected hemisphere (Fig. 2a) or anterograde expression in the SNpr (Fig. 2b) was detected. Our data suggest a modest, spatially restricted activation of both astrocytes and microglia in the area surrounding the injection site (Fig. 2c and d).
FIG. 1.
eGFP expression in rat striata. The right striata were injected with IPLV- or IDLV-GFAPp-eGFP 2 weeks before unilateral 6-OHDA lesioning at the same positions as for vector administration. Representative serial striatal sections from (a) IPLV- and (b) IDLV-eGFP-injected animals show both vector injection site and area of vector spread. Representative high-magnification images from (c) IPLV- and (d) IDLV-eGFP-injected striata, respectively, are shown. Cell-type identification by morphology and marker expression indicated that the majority of eGFP+ cells were astrocytes (GFAP+); no significant microglia (Iba1+) or neuron (NeuN+) transduction was observed. Nuclei were stained blue with DAPI; scale bar: (a and b) 2000 μm; (c and d) 100 μm; (insets in c and d) 20 μm. (e) eGFP levels evaluated 5 months postvector injection showed a twofold difference between IPLVs and IDLVs. Data were analyzed for statistical significance by two-tailed Student's t-test; error bars represent the S.E.M.; n=3 per group; **p<0.01. IDLV, integration-deficient lentiviral vector; IPLV, integration-proficient lentiviral vector.
FIG. 2.
Lack of anterograde eGFP transport and restricted glial activation. After striatal injection of IDLV-GFAPp-eGFP and unilateral 6-OHDA lesioning at the same site, (a) no IDLV-eGFP expression in the noninjected left hemisphere or (b) anterograde eGFP transport from the striatum to the SNpr (costained in red for TH+ cells) was detected. Glial activation was analyzed by GFAP+ or Iba1+staining (in red), respectively. Arrows indicate activated (c) astrocytes and (d) microglia surrounding the IDLV injection site, with images on right side additionally displaying eGFP production pattern. Nuclei were stained blue with DAPI. Scale bar: (a) 2000 μm; (b) 500 μm; (c and d) 1000 μm. Color images available online at www.liebertpub.com/hum
hGDNF neuroprotection on dopaminergic neurons in the SNpc of 6-OHDA-lesioned rats
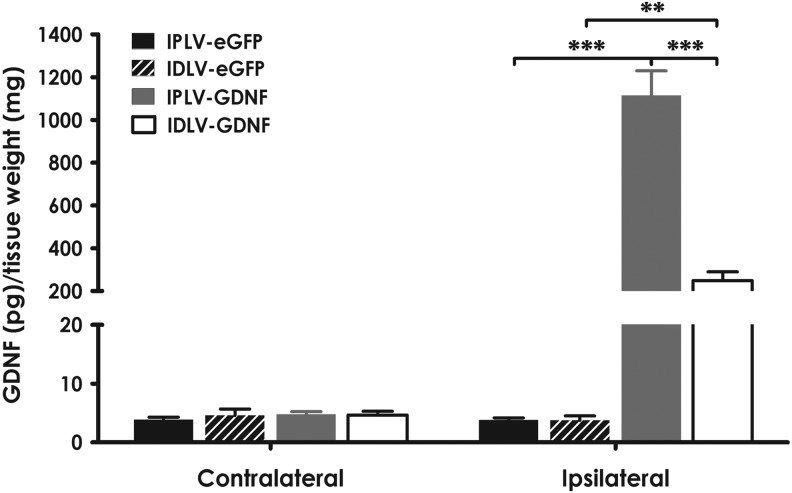
GDNF is naturally secreted by glial cells (Lin et al., 1993), and complications from neuronal transgene expression are possible (Jakobsson and Lundberg, 2006). Following this rationale, we employed an astrocyte-specific promoter, the glial fibrillary acidic protein promoter (GFAPp), to drive lentivector-mediated GDNF expression in our neuroprotection experiments. Animals were injected with IPLV- or IDLV-GFAPp-hGDNF vectors, using eGFP-expressing vectors as controls, followed 2 weeks later by 6-OHDA lesioning. Levels of hGDNF (measured by ELISA 5 months postinjection) in ipsilateral striata of rats receiving hGDNF vectors were 60–250-fold higher than in contralateral striata or in eGFP-treated rats. We also observed a fivefold difference in hGDNF levels between IPLV- and IDLV-hGDNF-treated groups, higher in the former (Fig. 3).
