Abstract
Candida albicans is a major cause of oropharyngeal, vulvovaginal and hematogenously disseminated candidiasis. Endocytosis of C. albicans hyphae by host cells is a prerequisite for tissue invasion. This internalization involves interactions between the fungal invasin Als3 and host E- or N-cadherin. Als3 shares some structural similarity with InlA, a major invasion protein of the bacterium Listeria monocytogenes. InlA mediates entry of L. monocytogenes into host cells through binding to E-cadherin. A role in internalization, for a non classical stimulation of the clathrin-dependent endocytosis machinery was recently highlighted. Based on the similarities between the C. albicans and L. monocytogenes invasion proteins, we studied the role of clathrin in the internalization of C. albicans. Using live-cell imaging and indirect immunofluorescence of epithelial cells infected with C. albicans, we observed that host E-cadherin, clathrin, dynamin and cortactin accumulated at sites of C. albicans internalization. Similarly, in endothelial cells, host N-cadherin, clathrin and cortactin accumulated at sites of fungal endocytosis. Furthermore, clathrin, dynamin or cortactin depletion strongly inhibited C. albicans internalization by epithelial cells. Finally, beads coated with Als3 were internalized in a clathrin-dependent manner. These data indicate that C. albicans, like L. monocytogenes, hijacks the clathrin-dependent endocytic machinery to invade host cells.
INTRODUCTION
Candida albicans is a dimorphic fungus that causes superficial oral or vaginal infections as well as life threatening disseminated candidiasis. The capacity of C. albicans to change from yeast to hyphae is an important virulence factor of this organism (Lo et al., 1997; Saville et al., 2003; Park et al., 2005). In vitro, C. albicans hyphae are endocytosed by oral epithelial and endothelial cells (Rotrosen et al., 1985; Drago et al., 2000) and this endocytosis is a prerequisite for the organism to damage host cells (Filler et al., 1995; Park et al., 2005). Endocytosis is induced by the interaction of the C. albicans Als3 adhesin with E-cadherin on epithelial cells and N-cadherin on endothelial cells. This interaction stimulates rearrangement of host cell actin, which is necessary for C. albicans invasion (Rotrosen et al., 1985; Filler et al., 1995; Phan et al., 2005; Phan et al., 2007). The first steps of this process are driven by the host cell since killed hyphae are internalized as efficiently as live hyphae even though they do not cause host cell damage (Filler et al., 1995; Phan et al., 2000; Park et al., 2005). However, the cellular machinery that mediates actin rearrangement and internalization of C. albicans hyphae remains unknown.
The Gram-positive bacterial pathogen Listeria monocytogenes is also able to invade host mammalian cells by interacting with E-cadherin (Mengaud et al., 1996a; Lecuit et al., 1999. Bonazzi et al 2009). The bacterial effector that recognizes E-cadherin is InlA (internalin), which belongs to the internalin family (Gaillard et al., 1991; Mengaud et al., 1996b; Mengaud et al., 1996a; Lecuit et al., 1997; Bierne et al., 2007). Interestingly, the cleft motif found at the amino-terminus of C. albicans Als3 is structurally similar to bacterial leucine-rich repeat domains from internalins (Schubert et al., 2002; Phan et al., 2007). Both InlA and Als3 induce pathogen uptake mediated by rearrangement of the actin cytoskeleton (Hamon et al., 2006). L. monocytogenes internalization has been extensively studied and the signal transduction pathway induced by the InlA/E-cadherin interaction is relatively well understood (Hamon et al., 2006; Bonazzi et al 2009). This pathway, which mimics the one that induces the formation of adherens junctions, involves the recruitment of β and α catenins, ARHGAP10, Arf6 and vezatin, as well as activation of the Arp2/3 complex (Lecuit et al., 2000; Sousa et al., 2004; Sousa et al., 2005; Hamon et al., 2006; Sousa et al., 2007). Recently, we have shown that L. monocytogenes invasion requires proteins normally involved in several endocytic pathways including clathrin, caveolin and dynamin (Veiga and Cossart, 2005; Veiga et al., 2007; Bonazzi et al., 2008). These results suggest that the endocytic machineries together with the actin cytoskeleton provide the force that actively engulfs invasive bacteria (Veiga and Cossart, 2005; Veiga et al., 2007). Clathrin-mediated endocytosis is linked to actin rearrangement by mechanisms that, although not fully understood, are known to require the presence of cortactin, an activator of the Arp2/3 complex (Veiga and Cossart, 2005; Sousa et al., 2007; Veiga et al., 2007).
Clathrin and its associated proteins are in fact involved in the internalization of a large variety of pathogens, including viruses and bacteria (Conner and Schmid, 2003; Marsh and Helenius, 2006; Veiga et al., 2007). However, it was previously unknown whether similar pathways mediated the internalization of fungi, which are considerably larger than viruses and bacteria. Based on the similarities of the C. albicans and L. monocytogenes models, we investigated whether clathrin plays a role in the internalization of this fungal pathogen.
RESULTS AND DISCUSSION
Clathrin is recruited at the C. albicans entry site
To address whether C. albicans exploits a clathrin-dependent mechanism to invade non-phagocytic host cells, we analyzed the interactions of a wild-type clinical isolate of C. albicans, strain SC5314, with both epithelial and endothelial cells. The human epithelial HEK293 and JEG-3 cell lines were used because they are known to endocytose L. monocytogenes by an E-cadherin-dependent mechanism (Sousa et al., 2005). Primary human umbilical-vein endothelial cells were used because they endocytose C. albicans via a N-cadherin-dependent mechanism (Phan et al., 2005). Both cell types were infected with C. albicans blastospores for 90 up to 120 min in RPMI 1640 medium at 37°C to allow the C. albicans cells to germinate and form hyphae, which were subsequently engulfed by the host cells in an actin-dependent manner (Filler et al., 1995; Park et al., 2005; Phan et al., 2007). Results presented in Figures 1A, 1B and Supplementary Figure S1 showed that fluorescently tagged clathrin, as well as endogenous clathrin, co-localized with endocytosed C. albicans hyphae in both epithelial and endothelial cells.
