Abstract
Simultaneous blockade of the CD40 and CD28 costimulatory pathways is an effective treatment strategy to promote allograft acceptance but does not lead to indefinite allograft survival. The immune mechanisms responsible for costimulation-independent rejection are not defined. Here we have studied the rejection responses of murine C57BL/6 recipients, which we show to be relatively resistant to inhibition by combined CD40/CD28 blockade. We demonstrate that asialo GM1+ CD8+ cells play a critical role in this costimulation blockade–resistant rejection. These results provide new insights into the costimulatory requirements for T-cell subsets and demonstrate for the first time that combined blockade of the CD40 and CD28 pathways does not adequately inhibit CD8-mediated skin allograft rejection. Furthermore, we provide evidence that asialo GM1 is a potentially important therapeutic target for CD8-dependent immune responses.
J. Clin. Invest. 104:1715–1722 (1999).
Introduction
Activated T cells play a central role in the immune response to transplanted tissues. Once activated, T cells may act as direct cytotoxic effectors or provide “help” for other cells that are important in the effector phase of the immune response. Definition of the T-cell subsets that are required to mediate rejection has been of long-standing interest to the transplant community. Numerous studies indicate that CD4+ T cells may play a crucial, if not obligatory, role in allograft rejection. For example, depletion of CD4+ T cells is sufficient to promote long-term acceptance of cardiac allografts in mice (1–3). More recently, it has been reported that CD4-deficient Balb/c mice permanently accept skin allografts (4). However, there is evidence in certain strains of mice and in humans that CD4 “help” is not always required to generate CD8+ cytotoxic T lymphocytes (CTL) (5, 6). Furthermore, in B6 and B10 mice, CD8+ T cells are sufficient to reject allografts (7).
Optimal T-cell proliferation and cytokine secretion require the delivery of signals via the T-cell receptor and costimulatory receptors. CD40 and CD28 serve as receptors for interrelated costimulatory pathways that are crucial for the development of many T-cell responses (8–11). Interest in the transplant community has been fueled by observations that simultaneous blockade of the CD40 and CD28 pathways provides a powerful means of inhibiting alloimmune responses in both rodents and primates (12, 13). However, this strategy does not always produce indefinite graft survival. For example, we have recently reported that rejection of skin xenografts can occur via CD40- and CD28-independent pathways (14).
In this report, we have examined the cellular mechanisms involved in costimulation blockade–resistant allograft rejection in C57BL/6 (B6) mice. We observed that addition of anti–asialo GM1 antibodies to combined blockade of the CD40 and CD28 pathways markedly delays skin allograft rejection. In subsequent experiments, we show that the effects of anti–asialo GM1 are not mediated through the depletion of natural killer (NK) cells, but rather by inhibition of CD8-dependent rejection. Furthermore, we demonstrate that treatment with anti–asialo GM1 significantly diminishes the expansion of CD8+ T cells in vivo. These results indicate that combined blockade of the CD40 and CD28 costimulatory pathways does not adequately inhibit CD8-mediated allograft rejection. Further, our data suggest that adjunctive therapies targeting alloreactive CD8 cells, such as anti–asialo GM1 treatment, may enhance the effectiveness of costimulation blockade–based therapies.
Methods
Mice.
Adult male BALB/c, C57BL/6, and C57BL/6Rag1–/– mice 6–8 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). B6 B2-microglobulin–/– mice and B6 class II–/– mice were purchased from Taconic Farms (Germantown, New York, USA).
Treatment protocols.
Skin graft recipients were treated with 500 μg each of hamster anti-murine CD40L antibody (MR1) and human CTLA4-Ig (provided by D.Hollenbaugh [Bristol Myers Squibb, Princeton, New Jersey, USA]) administered intraperitoneally on the day of transplantation (day 0) and on postoperative days 2, 4, and 6. Additional experimental groups were treated with 50 μL of polyclonal rabbit anti–asialo GM1 antisera (Wako Chemicals, Richmond, Virginia, USA) administered intraperitoneally or 200 μg of rabbit IgG (Sigma Chemical Co., St. Louis, Missouri, USA, or Jackson Immunochemicals, West Grove, Pennsylvania, USA) administered intraperitoneally on days 0, 4, 8, and 12; or 200 μg of anti-NK1.1 (PK136) administered intraperitoneally on days 0, 7, 14, and 21; or 200 μg of anti-γδ antibody (GL3) (gift of H. Kirk Ziegler, Department of Microbiology and Immunology, Emory University, Atlanta, Georgia) on day –2; or 100 μg rat anti-mouse CD8 (TIB105) or rat anti-mouse CD4 (GK1.5) administered intraperitoneally on days –3, –2, and –1, and weekly until the time of rejection.
Flow cytometry.
