Abstract
OBJECTIVES
Endoscopist fatigue potentially impacts colonoscopy. Fatigue is difficult to quantitate, but polyp detection rates between non-fatigued and fatigued time periods could represent a surrogate marker. We assessed whether timing variables impacted polyp detection rates at a busy tertiary care endoscopy suite.
METHODS
Consecutive patients undergoing colonoscopy were retrospectively identified. Indications, clinical demographics, pre-procedural, and procedural variables were extracted from chart review; colonoscopy findings were determined from the procedure reports. Three separate timing variables were assessed as surrogate markers for endoscopist fatigue: morning vs. afternoon procedures, start times throughout the day, and queue position, a unique variable that takes into account the number of procedures performed before the colonoscopy of interest. Univariate and multivariate analyses were performed to determine whether timing variables and other clinical, pre-procedural, and procedural variables predicted polyp detection.
RESULTS
During the 4-month study period, 1,083 outpatient colonoscopy procedures (57.5±0.5 years, 59.5% female) were identified, performed by 28 endoscopists (mean 38.7 procedures/endoscopist), with a mean polyp detection rate of 0.851/colonoscopy. At least, one adenoma was detected in 297 procedures (27.4%). A 12.4% reduction in mean detected polyps was detected between morning and afternoon procedures (0.90±0.06 vs. 0.76±0.06, P = 0.15). Using start time on a continuous scale, however, each elapsed hour in the day was associated with a 4.6% reduction in polyp detection (P = 0.005). When queue position was assessed, a 5.4% reduction in polyp detection was noted with each increase in queue position (P = 0.016). These results remained significant when controlled for each individual endoscopist.
CONCLUSIONS
Polyp detection rates decline as time passes during an endoscopist’s schedule, potentially from endoscopist fatigue. Queue position may be a novel surrogate measure for operator fatigue.
INTRODUCTION
The purpose of screening colonoscopy is to identify and resect adenomatous polyps, thereby halting their progression to adenocarcinoma (1–3). Consequently, colonoscopy can be an effective screening tool only if polyps are properly visualized and removed (3). Numerous factors affect the quality of colonoscopy as a screening tool, including the skill of the endoscopist, the adequacy of bowel cleansing, and patient-associated variables, such as body mass index, medications, and comorbid illness (4–8). Metrics have been proposed for quantification of some these factors; for instance, polyp miss rate is an accepted tool for measurement of endoscopist skill (9,10).
Recent reports suggest that endoscopist fatigue may be an additional factor that may impact the quality of colonoscopic polyp detection and removal (11,12). The timing of colonoscopy in the endoscopy schedule, specifically morning vs. later day or afternoon procedure times, has been proposed as a surrogate for endoscopist fatigue. The passage of time over the course of the endoscopist’s day has been proposed to correlate with the accumulation of fatigue, commensurate with the accrual of cognitive and perceptual errors with prolonged and repetitive activity (11). Morning colonoscopy has been shown to detect significantly more polyps than procedures performed later in the day or in the afternoon, even when controlling for covariates such as quality of bowel preparation (11,12). However, this method of using time of day as a surrogate for endoscopist fatigue is highly dependent on how colonoscopies are scheduled and is not applicable to endoscopists who perform these procedures solely during morning or afternoon sessions. Time of day also does not precisely account for the endoscopist’s accumulating fatigue caused by one or more procedures before a given colonoscopy.
Our aim in this study was to evaluate the impact of three different surrogates for endoscopist fatigue on colonoscopic polyp detection—morning vs. afternoon times of colonoscopy, hours elapsed in the endoscopist’s day till the start time of a given colonoscopy procedure, and a novel variable that we termed queue position—while adjusting for clinical and procedural variables that could impact polyp detection. We devised queue position as a means to quantify the procedural burden on an endoscopist before performing a given colonoscopy procedure, and is meant to measure endoscopist fatigue by taking into account all the procedures that the endoscopist performed that day before the given colonoscopy. All three surrogates of endoscopist fatigue were then applied to a consecutive cohort of patients presenting for colonoscopy over a 4-month period to determine the impact on polyp detection rates.
