Summary
As with many other bacterial species, the most commonly used method to assess staphylococcal biofilm formation in vitro is the microtiter plate assay. This assay is particularly useful for comparison of multiple strains including large-scale screens of mutant libraries. When such screens are applied to the coagulase-negative staphylococci in general, and Staphylococcus epidermidis in particular, they are relatively straightforward by comparison with microtiter plate assays used to assess biofilm formation in other bacterial species. However, in the case of clinical isolates of Staphylococcus aureus, including methicillin-resistant S. aureus, we have found it necessary to employ specific modifications including precoating of the wells of the microtiter plate with plasma proteins and supplementation of the medium with both salt and glucose. In this chapter, we describe the microtiter plate assay in the specific context of clinical isolates of S. aureus and the use of these modifications. A second in vitro method, which also is generally dependent on coating with plasma proteins and supplementation of the growth medium, is the use of flow cells. In this method, bacteria are allowed to attach to a surface and then monitored with respect to their ability to remain attached to the substrate and differentiate into mature biofilms under the constant pressure of fluid shear force. Although flow cells are not applicable to large-scale screens, we have found that they provide a more reproducible and accurate assessment of the capacity of S. aureus clinical isolates to form a biofilm. They also provide a means of analyzing structural differences in biofilm architecture and isolating bacteria and/or spent media for analysis of physiological and metabolic changes associated with the adaptive response to growth in a biofilm. While a primary focus of this chapter is on the use of in vitro assays to assess biofilm formation in clinical isolates of S. aureus, it is important to emphasize two additional considerations. First, it has become increasingly evident that biofilm formation in S. epiderimidis and S. aureus is not equivalent. Additionally, to date, most studies with S. aureus have been done with a very limited number of strains, almost all of which are derived from the NCTC strain designated 8325, and we have found that these strains are not representative of the most relevant clinical isolates. As with the specific elements of our flow cell system, we have written this chapter to reflect our focus on clinical isolates of S. aureus and the specific methods that we have found most reliable in that context. Second, as is often the case, in vitro methods do not necessarily reflect events that occur in vivo. Several in vivo methods to assess biofilm formation have been described, and these generally fall into one of two categories. The first focuses directly on staphylococcal diseases that are generally thought to include a biofilm component (e.g., endocarditis, osteomyelitis, septic arthritis). A discussion of these models is also beyond the scope of this chapter, but examples are easily found in the staphylococcal literature. The second approach uses some form of implanted device in an attempt to focus more directly on implant-associated biofilms. We use a model in which a small piece of Teflon catheter is implanted subcutaneously in mice and used as a substrate for colonization. We have the advantage of using bioluminescent derivatives of S. aureus clinical isolates and the IVIS® imaging system. However, because this system is not generally available, we restrict technical comments in this chapter to our use of an implanted catheter model evaluated by direct microbiological analysis of explanted catheters (2).
Keywords: Polysaccharide intercellular adhesin, poly-N-acetyl glucosamine, microbial surface components recognizing adhesive matrix molecules, flow cell, implant-associated biofilm
1. Introduction
Staphylococcus aureus is among the most prominent of all bacterial pathogens. It is a commensal inhabitant of a significant proportion of the healthy population, but it also has the capacity to cause a diverse array of infections ranging from relatively superficial skin infections to serious, life-threatening infections including endocarditis, pneumonia, and osteomyelitis. Many forms of staphylococcal infection are associated with the formation of a bacterial biofilm on either native tissues (e.g., cartilage, bone) or implanted biomaterials (e.g., catheters, orthopedic devices). For reasons that are not completely understood, this biofilm significantly impairs antimicrobial therapy even in those cases caused by strains that are not resistant to the relevant antibiotics (1,2). For this reason, considerable effort has been expended to define the specific staphylococcal factors that promote biofilm formation and/or persistence within a biofilm. The two most common in vitro methods are the microtiter plate assay and flow cells, while the most common in vivo method is the use of an implanted biomaterial that is either inoculated directly or preinoculated prior to implantation (3,4,5). Our application of these three methods with respect to clinical isolates of S. aureus is the specific focus of this chapter.