Analyses of nylon wool passaged splenocytes and peripheral blood were carried out using fluorochrome-conjugated antibodies (Armenian Hamster IgG2, Rat IgG2a, Mouse IgG2a, anti-CD4, anti-CD8, anti-γδ TCR, anti-β TCR, anti-NK1.1; all from PharMingen, San Diego, California, USA) or using primary antibodies (rabbit anti–asialo GM1 antisera [Wako Chemicals]) or rabbit IgG (Jackson Immunochemicals), followed by biotinylated donkey anti-rabbit IgG F(ab′)2 (Jackson Immunochemicals), followed by streptavidin-FITC (Jackson Immunochemicals) or TRI-COLOR (Caltag Laboratories, Burlingame, California, USA). Flow cytometry was performed using a FACScan, and data were analyzed using Cellquest software (both, Becton Dickinson Immunocytometry Systems, San Jose, California, USA).
Skin grafting.
Full thickness skin grafts (∼1 cm2) were transplanted on the dorsal thorax of recipient mice and secured with a plastic adhesive bandage for 7 days. Graft survival was then followed by daily visual inspection. Rejection was defined as the complete loss of viable epidermal graft tissue. Statistical analyses were performed using a Mann-Whitney U test.
Reconstitution of Rag1–/– mice.
Splenic and mesenteric lymph node cells were harvested from C57BL/6 mice. After red blood cell lysis, cells were incubated on ice for 20 minutes with rat anti-mouse CD11b (M1/70), anti-mouse CD45R (B220), and either anti-mouse CD4 (GK1.5) or anti-mouse CD8 (TIB105). Cells were then washed and incubated with goat anti-rat IgG biospheres (Biosource International, Camarillo, California, USA) (20:1 bead/target ratio) for 20 minutes on ice. Cells were then placed on a magnet for 15 minutes and collected, and viable cells were counted using trypan blue exclusion. Adequacy of T-cell subset depletion (<1% contaminating cells) was confirmed by flow cytometry. Recipient mice were adoptively transferred with 1 × 107 cells intravenously by penile vein injection.
CFSE staining.
Splenic and mesenteric lymph node cells were harvested from C57BL/6 mice. After red blood cell lysis and nylon wool passage, the cells were incubated in 10 μM CFSE (Molecular Probes, Eugene, Oregon, USA). After 10 minutes, the staining was halted by the addition of cold RPMI. Irradiated (18 Gy) Balb/c mice then received 3 × 107 CFSE-labeled cells intravenously by penile vein injection. Recipient mice were treated on the day before transfer and the day of transfer with either 50 μL anti–asialo GM1 antisera or 200 μg rabbit Ig. After 66 hours, spleens were harvested from the recipients, the red blood cells were lysed, and the remaining cells were stained for flow cytometry as already described.
Results
Rejection responses in B6 mice are relatively resistant to the effects of CD40/CD28 costimulation blockade.
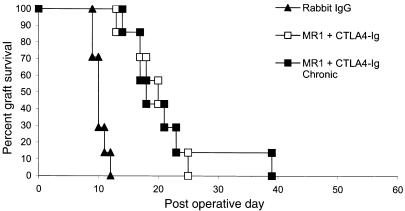
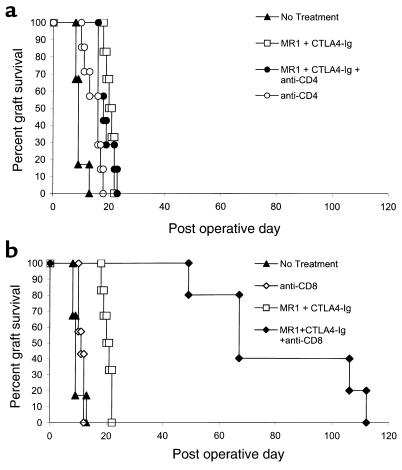
We have previously reported that allogeneic Balb/c (H-2d) skin grafts transplanted to C3H/HeJ (H-2k) recipients treated with anti-CD40L mAb (MR1) and CTLA4-Ig to inhibit the CD40 and CD28 costimulatory pathways have dramatically improved survival (median survival time [MST], 98 days) compared with untreated control animals or animals receiving either agent alone (MST 11 days) (ref. 13 and unpublished study). In an effort to explore the mechanisms involved in this prolonged graft survival, we undertook similar experiments using B6 (H-2b) recipients, with an aim to exploit the numerous gene knockout and transgenic strains that have been developed on this background. To our surprise, we found that similar treatment of B6 recipients with MR1 and CTLA4-Ig for 6 days after transplantation resulted in only minimal prolongation of Balb/c skin graft survival compared with untreated recipients (MST 20 versus 10 days) (Figure 1). To investigate whether this difference was due to insufficient blockade of the CD40 and CD28 pathways, we extended the costimulation blockade protocol with weekly injections of CTLA4-Ig and anti-CD40L until graft loss occurred. Despite chronic therapy, skin allograft survival was not significantly improved compared with the standard costimulation blockade protocol (MST 17 versus 20 days) (Figure 1). Thus, the relative resistance of rejection responses in B6 recipients to costimulation blockade suggests that B6 mice can effectively reject allografts via mechanisms that are independent of the CD40 and CD28 costimulatory pathways.
Figure 1.