METHODS
All patients presenting for outpatient colonoscopy at the endoscopy suite of the Division of Gastroenterology at Washington University Medical Center during a 4-month period (June to October 2007) were eligible for inclusion in this study. Exclusion criteria included patients <18 years of age, incomplete procedures with failure of cecal intubation, history of colorectal cancer, history of partial or complete colon resection, familial polyposis syndromes, and history of inflammatory bowel disease. Incomplete documentation of the procedure and inability to retrieve data on all variables of interest were further grounds for exclusion. This protocol was approved by the Human Research Protection Office (institutional review board) of Washington University School of Medicine.
Eligible patients were retrospectively identified from interrogation of the computerized endoscopic procedure records (Provation, Minneapolis, MN) by study investigators who did not participate in performance of the colonoscopic procedures (B.B.B. and N.G.). Their colonoscopy reports were electronically captured and scrutinized. Subsequently, electronic medical records were queried for additional data, once again by investigators who did not participate in colonoscopy (A.L., N.G., and J.M.I.). Data for 25 unique predictor variables were assessed, grouped into three main categories: clinical, pre-procedural, and procedural (Table 1).
Table 1.
Clinical, pre-procedural, and procedural variables for the study population
| Variable | Total | Morning | Afternoon |
|---|---|---|---|
| Number of colonoscopy procedures | 1,083 | 702 | 381 |
| Clinical | |||
| Demographics | |||
| Age, mean (years) | 57.5±0.4 | 58.3±0.4 | 56.0±0.7* |
| Gender, male (%) | 438 (40.4) | 296 (42.2) | 142 (37.3) |
| Race | |||
| White (%) | 659 (60.8) | 440 (62.7) | 219 (57.5) |
| Black (%) | 389 (35.9) | 240 (34.2) | 149 (39.1) |
| Other (%) | 35 (3.2) | 22 (3.1) | 13 (3.4) |
| Comorbidities | |||
| BMI, mean (kg/m2) | 29.3±0.2 | 29.1±0.3 | 29.6±0.4 |
| Smoking (%) | 245 (22.6) | 141 (20.1) | 104 (27.3)* |
| Alcohol (%) | 516 (47.6) | 326 (46.4) | 190 (49.9) |
| Heart disease (%) | 167 (15.4) | 111 (15.8) | 56 (14.7) |
| Psychiatric disease (%) | 215 (19.9) | 131 (18.7) | 84 (22.0) |
| Medications | |||
| Aspirin (%) | 314 (29.0) | 217 (30.9) | 97 (25.5) |
| NSAID (%) | 103 (9.5) | 69 (9.8) | 34 (8.9) |
| Psychoactive drugs (%) | 209 (19.3) | 132 (18.8) | 77 (20.2) |
| PPI (%) | 140 (12.9) | 100 (14.2) | 40 (10.5) |
| Pre-procedural | |||
| Previous colonoscopy (%) | 300 (27.7) | 197 (28.1) | 103 (27.0) |
| Indication | |||
| Screening (%) | 470 (43.4) | 305 (43.4) | 165 (43.3) |
| Surveillance (%) | 331 (30.6) | 235 (33.5) | 96 (25.2)* |
| Imaging (%) | 17 (1.6) | 10 (1.4) | 7 (1.8) |
| Bleeding (%) | 149 (13.8) | 88 (12.5) | 61 (16.0) |
| Other symptoms (%) | 116 (10.7) | 64 (9.1) | 52 (13.6)* |
| Bowel preparation method | |||
| PEG (%) | 999 (92.2) | 654 (93.2) | 345 (90.6) |
| Sodium phosphate (%) | 72 (6.6) | 42 (6.0) | 30 (7.9) |
| Magnesium citrate (%) | 4 (0.4) | 2 (0.3) | 2 (0.5) |
| 2-Day preparation (%) | 7 (0.6) | 4 (0.6) | 3 (0.8) |
| Dulcolax (%) | 1 (<0.1) | 0 (0) | 1 (0.3) |
| Bowel preparation quality, unadjusted score | |||
| Excellent (%) | 344 (31.8) | 234 (33.3) | 110 (28.9) |
| Good (%) | 418 (38.6) | 273 (38.9) | 145 (38.1) |
| Fair (%) | 250 (23.1) | 154 (21.9) | 96 (25.2) |
| Poor (%) | 62 (5.7) | 35 (5.0) | 27 (7.1) |
| Unsatisfactory (%) | 9 (0.8) | 6 (0.9) | 3 (0.8) |
| Bowel preparation quality, composite score | |||
| Acceptable (%) | 626 (57.8) | 410 (58.4) | 216 (56.7) |
| Unacceptable (%) | 457 (42.2) | 292 (41.6) | 165 (43.3) |
| Procedural | |||
| Number of endoscopists | 28 | 26 | 24 |
| Trainee involved (%) | 268 (24.7) | 166 (23.6) | 102 (26.8) |
| Ileal intubation (%) | 243 (22.4) | 159 (22.6) | 84 (22.0) |