In general, biofilm formation in all bacterial species involves four relatively distinct phases. The first phase is nonspecific interactions that promote the transient adherence to a substrate. These interactions are defined by general characteristics of the bacterium and the substrate (e.g., hydrophobicity). We have not investigated these interactions, but we have found that some microtiter plates work better than others, and this presumably reflects subtle differences in surface chemistry. With that in mind, we have included information regarding the specific components that we have found most reliable. The second phase is attachment to the substrate via specific bacterial adhesins. In S. aureus and many other Gram-positive pathogens, there is considerable evidence to suggest that this stage is mediated by the surface-exposed protein adhesins referred to as microbial surface components recognizing adhesive matrix molecules (MSCRAMMS) (6,7). This is consistent with the need to coat the substrate used for in vitro studies with plasma proteins. It should be noted that, in our experience, plasma coating is not necessary with Staphylococcus epidermidis, and there are exceptions to the rule in S. aureus (8). However, they are rare, and we have written this chapter to emphasize the rule rather than the exception. The third phase is the accumulation phase, in which bacteria adhere to each other in a fashion that ultimately results in the formation of a mature biofilm. In S. epidermidis, this is closely correlated with the presence and expression of the ica (intercellular adhesin) operon and the consequent production of the polysaccharide intercellular adhesin (9,10). The ica operon is present in most S. aureus isolates, and in some cases, it is required for biofilm formation (11). However, that is clearly not the case in all strains (12,13). Whether this reflects the existence of an alternative means of accumulation or simply the fact that polysaccharide intercellular adhesin production is important under growth conditions that are not reflected in current biofilm models remains unclear, but it should be noted that there is evidence that ica is preferentially expressed under in vivo growth conditions (14,15). This further emphasizes the need to verify the results of any in vitro biofilm assay using appropriate in vivo models, and it is for this reason that we have included a discussion of our murine model in this chapter. The fourth and final phase of biofilm formation is dispersal or release of bacteria from the biofilm. Although this can occur as a function of shear forces rather than any specific bacterial attribute, many bacteria also use specific mechanisms of dispersal, and in the case of S. aureus, there is evidence to suggest that production of phenol-soluble modulins (PSMs) may be important in that regard (16). As an example, there is convincing evidence that induction of the accessory gene regulator (agr), which results in expression of the PSM δ-toxin, results in detachment of S. aureus from mature biofilms (17). Indeed, studies from several laboratories have demonstrated that expression of agr is negatively correlated with biofilm formation in both S. aureus and S. epidermidis (8,18).
2. Materials
2.1. Microtiter Plate Biofilm Assay
Biofilm media: tryptic soy broth (#211822; BD Biosciences) supplemented with 3.0% NaCl and 0.5% dextrose.
Carbonate-bicarbonate buffer (C3041; Sigma, St. Louis, MO).
Lyophilized human plasma (P9523; Sigma): Prepare a 20% suspension by resuspending 5 mL of lyophilized human plasma in 20 mL of filter-sterilized carbonate-bicarbonate buffer (see Note 1).
Phosphate-buffered saline (PBS) (10X PBS stock): 1.37 M NaCl, 27 mM KCl, 100 mM Na2HPO4, 18 mM KH2PO4. Adjust the pH to 7.4 with HCl if necessary, and autoclave before storing at room temperature. Prepare a working solution by diluting 1 part with 9 parts of water.
Flat-bottomed polystyrene 96-well tissue culture plates (#3596; Corning, Corning NY).
2.2. Flow Cell Biofilm Assay
Stovall Flow Cell Kit (#FLCAS0001; Stovall Life Sciences).
10-L Polycarbonate media reservoir (#ACCFL0010 Stovall Life Sciences).
Lyophilized human plasma (P9523; Sigma).
Carbonate-bicarbonate buffer (C3041; Sigma).
Autoclavable tubing #EW-96429-42; Cole Parmer).
Luer-Lok™ syringes (#14-823-2B; Fisher).
Male and female Luer-Lok connectors.