Skin allograft rejection in B6 mice is relatively resistant to combined blockade of the CD40 and CD28 pathways. B6 recipients of Balb/c skin allografts treated with anti-CD40L (500 μg) and CTLA4-Ig (500 μg) on days 0, 2, 4, and 6 (MST 20 days; n = 7) had minimally prolonged survival compared with a control group that received no treatment (MST 10 days; n = 7; P < 0.01). Recipients treated with chronic costimulation blockade therapy (days 0, 2, 4, and 6 and weekly until rejection) (MST 17 days; n = 7) did not have prolonged survival compared with our standard CD40/CD28 blockade protocol.
Asialo GM1+ cells are important mediators of costimulation blockade–resistant skin allograft rejection.
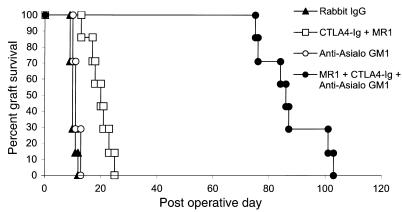
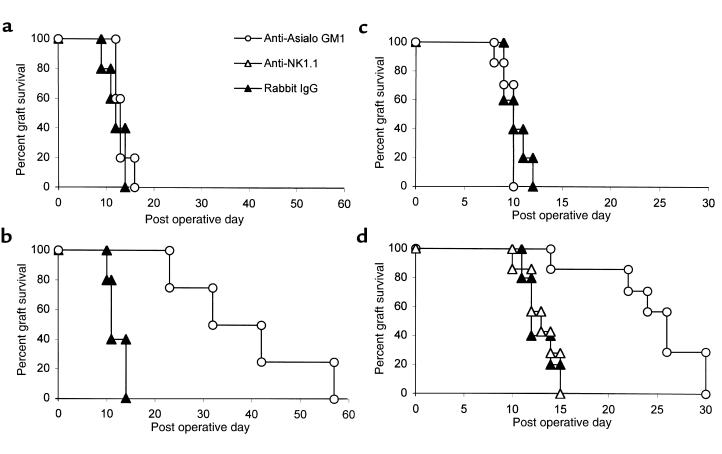
Although the acceptance of allografts and xenografts by T cell–deficient nude and SCID rodents with intact NK cell function indicates that NK cells are not sufficient to reject tissue allografts (15–17), NK cells play a prominent role in the rejection of allogeneic and xenogeneic bone marrow transplants (18–21) and infiltrate cardiac allografts during acute rejection responses (22). As an initial approach to explore the potential role of NK cells in costimulation blockade–resistant rejection, we used anti–asialo GM1 antibodies to deplete NK cells (23, 24). B6 recipients of Balb/c skin allografts were treated with anti-CD40L and CTLA4-Ig (costimulation blockade), anti–asialo GM1 antibodies alone, costimulation blockade plus anti–asialo GM1 antibodies, or control antibody (rabbit IgG) (Figure 2). Control-treated recipients promptly rejected their skin allografts, as did recipients treated with anti–asialo GM1 antibodies alone (MST 10 days and 11 days, respectively). As in our initial experiments, B6 recipients treated with costimulation blockade alone had only minimal prolongation of Balb/c skin allograft survival (MST 20 days). In contrast, allograft survival in recipients treated with costimulation blockade in addition to anti–asialo GM1 antibodies was dramatically prolonged compared with recipients treated with costimulation blockade alone (MST 86 versus 20 days; P < 0.01).
Figure 2.
Anti–asialo GM1 antibodies prolong skin allograft survival in B6 recipients treated with combined CD40/CD28 blockade. B6 recipients of Balb/c skin allografts treated with anti-CD40L, CTLA4-Ig (500 μg on days 0, 2, 4, and 6) and anti–asialo GM1 (50 μL on days 0, 4, 8, and 12) (MST 86 days; n = 7) had significantly prolonged graft survival compared with recipients treated with costimulation blockade alone (MST 20 days; n = 7; P < 0.01). Recipients treated with anti–asialo GM1 alone (MST 11 days; n = 7) did not have prolonged graft survival compared with control-treated (Rabbit IgG-treated) recipients (MST 10 days; n = 7).
Asialo GM1 is expressed on NK cells, NK T cells, and subsets of γδ T cells and CD8+ T cells in B6 mice.
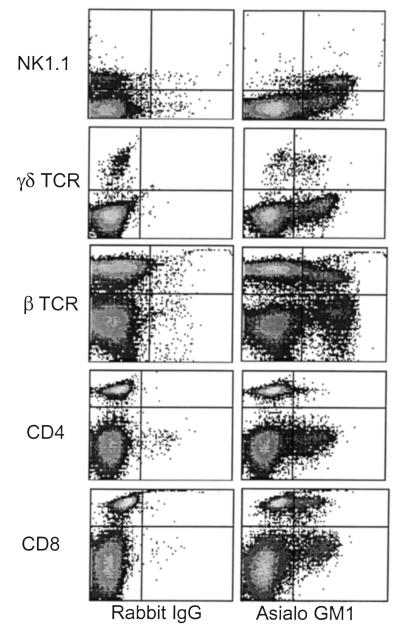
Although anti–asialo GM1 antibodies are widely used to deplete NK cells, it is known that asialo GM1 expression is not confined to NK cells. Therefore, we next studied the expression of asialo GM1+ cells in the B6 mouse. Two-color flow cytometric analysis of nylon wool passaged splenocytes demonstrated expression of asialo GM1 on most NK1.1+ cells (93%) and subsets of αβ (13%) and γδ (53%) T-lymphocytes. Further analysis demonstrated that approximately 20% of CD8+ T cells expressed asialo GM1, whereas approximately 1% of the CD4+ T cell population was asialo GM1+ (Figure 3). Thus, several distinct populations of cells within the B6 lymphocyte population express asialo GM1 and are potential targets of anti–asialo GM1–depleting antibodies.