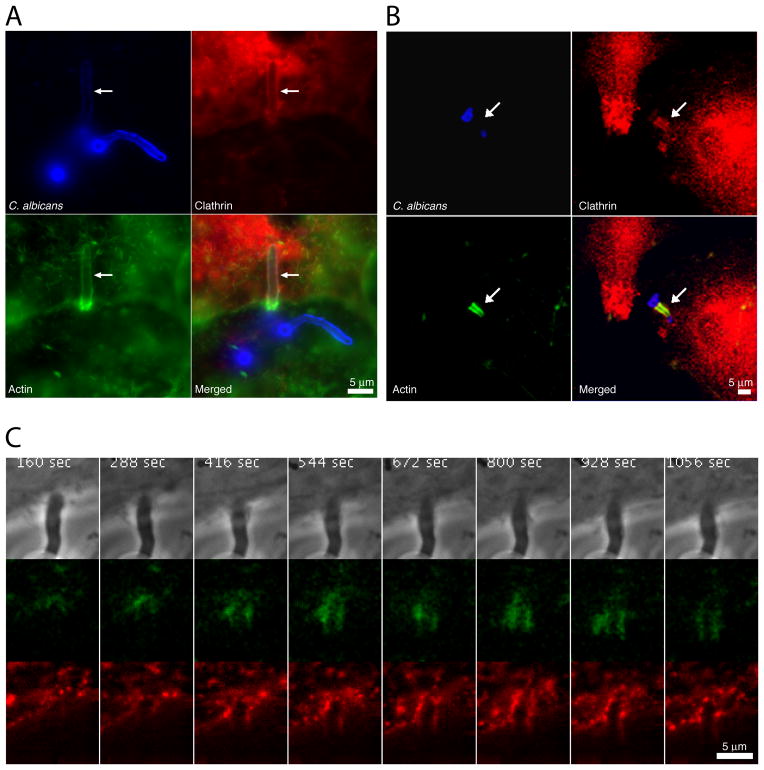
Figure 1.
Recruitment of clathrin during C. albicans internalization. (A) JEG-3 epithelial cells transformed with td-Tomato-LCa (clathrin light chain) were infected with the C. albicans SC5314 strain, shown in blue. Fluorescent clathrin is shown in red and actin detected with phalloidin in green. Arrows indicate internalized hyphae. Scale bar = 5μm. (B) Primary endothelial cells were infected with the C. albicans SC5314 strain, shown in blue. Clathrin, shown in red, was immunodetected using anti-clathrin heavy chain antibodies. Actin, shown in green, was detected with phalloidin. Arrows point internalized hyphae. Scale bar = 5μm. (C) Time series from HeLa cells transiently expressing tdTomato-LCa (red) and E-cadherin-GFP (green). Cells were infected with C. albicans SC5314 (phase). Recording started 32 min after infection. The figure shows one of every 16 acquisition frames from Supp. Movie 1. Scale bar = 5 μm.
The time course of recruitment of clathrin around C. albicans hyphae was also followed by live-cell imaging. HeLa cells expressing td-Tomato-LCa (clathrin light chain) and E-cadherin-GFP were infected for 30 min at 37°C and hyphal endocytosis was monitored during the subsequent 20 min. As shown in Figure 1C and Supplementary Movie 1, clathrin was recruited to the sites at which C. albicans hyphae entered the epithelial cells. Similar to what has been observed during bacterial internalization, the amount of clathrin that was recruited around the internalizing hyphae fluctuated over time. This pattern contrasts with the continuous, progressive accumulation of clathrin that is observed during typical clathrin-mediated endocytosis (Ehrlich et al., 2004; Boucrot et al., 2006; Massol et al., 2006).
The time course of E-cadherin recruitment during internalization was also determined. We found that E-cadherin co-localized with clathrin around hyphae that were internalized by epithelial cells (Figure 1C; Supplementary Movie S1). Interestingly, the amount of E-cadherin that accumulated around the hyphae also fluctuated over time, in parallel with clathrin.
Dynamin and cortactin are also recruited during C. albicans internalization
The GTPase dynamin is required for clathrin coated pits to pinch off the plasma membrane and form endocytic vesicles (Hinshaw, 2000; Kirchhausen, 2000; Conner and Schmid, 2003; Orth and McNiven, 2003; Ehrlich et al., 2004; Merrifield et al., 2005; Macia et al., 2006). Furthermore, dynamin is absolutely required for bacterial internalization (Veiga and Cossart, 2005; Veiga et al., 2007). During classical clathrin-dependent endocytosis and during bacterial internalization, dynamin is localized not only at the neck of vesicles pinching off the membrane but also around the entire vesicle (Stang et al., 2004; Veiga and Cossart, 2005; Veiga et al., 2007).
The endocytosis of C. albicans is unusual because hyphae are too large to be completely internalized by a single host cell. Frequently, the blastospore attached to the hypha remains extracellular. Therefore, the endocytic tube containing the penetrating hypha cannot pinch off the membrane as observed upon endocytosis of bacteria or smaller particles. Because dynamin is required for scission of endocytic vesicles, we investigated the distribution of dynamin during the endocytosis of C. albicans in GFP-dynamin expressing epithelial cells. After 120 min of infection, dynamin accumulated together with actin around C. albicans hyphae (Fig. 2 and Supp. Fig. S2).
Figure 2.
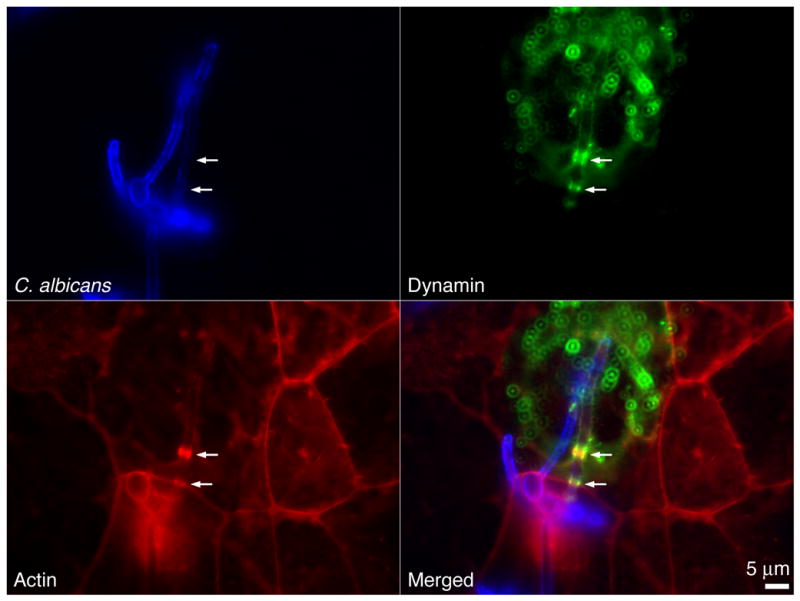
Localization of dynamin during C. albicans internalization. JEG-3 cells transformed with dynamin-GFP were infected for 120 min with C. albicans SC5314, shown in blue. Dynamin is shown in green and actin in red. Arrows mark internalized hyphae and accumulation at the entry site of both dynamin and actin. Scale bar = 5μm.
