Abstract
Objective:
Due to the challenges inherent in performing clinical trials in children, a systematic review of published clinical trials was performed to determine whether the efficacy of antiepileptic drugs (AEDs) in adults can be used to predict the efficacy of AEDs in the pediatric population.
Methods:
Medline/PubMed, EMBASE, and Cochrane library searches (1970–January 2010) were conducted for clinical trials of partial-onset seizures (POS) and primary generalized tonic-clonic seizures (PGTCS) in adults and in children <2 and 2–18 years. Independent epidemiologists used standardized search and study evaluation criteria to select eligible trials. Forest plots were used to investigate the relative strength of placebo-subtracted effect measures.
Results:
Among 30 adjunctive therapy POS trials in adults and children (2–18 years) that met evaluation criteria, effect measures were consistent between adults and children for gabapentin, lamotrigine, levetiracetam, oxcarbazepine, and topiramate. Placebo-subtracted median percent seizure reduction between baseline and treatment periods (ranging from 7.0% to 58.6% in adults and from 10.5% to 31.2% in children) was significant for 40/46 and 6/6 of the treatment groups studied. The ≥50% responder rate (ranging from 2.0% to 43.0% in adults and from 3.0% to 26.0% in children) was significant for 37/43 and 5/8 treatment groups. In children <2 years, an insufficient number of trials were eligible for analysis.
Conclusions:
This systematic review supports the extrapolation of efficacy results in adults to predict a similar adjunctive treatment response in 2- to 18-year-old children with POS.
Given the many challenges inherent in conducting clinical trials in children, drugs are often approved for adult use before pediatric development is completed. Consequently, children are often treated with off-label or unlicensed products for which efficacy, safety, and pharmacokinetics have not been established.1 Though certain types of seizures and epilepsy syndromes are specific to the pediatric population (such as infantile spasms) and thus require clinical study in age-appropriate groups, other seizure types (including partial and generalized) are common in both adults and children, and the antiepileptic drugs (AEDs) used to treat them may have similar effects. Therefore, extrapolation of efficacy data from adult AED clinical trials has been proposed as a predictive tool for assessing efficacy in children and has been suggested as an alternative to randomized, double-blind, placebo-controlled phase III trials in children.2,3 The analysis reported below evaluates the evidence for extrapolation of efficacy data from comparable adult AED clinical trials for pediatric use.
METHODS
Search strategy, selection criteria, and data abstraction.
This study was initiated in response to a regulatory request from the Pediatric Committee of the European Medicines Agency (EMA) as part of regulatory guidance in the pediatric approval process for brivaracetam, an AED being developed by UCB Pharma. Electronic searches of Medline/PubMed, EMBASE, and the Cochrane library were conducted for AED efficacy trials in the treatment of partial-onset seizures (POS) and primary generalized tonic-clonic seizures (PGTCS) in adults and children with idiopathic generalized epilepsy.
Search algorithms were tested and refined in order to establish predefined search criteria to yield a focused but comprehensive baseline of relevant articles. Separate searches and algorithms were performed for the <2-year age group and the 2- to 18-year age group due to such factors as age-related epilepsy syndromes and differences in trial design. Medline, EMBASE, and Cochrane library searches were conducted for publication dates through February 2010 using predefined criteria (appendix e-1 on the Neurology® Web site at www.neurology.org). Duplicate titles occurred as a result of searches of more than one database and due to slight differences in the entries of titles or authors in the different databases searched. These initial searches identified 4,893 titles. Duplicate titles were removed, yielding 3,280 (1,615 + 1,665) articles for screening. Two independent epidemiologists (W.C. and V.T.) sequentially screened these articles by title, key words, and abstract content. Full text was obtained if the trial met the following criteria: 1) interventional clinical trial of AEDs; 2) conducted after 1980, with the exception of controlled trials of carbamazepine and valproate since these AEDs were developed prior to 1980; 3) age of participants 2–70 or 0–2 years; 4) POS and PGTCS or terms that mapped to these seizure types; and 5) sample size ≥50 in trials examining participants age 2–70. None of the foreign language articles met all inclusion criteria to require full translation. Citations for conference proceedings and references with fewer than 3 pages and no abstract available were excluded. Full articles were then evaluated by W.C. and V.T. and included or excluded from the systematic review using a checklist developed from 3 standard evaluation tools: the CONSORT statement,4 Jadad criteria (a score ranging from 0 to 5 used to assess the methodologic quality of a clinical trial),5 and assessment criteria for evaluating the quality of clinical trial methodology and clinical relevance.6 Two pediatric neurologists (J.P. and O.D.) reviewed articles and confirmed the exclusions identified by the epidemiologist reviewers.