Three-way stopcock (#K75; Baxter Pharmaseal).
Tabletop incubator (LabLine Thermal Rocker: 14-512-30; Fisher) (see Note 2).
Peristaltic pump (Ismatec Low Flow, High Accuracy, 12 channel: #ACCFL0013; Stovall Life Sciences).
Insulin syringes (#14-841-31; Fisher).
2.3. Catheter-Based Model of In Vivo Biofilm Formation
Six- to eight-week-old NIH-Swiss female mice (see Note 3).
14-Gage Teflon iv catheters (#14-841-11 or similar; Fisher): Precut catheters into 1-cm segments and sterilize by autoclaving prior to surgery.
Vetbond™ tissue adhesive (#NC9259532; Fisher).
2,2,2-Tribromoethanol (TBE) (#T48402; Sigma-Aldrich) (see Note 4): Prepare a stock solution of TBE by mixing 25 g of TBE with 15.5 mL of tert-amyl alcohol (#152463; Sigma-Aldrich) in a dark bottle. Stir for 12–24 h at room temperature until the TBE is completely dissolved. Wrap the stock solution in foil and keep at room temperature (the stock solution is both hydroscopic and photosensitive). Prepare a working solution of TBE prior to surgery by mixing 0.5 mL of the TBE stock with 39.5 mL of PBS or 0.9% saline. Stir in a dark bottle until complete resuspension has occurred (this may take several hours). Filter sterilize the resuspended working solution and then store in the dark at 4°C. The working solution, stored properly, can be used for several months.
Fisher Scientific Sonic Dismembrator model 500 (#15-338-550) with 1.2-in. tapped horn (#15-338-56) and 1/8-in.-diameter microtip (#15-338-67).
PBS: Prepare as described in Subheading 2.1, item 4.
Tryptic soy agar (#236950; BD Biosciences).
3. Methods
3.1. Microtiter Plate Biofilm Assay
3.1.1. Day 1
Add 200 μL of 20% human plasma into the required number of wells and incubate overnight at 4°C.
Start overnight cultures of each test strain in biofilm medium (tryptic soy broth supplemented with 0.5% dextrose and 3.0% NaCl).
3.1.2. Day 2
Remove plasma from the wells by gentle aspiration with a sterile pipet tip. Care must be taken to avoid forceful suction of plasma from the well. Slowly and gently move the vacuum tip down the side of the well until all fluid has been removed. Take care not to aspirate the contents from the bottom of the well.
After ensuring that all overnight cultures grew to a comparable extent, dilute overnight cultures 1:200 in sterile biofilm medium (see Note 5)
Inoculate microtiter plate wells with 200 μL of diluted cultures. Fill the desired number of replicate wells for each strain. Include control wells consisting of sterile biofilm medium alone. Incubate the plate at 37°C without shaking for 24 h.
3.1.3. Day 3
Aspirate bacterial cultures from each well using the method described in Subheading 3.1.2., step 1. Wash the wells gently three times with 200 μL of sterile PBS.
Fix with 200 μL of 100% ethanol. Immediately aspirate off the ethanol, and let the microtiter plate dry for 10 min with the lid off in a sterile hood.
Stain the biofilm by adding 200 μL of crystal violet to each well for exactly 2 min. Gently aspirate off the crystal violet from each well.
Gently wash the wells three times with 200 μL of sterile PBS. Allow the plate to dry overnight with the lid on.
3.1.4. Day 4
Elute crystal violet by filling the wells with 100 μL of 100% ethanol for 10 min.
Gently pipet the eluted stain from each well into a new microtiter plate. Read the absorbance using an enzyme-linked immunosorbent assay plate reader at an absorbance of 595 nm (see Note 6).