Figure 3.
Asialo GM1 is expressed on NK cells and subsets of γδ T cells and αβ T cells in B6 mice. Nylon wool nonadherent splenocytes from B6 mice were analyzed for NK1.1, γδ TCR, αβ TCR, CD4, CD8, and asialo GM1 in the lymphoid compartment using 2-color flow cytometric analysis. All axes represent fluorescence intensity on a logarithmic scale. Asialo GM1 is expressed on approximately 93% of NK1.1+ cells, 53% of γδ T cells, 13% of αβ T cells, less than 1% of CD4+ T cells, and 20% of CD8+ T cells.
Neither NK cells nor γδ T cells play an important role in costimulation blockade–resistant rejection.
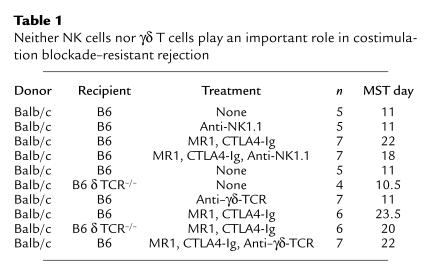
Next, we undertook experiments to identify the subsets of asialo GM1+ cells that are critical for costimulation blockade–resistant rejection. To explore the role of NK cells, we treated mice with an anti-NK1.1 antibody (PK136) alone or in combination with costimulation blockade (Table 1). In preliminary experiments, we confirmed that the anti-NK1.1 dose and frequency could maintain NK-cell depletion for more than 21 days (data not shown). Surprisingly, depletion of NK cells with anti-NK1.1 in combination with costimulation blockade failed to reproduce the effects observed with anti–asialo GM1 treatment. Similar experiments were performed to determine the role of γδ T cells using an anti-γδ TCR mAb (GL3) (25) or with B6 δ chain–/– mice that lack γδ T cells (Table 1). Again, when treated with costimulation blockade, neither of these groups had prolonged skin allograft survival compared with wild-type (control) mice treated with costimulation blockade (Table 1). Thus, neither NK1.1+ cells nor γδ T cells are critical for costimulation blockade–resistant allograft rejection in B6 mice.
Table 1.
Neither NK cells nor γδ T cells play an important role in costimulation blockade–resistant rejection
CD8+ T cells play a pivotal role in costimulation blockade–resistant rejection.
Because subsets of αβ T cells express asialo GM1, we undertook experiments to explore the role of CD4+ and CD8+ T cells in costimulation blockade–resistant rejection. For this, B6 recipients were depleted of CD4+ or CD8+ T cells with anti-CD4 (GK1.5) or anti-CD8 mAb (TIB105) or left undepleted. Recipients were then transplanted with Balb/c skin allografts and treated with costimulation blockade or received no treatment. As in previous experiments, undepleted B6 recipients treated with costimulation blockade had modestly prolonged allograft survival compared with untreated recipients (MST 20.5 versus 9 days) (Figure 4, a and b). Recipients depleted of CD4 cells treated with costimulation blockade did not have prolonged allograft survival compared with untreated CD4-depleted recipients (MST 18 versus 16 days) (Figure 4a). In contrast, recipients that had been depleted of CD8+ T cells and treated with costimulation blockade had markedly prolonged allograft survival (MST 67 days) compared with untreated CD8-depleted mice (MST 11 days) or undepleted mice treated with costimulation blockade (Figure 4b). Thus, these data suggest that while costimulation blockade is very effective in inhibiting CD4-dependent rejection responses, CD8+ T cells can aggressively mediate rejection independent of the CD40 and CD28 pathways in B6 recipients.
Figure 4.
CD8+ T cells mediate costimulation blockade–resistant rejection. B6 recipients were depleted of CD4 or CD8 cells with GK1.5 or TIB105, respectively (100 μg on days –3, –2, and –1 and weekly until rejection) or left undepleted. Recipient mice were transplanted with Balb/c skin allografts on day 0 and treated with costimulation blockade alone (CTLA4-Ig, anti-CD40L, 500 μg on days 0, 2, 4, and 6) or left untreated. (a) Costimulation blockade has no effect on CD8-mediated rejection. Untreated recipients depleted of CD4 cells (MST 16 days; n = 7) had minimally prolonged allograft survival compared with undepleted mice without treatment (MST 9 days; n = 6). Treatment with costimulation blockade did not prolong allograft survival in recipients depleted of CD4 cells (MST 18 days; n = 7) compared with costimulation blockade treatment of undepleted mice (MST 20.5 days; n = 6). (b) Costimulation blockade significantly inhibits CD4-mediated allograft rejection. In the same experiment as a, depicted on a separate graph for clarity, treatment of CD8-depleted recipients with costimulation blockade (MST 67 days; n = 5) significantly prolonged allograft survival compared with undepleted recipients treated with costimulation blockade (MST 20.5 days; n = 6; P < 0.01) or recipients treated with anti-CD8 alone (MST 11 days; n = 7).