| Duration, mean (min:s) | 21:30±00:18 | 21:18±0:22 | 21:48±0:31 |
| Type of sedation | |||
| Monitored anesthesia (%) | 716 (66.1) | 473 (67.4) | 243 (63.8) |
| Conscious sedation (%) | 367 (33.9) | 229 (32.6) | 138 (36.2) |
| Time of day (%) | |||
| Morning (%) | 702 (64.8) | ||
| Afternoon (%) | 381 (35.2) | ||
| Queue position, mean (%) | 3.692±0.069 | 2.748±0.002 | 5.430±0.006* |
BMI, body mass index; NSAID, non-steroidal anti-inflammatory drug; PEG, polyethylene glycol; PPI, proton-pump inhibitor.
Standard error of mean is reported for all mean values;
P<0.05 compared with morning procedures.
Patient scheduling
In our endoscopy laboratory, endoscopic procedures are scheduled on a ‘first come first served’ basis. The majority of patients are scheduled on an ‘open access’ basis, where the request for colonoscopy from treating physicians is processed by trained scheduling nurses, and assigned one of the first available endoscopy slots on any available endoscopist’s schedule. Clinician endoscopists with outpatient practices also call in their patients to the same scheduling nurses, and the same scheduling practices are followed. In particular, scheduling is random, and there is no attempt on the part of the scheduling nurses to schedule any particular colon cancer risk group or surveillance vs. screening indications into earlier or later slots. However, diabetic patients on insulin may be offered earlier slots for obvious reasons regardless of their risk status. Endoscopists are assigned an entire day at a time to work in the endoscopy laboratory. It is unusual for a particular endoscopist to only work part of the day. Therefore, the endoscopists do not vary by time of day.
Quality of bowel preparation
Bowel preparation regimens were implemented by the scheduling nurses, and input from the scheduled endoscopist was solicited on an as needed basis. The default regimen was polyethylene glycol, administered the day before colonoscopy. Alternate regimens included sodium phosphate (generally reserved for patients without cardiac, pulmonary, and renal comorbidities) and 2-day magnesium citrate regimens for repeat procedures in patients unable to tolerate or with poor outcomes from single-day bowel preparation.
Among the pre-procedural variables, bowel preparation quality, unadjusted score was extracted from each colonoscopy procedure report, in which each endoscopist used a forced drop-down menu to rate bowel preparation quality. This was converted to an ordinal score by using a predetermined Aronchick scale as follows: excellent, 1; good, 2; fair, 3; poor, 4; and unsatisfactory, 5. To more rigorously encapsulate the quality of bowel cleansing, bowel preparation quality, composite score was calculated using a previously reported formula (8). The composite score was a sum of three variables scored as follows:
Ordinal score by Aronchick scale was determined, as described previously. All bowel preparation scores ≤ 2 (excellent or good) were considered adequate and given a converted binary score of 0, whereas others were given a converted binary score of 1, indicating an unacceptable preparation.
Change in standard post-procedure recommendation for follow-up consequent to the quality of the bowel preparation. The recommendation for follow-up made after the colonoscopy was compared with accepted American Gastroenterological Association and American Society for Gastrointestinal Endoscopy recommendations for follow-up for each lesion identified, and follow-up intervals shorter than recommended were given a score of 1; follow-up intervals in accordance with society recommendations were scored 0. Procedure reports wherein the basis for shorter follow-up recommendations was not evident were individually discussed with the endoscopist on a case-by-case basis.