3.2. Flow Cell Biofilm Assay
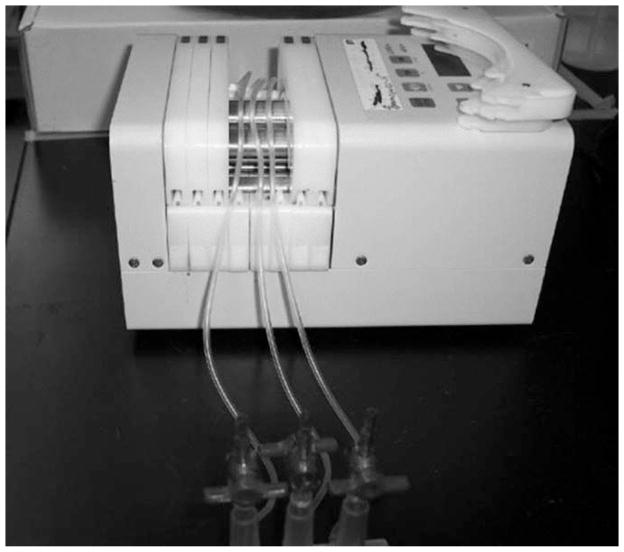
Diagram of the Stovall flow cell (Fig. 1).
Fig. 1.
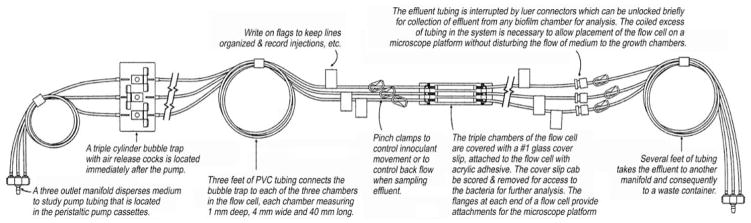
Diagram of Stovall flow cell.
3.2.1. Plasma Coating
Plasma coating should be performed in a sterile environmental hood if possible.
Resuspend 5 mL of lyophilized human plasma in 20 mL of carbonate-bicarbonate buffer.
Connect a sterile section of tubing fitted with a female Luer connector at one end to the flow cell output manifold. Connect a 20-mL Luer-Lok syringe to the female Luer connector (Fig. 2).
Connect a sterile section of tubing fitted with a female Luer connector at one end to the flow cell input manifold. Place this section of tubing into a sterile 50-mL beaker containing the resuspended 20% human plasma (Fig. 3).
Open all six pinch clamps on the flow cell apparatus. Slowly draw plasma into the flow cell tubing by exerting a slight pressure on the plunger of the syringe connected to the flow cell output manifold. Continue drawing plasma into the flow cell until each chamber is filled (see Note 7).
Close all six pinch clamps. To ensure sterility after drawing plasma into the flow cell, attach a 20-mL Luer-Lok syringe to the female Luer adapter connected to the flow cell input manifold. Rinse the connection with 70% isopropanol to remove residual plasma.
Incubate the entire flow cell apparatus at 4°C for 24 h.
Fig. 2.
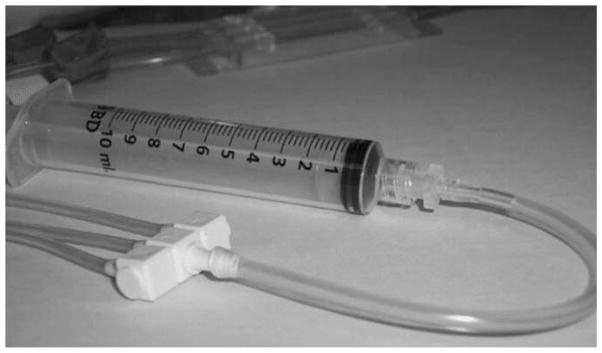
A 20-mL sterile “male” Luer-Lok fitted syringe is connected to a small (~6 in.) section of sterile tubing by means of a “female” Luer-Lok adapter. The other end of the sterile tubing is subsequently attached to the flow cell output manifold. This apparatus is used to introduce plasma into the flow cell circuit.
Fig. 3.
A small (~6 in.) section of sterile tubing containing a “female” Luer-Lok adapter at one end is connected to the flow cell input manifold. This “female” Luer-Lok-equipped end of the sterile tubing is subsequently placed in a 50-mL beaker containing 25 mL of resuspended 20% human plasma.