In addition to inducing vesicle scission, dynamin is involved in other cellular functions, which may also contribute to the endocytosis of microbial pathogens. For example, dynamin forms a complex with cortactin, which in turn activates the Arp2/3 complex (Krueger et al., 2003). The Arp2/3 complex mediates the polymerization of actin, which is required for endocytosis to occur (Engqvist-Goldstein and Drubin, 2003; Kaksonen et al., 2006). Consistent with this model, cortactin has been recently found to play a major role in E-cadherin dependent entry of L. monocytogenes (Sousa et al., 2007; Veiga et al., 2007). In addition, dynamin and the lymphocyte cortactin homolog Hs1 have been described to play a major role in the massive actin polymerization observed during the immune synapse formation (Billadeau et al., 2007). As actin rearrangements are involved in the internalization of C. albicans by epithelial and endothelial cells (Rotrosen et al., 1985; Filler et al., 1995; Park et al., 2005; Phan et al., 2005; Phan et al., 2007) we investigated the localization of cortactin during this process. As shown in Figure 3A and B, and Supplementary Figure S3A, cortactin accumulated with actin around C. albicans hyphae that were in the process of being internalized by either epithelial or endothelial cells. N-cadherin also accumulated with cortactin around organisms that were being endocytosed by endothelial cells (Figure 3C), suggesting that N-cadherin-mediated internalization of C. albicans occurs by the recruitment of cortactin. We also show as control a membrane protein (ICAM-I) not localizing with C. albicans during HEK293 infection (Supp. Fig. S3B).
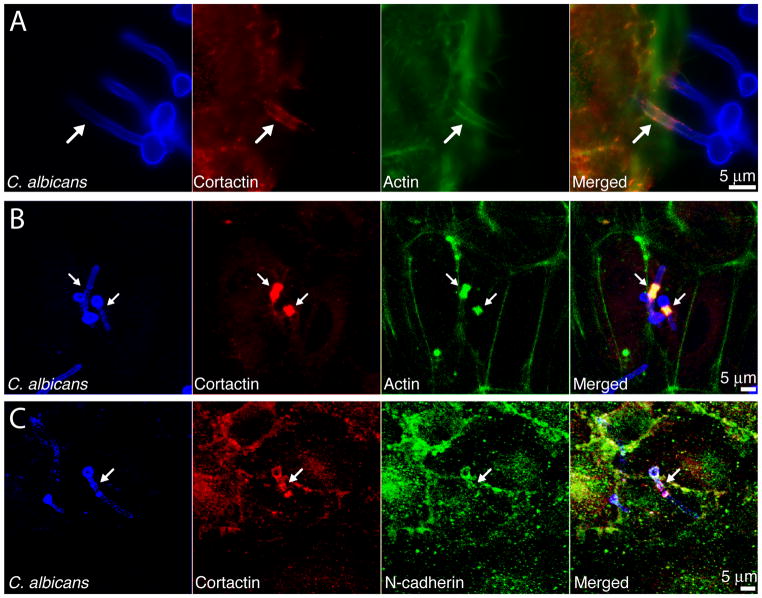
Figure 3.
Recruitment of cortactin to C. albicans entry site. (A) JEG-3 cells were infected with C. albicans SC5314, shown in blue. Cortactin, shown in red, was immunodetected using anti-cortactin antibodies. Actin, detected with phalloidin, is shown in green. Arrows mark internalized hyphae. Scale bar = 5μm. (B–C) Primary endothelial cells were infected with C. albicans SC5314, shown in blue. Cortactin was immunodetected with its respective antibody and is shown in red. Actin (B), detected with phalloidin, and N-cadherin (C), immunodetected with anti-N-cadherin antibody, are shown in green
Taken together, these results indicate that clathrin, dynamin and cortactin were recruited together with actin and either E-cadherin or N-cadherin at the C. albicans hyphae entry sites in host cells. These results suggest a clathrin-mediated-mechanism together with active remodeling of the actin cytoskeleton contributing to hyphal internalization.
Clathrin, dynamin, and cortactin are required for C. albicans hyphae internalization
To further examine the role of clathrin, dynamin and cortactin in the invasion of C. albicans, siRNA was used to knock-down (KD) these proteins in epithelial cells. Clathrin KD in endothelial cells was attempted but was unsuccessful (data not shown). We quantified the percentage of cell-associated C. albicans hyphae that were internalized by differential immunofluorescence labelling as described (Park et al., 2005) (Fig. 4A and Supplementary Fig. S3 and Supp Movie S2). At least 200 cell-associated C. albicans cells were counted per condition in each experiment and a minimum of 3 independent experiments were performed with two different epithelial cell lines. As shown in Figure 4, depletion of clathrin, dynamin or cortactin by siRNA significantly reduced the internalization of C. albicans (Fig. 4B). The depletion of each of these proteins was highly efficient and specific. KD of one protein did not influence the expression of any of the other proteins, and also had no effect on total actin or cadherin expression (Fig. 4C and Supp. Fig. S4A). As the observed decrease in hyphae internalization could be due to reduced levels of an Als3 receptor (i.e. E-cadherin) on the cell surface, we used flow cytometry to measure the E-cadherin surface expression in control and clathrin siRNA KD cells. As shown in Figure 5, clathrin KD cells expressed similar levels of E-cadherin on their surface as did control cells. Additionally we tested the role of AP-2 (the major clathrin adaptor at the plasma membrane). Depletion of AP-2 also significantly reduced hyphal internalization (Supp. Fig. S4B–C. These data demonstrate that clathrin, dynamin, and cortactin are required for host cell uptake of C. albicans.