In the 2- to 70-year age group, trials that were randomized, double-blind, and placebo-controlled were included in the analysis. As there are few such trials published in the literature in children <2 years of age, double-blind trials without placebo control for this age group were included in the analysis if dosage comparisons among medications could be made. Additionally, a minimum number of trial participants was not required in this age group for analysis. Trials of eslicarbazepine and lacosamide were excluded since there are no published data in children with which to compare the efficacy in adults. Drug withdrawal trials in which withdrawal was to “no therapy” were also excluded from the analysis.
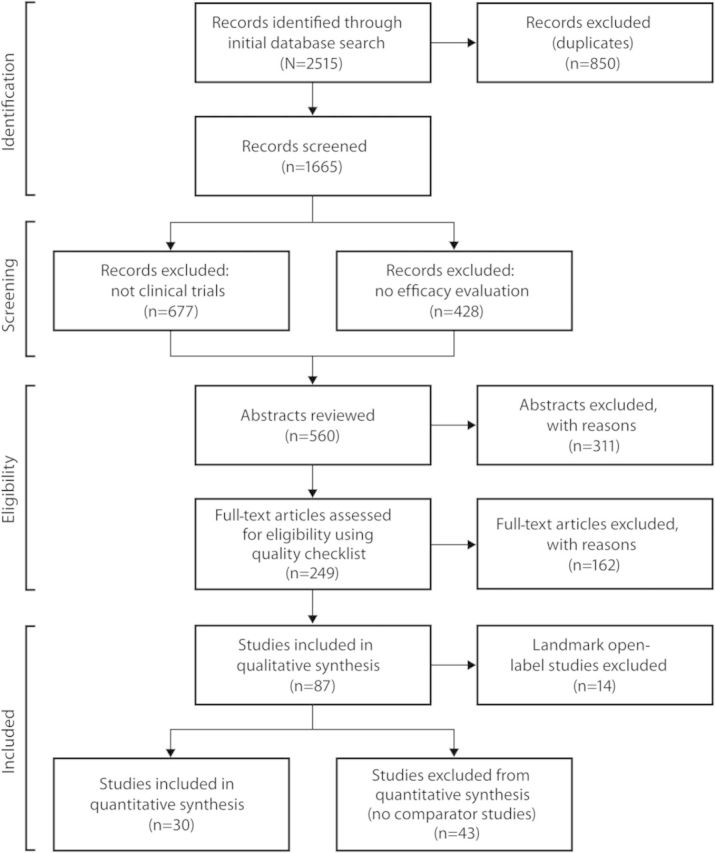
In the adult and 2- to 18-year age groups, initial searches yielded 2,515 titles (figure 1). After applying the inclusion and exclusion criteria, a total of 73 published trials were eligible for quantitative analysis, though only 30 were used since the remaining 43 were trials in adults for which no comparable trials in children were available. Quantitative analyses were conducted for adjunctive therapy trials in POS only as there were too few published trials for monotherapy in POS or for any therapy in PGTCS. The average Jadad score for the 30 trials used in the quantitative analysis was 3.5, with no score lower than 2 (Jadad Quality Score ranges from 0 to 5, with 0–2 considered low and 3–5 considered high). In the <2-year age group, the initial searches yielded 2,378 titles that, upon application of the inclusion and exclusion criteria, resulted in review of 196 full-length articles and 38 abstracts (figure e-1). Only 3 trials were randomized, double-blind, and placebo-controlled. A quantitative analysis was not performed for the trials in children <2 years since there were too few trials that met the predefined criteria for analysis.
