Abstract
Objective:
To test the hypothesis that patients with multiple sclerosis (MS) with intereye asymmetry on low contrast letter acuity, and thickness of the retinal nerve fiber layer (RNFL), would exhibit corresponding changes in cortical timing and amplitude responses on pattern reversal multifocal visual evoked potentials (mfVEP), contingent upon variable stimulus contrast.
Methods:
In a cross-sectional study, we investigated a cohort of 11 normal subjects and 40 patients with MS, 21 of whom had a history of acute optic neuritis (MS-AON) with an intereye asymmetry with respect to RNFL thickness, and on low contrast letter acuity performance. Pattern reversal mfVEP was performed at high (100%), low (33.3%), and very low (14.2%) Michelson-contrast levels.
Results:
Compared to baseline measures at 100% contrast, the mean amplitude of the mfVEP was reduced in MS-AON eyes, upon pattern-reversal stimulation at the 2 lower contrast levels (p < 0.0001). With respect to changes in timing responses, the intereye asymmetry was increased in the MS-AON patients upon lower contrast pattern-reversal stimulation (p < 0.0001 for 33.3% compared to 100%, and p < 0.001 for 14.2% compared to 100%). The fellow eye in 12 (57%; p < 0.001) of the patients with an abnormal eye, and a history of AON, revealed abnormal amplitude and timing responses upon low contrast stimulation (signifying unmasking of occult damage).
Conclusions:
Our findings support the hypothesis that mfVEP metric abnormalities are contingent upon contrast magnitude during pattern reversal stimulation. Further, this paradigm was capable of unmasking occult abnormalities in a significant number of apparently unaffected eyes.
Conventional visual evoked potential (VEP) studies can reveal the cardinal features of inflammatory demyelination within the anterior visual system, such as prolongation in the P-100 latency.1 This finding represents the pathophysiologic signature of conduction slowing, and represents one of the earliest demonstrable and objective features of acute optic neuritis (AON).2–4
The recent assessment of conventional VEP measures, with the application of a low contrast pattern-reversal method, represents a potentially practical refinement in the sensitivity and specificity of detecting demyelinating optic neuropathy.5 Nonetheless, conventional VEP testing yields a single summed potential from the entire visual field of stimulation, rendering this technique inadequately sensitive in detecting definitive albeit highly localized abnormalities. Multifocal VEP (mfVEP) has substantially enhanced the sensitivity of identifying such changes, even from highly discrete regions of the visual processing pathways.6–8
With the application of mfVEP, we tested the hypothesis that patients with MS with intereye asymmetry on low contrast letter acuity, and thickness measures of the retinal nerve fiber layer (RNFL) as measured by spectral domain optical coherence tomography (OCT), would exhibit corresponding side-side changes in cortical timing and amplitude responses on pattern-reversal mfVEP. Further, we also anticipated that the magnitude of these abnormal responses would be contingent upon defined levels of contrast stimulation.
METHODS
Patient characteristics and inclusion criteria.
Fifty-one subjects were recruited from the MS Center at UT Southwestern Medical Center from June to August 2010: 11 normal individuals (mean age = 35; 82% female) and 40 patients (mean age = 43; 71% female) with definite MS (as per the modified McDonald criteria), 21 of whom had a documented history of AON (MS-AON; all unilateral; 2 recurrent) at least 6 months prior to the onset of our studies (mean = 23 ± 11.43 months; range 8–54). Nineteen patients with MS had no history of AON (MS-NON), and no significant asymmetry with respect to low contrast acuity or RNFL thickness measures. Inclusion criteria for those with a history of AON required evidence of a left-right asymmetry in visual performance on corrected low contrast letter acuity charts (1.25 and 2.5%; ≥5 letters of acuity difference; a criteria associated with a significant change in RNFL thickness),3 and with respect to RNFL thickness, as measured by spectral domain OCT (Spectralis; Heidelberg, Germany). Specifically, for the AON affected eye, RNFL thickness had to be less than 1% of that predicted for a matched population (in either average RNFL thickness or in at least 1 quadrant of the RNFL). While the unaffected fellow eye (MS-FON) could have harbored subclinical disease activity, we required that both the average and quadrant RNFL thickness from this eye to be in the normal range. All patients with MS with a history of AON were treated with standard corticosteroid therapy regimens at the time of the acute visual syndrome.