FIG. 3.
hGDNF overexpression in injected striata. Five months after vector transduction and 6-OHDA lesioning, rat striata were harvested. Levels of hGDNF measured by ELISA showed no differences in the contralateral hemispheres between groups. In contrast, the levels of hGDNF detected in the ipsilateral hemispheres of hGDNF-treated groups (n=10) were significantly higher than those in eGFP-treated groups (n=4). There was also a significant difference between IPLV- and IDLV-hGDNF-treated groups. Statistical analysis with one-way ANOVA and Bonferroni's post hoc test. Error bars represent the S.E.M.; **p<0.01, ***p<0.001.
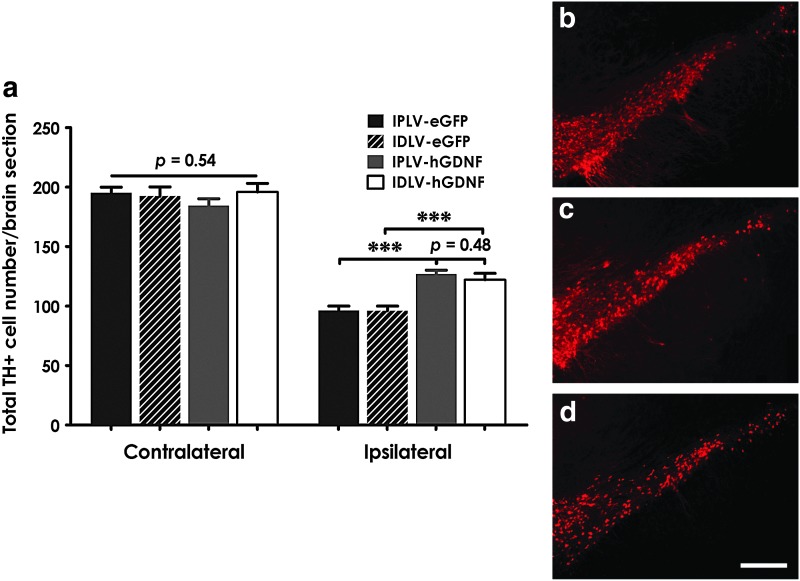
We investigated the potential neuroprotective effects of hGDNF on dopaminergic neurons by estimating the loss of TH+ cells in the SNpc 5 months after vector injection and lesioning. After a 10 μg striatal injection of 6-OHDA, a 50% reduction from normal levels of dopaminergic cell bodies was detected in the ipsilateral hemisphere of control (eGFP-treated) groups. The reduction was only 30% in hGDNF-treated animals, with no statistical difference between IPLVs and IDLVs despite the 5-fold difference in hGDNF levels (p=0.48; Fig. 4a). Figure 4b–d show representative images of the SN with no damage in the contralateral hemisphere, 30% TH+ cell loss in the ipsilateral hemisphere of hGDNF-treated groups, and 50% TH+ cell loss in the ipsilateral hemisphere of eGFP-treated groups, respectively. These data indicate an effective neuroprotection by hGDNF on dopaminergic neurons regardless of lentiviral integration proficiency.
FIG. 4.