Figure 1. Algorithm for identification of clinical trials in adults and children 2–18 years of age.
Effect measures, quantitative analysis, and statistical methods.
The most commonly reported efficacy measures in the trials selected for analysis were 1) median percent reduction in seizure frequency from baseline and 2) percentage of patients who experienced a ≥50% reduction in seizure frequency from baseline (≥50% responder rate). These measures were abstracted for patients who received adjunctive drug treatment and for those who received placebo.
Based on the 2 abstracted efficacy measures, placebo-subtracted effect measures were calculated for each trial as the difference in group efficacy measure between drug-treated patients and placebo-treated patients. Standard errors were estimated for each trial and 95% confidence intervals (CIs) for the effect measures were calculated. Statistical analyses were performed and forest plots were constructed with a statistical analysis package for research synthesis (Comprehensive Meta Analysis version 2).
RESULTS
Age 2–18 years and comparable adult trials.
Characteristics of the 30 trials eligible for quantitative analysis in the 2- to 18-year age group are shown in the table, with the references identified in appendix e-2. All trials were for the treatment of POS (5 gabapentin, 6 lamotrigine, 10 levetiracetam, 2 oxcarbazepine, and 7 topiramate), with 6 trials conducted in children and 24 comparable clinical trials conducted in adults.
Table.
Characteristics of the 30 studies in adjunctive POS among adults and children 2–18 years