Anti–asialo GM1 inhibits allograft rejection mediated by CD8+, but not CD4+, T cells.
Taken together, the preceding experiments suggested that the important target of the anti–asialo GM1 antibodies was the CD8+ T cell rather than the NK cell. To assess directly whether anti–asialo GM1 antibodies could inhibit rejection mediated by CD8+ T cells, we reconstituted B6 Rag1–/– mice with CD4 or CD8 cell subsets and treated with anti–asialo GM1 or control antibody (rabbit Ig) for 12 days after transplant. Recipient mice reconstituted with CD4 cells promptly rejected allografts when treated with either rabbit IgG or anti–asialo GM1 (MST 12 versus 13 days) (Figure 5a). In contrast, recipients reconstituted with CD8 cell subsets promptly rejected Balb/c skin allografts when treated with rabbit IgG (MST 11 days), but they had significantly prolonged survival when treated with anti–asialo GM1 antibodies (MST 37 days; P < 0.02) (Figure 5b).
Figure 5.
Anti–asialo GM1 delays skin allograft rejection mediated by CD8+ T cells. (a and b) B6 Rag1–/– recipients were reconstituted with 107 B6 CD4+ or CD8+ T cells and transplanted with Balb/c skin allografts 2 days later. (a) Recipients reconstituted with B6 CD4+ T cells promptly rejected Balb/c skin allografts when treated with rabbit Ig (MST 12 days; n = 5) or anti–asialo GM1 (MST 13 days; n = 5). (b) Recipients reconstituted with CD8+ T cells treated with rabbit Ig (MST 11 days; n = 5) promptly rejected Balb/c skin allografts. Recipients reconstituted with CD8 cells treated with anti–asialo GM1 had significantly prolonged allograft survival (MST 37 days; n = 4; P < 0.02). (c) B6 B2-microglobulin–/– promptly rejected Balb/c skin allografts when treated with rabbit IgG (MST 10 days; n = 5) or anti–asialo GM1 (MST 10 days; n = 7). (d) Balb/c skin graft survival was significantly prolonged in B6 class II–/– recipients treated with anti–asialo GM1 (MST 26 days; n = 7) compared with recipients treated with rabbit Ig (MST 12 days; n = 5; P < 0.01). Treatment of B6 class II–/– recipients with anti-NK1.1 did not significantly prolong graft survival (MST 13 days; n = 7).
As an alternative approach to study the effects of anti–asialo GM1 antibodies on CD4- and CD8-mediated rejection responses, we used MHC class II–/– mice and B2-microglobulin–/– mice, which are deficient in CD4+ and CD8+ T cells, respectively. Consistent with our data from the adoptive transfer model, treatment with anti–asialo GM1 significantly extended Balb/c skin allograft survival in B6 class II–/– recipients (MST 26 versus 12 days in rabbit Ig–treated animals; P < 0.01) (Figure 5d), but had no effect on rejection in B6 B2-microglobulin–/– recipients (MST 10 days) (Figure 5c). To ensure that the effect of anti–asialo GM1 on CD8-mediated rejection was not due to depletion of NK cells, recipient B6 class II–/– mice were also treated with anti-NK1.1 antibody. As expected, depletion of NK1.1+ cells had no effect on CD8- mediated skin allograft rejection (MST 13 days) (Figure 5d).
Anti–asialo GM1 prevents the expansion of CD8+ T cells in vivo.
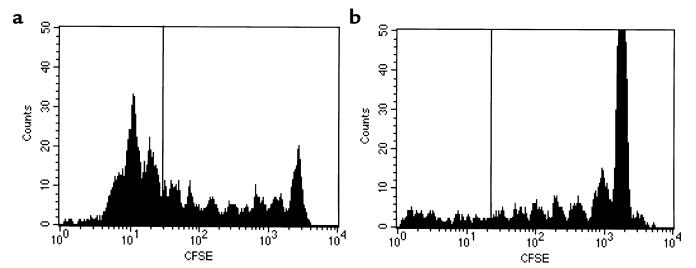
To investigate the mechanism by which anti–asialo GM1 antibodies inhibit CD8-mediated allograft rejection responses, we studied the effects of this antibody on the expansion of CD8+ T cells in vivo in a GvHD model using the fluorescent dye CFSE (26). Cells labeled with CFSE lose half of their fluorescence each time they divide, and thus cell divisions can be tracked in vivo using flow cytometry (27, 28). CFSE-labeled B6 T cells were adoptively transferred into lethally irradiated Balb/c recipients that were treated with either anti–asialo GM1 antibodies or rabbit Ig control antibody. Recipient splenocytes were harvested 66 hours after adoptive transfer and analyzed by flow cytometry for CD8 and CFSE fluorescence.