Effect of hGDNF expression on survival of dopaminergic neurons in the SNpc. (a) Five months after vector transduction and 6-OHDA lesioning, counts of TH+ cells in the SNpc from contralateral and ipsilateral striata demonstrated similar cell survival in the contralateral SNpc of all groups and the loss of about 50% TH+ cells in the ipsilateral SNpc of eGFP-treated groups (n=10). Animals receiving hGDNF vectors showed enhanced cell survival, with 70% TH+ cells remaining in both IPLV- and IDLV-treated groups (n=10). (b–d) Representative images of the SN with (b) no damage in the contralateral hemisphere; (c) 30% TH+ cell loss in the ipsilateral hemisphere of hGDNF-treated groups; and (d) 50% TH+ cell loss in the ipsilateral hemisphere of eGFP-treated groups. Red cells are TH+; scale bar=500 μm. Statistical analysis with one-way ANOVA and Bonferroni's post hoc test. Error bars represent the S.E.M.; **p<0.01, ***p<0.001. Color images available online at www.liebertpub.com/hum
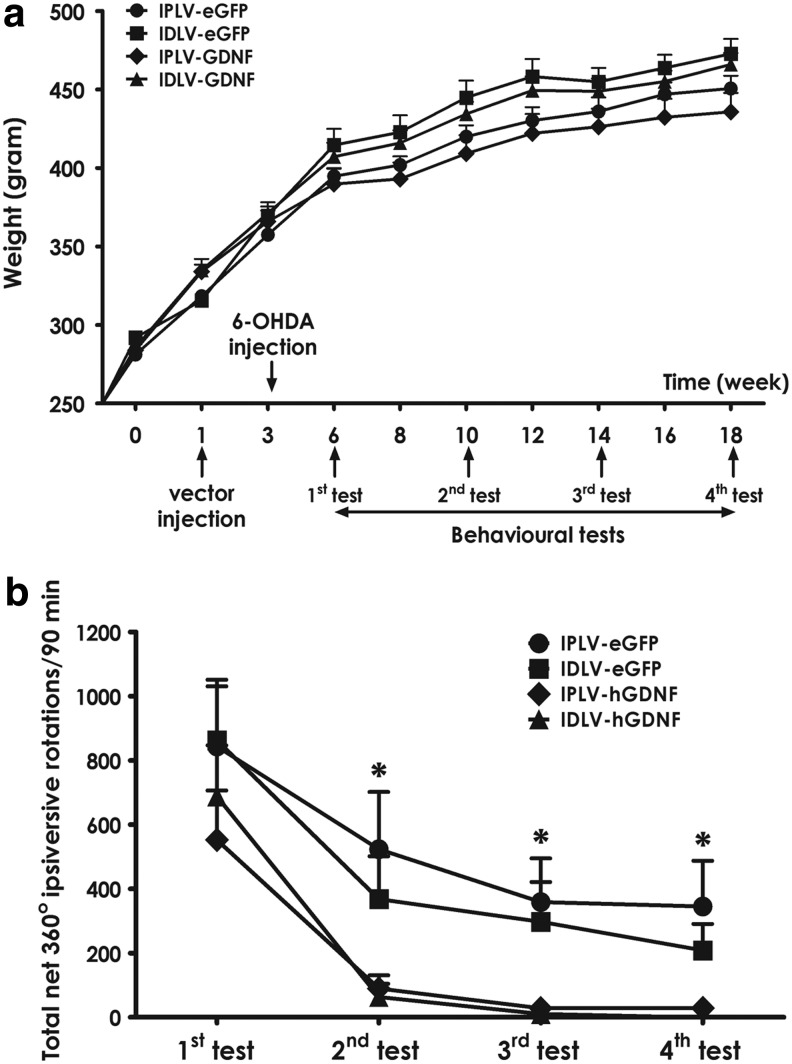
Behavioral recovery of hGDNF-treated rats
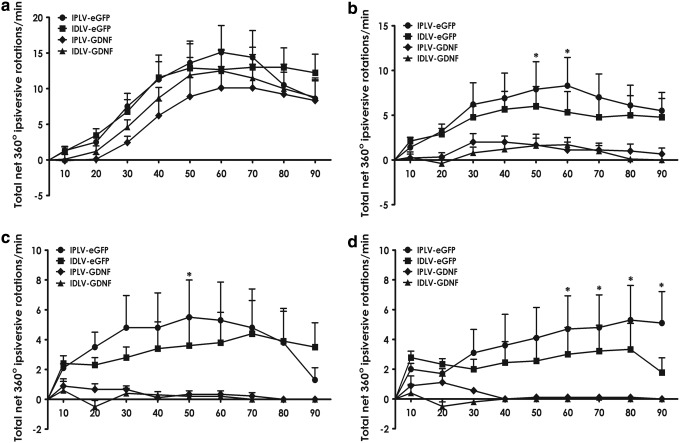
The vector-injected and 6-OHDA-lesioned animals were subjected to amphetamine-induced rotational tests performed monthly, starting from 3 weeks postlesioning. Animals showed a normal weight gain with no difference between groups (p=0.59; Fig. 5a). A time-dependent reduction in number of rotations was observed in all groups (Fig. 5b). However, an extensive recovery, with 98% reduction in rotational turns by the third test, was only seen in hGDNF-treated animals, regardless of vector integration proficiency. In contrast, those injected with eGFP vectors presented only 66% reduction. Furthermore, we detected significant differences in rotational behavior between eGFP- and hGDNF-treated groups within tests, starting from the second test. By quantifying the number of rotations at 10 min intervals, we noted that these differences occurred from minute 50 onward (Fig. 6a–d). These results demonstrate that higher hGDNF expression resulting from IPLV administration (Fig. 3) does not lead to any significant difference in the behavioral recovery compared with IDLVs (p=0.36).