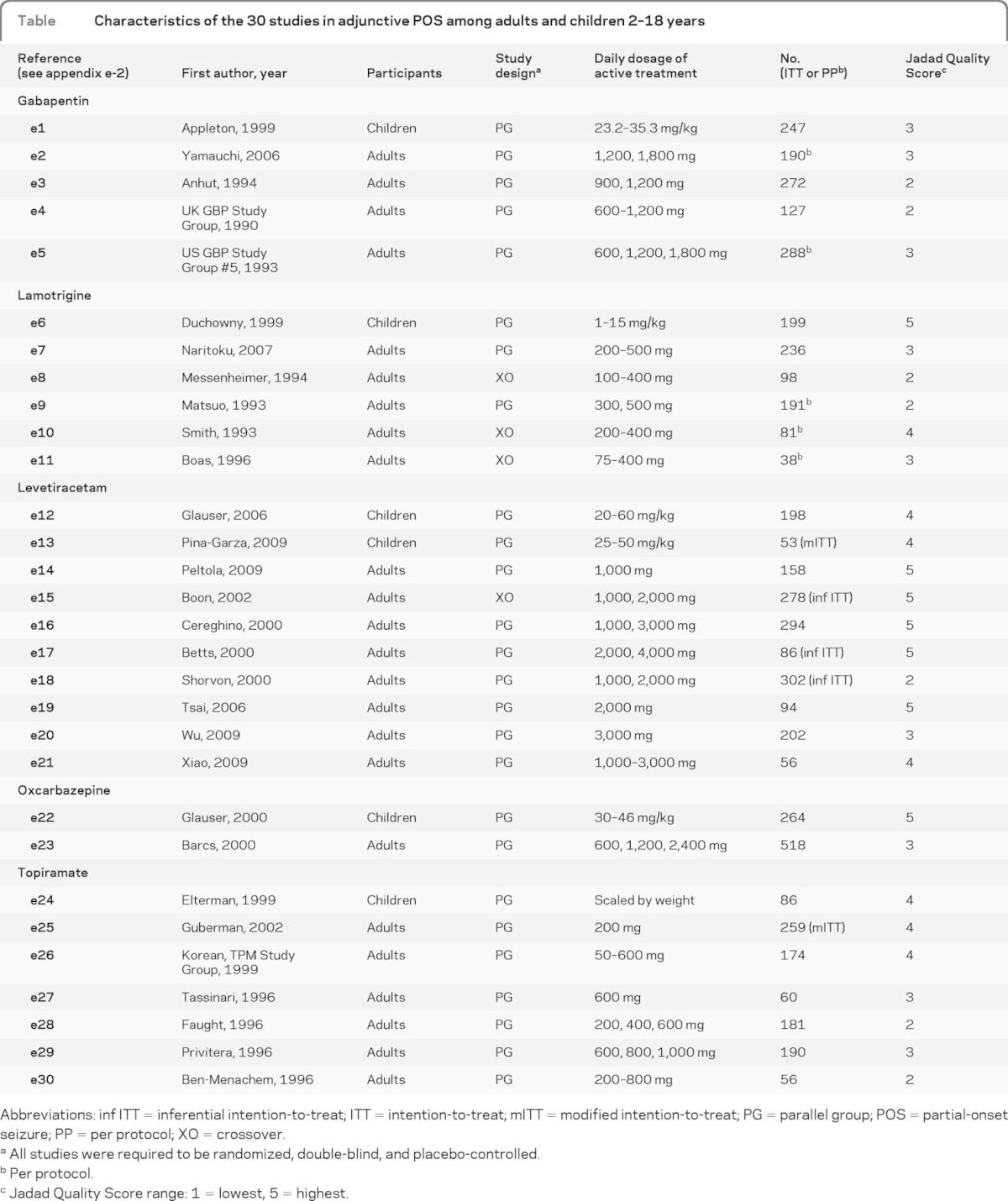
Abbreviations: inf ITT = inferential intention-to-treat; ITT = intention-to-treat; mITT = modified intention-to-treat; PG = parallel group; POS = partial-onset seizure; PP = per protocol; XO = crossover.
All studies were required to be randomized, double-blind, and placebo-controlled.
Per protocol.
Jadad Quality Score range: 1 = lowest, 5 = highest.
Adjunctive therapy for POS.
The placebo-subtracted effect measures based on median percent seizure reduction in trials conducted in children were comparable to those in adults for adjunctive POS therapy with gabapentin, lamotrigine, levetiracetam, oxcarbazepine, and topiramate. Forest plots were created for these 5 drugs from 6 clinical trials and 6 treatment groups in children and from 24 clinical trials and 46 treatment groups in adults (figure 2). These effect measures ranged from 10.5% to 31.2% in children and from 7.0% to 58.6% in adults and were significantly greater than zero for 6 of 6 treatment groups in children and for 40 of 46 in adults. Though the CIs for 6 treatment groups in adults were outside the range of “favors treatment,” the mean differences between baseline and treatment period for each regimen were within the range. Overall, the effect measures based on median percent seizure reduction were consistent from trial to trial and were comparable between adult and pediatric studies for all 5 AEDs, as indicated by the overlapping CIs.
Figure 2. Efficacy comparison of differences in median % seizure reduction between baseline and treatment periods by drug for children and adults.