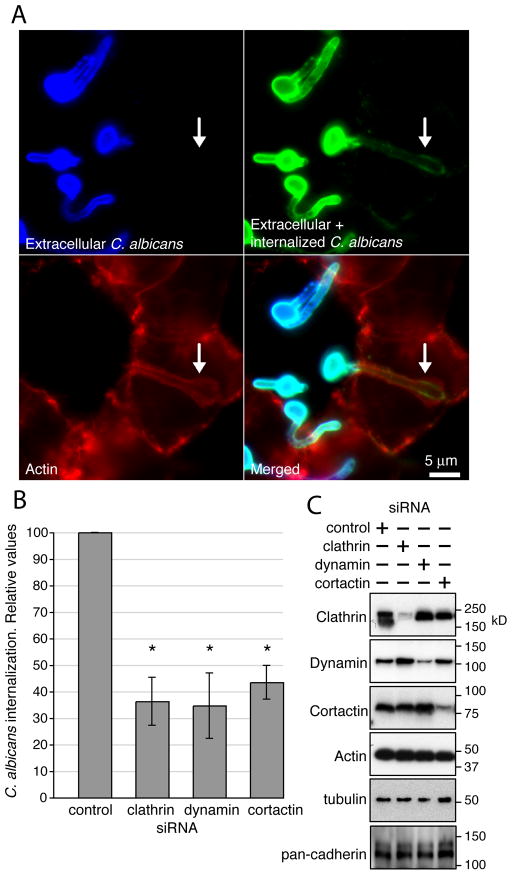
Figure 4.
Role of clathrin, dynamin, and cortactin in C. albicans internalization. (A) Extracellular C. albicans, immunodetected before permeabilization of epithelial cells are shown in blue. All C. albicans (extracellular and internalized) detected after permeablization are shown in green. Actin is shown in red. Arrows mark an internalized hypha. Scale bar = 5μm. (B) Clathrin, dynamin or cortactin depletion significantly decreased the endocytosis of C. albicans. HEK293 cells knocked-down (KD) by siRNA for the indicated proteins were infected with C. albicans SC5314. Fungal internalization ratio was measured by differential immunofluorescence labeling. Organisms that were at least partly internalized were counted as endocytosed. Data were normalized to control siRNA (RNA not targeting any cellular mRNA)-treated cells. Similar data were obtained in JEG-3 cells, but the siRNA treatment was more efficient using HEK293 cells (data not shown). Results are mean ± standard deviation of 3 independent experiments. * P < 0.05 compared to control. (C) Protein KD by siRNA was tested by Western-blot. + corresponds to samples treated with siRNA against the indicated proteins. Actin and tubulin are shown as a loading control. KD of clathrin or dynamin did not affect the expression of cadherins.
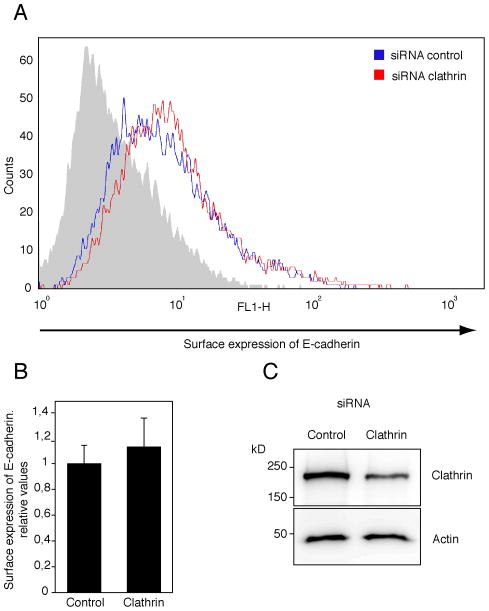
Figure 5.
Surface expression of E-cadherin. (A) A representative of 6 independent flow cytometry assays is shown. Surface exposed E-cadherin was immunodetected in non-permeabized epithelial cells using a Alexa Fluor 488-labelled anti E-cadherin antibody (see methods) in control (blue line) and clathrin knock-down (KD) (red line) cells. Grey histogram shows the fluorescent background observed using a non-specific antibody Alexa Fluor 488-labeled. (B) Combined results from flow cytometry assays (as in A). The number of events (i.e. cells) with fluorescence signal above 101 are represented normalized to the control. As shown, clathrin depletion did not affect E-cadherin expression on HEK293 cell surface. Results are mean ± standard deviation of 6 independent experiments. (C) Protein KD by siRNA in HEK293 cells was verified by immunoblotting with antibodies against clathrin and β-actin (as loading control). Clathrin siRNA KD data were normalized to control siRNA (RNA not targeting any cellular mRNA). Clathrin KD cells expressed similar levels of dynamin as did control cells (see Supp Fig. 4).
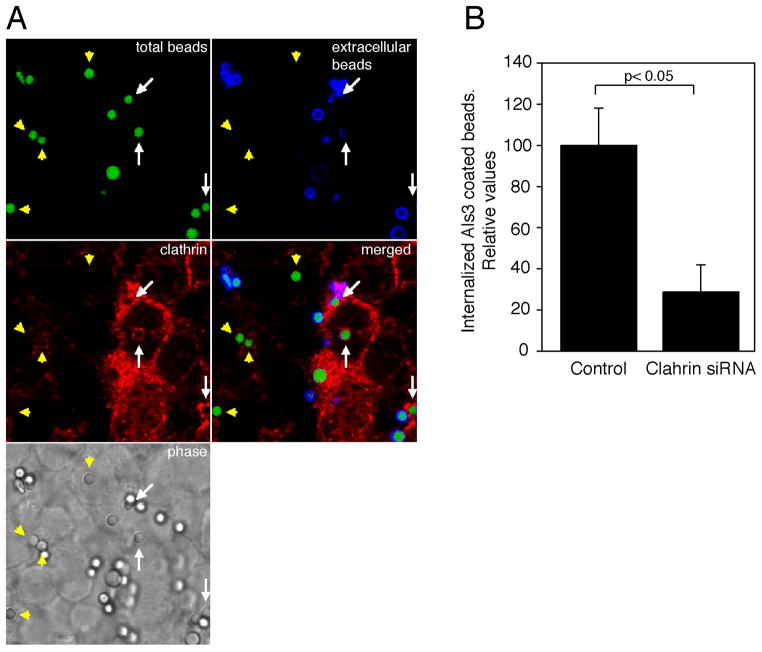
To further study the recruitment of clathrin and its role in the C. albicans invasion via Als3 in the absence of any other fungal factor, we used latex beads that had been coated with the recombinant N-terminal region of Als3. As reported previously (Phan et al., 2007), these beads were internalized by both epithelial and endothelial cells. Moreover clathrin accumulated around the Als3-coated beads during internalization (Fig. 6A, and Supp. Fig. S5). Similar to what was observed during the internalization of hyphae, this accumulation of clathrin was not continuous and varied in amount around the beads as they entered the cells. In contrast, very few BSA-coated beads were internalized, and clathrin did not accumulate around them (data not shown). Similarly to the hypha internalization data, we observed a dramatic reduction in number of Als3-coated beads that were internalized by epithelial cells when the level of clathrin was reduced by siRNA (Fig. 6B). Therefore, clathrin plays a key role in Als3-mediated internalization. (Fig. 6B).