FIG. 5.
hGDNF-mediated improvement in amphetamine-induced rotational movement. (a) Time-scale of the experiment superimposed on a weight gain chart, with no statistical difference in weight between groups at each time point. (b) After 30 min acclimation, animals were injected with amphetamine and net 360° rotations were scored for 90 min. hGDNF-treated groups showed a significantly improved recovery of behavior starting from the second monthly test, with similar improvements regardless of vector integration proficiency. Data were analyzed for statistical significance by two-way ANOVA and Bonferroni's post hoc test. Error bars represent the S.E.M.; n=10 per group; *p<0.05.
FIG. 6.
Timing of hGDNF-mediated improvement in rotational behavior in lesioned rats. (a–d) After 30 min acclimation, animals were injected with amphetamine and net 360° rotations were scored every 10 min for 90 min in monthly rotational tests. The improved behavior in hGDNF-treated groups noted from the second test is because of lower rotation numbers from min 50. Data were analyzed for statistical significance by two-way ANOVA and Bonferroni's post hoc test. Error bars represent the S.E.M.; n=10 per group; *p<0.05.
Discussion
Since we and others first reported safe and efficient in vivo transduction with IDLVs (Philippe et al., 2006; Yáñez-Muñoz et al., 2006), there has been scope for application to gene therapy for a major disease. Here we have investigated IDLV transduction for PD gene therapy in an animal model, in direct comparison with the commonly used IPLVs. Our data demonstrate that IDLVs injected ahead of striatal lesioning support long-lasting transduction of CNS cells for at least 5 months postadministration. We report a sustained therapeutic effect in this animal model after such IDLV-mediated neuroprotection, which was not significantly different to that of their integrating counterparts and persisted for the duration of the experiment.
Lentiviral vectors are an attractive system for gene therapy, with many positive features derived from their biology and extensive vector development, such as large transgene capacity of up to 8 kb, low immunogenicity (Mátrai et al., 2010), enhanced cell-specific targeting through pseudotyping (Cannon et al., 2011), and relatively simple production (Dull et al., 1998). Moreover, they can transduce many cell types of the CNS (Jakobsson et al., 2003), including dividing as well as nondividing cells, with stable long-term expression of transgene(s) (Cockrell and Kafri, 2007). Effective transgene expression can be obtained at low vector doses, 3–4 logs lower than AAVs, which have been widely used in gene therapy for PD treatment. Using lentiviral vectors, therefore, may be a significant advantage considering the potential of side effects that may be associated with high vector doses (Bartus et al., 2011; Drinkut et al., 2012). However, a significant obstacle in the routine clinical use of current IPLVs is the potential for insertional mutagenesis and subsequent oncogenesis caused by integration of the viral genome into the host cell genome (Biasco et al., 2012). This risk could be addressed by using IDLVs if the target cell population does not divide significantly (Wanisch and Yáñez-Muñoz, 2009; Banasik and McCray, 2010).
In the current work, we have assessed striatal integration of stereotaxically injected lentiviral vectors in normal rats or rats previously lesioned with 6-OHDA. Using LAM-PCR followed by pyrosequencing, we have observed only marginal integration levels of IDLVs, in agreement with previous analyses of IDLV integration in vivo (Yáñez-Muñoz et al., 2006; Mátrai et al., 2011; Chick et al., 2012). Extending previous observations, we noticed that such residual integration did not change significantly in the presence of the striatal lesion.