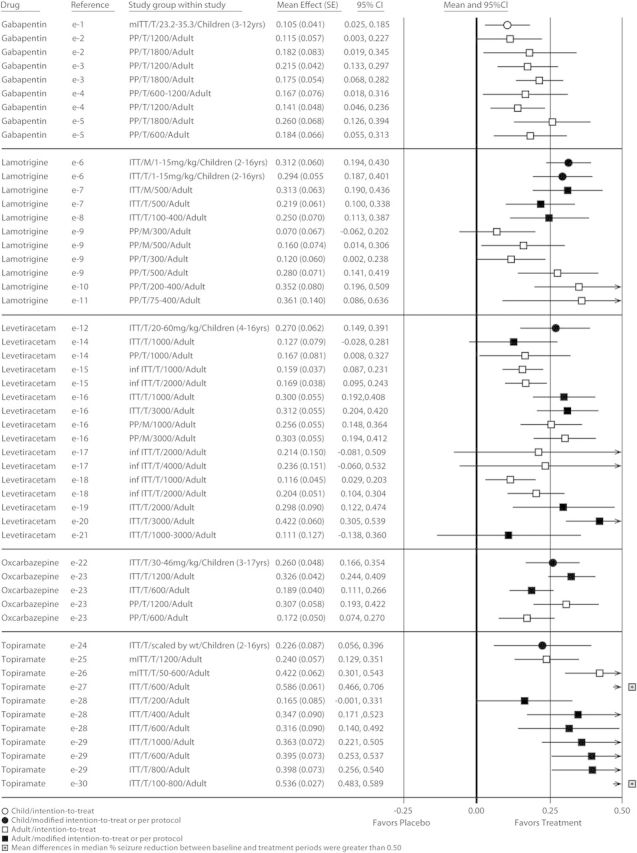
A single row in the forest plot represents 1 trial or 1 trial regimen. In these plots, each effect measure is bounded by a confidence interval (CI). The null hypothesis for these analyses—that there would be no difference between drug-treated patients and those who received placebo—would yield a value of zero for each effect measure, indicated by the center line of the plot. An effect measure to the right of center favors drug treatment, while an effect measure to the left of center favors placebo. The CIs indicate the precision of that effect measure estimate. If the interval includes zero, this indicates that the p value was >0.05. inf ITT = inferential intention-to-treat; ITT = intention-to-treat; mITT = modified intention-to-treat; PP = per protocol.
The placebo-subtracted effect measures based on the ≥50% responder rate in trials conducted in children were comparable to those in adults for adjunctive POS therapy with gabapentin, lamotrigine, levetiracetam, oxcarbazepine, and topiramate. Forest plots were created for these 5 drugs from 6 clinical trials and 8 regimens in children and from 22 clinical trials and 43 regimens in adults (figure 3). Effect measures ranging from 3.0% to 26.0% in children and from 2.0% to 43.0% in adults were significantly greater than zero for 5 of 8 regimens and for 37 of 43 regimens, respectively, indicating that they were reasonably consistent from trial to trial and were comparable between adult and pediatric studies for all 5 AEDs. For the nonsignificant treatment groups, mean differences in ≥50% responder rates between baseline and treatment period in both children and adults were within the range of “favors treatment,” but the CIs reached beyond that margin.
Figure 3. Efficacy comparison of differences in ≥50% reduction in seizure frequency between baseline and treatment periods by drug for children and adults.
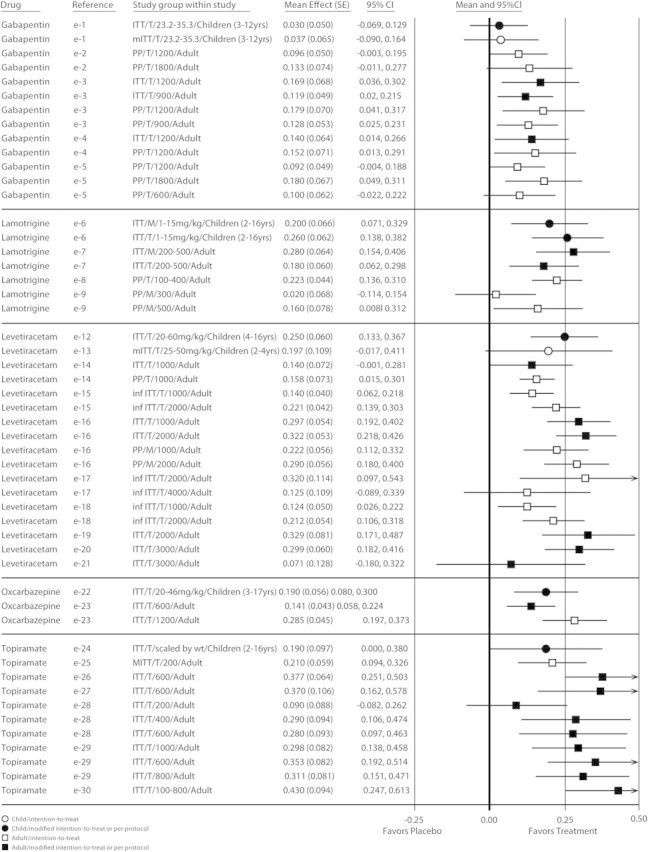
A single row in the forest plot represents 1 trial or 1 trial regimen. In these plots, each effect measure is bounded by a confidence interval (CI). The null hypothesis for these analyses—that there would be no difference between drug-treated patients and those who received placebo—would yield a value of zero for each effect measure, indicated by the center line of the plot. An effect measure to the right of center favors drug treatment, while an effect measure to the left of center favors placebo. The CIs indicate the precision of that effect measure estimate. If the interval includes zero, this indicates that the p value was >0.05. inf ITT = inferential intention-to-treat; ITT = intention-to-treat; mITT = modified intention-to-treat; PP = per protocol.