As demonstrated in Figure 6a, control mice treated with rabbit Ig had a significant population of CD8+ cells that had undergone more than 6 divisions. In contrast, mice treated with anti–asialo GM1 (Figure 6b) demonstrated an almost complete absence of CD8 cells that had divided more than 6 times. These data suggest that anti–asialo GM1 antibodies either inhibit cellular division or selectively deplete dividing CD8+ T cells. In other experiments, we have observed that expression of asialo GM1 increases with the number of cell divisions in this model (data not shown). Because anti–asialo GM1 antibodies effectively deplete NK cells, we hypothesize that treatment with anti–asialo GM1 may also deplete activated CD8 cells undergoing multiple rounds of division and, thus, may be a useful strategy to inhibit CD8-mediated immune responses in vivo. In this regard, we have observed using flow cytometry that treatment with anti–asialo GM1 specifically depletes B6 mice of asialo GM1+CD8+ cells while leaving the bulk of the CD8 population intact (data not shown).
Figure 6.
Anti–asialo GM1 prevents CD8+ T cell expansion. CFSE-labeled B6 T cells were adoptively transferred into irradiated (18 Gy) Balb/c recipients treated with rabbit IgG or anti–asialo GM1. (a) Flow cytometric analysis of cells harvested from rabbit IgG–treated mice 66 hours after transfer demonstrates expansion in the CD8 gate, including cells that have undergone more than 6 divisions (left of line). (b) Cells harvested from mice treated with anti–asialo GM1 show considerably less CD8 expansion and an almost complete absence of cells that have undergone more than 6 divisions. Histograms of CFSE fluorescence are gated on CD8 cells and depict data from 1 mouse representative of 5.
Discussion
These studies define limitations in the effectiveness of costimulation blockade therapies that may prove important as these strategies reach clinical trials. Although combined blockade of the CD40 and CD28 pathways significantly inhibits allograft rejection in some recipient strains of mice, we have shown that this strategy is relatively ineffective in promoting skin allograft acceptance in B6 mice due to inadequate blockade of CD8-mediated rejection responses.
Our data also indicate that under certain circumstances, CD8+ T cells are able to reject allografts in the absence of CD4+ T cells. Although Krieger et al., using Balb/c recipients, have published compelling data that CD4+ cells are necessary and sufficient to mediate heart and skin allograft rejection in some settings (4), the data presented here using antibody depletion suggest that CD8 cells are able to mediate skin graft rejection in B6 recipients. The ability of CD8 cells to mediate graft rejection in this model is further supported by our observation that B6 CD4–/– recipients reject skin allografts (MST 14.5 days; data not shown) with kinetics similar to those shown here for recipients depleted of CD4+ cells.
Although the data presented here are limited to B6 recipients of skin grafts, recent observations suggest that costimulation blockade–resistant rejection occurs in other models as well. In experiments in other strains, we have found that DBA/2 recipients, like C3H/HeJ recipients, enjoy a marked increase in skin graft survival when treated with costimulation blockade, whereas costimulation blockade–treated Balb/c recipients reject skin grafts with kinetics similar to B6 mice (unpublished study). Further, while we and others have reported impressive prolongation of cardiac allograft survival due to blockade of the CD40 and/or CD28 pathways, Newell et al. have recently demonstrated the rejection of small bowel allografts by CD8+ T cells in a CD28 independent manner (29). Thus it appears that the costimulation requirements of murine CD4+ and CD8+ T cells may depend on both the genetic background of the recipient and the organ being transplanted.
It seems likely that in outbred human populations, the activation requirements of CD4+ and CD8+ T cell subsets may also be heterogeneous. Indeed, the expression of CD28 on CD8+ T cells shows dramatic individual variation in humans, ranging from 18% to 87% of peripheral blood CD8+ T cells (30). In human transplant recipients, heterogeneity in the responsiveness to conventional immunosuppression is well recognized but poorly understood (31). Thus, the response to combined CD40/CD28 blockade may also prove to be variable in humans, and adjunctive therapies targeting costimulation blockade–resistant rejection may be necessary.
Asialo GM1+ CD8+ T cells represent a potential therapeutic target.
Although we initially hypothesized that anti–asialo GM1 antibodies augmented costimulation blockade therapy by depleting NK cells, our experiments using costimulation blockade in mice depleted of various leukocyte subsets suggest that anti–asialo GM1 mediates its effects on allograft rejection through the depletion of alloreactive CD8+ T cells, not NK cells. Alternatively, anti–asialo GM1 antibodies may deplete or interfere with the function of another asialo GM1+ cell required for CD8-dependent rejection responses (e.g., an antigen-presenting cell [APC] or effector cell); however, we consider this second hypothesis less likely, as anti–asialo GM1 antibodies prevent CD8+ T cell expansion and so selectively inhibit CD8-mediated rejection responses while sparing CD4-mediated rejection responses.