Due to the lack of integration, transgene expression induced after IDLV administration can be reduced by cell division, though a similar disadvantage is well known in the equally replication-deficient AAVs. In addition, although transcriptional targeting through the use of cell-type-specific promoters may be beneficial (Jakobsson et al., 2003; Hioki et al., 2007; Li et al., 2010), the GFAPp promoter may be less efficient in an episomal configuration, as observed with some promoters (Wanisch and Yáñez-Muñoz, 2009). Astrocyte proliferation stimulated after CNS injury would therefore be predicted to result in less effective astrocytic eGFP or hGDNF expression after transduction with IDLV-GFAPp than IPLV-GFAPp. However, the stimulation of proliferation is temporary (Drinkut et al., 2012) and rarely observed in the pathophysiology of PD (Mirza et al., 2000). Indeed the results from this study indicate that proliferation and episomal expression were no major obstacles, with IDLV vectors driven by GFAPp expressing moderately lower levels of eGFP (twofold) and hGDNF (fivefold) than their integrating counterparts. Moreover, high levels of hGDNF expression may be disadvantageous (Georgievska et al., 2004). Using lentiviral vector-mediated intrastriatal delivery, these authors demonstrated that hGDNF levels above 700 pg/mg tissue weight caused a downregulation of striatal TH levels and a significant reduction in mRNA level of this enzyme in the SN. Although the reductions might be restored 4–8 weeks later (Cohen et al., 2011), it is unclear whether these changes may in turn affect striatal dopamine levels. The IDLVs here provided safe levels of hGDNF that were about threefold lower than the reported overexpression threshold. Importantly, the beneficial therapeutic effects of IDLV-hGDNF on survival of nigral dopaminergic cells were as efficient as those from IPLVs, which surpassed the 700 pg/mg threshold.
Numerous studies (Jakobsson et al., 2003; Ciesielska et al., 2011; Richardson et al., 2011; Drinkut et al., 2012) have previously reported that striatal transgene expression targeted to neurons resulted in transgene products in the SNpc/pr, the globus pallidus, and the subthalamic nucleus, in both lesioned and unlesioned hemispheres. This was possibly because of retrograde delivery from striatal dopaminergic nerve terminals to the cell bodies in the SNpc (Tomac et al., 1995) and anterograde axonal transport from striatal neurons to the afferent regions via the indirect pathway, plus axonal sprouting between the two striatal hemispheres (Ciesielska et al., 2011; Richardson et al., 2011). These off-target effects may provide some advantages in GDNF application for PD treatment, but levels of GDNF transported indirectly to these afferent regions are unpredictable, particularly when higher vector doses or longer expression times are required (Bartus et al., 2011; Drinkut et al., 2012). It also remains unclear what the adequate level of GDNF required by these regions is in order to provide therapeutic effects and simultaneously minimize side effects (Georgievska et al., 2004). In the light of the possible complications from neuronal transgene expression, we chose to target expression to astrocytes (Jakobsson and Lundberg, 2006). While neuronal axons can be several centimeters long, astrocytic protrusions rarely extend more than 20 μm from the cell body. Such a natural physical barrier may diminish off-target expression of transgene(s). In fact, the present study demonstrated astrocytic-eGFP expression targeted by IPLV- or IDLV-GFAPp vectors mainly in the injected striatum; a small proportion of cells in the white matter were transduced, presumably as a result of the backflow that occurs after withdrawal of the injection needle (Jakobsson et al., 2003). Such an approach may therefore lead to maximal transgene expression in the required brain region, while preventing off-target effects resulting from ectopic expression in afferent regions. Moreover, since astrocytes are the major source of GDNF upon brain injury and may be responsible for maintenance of GDNF levels in the SN of PD brains (Mogi et al., 2001; Saavedra et al., 2008), astrocytic-hGDNF expression by the lentiviral vector GFAPp would be beneficial as demonstrated in this study.
One further advantage of lentiviral vectors is the minimal stimulation of the immune response that may occur after transgene delivery (Abordo-Adesida et al., 2005; Mátrai et al., 2010). Indeed, we detected only an elevated density of activated microglia and astrocytes surrounding the needle tract, which most likely reflects physical damage after injection of vectors and/or 6-OHDA rather than immune system activation after cell transduction (Batchelor et al., 1999). This finding is consistent with previous studies (Jakobsson et al., 2003; Drinkut et al., 2012) regardless of whether lentiviral vectors or AAVs were used. In the current study, the astrogliosis was prolonged up to 5 months, the end of the experiment, whereas Drinkut et al. (2012) reported that activated cells disappeared 3 months postinjection. A possible explanation can be that in our study both vectors and 6-OHDA were administered into the same location, with concomitant doubling of physical trauma to the brain regions after repeated injections. As such, our approach may cause more physical damage than vector injection into the brain separated from intraperitoneal injection of MPTP in the previous study.