As we were not aware of any similar mfVEP studies utilizing variable contrast stimulation, we chose the simplest method (Michelson) to decrease contrast on a very accurate scale that is available with the microdisplay stimulator (appendix e-1 on the Neurology® Web site at www.neurology.org).
Pattern reversal mfVEP (VERIS; EDI, Redwood City, CA) was performed on both eyes monocularly at high (100%), low (33.3%), and very low (14.2%) Michelson contrast levels, in order to provide evidence of a gradient or differential effect of visual cortical responses, similar to patient performance on high and low contrast letter acuity charts.5 We analyzed mean amplitude and timing response asymmetry across 60 sectors of cortical responses. In order to interrogate the intereye differences spanning the 60 sectors (organized as rings), waveform metrics were transformed into pseudocolor 2D representations through the VERIS software.
Analysis of our various datasets included the application of the Student t test for comparison of mean metric values. Further, given the non-normal distribution of our data, we utilized the Wilcoxon rank sum test for the investigation of significant differences between paired comparisons (e.g., cortical responses at 100% vs 33.3% contrast stimulation). Level of significance was set at p = 0.05.
Standard protocol approvals, registrations, and patient consents.
All participants provided informed and written consent prior to the beginning of study procedures. Consent was obtained according to the Declaration of Helsinki. The protocol was approved by the Investigational Review Board of the University of Texas Southwestern Medical Center.
RESULTS
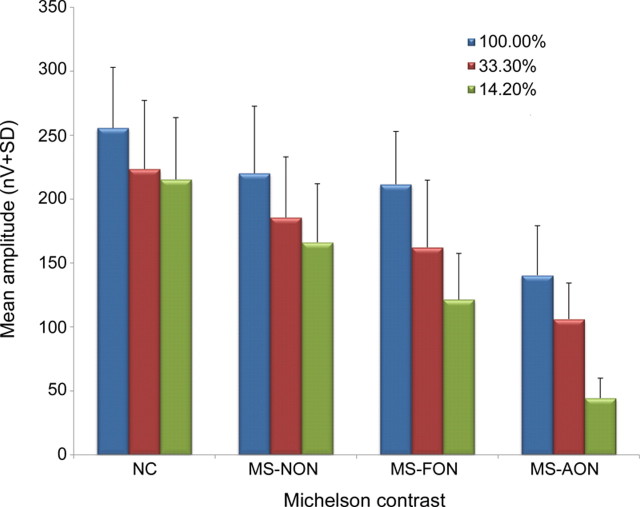
In normal individuals, changes in the magnitude of contrast stimulation yielded mfVEP waveforms from the 2 eyes that remained coregistered without significant asymmetry. All patients with MS with a history of unilateral AON that met our inclusion criteria exhibited evidence of waveform asymmetry with respect to amplitude, timing responses, or both. When compared to black on white high contrast stimulation, 90% (19/21) of patients with MS with a history of AON exhibited amplitude attenuation or prolongation in cortical timing responses upon low and very low contrast pattern reversal stimulation (p < 0.0001) (figure 1). Further, compared to baseline measures at 100% contrast, the mean amplitude of the mfVEP was attenuated in MS-AON eyes upon pattern-reversal stimulation at the 2 lower contrast levels (p < 0.0001) (figure 2).
Figure 1. Variable contrast pattern-reversal stimulation.