POS monotherapy and PGTCS adjunctive or monotherapy.
Only 1 of 3 randomized, double-blind, dose-controlled monotherapy trials in POS that contained pediatric participants met inclusion criteria and provided data that could be separated by age groups,7 and a single trial of PGTCS with data from adults and children was eligible for review.8 Due to the paucity of eligible trials, a quantitative analysis for monotherapy trials in POS or for any therapy in PGTCS could not be performed.
Age <2 years.
Only 3 trials of the 1,615 unique titles identified in the initial searches for the <2-year age group met inclusion criteria (figure e-1). One trial conducted with levetiracetam9 showed positive results compared to placebo, while the other 2 trials (1 with lamotrigine10 and the other with topiramate11) showed effects that were not significantly different from placebo. No attempt was made to quantitatively analyze these studies due to the few trials eligible for analysis.
DISCUSSION
It is commonly accepted that all medication, whether provided to pediatric patients or adults, should be appropriately and extensively evaluated. Ideally, a large-scale, well-controlled, longer-term randomized phase III clinical trial would provide the best supportive evidence for the efficacy, safety, and pharmacokinetic profile of a particular therapeutic agent for the pediatric population; however, conducting clinical trials in children can be clinically, operationally, and ethically challenging (e.g., difficulties in subject recruitment, obtaining informed consent, biological sampling, ethical concerns), and should be avoided particularly when the disease or disorder is comparable to the adult condition.3 An alternative to performing controlled efficacy trials in children is to predict efficacy based on well-controlled trials in adults, and determine dose range, tolerability, and safety in children by conducting appropriate pharmacokinetic and safety studies only in children.
Recognizing the need for alternatives to placebo-controlled trials in children, both the US Food and Drug Administration (FDA) and the EMA have explored methods to authorize the use of new medicines through data extrapolation from adult clinical trials. In 1994, the FDA published a final rule in the Federal Register regarding the “Pediatric Use” subsection of the labeling for human prescription drugs.12 Under this rule, the FDA “may approve a drug for pediatric use based on adequate and well-controlled studies in adults, with other information supporting pediatric use. In such cases, the agency will have concluded that the course of the disease and effects of the drug are sufficiently similar in adults and the pediatric population to permit extrapolation from the adult efficacy data to pediatric patients.”12 In 2006, the European Parliament and the Council for the European Union introduced new legislation governing the development and authorization of medicines for use in children (age 0–17 years) that allows extrapolation of data from comparable adult clinical trials.13 Such methods have been endorsed by the International Committee on Harmonization. According to International Committee on Harmonization E11, “when a medicinal product is to be used in the pediatric population for the same indication(s) as those studied and approved in adults, the disease process is similar in adults and pediatric patients, and the outcome of therapy is likely to be comparable, extrapolation from adult efficacy data may be appropriate.”2
Extrapolation of efficacy data from adults to the pediatric population for AEDs in particular has been supported by the NIH. In a 1996 consensus statement, the NIH included the recommendation for data extrapolation from adults to children for use in partial seizures provided that safety and pharmacokinetic considerations are appropriate.14 Furthermore, the conclusions from an EMA-sponsored Pediatric Epilepsy Group Experts Meeting in 200915 stated that “there was general agreement that results of studies for POS in adults could be extrapolated down to the age of about 4 years.” Subcommittees of the American Academy of Neurology and the American Epilepsy Society16 have also supported this approach for adjunctive therapy in children older than 2 years with refractory partial seizures. Finally, in a recent review of AED development in children,3 no difference was observed in the results from large randomized clinical trials in adults and children that used the common and sensitive primary efficacy parameter of percentage of seizure decrease vs baseline. The authors concluded that “For focal epilepsy, it therefore seems logical to extrapolate efficacy data from adults to children aged 2 years and above,” but noted that trials in infants and trials for epileptic encephalopathies are required and that safety trials are required for all pediatric age groups.3