The neuroprotective effects of GDNF were also confirmed here through an improvement in amphetamine-induced rotational asymmetry of treated animals. Rats with a partial 6-OHDA lesion (<70% nigral dopaminergic cell loss) improve their rotational behavior over time, returning to nearly normal by about week 16 postlesioning (Stanic et al., 2003). This may be a consequence of regeneration of striatal dopaminergic terminals induced by GDNF or BDNF secreted from activated microglia in the injured striatum (Batchelor et al., 1999). Such findings may explain the reduction in the number of rotations in all animal groups observed here. However, only those animals injected with hGDNF-expressing vectors displayed an essentially complete recovery of behavior, which started from week 9 after vector injection. This recovery occurred regardless of vector integration proficiency.
In the present study, we chose to test a neuroprotection approach in which hGDNF lentivectors were administered before 6-OHDA lesioning. This strategy should be optimal for prevention of axonal retraction and subsequent neuronal death and indeed rendered a positive outcome. However, in PD patients the effects of neurotrophic factor therapy may also include neurorestoration of damaged neurons and neuroregeneration with new neurons in the injured regions. Therefore, further assessment of the effectiveness of IDLV-hGDNF expression for PD should include an experimental strategy in which 6-OHDA lesioning precedes the injection of therapeutic vector.
In summary, our study demonstrates IDLV-mediated neuroprotection in rats when intrastriatal hGDNF expression precedes 6-OHDA lesioning. These data also show promoter-mediated cell type specificity from IDLVs, with a focus on effective astrocytic transgene expression. We demonstrate both efficient delivery and long-lasting expression of a therapeutically relevant transgene by IDLVs at low dose. We also demonstrate lack of significant IDLV integration in this PD lesion model, supporting these safer vectors as potential tools in gene therapy for PD treatment, and by extension for other major diseases of the CNS. Our data warrant further exploration of IDLVs in PD-relevant models, in particular nonhuman primates, before considering clinical application. Demonstration of safety and efficacy in such models would strongly support the use of IDLVs in first-in-man studies.
Supplementary Material
Acknowledgments
The authors thank C. Lundberg (Wallenberg Neuroscience Center, Lund University, Sweden) for plasmid pHR′-hGFAPp-hGDNF-W. Steffanie Wiltcher assisted with the scoring of the behavioral experiment. This work received financial support from the 6th EU Framework Programme (Clinigene; Grant Agreement No. 18933), the 7th EU Framework Programme (Neugene; Grant Agreement No. 222925), and a studentship from Royal Holloway—University of London and University of Medicine and Pharmacy at Ho Chi Minh city, Vietnam.
Author Contributions
R.J.Y.-M. conceived and directed the project; R.J.Y.-M., M.B., M.S., and C.v.K. designed the experiments; N.B.L.-N., M.B., M.S., and C.C.B. performed all experiments; all authors contributed to data analysis; N.B.L.-N., M.B., and R.J.Y.-M. wrote the article, with input from all authors.
Author Disclosure Statement
The authors declared no conflict of interest.
References
- Abordo-Adesida E., Follenzi A., Barcia C., et al. (2005). Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum. Gene Ther. 16, 741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A., Biasco L., Scaramuzza S., et al. (2013). Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341, 1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolonia L., Waddington S.N., Fernandes C., et al. (2007). Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol. Ther. 15, 1947–1954 [DOI] [PubMed] [Google Scholar]
- Arens A., Appelt J.U., Bartholomae C., et al. (2012). Bioinformatical clonality analysis of next generation sequencing derived viral vector integration sites. Hum. Gene Ther. Methods. 23, 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasik M.B., and McCray P.B., Jr. (2010). Integrase-defective lentiviral vectors: progress and applications. Gene Ther. 17, 150–157 [DOI] [PubMed] [Google Scholar]
- Bartus R.T., Brown L., Wilson A., et al. (2011). Properly scaled and targeted AAV2-NRTN (neurturin) to the substantia nigra is safe, effective and causes no weight loss: support for nigral targeting in Parkinson's disease. Neurobiol. Disease 44, 38–52 [DOI] [PubMed] [Google Scholar]