Adequate visualization of the entire colonic mucosa and endoscopists’ confidence in adequacy of the examination. When available, these were extracted from the procedure reports, wherein a drop-down menu allows the endoscopist to designate whether the preparation was good enough to identify polyps <5 mm in size. When this designation or other statements describing the endoscopists’ confidence in the adequacy of the examination were not explicit in the report, the procedure report was directly discussed with the endoscopist for clarification. This was also scored in binary fashion, 0 indicating adequate visualization and good confidence that the examination was adequate, and 1 indicating incomplete visualization or lack of confidence in an adequate examination.
With the sum of these three components, a composite score of “acceptable” was given if the sum was 0; otherwise, a sum of ≥ 1 was given a composite score of “unacceptable” (8).
Timing of colonoscopy
Among the procedural variables, timing was the primary predictor variable of interest and was defined in three different ways for each colonoscopy. First, “time of day” was either designated morning (encapsulating colonoscopies starting between 0700 and 1159 hours) or afternoon (encapsulating colonoscopies starting between 1200 and 1700 hours). Second, “start time” was the exact time at which each colonoscopy began, coded into one of 10 pre-specified hour-long blocks (0700–0759 hours, 0800–0859 hours, and so on). Third, “queue position” was a novel variable that we defined as the number of preceding procedures performed by the endoscopist on that particular day, plus 1 (Figure 1).
Figure 1.
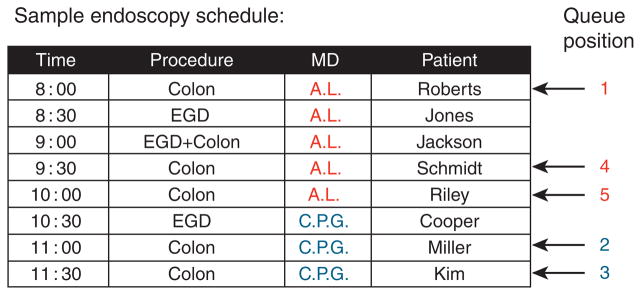
Demonstration of queue position using a sample endoscopy schedule. Queue position was defined as the number of preceding procedures performed by the operator on the day plus 1. Queue position values for each operator are shown on the right for each colonoscopy in the sample schedule. EGD, esophagogastroduodenoscopy.
Variables assessed
The outcome variable for each colonoscopy was the number of detected polyps. This consisted of all polyps reported, resected, and sent for histopathology. An attempt was made to determine polyp histology, but as polyps from colonic segments (and sometimes larger sections of the colon) were often placed in the same pathology jar, a ‘per polyp’ histopathological analysis was not feasible. The proportion of colonoscopic examinations with at least one adenoma detected was assessed. Continuous variables assessed included age, body mass index, and bowel preparation quality score. Binary variables included gender, smoking status, alcohol consumption, heart disease, psychiatric morbidity, use of aspirin, use of non-steroidal anti-inflammatory drugs, use of proton-pump inhibitor, history of previous colonoscopy, use of general anesthesia vs. conscious sedation, and the presence of a gastroenterology trainee. Factor variables included ethnicity, indication for colonoscopy, and bowel preparation type.
Statistical analysis
Descriptive statistics were calculated for the entire study population for all 25 predictor variables. All data are presented as mean±standard error of mean, unless otherwise specified. For each variable, values for morning and afternoon colonoscopy procedures were calculated and compared. The Student’s t-test was used for univariate comparison of continuous parametric variables, and the Fisher’s exact test or χ2-test for categorical variables. Rigorous comparison of the mean number of detected polyps per colonoscopy for all three timing variables required multivariate analysis to account for covariates. General linear modeling with a quasi-Poisson link function (overdispersion parameter = 3) was used to determine covariates by regressing the number of detected polyps onto all predictor variables not related to time. Our approach was appropriate for the decaying, Poisson-like distribution expected for the number of colonoscopies and polyps with increasing values of time and queue position.