This review was conducted in response to a regulatory request for a particular AED (brivaracetam). The results of the review, however, suggest a more universal application to pediatric drug development. Despite the fact that the number of pediatric trials in this systematic review is small, the findings presented here are based on well-controlled and well-conducted trials in which efficacy outcomes were observed to be similar for all AEDs included in the analysis. Though some data are available for gabapentin suggesting that values for responder rates and median percent seizure reduction are lower in children than in adults, these data are available only in the product label and not in the published literature, thus they were not included in this analysis. This situation brings to light the possible inherent limitations of this analysis, including 1) differences in clinical trial methodology and reporting of results, 2) varying populations between adult and pediatric trials (e.g., possibility of presence of benign epilepsies of childhood in pediatric studies with no appropriate adult comparison), 3) differences in analysis subsets (intention-to-treat, modified intention-to-treat), and 4) differing dosage and length of treatment periods reported. Due to such differences between the adult and pediatric studies of this systematic review of published trial data, there is a possibility that in clinical practice the AEDs included in the analysis could possibly be less effective in children than in adults; however, within the confines of the recognized limitations, the results presented here are robust and consistent and provide evidence for a possible alternative approach to conducting controlled trials in children with POS in order to establish AED efficacy.
This analysis supports the extrapolation of efficacy from adult AED clinical trials in POS to predict efficacy in children 2–18 years of age with POS, excluding epilepsies known to only occur in children (i.e., benign epilepsies of childhood). This evidence-based review, which represents a collaborative approach between regulators, industry, and academia, may be helpful in expediting drug development for children with epilepsy.
Supplementary Material
ACKNOWLEDGMENT
Publication management in the form of publication coordination was provided by Elizabeth Hackler, PhD, employee of UCB Pharma. Editorial support in the form of technical writing and editing was provided by Kristen A. Andersen, PhD, of Prescott Medical Communications Group, Chicago, IL.
GLOSSARY
- AED
antiepileptic drug
- CI
confidence interval
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- PGTCS
primary generalized tonic-clonic seizure
- POS
partial-onset seizure
Footnotes
Editorial, page 1420
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
J.M. Pellock contributed to the design and conceptualization of the study, interpretation of the data analysis, and the drafting and revising of the manuscript. W.J. Carman contributed to the design of the study, the analysis and interpretation of the data, as well as the drafting and revising of the manuscript. V. Thyagarajan contributed to the design of the study, the analysis and interpretation of the data, and the drafting and revising of the manuscript. T. Daniels contributed to the conceptualization of the study, the interpretation of the data, and the revising of the manuscript. D.L. Morris contributed to the conceptualization of the study, the interpretation of the data, and the revising of the manuscript. O. D'Cruz contributed to the design and conceptualization of the study, interpretation of the data analysis, and the drafting and revising of the manuscript.