- Batchelor P.E., Liberatore G.T., Wong J.Y., et al (1999). Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J. Neurosci. 19, 1708–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasco L., Baricordi C., and Aiuti A. (2012). Retroviral integrations in gene therapy trials. Mol. Ther. 20, 709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A., Bartolomae C.C., Cesana D., et al. (2011). Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood 117, 5332–5339 [DOI] [PubMed] [Google Scholar]
- Bjorklund A., Kirik D., Rosenblad C., et al. (2000). Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 886, 82–98 [DOI] [PubMed] [Google Scholar]
- Broadstock M., and Yáñez-Muñoz R.J. (2012). Challenges for gene therapy of CNS disorders and implications for Parkinson's disease therapies. Hum. Gene Ther. 23, 340–343 [DOI] [PubMed] [Google Scholar]
- Cannon J.R., Sew T., Montero L., et al. (2011). Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Exp. Neurol. 228, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., He Y., and Yang K. (2005). Gene therapy for Parkinson's disease: progress and challenges. Curr. Gene Ther. 5, 71–80 [DOI] [PubMed] [Google Scholar]
- Chick H.E., Nowrouzi A., Fronza R., et al. (2012). Integrase-deficient lentiviral vectors mediate efficient gene transfer to human vascular smooth muscle cells with minimal genotoxic risk. Hum. Gene Ther. 23, 1247–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielska A., Mittermeyer G., Hadaczek P., et al. (2011). Anterograde axonal transport of AAV2-GDNF in rat basal ganglia. Mol. Ther. 19, 922–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell A.S., and Kafri T. (2007). Gene delivery by lentivirus vectors. Mol. Biotechnol. 36, 184–204 [DOI] [PubMed] [Google Scholar]
- Cohen A.D., Zigmond M.J., and Smith A.D. (2011). Effects of intrastriatal GDNF on the response of dopamine neurons to 6-hydroxydopamine: time course of protection and neurorestoration. Brain Res. 1370, 80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condiotti R., Goldenberg D., Giladi H., et al. (2013). Transduction of fetal mice with a feline lentiviral vector induces liver tumors which exhibit an E2F activation signature. Mol. Ther. 22, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkut A., Tereshchenko Y., Schulz J.B., et al (2012). Efficient gene therapy for Parkinson's disease using astrocytes as hosts for localized neurotrophic factor delivery. Mol. Ther. 20, 534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T., Zufferey R., Kelly M., et al. (1998). A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72, 8463–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslamboli A., Cummings R.M., Ridley R.M., et al (2003). Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey (Callithrix jacchus). Exp. Neurol. 184, 536–548 [DOI] [PubMed] [Google Scholar]
- Georgievska B., Kirik D., and Bjorklund A. (2004). Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J. Neurosci. 24, 6437–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.S., Patel N.K., Hotton G.R., et al (2003). Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 9, 589–595 [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., Von Kalle C., Schmidt M., et al. (2003). A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 348, 255–256 [DOI] [PubMed] [Google Scholar]
- Hefti F., Melamed E., Sahakian B.J., and Wurtman R.J. (1980). Circling behavior in rats with partial, unilateral nigro-striatal lesions: effect of amphetamine, apomorphine, and DOPA. Pharmacol. Biochem. Behav. 12, 185–188 [DOI] [PubMed] [Google Scholar]
- Hioki H., Kameda H., Nakamura H., et al. (2007). Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Ther. 14, 872–882 [DOI] [PubMed] [Google Scholar]
- Hutson T.H., Verhaagen J., Yáñez-Muñoz R.J., and Moon L.D. (2012). Corticospinal tract transduction: a comparison of seven adeno-associated viral vector serotypes and a non-integrating lentiviral vector. Gene Ther. 19, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson J., and Lundberg C. (2006). Lentiviral vectors for use in the central nervous system. Mol. Ther. 13, 484–493 [DOI] [PubMed] [Google Scholar]
- Jakobsson J., Ericson C., Jansson M., et al. (2003). Targeted transgene expression in rat brain using lentiviral vectors. J. Neurosci. Res. 73, 876–885 [DOI] [PubMed] [Google Scholar]
- Kirik D., Rosenblad C., and Bjorklund A. (2000). Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur. J. Neurosci. 12, 3871–3882 [DOI] [PubMed] [Google Scholar]
- Lang A.E., Gill S., Patel N.K., et al (2006). Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 59, 459–466 [DOI] [PubMed] [Google Scholar]