3.2.2. Establishing Flow of Medium
Prior to sterilization of the media reservoir containing a sufficient quantity of biofilm medium (see Note 8), attach a three-way stopcock to the external tubing connected to the media reservoir (Fig. 4, inset). Wrap the stopcock in foil to ensure sterility once autoclaved. Confirm that the stopcock is in the “off” position such that the biofilm medium cannot exit the reservoir during sterilization.
Using aseptic technique, carefully remove the 20-mL Luer-Lok syringe and female Luer adapter from the tubing section connected to the flow cell input manifold. Replace the female Luer adapter with a threaded male adaptor. Connect the threaded male Luer adapter to the three-way stopcock attached to the sterile media reservoir (Fig. 5).
Remove the 20-mL Luer-Lok syringe from the section of tubing connected to the flow cell output manifold. Insert this section of tubing into a vessel suitable for collecting flow cell effluent waste (see Note 9). Connect the flow cell to a peristaltic pump by placing each of the three pieces of tubing between the flow cell input manifold and the bubble trap apparatus in adjacent pump channels (Fig. 6).
Open all six pinch clamps on the flow cell apparatus. Turn the three-way stopcock connected to the media reservoir to the “on” position, such that the medium can now enter the flow cell apparatus.
After ensuring that all tubing connections are intact, turn on the peristaltic pump at a rate of 1.5 mL/min (approx 0.5 mL/min per flow cell chamber). Prepare the bubble trap apparatus by turning one of the bubble trap stopcocks to the “on” (vertical) position until sterile medium has filled approximately half of the bubble trap (lower right). Repeat this process for the remaining two bubble traps and then return the bubble trap stopcock to the “off” (horizontal) position (Fig. 7).
Allow fresh biofilm medium to flow through the flow cell apparatus for 20–30 min to remove all the plasma. If bubbles have accumulated in the flow cell chambers, they can be removed by turning the chamber vertically and lightly tapping on the surface. Remove all bubbles in or near the flow cell chamber before inoculation.
Fig. 4.
Prior to sterilization of the media reservoir, a three-way stopcock is fitted to the external tubing and placed in the “off” position.
Fig. 5.
The “female” Luer-Lok adapter on the section of tubing connected to the flow cell input manifold is removed and replaced with a “male” Luer-Lok adapter. The flow cell may now be aseptically connected to the sterilized media reservoir by means of the three-way stopcock.
Fig. 6.
The flow cell is connected to a peristaltic pump by placing each of the three pieces of tubing between the flow cell input manifold and the bubble trap apparatus into adjacent pump channels.
Fig. 7.

Bubble traps in the flow cell apparatus are sequentially filled by turning a bubble trap stopcock to the “on” position. Each bubble trap cylinder is filled approximately half full with medium, and then the bubble trap stopcock is returned to the “off” position. This process is repeated for each of three bubble traps per flow cell.
3.2.3. Inoculation of Flow Cell Chambers
Prepare strains by setting up an overnight broth culture in biofilm medium. Prior to inoculation of the flow cell, standardize each culture to be tested based on spectrophotometer readings (see Note 10).
Turn off the peristaltic pump and close all pinch clamps on the flow cell apparatus.
Clean the section of tubing between the upstream pinch clamps and the flow cell chamber with a sterile alcohol pad to prepare for inoculation. Apply a small piece of self-sealing tape (included in the Stovall Flow Cell Kit) to this section of tubing, and clean the tape with a sterile alcohol pad (Fig. 8).
Draw up 0.5 mL of each standardized bacterial culture into an insulin syringe. Working on one chamber at a time, carefully insert the needle through the self-sealing tape and into the lumen of the flow cell tubing (Fig. 9).
Open the downstream pinch clamp, and slowly inject the bacterial suspension making sure that the turbid suspension fills the flow cell chamber. Take care not to introduce bubbles into the chamber. After injection, carefully remove the needle and clean the self-sealing tape with a sterile alcohol pad once more. Close the downstream pinch clamp. Repeat this process for each strain in the respective flow cell chambers.