The biologic significance of asialo GM1 expression by a subset of CD8+ T cells is not clear. It remains controversial whether asialo GM1 expression represents a marker of CD8+ T-cell activation or identifies a distinct population of killer T cells with unique functional properties (32–37). Our observations that asialo GM1 expression increases with the number of cell divisions (data not shown) and that anti–asialo GM1 can prevent CD8 expansion while sparing nondividing CD8 cells suggest that asialo GM1 is indeed an activation marker. These results suggest that asialo GM1 may represent a novel therapeutic target to interrupt allograft rejection responses or other pathological immune responses mediated by CD8+ T cells. Further studies on the distribution of asialo GM1 on human leukocytes will be necessary to determine the therapeutic potential of such an approach.
Costimulation blockade–resistant rejection has important biologic and clinical implications.
These studies also add to our understanding of the costimulatory requirements of CD4+ and CD8+ T cells during allograft rejection responses. Previous reports have indicated that CD4+ T-cell responses to a variety of environmental and self-antigens are critically dependent on CD40L (8, 38–40). Our data indicating that CD40/CD28 blockade is markedly more effective in B6 recipients that have been depleted of CD8+ T cells are consistent with these reports and suggest that these pathways are also important for CD4-mediated rejection responses.
The importance of CD28 and CD40 costimulation in the development of CD8+ T-cell responses is less clear. In some settings, such as the response to lymphocytic choriomeningitis virus (LCMV), it appears that neither CD40 nor CD28 is required for CTL development or viral clearance (41, 42). However, several recent reports have indicated that for helper-dependent CD8 responses, CD40L plays a crucial role in licensing APCs to arm effector CD8+ T cells to mediate killing (43–45). Our data are most consistent with the studies of the response to LCMV, in that it appears that CD8-mediated rejection in B6 mice requires neither the CD40 nor CD28 pathway. It is possible that other interactions such as 41BB/41BBL and fas/fasL (46–48) can provide costimulation for CD8+ T cells. Alternatively, recent evidence indicates that in the setting of high-affinity T-cell receptor-MHC peptide interactions, CD8+ T cells can be activated in vitro in the absence of costimulation (49, 50). While further study of the biology of CD8+ T cells will be needed, it is clear that the development of therapeutic strategies targeting CD8+ T cells, such as anti–asialo GM1 antibodies, may enhance the effectiveness of costimulation blockade therapies.
Acknowledgments
We thank D. Hollenbaugh, R. Peach, and A. Aruffo (Bristol-Myers Squibb) for providing MR1 and CTLA4-Ig. This research was supported by grants from the National Institutes of Health and from the Carlos and Marguerite Mason Trust.
Footnotes
Joel Trambley and Adam W. Bingaman contributed equally to this work.
References
- 1.Pearson T, Darby CR, Bushell AR, Morris PJ, Wood KJ. The assessment of transplantation tolerance induced by anti-CD4 monoclonal antibody in the murine model. Transplantation. 1993;55:361–367. doi: 10.1097/00007890-199302000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Qin S, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 3.Wood KJ, Pearson TC, Darby C, Morris PJ. CD4: a potential target molecule for immunosuppressive therapy and tolerance induction. Transplant Rev. 1991;5:150–164. [Google Scholar]
- 4.Krieger NR, Yin DP, Fathman CG. CD4+ but not CD8+ cells are essential for allorejection. J Exp Med. 1996;184:2013–2018. doi: 10.1084/jem.184.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young JW, Steinman RM. Dendritic cells stimulate primary human cytolytic lymphocyte responses in the absence of CD4+ helper cells. J Exp Med. 1990;171:1315–1332. doi: 10.1084/jem.171.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inaba K, Young JW, Steinman RM. Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J Exp Med. 1987;166:182–194. doi: 10.1084/jem.166.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall BM. Cells mediating allograft rejection. Transplantation. 1991;51:1141–1151. doi: 10.1097/00007890-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 9.van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 10.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- 12.Kirk AD, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen CP, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 14.Elwood ET, et al. Prolonged acceptance of skin and cardiac xenografts with combined CD40 and CD28 pathway blockade. Transplantation. 1998;65:1422–1428. doi: 10.1097/00007890-199806150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Bolton EM, Gracie JA, Briggs JD, Kampinga J, Bradley JA. Cellular requirements for renal allograft rejection in the athymic nude rat. J Exp Med. 1989;169:1931–1946. doi: 10.1084/jem.169.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dallman MJ, Mason DW. Role of thymus-derived and thymus-independent cells in murine skin allograft rejection. Transplantation. 1982;33:221–223. doi: 10.1097/00007890-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Van Buskirk AM, Wakely ME, Orosz CG. Acute rejection of cardiac allografts by noncytolytic CD4(+) T cell populations. Transplantation. 1996;62:300–302. doi: 10.1097/00007890-199607270-00026. [DOI] [PubMed] [Google Scholar]