Variables that achieved statistical significance (P ≤ 0.05) on univariate analysis were retained for multivariate regression. After selecting covariates, hierarchical modeling (also known as mixed modeling) was employed to allow the intercept of the model to vary depending on the endoscopist. This procedure allowed removal of inter-endoscopist variance from our modeling, which reduced the errors on the remaining covariates. Last, time of day (morning vs. afternoon), start time (organized into hour-long time blocks), and queue position were added in to the model in separate analyses to determine the relationship of each of these time-related variables to the number of detected polyps. Analyses were performed separately because of the anticipated covariance between the three time-related variables. All statistical calculations were performed using SAS (Version 9.1 for Windows; SAS Institute, Cary, NC) and R programming language (Bell Laboratories, www.r-project.org).
RESULTS
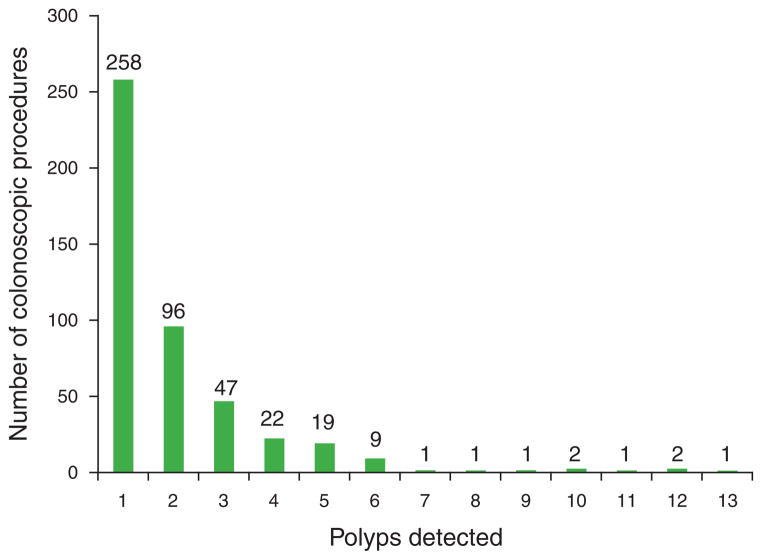
During the 4-month study period, 1,418 outpatient colonoscopy procedures were recorded in our institution’s electronic database. After application of exclusion criteria, a total of 1,083 colonoscopy procedures were included in our study (Table 1). The mean age of the patients was 57.5±0.4 years, and 59.5% were female. The majority of the patients were Caucasian (60.8%), followed by African-American (35.9%); other races (Hispanic, Asian, Native American, and other) accounted for <0.1% each of the total patients. Colorectal cancer screening or polyp surveillance was the sole indication for colonoscopy in 74.0%. Unadjusted bowel preparation quality scores were rated excellent or good in 70.4%; composite scores were acceptable in 57.8%. A trainee directly participated in 268 colonoscopies (24.7%). The mean duration of colonoscopy was 21.5±0.2 min. The majority of the procedures (64.8%) were performed in the morning, and the remaining 35.2% were in the afternoon. The mean queue position was 3.7±0.07. There were 28 endoscopists included in the study, and each performed a mean of 38.7 procedures (range 1–120, median 28.5). A total of 922 polyps were detected in the study cohort, with a mean of 0.851±0.05 polyps detected per colonoscopy (Figure 2); 460 (42.5%) colonoscopic examinations had at least one polyp detected, and 297 (27.4%) had at least one adenoma on histopathology.
Figure 2.
Proportions of colonoscopic procedures with polyps detected, stratified by numbers of polyps resected. The exact number of procedures associated with each number of detected polyps is shown above each bar. No polyps were detected in 623 procedures.
All investigated variables were compared between morning and afternoon colonoscopy procedures (Table 1); afternoon procedures were associated with slightly younger patients, more smokers, a lower likelihood of surveillance procedures, and higher likelihood of bleeding symptoms (P ≤ 0.02 for each). Although the proportion of colonoscopies with each respective unadjusted bowel preparation quality score (‘excellent’ through ‘unsatisfactory’) did not differ significantly between morning and afternoon colonoscopies, the mean unadjusted scores were significantly different, 2.011 and 2.129, respectively, (P = 0.048). Using the composite bowel preparation quality score, 410 colonoscopies (58.4%) had acceptable scores in the morning, whereas 216 colonoscopies (56.7%) had acceptable scores in the afternoon (P = 0.554). As expected, mean queue position was significantly different, 2.7 in the morning and 5.4 in the afternoon (P<0.001).