After inoculation, place the flow cell chamber upside down in an incubator to allow bacteria to attach (Fig. 10). Use a small weight to stabilize the flow cell in a flat position. Ensure that the upstream and downstream tubing is not pinched as it enters or exits the incubator (Fig. 11).
Incubate the inoculated flow cell with the flow off for 1 h at 37°C.
Return the flow chamber to the upright position and start the peristaltic pump at a flow rate of 1.5 mL/min (see Note 11). Incubate the flow cell at 37°C for the remainder of the experiment.
Observe the bubble trap periodically to ensure that it is approximately half full, adjusting the stopcocks as necessary to allow more medium to enter the cylinder.
Fig. 8.
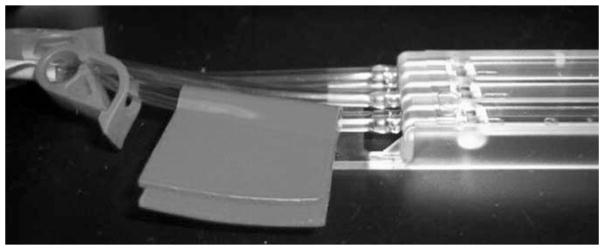
Tubing just upstream of the flow cell chamber is prepared for inoculation by cleansing with a sterile alcohol pad followed by application of self-sealing tape (included in the Stovall Flow Cell Kit).
Fig. 9.
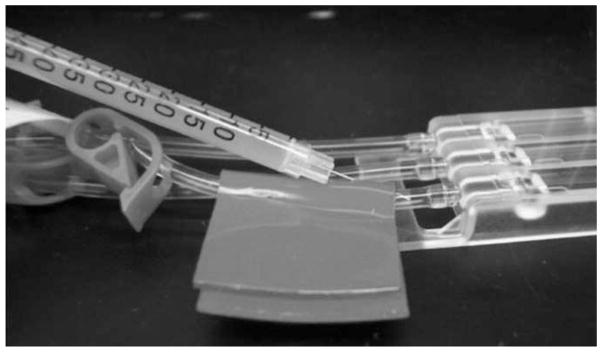
The needle of an insulin syringe containing the flow cell inoculum is carefully inserted through the self-sealing tape and into the lumen of the tubing just upstream of the flow cell chamber. The bacterial inoculum (0.5 mL) is slowly introduced into the flow cell chamber while the upstream pinch clamp is closed and the downstream pinch clamp is open. After full injection of the inoculum, the injection site is cleaned with a sterile alcohol pad, and the downstream pinch clamp is closed.
Fig. 10.
After inoculation, the flow cell chamber is placed upside down in a 37°C incubator. Growth at 37°C is maintained without medium flow for 1 h, after which the flow cell is returned to an upright position and medium flow is resumed.
Fig. 11.
The flow cell tubing should not be impacted as it enters or exits the incubator. The flow cell chamber should be level with the incubator surface at all times throughout the experiment (a small weight may be used for this purpose).
3.3. Catheter-Based Model of In Vivo Biofilm Formation
3.3.1. Preparation of Bacterial Inocula
Grow each bacterial strain at 37°C with constant aeration to the desired concentration, as measured by optical density (OD) (see Note 12). Harvest bacterial cells by centrifugation and resuspend in PBS containing 10% dimethyl sulfoxide and 5% bovine serum albumin.
After determining viable colony counts by plating on suitable growth medium, store aliquots at −80°C.
Prior to injection, thaw the aliquots on ice and wash twice with sterile PBS.
3.3.2. Implantation of sc Catheter Segments
Anesthetize mice by injecting 0.5–0.7 cc of TBE intraperitoneally (approx 0.4–0.75 mg of TBE/g of body weight). Induction of anesthesia should occur within 5–15 min.
After ensuring adequate anesthesia, shave the dorsal flanks of each mouse. Clean the shaved areas first with Betadyne and then with alcohol. Allow the area to dry before making incisions.
Make a small (~1 cm) incision in the shaved area over one flank by lifting the skin and cutting with surgical scissors. Using forceps or a blunt probe, insert the catheter segment into the incision and approx 3 cm cephalad into the sc space. Ensure that the catheter does not shift back toward the incision site. Close the wound with surgical adhesive. Repeat this process for the other flank (i.e., two catheters per mouse).