- Li M., Husic N., Lin Y., et al. (2010). Optimal promoter usage for lentiviral vector-mediated transduction of cultured central nervous system cells. J. Neurosci. Methods 189, 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.F., Doherty D.H., Lile J.D., et al (1993). GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132 [DOI] [PubMed] [Google Scholar]
- Lyons K.E., and Pahwa R. (2011). Diagnosis and initiation of treatment in Parkinson's disease. Int. J. Neurosci. 121Suppl 2, 27–36 [DOI] [PubMed] [Google Scholar]
- Marks W.J., Jr., Ostrem J.L., Verhagen L., et al. (2008). Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 7, 400–408 [DOI] [PubMed] [Google Scholar]
- Marks W.J., Jr., Bartus R.T., Siffert J., et al. (2010). Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 9, 1164–1172 [DOI] [PubMed] [Google Scholar]
- Mátrai J., Chuah M.K., and Vandendriessche T. (2010). Recent advances in lentiviral vector development and applications. Mol. Ther. 18, 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátrai J., Cantore A., Bartholomae C.C., et al (2011). Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology 53, 1696–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza B., Hadberg H., Thomsen P., and Moos T. (2000). The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson's disease. Neuroscience 95, 425–432 [DOI] [PubMed] [Google Scholar]
- Mogi M., Togari A., Kondo T., et al. (2001). Glial cell line-derived neurotrophic factor in the substantia nigra from control and parkinsonian brains. Neurosci. Lett. 300, 179–181 [DOI] [PubMed] [Google Scholar]
- Nowrouzi A., Cheung W.T., Li T., et al. (2013). The fetal mouse is a sensitive genotoxicity model that exposes lentiviral-associated mutagenesis resulting in liver oncogenesis. Mol. Ther. 21, 324–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruzynski A., Arens A., Gabriel R., et al. (2010). Genome-wide high-throughput integrome analyses by nrLAM-PCR and next-generation sequencing. Nat. Protoc. 5, 1379–1395 [DOI] [PubMed] [Google Scholar]
- Pascual A., Hidalgo-Figueroa M., Piruat J.I., et al (2008). Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat. Neurosci. 11, 755–761 [DOI] [PubMed] [Google Scholar]
- Peluffo H., Foster E., Ahmed S.G., et al (2013). Efficient gene expression from integration-deficient lentiviral vectors in the spinal cord. Gene Ther. 20, 645–657 [DOI] [PubMed] [Google Scholar]
- Philippe S., Sarkis C., Barkats M., et al. (2006). Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc. Natl. Acad. Sci. USA 103, 17684–17689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim A.A., Wong A.M., Howe S.J., et al (2009). Efficient gene delivery to the adult and fetal CNS using pseudotyped non-integrating lentiviral vectors. Gene Ther. 16, 509–520 [DOI] [PubMed] [Google Scholar]
- Richardson R.M., Kells A.P., Rosenbluth K.H., et al (2011). Interventional MRI-guided putaminal delivery of AAV2-GDNF for a planned clinical trial in Parkinson's disease. Mol. Ther. 19, 1048–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra A., Baltazar G., and Duarte E.P. (2008). Driving GDNF expression: the green and the red traffic lights. Prog. Neurobiol. 86, 186–215 [DOI] [PubMed] [Google Scholar]
- Schmidt M., Schwarzwaelder K., Bartholomae C., et al. (2007). High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR). Nat. Methods 4, 1051–1057 [DOI] [PubMed] [Google Scholar]
- Stanic D., Parish C.L., Zhu W.M., et al (2003). Changes in function and ultrastructure of striatal dopaminergic terminals that regenerate following partial lesions of the SNpc. J. Neurochem. 86, 329–343 [DOI] [PubMed] [Google Scholar]
- Themis M., Waddington S.N., Schmidt M., et al. (2005). Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol. Ther. 12, 763–771 [DOI] [PubMed] [Google Scholar]
- Tomac A., Widenfalk J., Lin L.F., et al (1995). Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc. Natl. Acad. Sci. USA 92, 8274–8278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth G., Yang H., Anguelov R.A., et al (2002). Gene transfer of glial cell-derived neurotrophic factor and cardiotrophin-1 protects PC12 cells from injury: involvement of the phosphatidylinositol 3-kinase and mitogen-activated protein kinase kinase pathways. J.Neurosci. Res. 69, 622–632 [DOI] [PubMed] [Google Scholar]
- Wanisch K., and Yáñez-Muñoz R.J. (2009). Integration-deficient lentiviral vectors: a slow coming of age. Mol. Ther. 17, 1316–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Muñoz R.J., Balaggan K.S., Macneil A., et al. (2006). Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 12, 348–353 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.