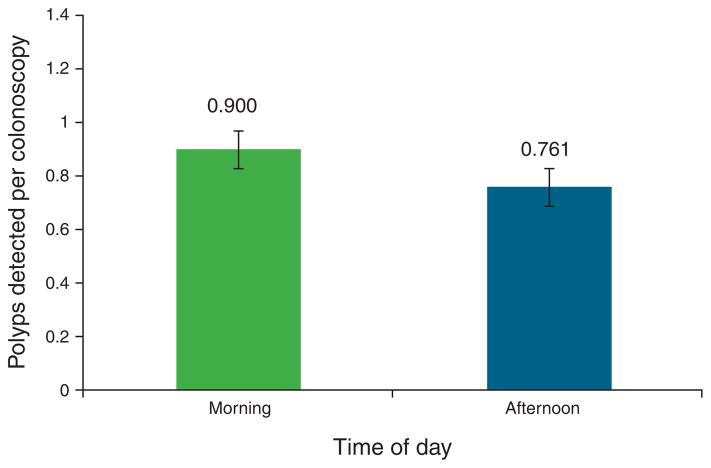
Comparing morning vs. afternoon procedure times, the mean number of detected polyps per colonoscopy was higher in the morning (0.90±0.06) than in the afternoon (0.76±0.06), but this did not reach statistical significance (P = 0.12; Figure 3). On multivariate analysis, afternoon colonoscopy was associated with a mean 12.4% reduction in detected polyps, but this did not reach statistical significance (P = 0.068; Table 2). Seven non-timing variables predicted increased polyp detection (increasing age, male gender, Hispanic race, increasing body mass index, smoking, surveillance as indication, and history of previous colonoscopy), whereas three non-timing variables predicted decreased polyp detection (alcohol consumption, non-bleeding symptoms as indication, and sodium phosphate as bowel preparation method; Table 2). Of note, both unadjusted bowel preparation quality score and composite bowel preparation quality score were not statistically significant as predictors of polyp detection.
Figure 3.
Mean number of polyps detected per colonoscopy, stratified by time of day. Morning procedures were performed between 0700 and 1159 hours, afternoon procedures were performed after 1200 hours through the end of the endoscopist’s routine day. Although the mean number of detected polyps was higher in the morning, the difference was not statistically significant (P = 0.116).
Table 2.
Independent predictors of polyp detection by timing variable using multivariate analysis
| Timing variable | Time of day
|
Start time
|
Queue position
|
|||
|---|---|---|---|---|---|---|
| Change in detected polyps | P value | Change in detected polyps | P value | Change in detected polyps | P value | |
| 1. Timing (%) | −12.4a | 0.068 | −4.6b | 0.005 | −5.4c | 0.016 |
|
| ||||||
| 2. Age, per 10-year increase (%) | +20.8 | <0.001 | +21.2 | <0.001 | +21.5 | <0.001 |
|
| ||||||
| 3. Gender, male (%) | +50.2 | <0.001 | +50.5 | <0.001 | +50.6 | <0.001 |
|
| ||||||
| 4. Race, Hispanic (%) | +233.8 | <0.001 | +238.2 | <0.001 | +228.0 | <0.001 |
|
| ||||||
| 5. BMI, per 0.5 kg/m2 increase (%) | +1.2 | <0.001 | +1.3 | <0.001 | +1.3 | <0.001 |
|
| ||||||
| 6. Smoking (%) | +103.7 | <0.001 | +104.5 | <0.001 | +103.1 | <0.001 |
|
| ||||||
| 7. Alcohol (%) | −15.2 | 0.019 | −15.4 | 0.017 | −14.8 | 0.019 |
|
| ||||||
| 8. Indication, surveillance (%) | +51.5 | <0.001 | +49.7 | <0.001 | +50.3 | <0.001 |
|
| ||||||
| 9. Indication, non-bleeding symptoms (%) | −32.8 | 0.010 | −33.0 | 0.009 | −33.2 | 0.010 |
|
| ||||||
| 10. History of previous colonoscopy (%) | +57.0 | <0.001 | +54.7 | <0.001 | +55.2 | <0.001 |
|
| ||||||
| 11. Preparation type, sodium phosphate | −35.3% | 0.009 | −35.5% | 0.008 | −35.8% | 0.009 |
BMI, body mass index.