3.3.3. Inoculation of Catheter Lumen
Prepare inocula by filling insulin syringes with 100 μL of bacterial suspension consisting of the desired number of colony-forming units.
Ensure that the surgical wound is closed and that the adhesive is completely dry before inoculation (see Note 13).
Working from the cephalad side of the catheter, carefully insert the needle subcutaneously and into the lumen of the catheter. It is helpful to use one hand to secure the catheter and surrounding skin while manipulating the syringe with the other hand.
Slowly inject the bacterial suspension into the lumen of the catheter. Forceful injections will increase the chances of inoculating outside of the catheter.
Carefully remove the needle and gently clean the injection site with isopropanol.
Monitor infected mice for signs of distress until awake and mobile (see Note 14).
3.3.4. Assessment of Catheter Infection
At the desired time point(s), humanely euthanize mice according to the protocols approved at the researcher’s institution.
Using aseptic technique, make a small incision with surgical scissors and carefully remove each catheter from the sc space using sterile forceps.
To remove nonadherent or loosely adherent bacteria from the catheter, carefully dunk the catheter into sterile PBS three times before placing it into a sterile container containing 5 mL of sterile PBS.
Sonicate the explanted catheters to remove adherent bacteria. We have found that 5 min of sonication (using the Fisher Sonic Dismembrator at a setting of 2) is sufficient to remove a prototypic clinical isolate of S. aureus from both 2- and 10-d-old catheter-associated biofilms (see Note 15).
Make serial dilutions of each sample and plate on an appropriate medium to obtain quantitative colony counts. Correct for the dilution factor and the volume plated to determine the total number of bacteria recovered per explanted catheter.
Footnotes
This formulation provides enough plasma to coat just over one full plate (125 wells). While this adds considerable expense to the protocol, we have tried alternative concentrations (as low as 5%) and have found that this increases variability between wells. Nevertheless, variability is unavoidable, and for this reason it is mandatory that all assays be done in replicates. We typically employ at least four and sometimes eight wells per test strain.
This incubator has a heated platform with a removable cover, which allows us to do our assays on the benchtop while maintaining 37°C in the flow cell itself. A standard laboratory incubator can be used assuming it can accommodate all components of the flow cell system or has ports that can be used to extend the tubing from the media reservoir/pump to the spent-medium collection vessel.
There are a number of suitable choices for murine species. We chose to use NIH-Swiss mice based on studies indicating that these mice are also an appropriate choice for our other experiments investigating the pathogenesis of staphylococcal septic arthritis.
There are a number of options for murine anesthesia. We have found that administration of TBE results in a rapid and predictable anesthesia with a relatively low incidence of adverse reactions or overdose.
If cultures did not grow comparably, it may be necessary to make appropriate modifications to the starting dilution. Note that we have also tried alternative starting densities for these assays and have found that a 1:200 dilution, which corresponds to an OD (560 nm) of approx 0.05, yields the most reproducible results.
It may be necessary to dilute the eluted stain in PBS in order to obtain an absorbance value within the linear range of the plate reader. Results can be expressed in terms of absolute absorbance value, but we often express our results relative to a well-characterized reference strain. This is particularly appropriate when screening mutants generated in the reference strain.
To avoid wasting plasma, do not open the stopcocks on the bubble trap at this time. If bubbles accumulate during this step, ensure that they do not remain inside the flow chamber itself before incubating at 4°C. Bubbles inside the tubing are acceptable at this point.
A sufficiently sized sterile media reservoir must be used to ensure that the flow of medium is not compromised once established. The 10-L media reservoir from Stovall (Fig. 4) comes prefitted with tubing and is highly recommended. Ensure that the media reservoir is sufficiently sterilized by autoclaving, because the biofilm medium in the reservoir can become contaminated quite easily.
Alternatively, the flow cell output manifold may be removed, and each of the three flow cell chamber effluents may be collected individually for subsequent analyses.