Figure 6.
Role of clathrin in the internalization of Als3-coated beads. (A) Als3-coated beads were incubated with HEK293 cells for 45 min. Extracellular beads were immunodetected before permeabilization (blue) using anti-Als3 antibodies. Total beads (intracellular and extracellular) are shown in green. In the merged image extracellular beads are shown in cyan and intracellular beads in green and are tagged with arrowheads. Arrows indicate beads that are in the process of being internalized (only a portion of the bead can be seen in blue). It is clear that clathrin surrounds internalizing beads. (B) Clathrin depletion significantly decreased the endocytosis of Als3-coated beads. HEK293 cells knocked-down (KD) by siRNA for clathrin or treated with control siRNA were incubated with Als3-coated beads. The number of internalized beads was measured by differential immunofluorescence labeling. We compared the ratio between internalized and non-internalized beads in control or clathrin KD cells. Data were normalized to control siRNA (RNA not targeting any cellular mRNA)-treated cells. Results are mean ± standard deviation of 4 independent experiments. * P < 0.05 compared to control. Very few beads coated with BSA were internalized and clathrin did not accumulate around them (data not shown)
In conclusion, our results indicate that the internalization of C. albicans hyphae by host cells following Als3-cadherin interaction is mediated by the combined interactions of clathrin, dynamin and cortactin. These proteins in turn induce actin cytoskeleton remodeling, which results in endocytosis of the hyphae. Importantly, our results highlight the role of a clathrin-dependent mechanism in the entry of large pathogens. Similar to what has been observed during viral and bacterial infections, the clathrin-dependent machinery, in coordination with cytoskeleton rearrangements, seems to provide the necessary force to internalize the different pathogenic particles. Thus, clathrin appears to be a common host cell target for a variety of microbial pathogens, including fungi.
MATERIALS AND METHODS
Cells, fungi, and growth conditions
The human epithelial cell lines HeLa (ATCC number CCL-2), JEG-3 (ATCC number HTB-36), and HEK293 (ATCC number CRL-157) were grown as recommended by the ATCC. Endothelial cells were harvested from umbilical cord veins and maintained as previously described (Filler et al., 1995). C. albicans SC5314 (Gillum et al., 1984) was routinely grown at 30°C on minimal medium (0.67% yeast nitrogen base without amino acids, 0.4% glucose, pH 5.4). To induce hyphal formation, blastospores were incubated at 37°C in RPMI 1640 (Gibco) medium buffered with 50 mM Hepes pH 7.3.
Plasmids
The plasmid encoding dynamin2-GFP (isoform aa) was a gift from Prof. Mark A. McNiven (Cao et al., 1998). Plasmids encoding tdTomato-LCa (Massol et al., 2006) and GFP-LCa (Ehrlich et al., 2004) were gifts from Prof. Tomás Kirchhausen. The plasmid encoding E-cadherin-GFP was a gift from Prof. W. James Nelson (Yamada et al., 2005).
Antibodies and reagents
Alexa Fluor 488–, 546–, and 647–conjugated phalloidin, Alexa Fluor 488–, 546–, and 647–conjugated goat anti–rabbit and goat anti–mouse antibodies were purchased from Molecular Probes. Other antibodies used were: mouse monoclonal (mAb) anti-β-actin (AC15; Sigma), anti-clathrin heavy chain mAb (BD Pharmingen), rabbit polyclonal antibody (pAb) anti-dynamin2 (Calbiochem), anti-pan-cadherin pAb (Santa Cruz Biotechnologies), anti-cortactin mAb (4F11; Upstate), anti-cortactin (sc-11408, Santa Cruz Biotechnology), anti-C. albicans pAb (B65411R; Biodesign International), anti-tubulin mAb (Sigma Aldrich), or anti-N-cadherin mAb (clone 32, BD Biosciences), anti-CD324 (E-cadherin) Alexa Fluor 488-labeled Ab (324109, BioLegend), supernatant from the mouse myeloma P3-X63Ag8 (X63), anti-Als3 pAb (Phan et al., 2007) and green fluorescent latex beads coated with either the recombinant N-terminal region of Als3 or BSA were produced as described previously (Phan et al., 2007). When needed, anti-Candida antibodies were conjugated with Alexa Fluor-647 before proceeding with the immunolabelling.
Internalization assays
Internalization of C. albicans hyphae and Als3-coated beads was quantified as previously described with some modifications (Park et al., 2005). HEK293 or JEG-3 epithelial cells were infected with 105 blastospores of C. albicans SC5314 in RPMI 1640 for 120 min at 37°C. The samples were first incubated at 4°C with an anti-C. albicans pAb (1:50), washed with PBS fixed for 30 min with 3% paraformaldehyde (PFA) in PBS and incubated with Alexa Fluor 647-conjugated secondary antibodies. Cells were then permeabilized for 5 min with 0.5% Triton X-100 in PBS and incubated with anti-C. albicans pAb and with Alexa Fluor 488-conjugated secondary antibodies. Actin was stained using Alexa Fluor 546-conjugated phalloidin. C. albicans cells that stained with Alexa Fluor 647 and Alexa Fluor 488 were recorded as internalized while those staining with Alexa Fluor 647 only were counted as adherent, extracellular. Organisms that were partly engulfed were counted as internalized. To measure the internalization of Als3-coated latex beads, the epithelial cells were incubated with 3×105 beads ml−1 in RPMI 1640 for 45 min at 37°C. They were fixed in 3% PFA in PBS and then the non-internalized beads were detected with a polyclonal rabbit anti-Als3 antibody (Phan et al 2007) before permeabilization followed by an Alexa Fluor 647-conjugated secondary antibody.