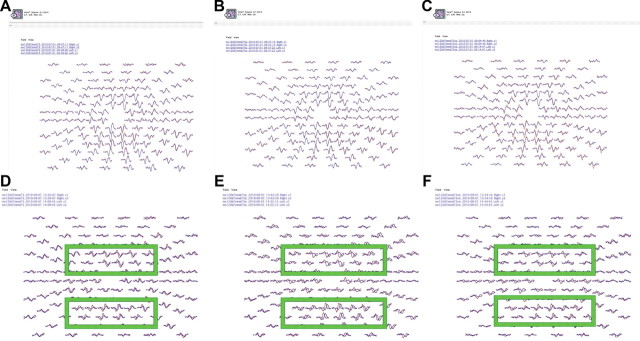
(A–C) Multifocal visual evoked potential (mfVEP) responses in the right (red) and left (blue) eyes from a normal subject at high (100%), low (33.3%), and very low (14.2%) Michelson-contrast, pattern-reversal stimulation. Note that the waveforms derived from the 2 eyes are superimposed with near complete coregistration. (D–F) mfVEP cortical responses induced with the same sequence of variable contrast, pattern-reversal stimulation, from a patient with a history of left optic neuritis. Note that with low contrast stimulation, there is a corresponding prolongation in the timing responses of the visual cortical responses in the left eye (blue mfVEP responses), most prominently illustrated by the central field clusters within the green boxes. Further, at very low contrast stimulation (F), both timing response prolongation and amplitude attenuation is observed. These findings are in keeping with the established pathophysiologic principles of multiple sclerosis–related demyelination.
Figure 2. Mean amplitudes of multifocal visual evoked potential (mfVEP) responses.
This figure demonstrates the mean amplitudes of the mfVEP (means ± SD) in nV, across different levels of Michelson contrast pattern-reversal stimulation. Eyes from patients with multiple sclerosis with a history of AON (MS-AON) exhibited a significant attenuation in the mean amplitude responses to 100%, 33.3%, and 14.2% Michelson contrast pattern-reversal stimulation, when compared normal subjects (p < 0.0001 for all), and with respect to 33.3% and 14.2% contrast stimulation when compared to100% Michelson contrast stimulation (p < 0.0001 for both). Significant attenuation was also observed in 42% of eyes from MS-NON patients, and 57% of MS-FON eyes (p < 0.001 for both). A modest and insignificant reduction in mean amplitude response was observed in normal subject eyes with low and very low contrast stimulation. MS-FON = unaffected fellow eye; NC = normal control.
Sixty discrete cortical response sectors were organized into concentric rings and transformed into pseudocolor 2D topographic maps that illustrate either interocular waveform homology vs response predominance of the more normal eye (figure 3). The fellow eye (MS-FON) in 12 (57%; p < 0.001) of the patients with an abnormal eye and a history of AON revealed abnormal amplitude and timing responses upon low contrast stimulation (signifying unmasking of occult damage), thereby reducing the color saturation (less predominance of the dominant eye mfVEP waveforms) of the cortical response sectors. Of the 19 patients with MS without a history of acute optic neuritis (and without asymmetry with respect to low contrast letter acuity or RNFL thickness), 42% demonstrated progressive desynchronization of the mfVEP waveforms in response to lower contrast pattern-reversal stimulation (p < 0.001). Similarly, at lower contrast levels, the 60-sector pseudocolor 2D waveform maps confirmed greater intereye differences.
Figure 3. 2D Pseudocolor multifocal visual evoked potential (mfVEP) waveform topography maps.