Afternoon procedures.
Per hour elapsed.
Queue position.
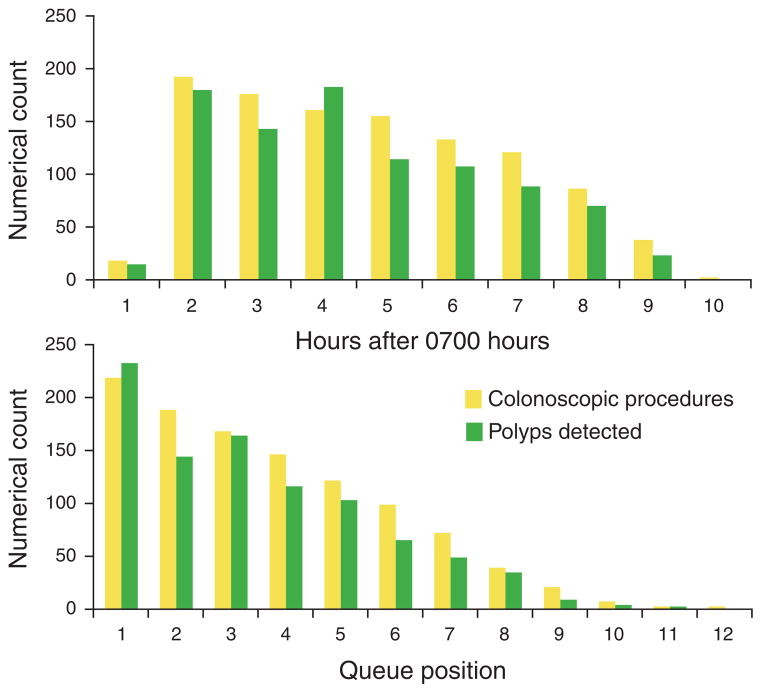
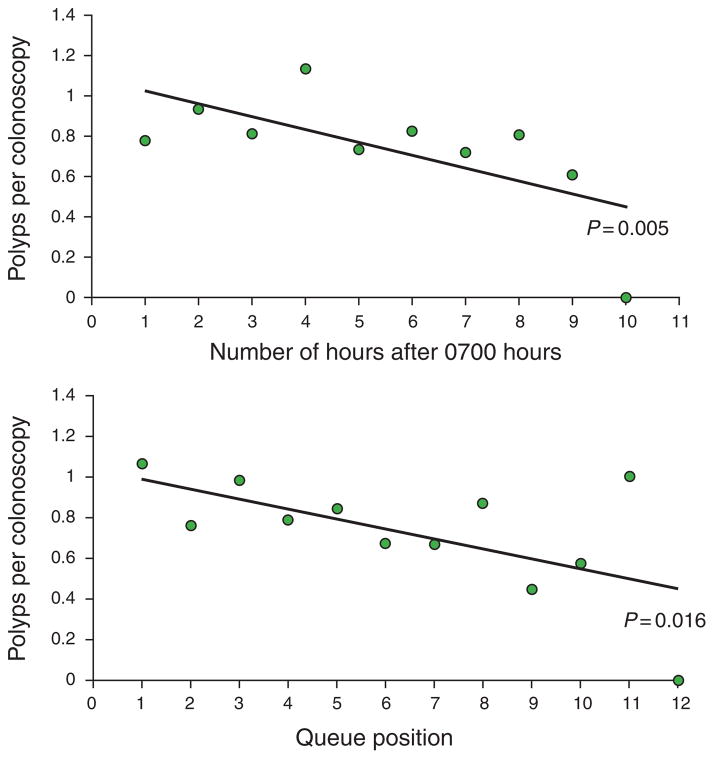
Using colonoscopy start time on a continuous scale, the distribution of polyps and colonoscopies hour-by-hour is shown in Figure 4. Multivariate analysis using this parameter demonstrated that each hour elapsed during the day was associated with a 4.6% reduction in the mean number of polyps detected, which was statistically significant (P = 0.005; Table 2, Figure 5). Results for non-timing variables that predicted changes in polyp detection rates were similar to multivariate analysis using morning vs. afternoon time of day described above.