Images were acquired with a fluorescence inverted microscope (Axiovert 135; Carl Zeiss MicroImaging, Inc.) equipped with a cooled charge-coupled device camera (MicroMax 5 MHz; Princetown Instruments) driven by the Metamorph Imaging System software (Universal Imaging Corp). For figure assembly, the images were treated using ImageJ 1.38x (http://rsb.info.nih.gov/ij/index.html).
Time-lapse wide-field microscopy
HeLa cells expressing E-cadherin-GFP and td-Tomato-LCa (clathrin light chain) were infected with 105 blastospores of C. albicans SC5314 in RPMI 1640 for 30 min at 37°C, then hyphal entry was monitored for 20 additional min. Images were acquired with a motorized inverted fluorescence microscope (Axiovert 200I, Carl Zeiss MicroImaging, Inc.) equipped with a temperature-controlled stage using 100x lenses (Carl Zeiss, Inc). Fluorescent illumination was driven by an ultrahigh-speed wavelength switcher Lambda DG4 (Sutter Instrument) equipped with a 175 W xenon arc lamp and excitation filters for GFP (Excitation=480 – Emission=525) and DsRed (Excitation= 565 – Emission=620) (Chroma Technology). Emission filters were selected using a high-speed Lambda 10 filter wheel (Sutter Instrument). Images were acquired with exposure times between 100 and 500 milliseconds with a cooled, digital, charge-coupled device camera (CoolSNAPHQ, Photometrics). All devices were controlled by the MetaMorph Imaging System software (Universal Imaging). Resulting images were treated using ImageJ 1.38x (http://rsb.info.nih.gov/ij/index.html).
Immunolabelling
Epithelial or endothelial cells were infected with 105 blastospores of C. albicans SC5314 suspended in RPMI 1640 medium. After 90 min or 120 min for endothelial and epithelial cells respectively, cells were rinsed and fixed in 3% PFA in PBS. Next, host cells were permeabilized for 5 min with 0.5% Triton X-100 in PBS and then incubated with an anti-C. albicans pAb (1:200) and with anti-clathrin, anti-cortactin and/or anti-N-cadherin antibodies. Alternatively, cells were transfected with tdTomato-LCa (fluorescent clathrin light chain), LCa-GFP or dynamin-GFP 24 h prior to infection. Actin was stained using Alexa Fluor-conjugated phalloidin. Fluorescent secondary antibodies (Alexa Fluor-conjugated) were used for differential detection of C. albicans and clathrin, or dynamin or cortactin.
Confocal microscopy
Confocal images of the epithelial cells were acquired using a laser scanning confocal microscope (Zeiss LSM510) using 100x lenses (Carl Zeiss, Inc.) under control of LSM (Carl Zeiss, Inc), or using a Leica TCS-SP5 confocal microscope (63x lenses) under the control of Leica LAS AF. The images were treated using ImageJ (1.38x; http://rsb.info.nih.gov/ij/index.html), Imaris (Bitplane scientific solutions), and LSM (Carl Zeiss, Inc). Confocal images of the endothelial cells were acquired using a Leica laser scanning confocal microscope (Leica Microsystems). Optical sections were collected along the z-axis and merged to produce the final image.
RNAi assays
Double stranded RNA against clathrin heavy chain (s 5′-GGC CCA GGU GGU AAU CAU Utt-3′, as 5′-AAU GAU UAC CAC CUG GGC Ctg-3′), dynamin II (s 5′ GGA GAU UGA AGC AGA GAC Ctt-3′, as 5′-GGU CUC UGC UUC AAU CUC Ctg-3′) and cortactin (s, 5′-GGG AGA AUG UCU UUC AAG ATT-3′; as, 5′-UCU UGA AAG ACA UUC UCC CTC-3′) were purchased from Ambion and Eurogentec. Control RNA (Silencer Negative Control 1 siRNA) was purchased from Ambion and siCONTROL Non-Targeting siRNA Pool was purchased from Dharmacon. For clathrin, additional On-Target SMART pool against clathrin heavy chain (Dharmacon) was also purchased. Transfections were performed using Oligofectamine (Invitrogen) for HeLa and HEK293 cells and Dharmafect1 (Dharmacon) for JEG-3 and HEK293 cells. These reagents were used as recommended by the manufacturers. Cells were tested 72 hours after transfections.
Flow cytometry assays
E-cadherin expression was analyzed by direct immunofluorescence using an anti-human CD324 (E-cadherin) Alexa Fluor 488-labeled Ab (324109, BioLegend). Supernatant from the mouse myeloma P3-X63Ag8 (X63) was used as negative control followed by a secondary Alexa Fluor 488-labeled-Ab (Molecular Probes). All incubations were done at 4o C in PBS with 2% BSA, 1% FCS in the presence of 50 μg/ml of poly-human Ig for Fc receptor blocking. Fluorescence was analyzed in a FACSCalibur flow cytometer (BD-Biosciences); cells were gated by forward and side scatter based on wild-type cell size and shape, and mean fluorescence intensity of 1–2 × 104 labeled cells was calculated using CellquestPro software (BD-Biosciences).
Statistical analysis
Differences in the endocytosis of C. albicans by epithelial cells treated with the different siRNAs was determined by Student’s T test. In other analyses, the difference between groups was analyzed by the Mann-Whitney U test. P values of 0.05 or less were considered significant. Unless otherwise stated, all experiments were performed atast five times, and the data are given as mean values ± SD.
Supplementary Material
Recruitment of clathrin during C. albicans infection. HEK293 cells transformed with GFP-LCa (clathrin light chain), shown in green, were infected for 120 min with C. albicans SC5314, shown in blue. Actin, detected with fluorescent phalloidin is shown in red. Arrows point internalized hyphae. Scale bar = 5μm.
Recruitment of dynamin in human epithelial cells around endocytosed C. albicans. HEK293 wild-type cells were infected with C. albicans SC5314 strain, shown in blue. Dynamin-2, shown in green, was immunodetected using anti-dynamin-2 antibodies. Actin, shown in red, was detected with phalloidin. Arrows point internalizing hyphae. Scale bar = 10 μm.