2D Pseudocolor topography maps for the analysis of ring sectors. Briefly, gray indicates that both eyes are in the noise level of the device, and therefore no analytical conclusions can be rendered. White represents waveforms that are strong and equally balanced between the 2 eyes (expected in normal subjects). Red sectors designate dominance of the right eye waveforms, whereas blue constitutes left eye dominance. (A) Topography maps are presented from a normal subject in response to 100%, 33.3%, and 14.2% Michelson contrast stimulation (left to right). Observe the predominant gray and white saturation, without evident discordance between the 2 eyes (i.e., no dominant red or blue saturation). (B) In a patient with left optic neuritis, we observe progressive dominance of the right eye (red) waveforms as the sequence of stimulation transitions from 100% Michelson contrast (map on the left), 33.3% (center), and 14.2% (on the right). With reduction of the magnitude of the contrast stimulation, note the intensification of the red saturation, signifying worsening of the left eye mfVEP waveforms. (C) In a patient with left optic neuritis, note the more subtle right eye (red) dominance at 100% contrast stimulation, whereas at 33.3% stimulation there is increased sector dominance of the right eye (implicating more prominent left eye mfVEP waveform abnormalities), in addition to the emergence of some blue saturation intensity (signifying the unmasking of occult right eye mfVEP waveform abnormalities). At the 14.2% contrast level of stimulation, there is diffuse reduction of red saturation, further implicating the abnormalities of both the left and right waveform abnormalities, albeit still with milder changes in the right vs the left eye (hence the persistence of red saturation).
With respect to changes in timing responses (analogous to latency), the intereye asymmetry was substantially worsened in the MS-AON patients upon lower contrast pattern-reversal stimulation (p < 0.0001 for 33.3% compared to 100%, and p < 0.001 for 14.2% compared to 100%). Conspicuously, the mean asymmetry in these patients was actually more prominent upon pattern-reversal stimulation at 33.3% stimulation than at 14.2% stimulation (61.5 ± 13.3 msec vs 49.8 ± 15.2 msec, respectively). We hypothesize that upon the lowest level of contrast stimulation, occult damage in the apparently unaffected fellow eye (MS-FON) is unmasked.
DISCUSSION
In a longitudinal study of mfVEP in AON, an asymmetry analysis strategy was applied in order to confirm the utility of intrasubject, intereye comparisons, as a valid method to potentially overcome, or at least mitigate, the heterogeneity of responses across different patients.7,9 Alternately, our pilot investigation was cross-sectional in nature, and aimed at characterizing the effects that low contrast visual stimulation exerts on visual system cortical responses. The results reported herein are similar to the outcomes observed with low contrast visual acuity testing in MS cohorts.3,10
An important limitation of our application of mfVEP to address our hypothesis-driven question is related to the heterogeneity of responses across subjects, at least partly related to corresponding variability in visual cortical anatomy. As such, we purposely formulated stringent function and structure inclusion criteria, specifying that MS-AON patients exhibit evidence of interocular differences in both low contrast acuity and RNFL thickness.
We confirmed our principal hypothesis that the magnitude of mfVEP amplitude and timing response abnormalities would be contingent upon precisely defined levels of contrast, during pattern-reversal stimulation, in those patients with MS with unilaterally affected eyes. We also underscore the observation that the variable contrast, pattern-reversal stimulation paradigm was capable of also unmasking similar abnormalities in a significant number of apparently unaffected eyes.
Supplementary Material
ACKNOWLEDGMENT
The authors thank their patients for participating in our studies.
GLOSSARY
- AON
acute optic neuritis
- mfVEP
multifocal visual evoked potential
- MS
multiple sclerosis
- MS-AON
multiple sclerosis with a history of acute optic neuritis
- MS-FON
unaffected fellow eye
- OCT
optical coherence tomography
- RNFL
retinal nerve fiber layer
- VEP
visual evoked potential
Footnotes
Editorial, page 732
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Audrey Frohman was involved in the formulation, design, and execution of the study. She participated in the analysis of the data, preparing the manuscript, and its final revision. Zane Schnurman was involved in the formulation, design, and execution of the study. He participated in the analysis of the data, preparing the manuscript, and its final revision. Amy and Darrel Conger contributed to the study through data collection and analysis and with respect to assistance with the editing and revision of the manuscript. Shin Beh was involved in the formulation of the study, execution of the studies on patients and control subjects, and in the data analysis and preparation of the manuscript. Benjamin Greenberg contributed by helping with the design and execution of the study. He helped with the editing and writing of the manuscript. Erich Sutter was involved in all aspects of this investigation from the programming of the mfVEP software to the analysis and preparation of the manuscript. Peter Calabresi contributed to all aspects of the study. Laura Balcer contributed to all aspects of the study. Teresa Frohman is the Director of the Eye Testing Laboratory at the MS Program. She contributed to all aspects of the study. Elliot Frohman is the senior author and contributed to all aspects of the study.