With our prototype clinical isolate (UAMS-1) and its corresponding mutants, we typically use 0.5 mL of a standardized overnight culture to inoculate each flow cell (8).
Flow rate is an experimentally defined parameter. Setting a flow rate that is too slow will result in planktonic growth within the flow chamber, owing to a failure to remove nonadherent cells. Setting a flow rate that is too fast will prevent biofilm growth, owing to the presence of high shear forces. It may be necessary to set up multiple experiments with varying flow rates, especially when comparing S. aureus mutants thought to be impaired in biofilm formation.
The relationship between OD and viable cell counts depends on many factors and should be determined empirically for each isolate of S. aureus.
It is highly recommended that infected mice be sufficiently anesthetized such that after inoculation the bacterial suspension can settle in the lumen of the catheter without being disturbed by movement of the mouse.
Signs of distress in mice include any of the following: rapid breathing rate; slow, shallow, or labored breathing; rapid weight loss; ruffled fur or rough hair coat; hunched posture; difficulty moving; hypothermia or hyperthermia; anorexia; diarrhea; or constipation.
Since strains of S. aureus have varying capacities for both adherence and persistence within a biofilm, it is highly recommended that additional mice be included in the experimental protocol for the purpose of determining the appropriate amount of sonication. Using aseptic technique, sonicate the catheter in 5 mL of PBS for 30 s (this volume of PBS allows sufficient space for the sonicating tip to operate without touching the catheter segment). Remove 100 μL for serial dilution and subsequent determination of colony-forming units. Repeat this step after another 30 s of sonication.
References
- 1.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 3.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983;40:407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rupp ME, Ulphani JS, Fey PD, Mack D. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect Immun. 1999;67:2656–2659. doi: 10.1128/iai.67.5.2656-2659.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadurugamuwa JL, Sin L, Albert E, et al. Direct continuous method for monitoring biofilm infection in a mouse model. Infect Immun. 2003;71:882–890. doi: 10.1128/IAI.71.2.882-890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 7.Sillanpaa J, Xu Y, Nallapareddy SR, Murray BE, Hook M. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology. 2004;150:2069–2078. doi: 10.1099/mic.0.27074-0. [DOI] [PubMed] [Google Scholar]
- 8.Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun. 2003;71:4206–4211. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cafiso V, Bertuccio T, Santagati M, et al. Presence of the ica operon in clinical isolates of Staphylococcus epidermidis and its role in biofilm production. Clin Microbiol Infect. 2004;10:1081–1088. doi: 10.1111/j.1469-0691.2004.01024.x. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick F, Humphreys H, O’Gara JP. The genetics of staphylococcal biofilm formation—will a greater understanding of pathogenesis lead to better management of device-related infection? Clin Microbiol Infect. 2005;11:967–973. doi: 10.1111/j.1469-0691.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 11.Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. The intracellular adhesin (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beenken KE, Dunman PM, McAleese F, et al. Global gene expression in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:4665–4684. doi: 10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzpatrick F, Humpreys H, O’Gara JP. Evidence for icaADBC-independent biofilm development mechanism in methicillin-resistant Staphylococcus aureus clinical isolates. J Clin Microbiol. 2005;43:1973–1976. doi: 10.1128/JCM.43.4.1973-1976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fluckiger U, Ulrich M, Steinhuber A, et al. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect Immun. 2005;73:1811–1819. doi: 10.1128/IAI.73.3.1811-1819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenney D, Pouliot KL, Wang Y, et al. Broadly protective vaccine for Staphylococcus aureus based on an in vivo–expressed antigen. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 16.Yao Y, Sturdevandt DE, Otto M. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J Infect Dis. 2005;191:289–298. doi: 10.1086/426945. [DOI] [PubMed] [Google Scholar]
- 17.Yarwood JM, Bartels DJ, Volper EM, Greenberg EP. Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuong C, Gerke C, Somerville GA, Fischer ER, Otto M. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J Infect Dis. 2003;188:706–718. doi: 10.1086/377239. [DOI] [PubMed] [Google Scholar]