(A) Localization of cortactin during C. albicans infection. HEK293 cells were infected with C. albicans SC5314. Extracellular C. albicans, immunodetected before permeabilization are shown in blue. All C. albicans (extracellular and internalized) detected after permeablization are shown in green. Cortactin is shown in red. Arrows mark an internalized hypha, which is surrounded by cortactin. Scale bar = 5μm. (B) ICAM-I (used as control membrane protein) did not localize with C. albicans hyphae during infection of HEK293 cells. Infection was performed exactly as shown in Figs. 1–3. Extracellular C. albicans, immunodetected before permeabilization of epithelial cells are shown in blue. All C. albicans (extracellular and internalized) detected after permeablization are shown in green. ICAM-I is shown in red.
(A) Depletion of clathrin did not change the dynamin content of epithelial cells. The amount of epithelial cell dynamin was measured by Western-Blotting in clathrin KD and in control cells. The ratio between dynamin expression levels in control and clathrin siRNA KD cells is shown. Results are the mean ± SD of 6 independent experiments. (B) AP-2 depletion significantly decreased the endocytosis of C. albicans. HEK293 cells knocked-down (KD) using siRNA to different sequences targeting AP-2 and infected with C. albicans SC5314. Fungal internalization ratio was measured by differential immunofluorescence labeling. Organisms that were at least partly internalized were counted as internalized. Data were normalized versus control siRNA (RNA not targeting any cellular mRNA)-treated cells. Results are mean ± standard deviation of 3 independent experiments. (C) Protein KD by siRNA was tested by Western-blot. Tubulin is shown as a loading control.
Clathrin and cortactin localize with Als3-coated beads that were internalized by endothelial cells. Als3-coated beads (green) were incubated with primary endothelial cells for 45 min. Endogenous clathrin (A) or cortactin (B) were immunodetected after cellular permeabilization and are shown in red. Arrows indicate beads that are surrounded by clathrin or cortactin.
Time lapse series where images were acquired every ~ 4 s with a widefield microscope from HeLa cells transiently expressing tdTomato-LCa (red) and E-cadherin-GFP (green) infected with C. albicans SC5314 (phase). Time is shown in seconds. Scale bar corresponds to 5 μm.
3D rendering of confocal images showing internalized hyphae. HEK293 cells were infected with C. albicans (SC5314) during 120 min in RPMI medium at 37°C. Extracellular C. albicans were detected before permeabilization (blue). All C. albicans (extracellular and internalized) detected after permeablization are shown in green. The left panel shows extracellular C. albicans, while the right panel shows both intracellular and extracellular C. albicans. Actin is shown in both panels in red. 0.17 μm confocal slice images were acquired. Image rendering were performed using Imaris and ImageJ.
Acknowledgments
We are grateful to Prof. Mark A. McNiven, Prof. Tomás Kirchhausen and Prof. W. James Nelson for provided us reagents. We thank Quynh Trang Phan for assistance with tissue culture and the perinatal nurses at the Harbor-UCLA Medical Center Pediatric Clinical Research Center for collection of umbilical cords. EMR was the recipient of a European Union post-doctoral fellowship (Galar Fungail 2 MRTN-CT-2003-504148) MGD was supported by Spanish FIS 05/1999, EV was a holder of French “Jeune Chercheur” contract from INSERM, PC is an Howard Hughes International Scholar. This work was supported in part by grants ERA-NET PathoGenoMics (SPATELIS) from the 6th framework program of EU, M01RR00425, R01AI054928, R01AI19990, and R01DE017088 from the National Institutes of Health, U.S.A, FIS 05/1999 from the Spanish Ministry of Health and Ramón y Cajal program from the Spanish Ministry of Science and Innovation.
References
- Bierne H, Sabet C, Personnic N, Cossart P. Internalins: a complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes Infect. 2007;9:1156–1166. doi: 10.1016/j.micinf.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- Bonazzi M, Lecuit M, Cossart P. Listeria monocytogenes internalin-E-cadherin interactions: from structure to pathogenesis. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01293.x. (in Press) [DOI] [PubMed] [Google Scholar]
- Bonazzi M, Veiga E, Cerda JP, Cossart P. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalisation of Listeria monocytogenes. Cell Microbiol. 2008;10:2208–2222. doi: 10.1111/j.1462-5822.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- Boucrot E, Saffarian S, Massol R, Kirchhausen T, Ehrlich M. Role of lipids and actin in the formation of clathrin-coated pits. Exp Cell Res. 2006;312:4036–4048. doi: 10.1016/j.yexcr.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Drago L, Mombelli B, De Vecchi E, Bonaccorso C, Fassina MC, Gismondo MR. Candida albicans cellular internalization: a new pathogenic factor? Int J Antimicrob Agents. 2000;16:545–547. doi: 10.1016/s0924-8579(00)00296-x. [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- Filler SG, Swerdloff JN, Hobbs C, Luckett PM. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard JL, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol. 2006;4:423–434. doi: 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol. 2000;1:187–198. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- Krueger EW, Orth JD, Cao H, McNiven MA. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell. 2003;14:1085–1096. doi: 10.1091/mbc.E02-08-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Ohayon H, Braun L, Mengaud J, Cossart P. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect Immun. 1997;65:5309–5319. doi: 10.1128/iai.65.12.5309-5319.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. Embo J. 1999;18:3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Hurme R, Pizarro-Cerda J, Ohayon H, Geiger B, Cossart P. A role for alpha-and beta-catenins in bacterial uptake. Proc Natl Acad Sci U S A. 2000;97:10008–10013. doi: 10.1073/pnas.97.18.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol RH, Boll W, Griffin AM, Kirchhausen T. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci U S A. 2006;103:10265–10270. doi: 10.1073/pnas.0603369103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996a;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- Mengaud J, Lecuit M, Lebrun M, Nato F, Mazie JC, Cossart P. Antibodies to the leucine-rich repeat region of internalin block entry of Listeria monocytogenes into cells expressing E-cadherin. Infect Immun. 1996b;64:5430–5433. doi: 10.1128/iai.64.12.5430-5433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Orth JD, McNiven MA. Dynamin at the actin-membrane interface. Curr Opin Cell Biol. 2003;15:31–39. doi: 10.1016/s0955-0674(02)00010-8. [DOI] [PubMed] [Google Scholar]
- Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, JEE, Filler SG. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol. 2005;7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Phan QT, Belanger PH, Filler SG. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun. 2000;68:3485–3490. doi: 10.1128/iai.68.6.3485-3490.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan QT, Fratti RA, Prasadarao NV, Edwards JE, Jr, Filler SG. N-cadherin Mediates Endocytosis of Candida albicans by Endothelial Cells. J Biol Chem. 2005;280:10455–10461. doi: 10.1074/jbc.M412592200. [DOI] [PubMed] [Google Scholar]
- Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, et al. Als3 Is a Candida albicans Invasin That Binds to Cadherins and Induces Endocytosis by Host Cells. PLoS Biol. 2007;5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D, Edwards JE, Jr, Gibson TR, Moore JC, Cohen AH, Green I. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis. 1985;152:1264–1274. doi: 10.1093/infdis/152.6.1264. [DOI] [PubMed] [Google Scholar]
- Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert WD, Urbanke C, Ziehm T, Beier V, Machner MP, Domann E, et al. Structure of Internalin, a Major Invasion Protein of Listeria monocytogenes, in Complex with Its Human Receptor E-Cadherin. Cell. 2002;111:825–836. doi: 10.1016/s0092-8674(02)01136-4. [DOI] [PubMed] [Google Scholar]
- Sousa S, Cabanes D, El-Amraoui A, Petit C, Lecuit M, Cossart P. Unconventional myosin VIIa and vezatin, two proteins crucial for Listeria entry into epithelial cells. J Cell Sci. 2004;117:2121–2130. doi: 10.1242/jcs.01066. [DOI] [PubMed] [Google Scholar]
- Sousa S, Cabanes D, Bougneres L, Lecuit M, Sansonetti P, Tran-Van-Nhieu G, Cossart P. Src, cortactin and Arp2/3 complex are required for E-cadherin-mediated internalization of Listeria into cells. Cell Microbiol. 2007;9:2629–2643. doi: 10.1111/j.1462-5822.2007.00984.x. [DOI] [PubMed] [Google Scholar]
- Sousa S, Cabanes D, Archambaud C, Colland F, Lemichez E, Popoff M, et al. ARHGAP10 is necessary for alpha-catenin recruitment at adherens junctions and for Listeria invasion. Nat Cell Biol. 2005;7:954–960. doi: 10.1038/ncb1308. [DOI] [PubMed] [Google Scholar]
- Stang E, Blystad FD, Kazazic M, Bertelsen V, Brodahl T, Raiborg C, et al. Cbl-dependent Ubiquitination Is Required for Progression of EGF Receptors into Clathrin-coated Pits. Mol Biol Cell. 2004;15:3591–3604. doi: 10.1091/mbc.E04-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- Veiga E, Guttman JA, Bonazzi M, Boucrot E, Toledo-Arana A, Lin AE, et al. Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell Host Microbe. 2007;2:340–351. doi: 10.1016/j.chom.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recruitment of clathrin during C. albicans infection. HEK293 cells transformed with GFP-LCa (clathrin light chain), shown in green, were infected for 120 min with C. albicans SC5314, shown in blue. Actin, detected with fluorescent phalloidin is shown in red. Arrows point internalized hyphae. Scale bar = 5μm.
Recruitment of dynamin in human epithelial cells around endocytosed C. albicans. HEK293 wild-type cells were infected with C. albicans SC5314 strain, shown in blue. Dynamin-2, shown in green, was immunodetected using anti-dynamin-2 antibodies. Actin, shown in red, was detected with phalloidin. Arrows point internalizing hyphae. Scale bar = 10 μm.
(A) Localization of cortactin during C. albicans infection. HEK293 cells were infected with C. albicans SC5314. Extracellular C. albicans, immunodetected before permeabilization are shown in blue. All C. albicans (extracellular and internalized) detected after permeablization are shown in green. Cortactin is shown in red. Arrows mark an internalized hypha, which is surrounded by cortactin. Scale bar = 5μm. (B) ICAM-I (used as control membrane protein) did not localize with C. albicans hyphae during infection of HEK293 cells. Infection was performed exactly as shown in Figs. 1–3. Extracellular C. albicans, immunodetected before permeabilization of epithelial cells are shown in blue. All C. albicans (extracellular and internalized) detected after permeablization are shown in green. ICAM-I is shown in red.
(A) Depletion of clathrin did not change the dynamin content of epithelial cells. The amount of epithelial cell dynamin was measured by Western-Blotting in clathrin KD and in control cells. The ratio between dynamin expression levels in control and clathrin siRNA KD cells is shown. Results are the mean ± SD of 6 independent experiments. (B) AP-2 depletion significantly decreased the endocytosis of C. albicans. HEK293 cells knocked-down (KD) using siRNA to different sequences targeting AP-2 and infected with C. albicans SC5314. Fungal internalization ratio was measured by differential immunofluorescence labeling. Organisms that were at least partly internalized were counted as internalized. Data were normalized versus control siRNA (RNA not targeting any cellular mRNA)-treated cells. Results are mean ± standard deviation of 3 independent experiments. (C) Protein KD by siRNA was tested by Western-blot. Tubulin is shown as a loading control.
Clathrin and cortactin localize with Als3-coated beads that were internalized by endothelial cells. Als3-coated beads (green) were incubated with primary endothelial cells for 45 min. Endogenous clathrin (A) or cortactin (B) were immunodetected after cellular permeabilization and are shown in red. Arrows indicate beads that are surrounded by clathrin or cortactin.
Time lapse series where images were acquired every ~ 4 s with a widefield microscope from HeLa cells transiently expressing tdTomato-LCa (red) and E-cadherin-GFP (green) infected with C. albicans SC5314 (phase). Time is shown in seconds. Scale bar corresponds to 5 μm.
3D rendering of confocal images showing internalized hyphae. HEK293 cells were infected with C. albicans (SC5314) during 120 min in RPMI medium at 37°C. Extracellular C. albicans were detected before permeabilization (blue). All C. albicans (extracellular and internalized) detected after permeablization are shown in green. The left panel shows extracellular C. albicans, while the right panel shows both intracellular and extracellular C. albicans. Actin is shown in both panels in red. 0.17 μm confocal slice images were acquired. Image rendering were performed using Imaris and ImageJ.