DISCLOSURE
A. Frohman, Z. Schnurman, A. Conger, D. Conger, and S. Beh report no disclosures. B. Greenberg has received grants from the Guthy Jackson Charitable Foundation, Accelerated Cure Project, and Ampliummune. He has received consulting fees from Diogenix, Sanofi Aventis, and the Greater Good Foundation. He also holds an equity position in Diogenix. He has received speaker honoraria from Serono, the MS Association of America, the National MS Society, and the American Academy of Neurology. E. Sutter holds an equity position in Electrodiagnostic Imaging. P. Calabresi has provided consultation services to Novartis, EMD-Serono, Teva, Biogen-IDEC; and has received grant support from EMD-Serono, Teva, Biogen-IDEC, Genentech, Bayer, Abbott, and Vertex. L. Balcer has received honoraria for consulting on development of visual outcomes for MS trials from Biogen-Idec and Bayer. She is on a clinical trial advisory board for Biogen-Idec. T. Frohman has received speaker fees from Biogen Idec and TEVA. E. Frohman has received speaking and consulting fees from Biogen Idec, TEVA Neuroscience, Acorda, Bayer, and Novartis. He has received consulting fees from Biogen Idec, TEVA Neuroscience, Acorda, Novartis, and Abbott Laboratories. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Gronseth GS, Ashman EJ. Practice parameter: the usefulness of evoked potentials in identifying clinically silent lesions in patients with suspected multiple sclerosis (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;54:1720–1725 [DOI] [PubMed] [Google Scholar]
- 2.Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol 2006;59:963–969 [DOI] [PubMed] [Google Scholar]
- 3.Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006;113:324–334 [DOI] [PubMed] [Google Scholar]
- 4.Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer L. Imaging retinal architecture in multiple sclerosis with optical coherence tomography. Nat Neurol 2008;4:664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thurtell MJ, Bala E, Yaniglos SS, Rucker JC, Peachey NS, Leigh RJ. Evaluation of optic neuropathy in multiple sclerosis using low-contrast visual evoked potentials. Neurology 2009;73:1849–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klistorner A, Arvind H, Nguyen T, et al. Multifocal VEP and OCT in optic neuritis: a topographical study of the structure-function relationship. Doc Ophthalmol 2009;118:129–137 [DOI] [PubMed] [Google Scholar]
- 7.Klistorner A, Arvind H, Garrick R, Graham SL, Paine M, Yiannikas C. Interrelationship of optical coherence tomography and multifocal visual-evoked potentials after optic neuritis. Invest Ophthalmol Vis Sci 2010;51:2770–2777 [DOI] [PubMed] [Google Scholar]
- 8.Fraser C, Klistorner A, Graham S, Garrick R, Billson F, Grigg J. Multifocal visual evoked potential latency analysis: predicting progression to multiple sclerosis. Arch Neurol 2006;63:847–850 [DOI] [PubMed] [Google Scholar]
- 9.Klistorner A, Graham S, Fraser C, et al. Electrophysiological evidence for heterogeneity of lesions in optic neuritis. Invest Ophthalmol Vis Sci 2007;48:4549–556 [DOI] [PubMed] [Google Scholar]
- 10.Costello F, Hodge W, Pan YI, et al. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler 2008;14:893–905 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.