- 18.Ohlen C, et al. Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science. 1989;246:666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- 19.Lee LA, Sergio JJ, Sykes M. Natural killer cells weakly resist engraftment of allogeneic, long-term, multilineage-repopulating hematopoietic stem cells. Transplantation. 1996;61:125–132. doi: 10.1097/00007890-199601150-00024. [DOI] [PubMed] [Google Scholar]
- 20.Gritsch HA, et al. The importance of nonimmune factors in reconstitution by discordant xenogeneic hematopoietic cells. Transplantation. 1994;57:906–917. doi: 10.1097/00007890-199403270-00024. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, et al. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med. 1996;2:1211–1216. doi: 10.1038/nm1196-1211. [DOI] [PubMed] [Google Scholar]
- 22.Petersson E, et al. Allogeneic heart transplantation activates alloreactive NK cells. Cell Immunol. 1997;175:25–32. doi: 10.1006/cimm.1996.1031. [DOI] [PubMed] [Google Scholar]
- 23.Kasai M, Iwamori M, Nagai Y, Okumura K, Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980;10:175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, et al. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med. 1996;2:1211–1216. doi: 10.1038/nm1196-1211. [DOI] [PubMed] [Google Scholar]
- 25.Skeen MJ, Ziegler HK. Induction of murine peritoneal gamma/delta T cells and their role in resistance to bacterial infection. J Exp Med. 1993;178:971–984. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song HK, et al. Cutting edge: alloimmune responses against major and minor histocompatibility antigens: distinct division kinetics and requirement for CD28 costimulation. J Immunol. 1999;162:2467–2471. [PubMed] [Google Scholar]
- 27.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newell KA, et al. Cutting edge: blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J Immunol. 1999;163:2358–2362. [PubMed] [Google Scholar]
- 30.Sfikakis PP, et al. CD28 expression on T cell subsets in vivo and CD28-mediated T cell response in vitro in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:649–654. doi: 10.1002/art.1780380512. [DOI] [PubMed] [Google Scholar]
- 31.Kasiske BL, et al. The effect of race on access and outcome in transplantation [see comments] N Engl J Med. 1991;324:302–307. doi: 10.1056/NEJM199101313240505. [DOI] [PubMed] [Google Scholar]
- 32.Lee U, Santa K, Habu S, Nishimura T. Murine asialo GM1+CD8+ T cells as novel interleukin-12-responsive killer T cell precursors. Jpn J Cancer Res. 1996;87:429–432. doi: 10.1111/j.1349-7006.1996.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ting CC, Hargrove ME, Wunderlich J, Loh NN. Differential expression of asialo GM1 on alloreactive cytotoxic T lymphocytes and lymphokine-activated killer cells. Cell Immunol. 1987;104:115–125. doi: 10.1016/0008-8749(87)90012-8. [DOI] [PubMed] [Google Scholar]
- 34.Charley MR, Mikhael A, Hackett J, Kumar V, Bennett M. Mechanism of anti–asialo GM1 prevention of graft-vs-host disease: identification of allo-antigen activated T cells. J Invest Dermatol. 1988;91:202–206. doi: 10.1111/1523-1747.ep12464858. [DOI] [PubMed] [Google Scholar]
- 35.Stout RD, Schwarting GA, Suttles J. Evidence that expression of asialo-GM1 may be associated with cell activation. Correlation of asialo-GM1 expression with increased total cellular RNA and protein content in normal thymocyte and spleen cell populations. J Immunol. 1987;139:2123–2129. [PubMed] [Google Scholar]
- 36.Stout RD, Suttles J. T cells bearing the CD44hi “memory” phenotype display characteristics of activated cells in G1 stage of cell cycle. Cell Immunol. 1992;141:433–443. doi: 10.1016/0008-8749(92)90161-h. [DOI] [PubMed] [Google Scholar]
- 37.Ting CC, Bluestone JA, Hargrove ME, Loh NN. Expression and function of asialo GM1 in alloreactive cytotoxic T lymphocytes. J Immunol. 1986;137:2100–2106. [PubMed] [Google Scholar]
- 38.Grewal IS, et al. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 39.Early GS, Zhao W, Burns CM. Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand black × New Zealand white mice. Response correlates with the absence of an anti-antibody response. J Immunol. 1996;157:3159–3164. [PubMed] [Google Scholar]
- 40.Campbell KA, et al. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 41.Whitmire JK, Slifka MK, Grewal IS, Flavell RA, Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection [published erratum appears in J. Virol. 1997;71:1736] J Virol. 1996;70:8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahinian A, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 43.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 44.Bennett SRM, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 45.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 46.Shuford WW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki I, Fink PJ. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187:123–128. doi: 10.1084/jem.187.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28- T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 49.Luxembourg AT, et al. Requirements for stimulating naive CD8+ T cells via signal 1 alone. J Immunol. 1998;161:5226–5235. [PubMed] [Google Scholar]
- 50.Goldstein JS, Chen T, Brunswick M, Mostowsky H, Kozlowski S. Purified MHC class I and peptide complexes activate naive CD8+ T cells independently of the CD28/B7 and LFA-1/ICAM-1 costimulatory interactions. J Immunol. 1998;160:3180–3187. [PubMed] [Google Scholar]