DISCLOSURE
J.M. Pellock has received grants and research support and is a paid consultant for the NIH/NINDS, Eisai, GSK, King Pharmaceuticals, KV Pharmaceuticals, Marinus Pharmaceuticals, Neuropace, Ortho McNeil Johnson & Johnson, Lundbeck, Pfizer, Questcor, Sepracor, UCB Pharmaceuticals, and Valeant. Dr. Pellock also has served as an advisory board member, consultant, or lecturer, with all grants, research support, consultant fees and honoraria paid to Virginia Commonwealth University or to the physician practice plan (MCV Physicians). W.J. Carman is an employee of OptumInsight, Waltham, MA, Epidemiology, contracted by UCB Pharma to perform the systematic review. V. Thyagarajan is an employee of OptumInsight, Waltham, MA, Epidemiology, contracted by UCB Pharma to perform the systematic review. T. Daniels is an employee of UCB Pharma, Raleigh, NC. D.L. Morris is an employee of UCB Pharma, Raleigh, NC. O. D'Cruz is an employee of UCB Pharma, Raleigh, NC. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rocchi F, Tomasi P.The development of medicines for children: part of a series on pediatric pharmacology, guest edited by Gianvincenzo Zuccotti, Emilio Clementi, and Massimo Molteni.Pharmacol Res 2011;64:169–175 [DOI] [PubMed] [Google Scholar]
- 2.ICH Topic E 11 Clinical Investigation of Medicinal Products in the Paediatric Population: Note for Guidance on Clinical Investigation of Medicinal Products in the Paediatric Population (CPMP/ICH/2711/99). [Accessed December 1, 2011]. Available at: www.ema.europa.eu/pdfs/human/ich/271199en.pdf.
- 3.Chiron C, Dulac O, Pons G.Antiepileptic drug development in children: considerations for a revisited strategy.Drugs 2008;68:17–25 [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, Altman DG.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement.PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 6.Cho MK, Bero LA.Instruments for assessing the quality of drug studies published in the medical literature.JAMA 1994;272:101–104 [PubMed] [Google Scholar]
- 7.Glauser TA, Dlugos DJ, Dodson WE, Grinspan A, Wang S, Wu SC.Topiramate monotherapy in newly diagnosed epilepsy in children and adolescents.J Child Neurol 2007;22:693–699 [DOI] [PubMed] [Google Scholar]
- 8.Biton V, Montouris GD, Ritter F, et al. A randomized, placebo-controlled study of topiramate in primary generalized tonic-clonic seizures: Topiramate YTC Study Group.Neurology 1999;52:1330–1337 [DOI] [PubMed] [Google Scholar]
- 9.Pina-Garza JE, Nordli DR, Jr, Rating D, Yang H, Schiemann-Delgado J, Duncan B.Adjunctive levetiracetam in infants and young children with refractory partial-onset seizures.Epilepsia 2009;50:1141–1149 [DOI] [PubMed] [Google Scholar]
- 10.Pina-Garza JE, Levisohn P, Gucuyener K, et al. Adjunctive lamotrigine for partial seizures in patients aged 1 to 24 months.Neurology 2008;70:2099–2108 [DOI] [PubMed] [Google Scholar]
- 11.Novotny E, Renfroe B, Yardi N, et al. Randomized trial of adjunctive topiramate therapy in infants with refractory partial seizures.Neurology 2010;74:714–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.59 FR specific requirements on content and format of labeling for human prescription drugs; revision of “pediatric use” subsection in the labeling. In: Department of Health and Human Services FaDAH, ed. Volume 59, Issue 238 Vol Final Rule. Office of the Federal Register,National Archives and Records Administration;1994 [Google Scholar]
- 13.Regulation (EC) No 1901/2006 on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. In: Union TEPaTCotE, ed. Vol Final Rule. Official J Eur Union2006 [Google Scholar]
- 14.Sheridan PH, Jacobs MP.The development of antiepileptic drugs for children: report from the NIH workshop, Bethesda, Maryland, February 17–18, 1994. Epilepsy Res 1996;23:87–92 [DOI] [PubMed] [Google Scholar]
- 15.Conclusions of the Paediatric Epilepsy Experts Group Meeting, London 1 September 2009. EMA/153272 Vol Final Rule: Human Medicines Development and Education;2010Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/events/2010/05/event_detail_000255.jsp&mid=WC0b01ac058004d5c3&jsenabled=true#.Accessed December 2, 2011
- 16.French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society.Neurology 2004;62:1261–1273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