Figure 4.
Distribution of colonoscopic procedures and polyps detected by each elapsed hour of the day (top graph) and by queue position (bottom graph). Each elapsed hour resulted in a 4.6% reduction in polyp detection (P = 0.005). Each increase in queue position was associated with a 5.4% reduction in polyp detection (P = 0.016).
Figure 5.
Reduction in detection of polyps by time elapsed (top graph) and by queue position (bottom graph). The change in polyp detection was statistically significant with both comparisons, and each timing variable (time elapsed, queue position) was an independent predictor of polyp detection on multivariate analysis. The regression line was generated by multivariate modeling in each instance.
Using queue position, the distribution of polyps and colonoscopies for increasing queue position is shown in Figure 4. Multivariate analysis using queue position demonstrated that each increase in queue position was associated with a 5.4% reduction in the mean number of polyps detected (P = 0.016; Table 2, Figure 5). Again, results of non-timing variables that predicted changes in polyp detection were similar to multivariate analysis using morning vs. afternoon time of day. Notably, hierarchical analysis in our multivariate modeling accounted for variations between individual endoscopists performing the procedures; in other words, our results held true independent of individual endoscopists or trainee involvement in the procedures. Secondary multivariate analysis including only endoscopists who performed ≥ 10 procedures did not alter our results and conclusions.
Finally, the proportion of colonoscopic examinations with at least one adenoma detected was analyzed. Marginally fewer afternoon procedures had at least one adenoma detected (26.2 vs. 28.1%, P = 0.56). Using queue position, the proportion of colonoscopic examinations with at least one adenoma detected was highest for the first queue position (30.6%), following which numbers declined (28.1–22.2% for subsequent queue positions through queue position 7). However, the differences were not statistically significant (P = 0.44). The overall numbers of procedures for queue positions ≥ 8 were too small to make meaningful comparisons. Further rigorous statistical analysis could not be performed, as the precise numbers of adenomas detected in each patient could not be ascertained because of the retrospective nature of the study.
DISCUSSION
In this large study involving a substantial number of colonoscopic procedures and endoscopists at a tertiary care medical center, we have demonstrated that progressively later colonoscopy start times and increasing numbers of preceding endoscopic procedures before a given colonoscopic procedure significantly diminish polyp detection, even after adjusting for clinical, endoscopist, and procedural variables. To our knowledge, this is the first report using the number of procedures before a given colonoscopy, which we termed queue position, as a marker of endoscopist fatigue. The reduction in polyp detection with increasing queue position was substantial. We demonstrated 5.4% reduction in mean number of polyps detected for each increase in queue position. The highest number of procedures performed in a single day by a single endoscopist was 12 in our cohort; the endoscopist’s twelfth procedure of the day would detect, on average, 45.7% fewer polyps than the first procedure of the day.
Fatigue is a difficult entity to measure and cannot be easily quantified objectively, because of its high subjectivity. Hence, similar to other reports in the literature, we utilized time-related variables as surrogates of fatigue (11,12). Compared with time of day or start time, we reasoned that queue position could be a potentially more robust metric for fatigue. Queue position circumvents variations in scheduling between endoscopists. Moreover, it is reasonable to assume that when comparing two endoscopists performing separate colonoscopies at approximately the same time, physical and cognitive fatigue would have a higher impact on performance on the endoscopist who performed more procedures before the given colonoscopy. We believe our study contributes to the field of endoscopist fatigue not only by demonstrating a lower polyp detection rate later in the day but also by adding a new metric in the form of queue position that can be easily used in assessing the role of procedure burden in outcome.
Consistent with previous reports, upon analysis with time on a continuous scale, we were also able to document that progressively later start times were associated with decreasing polyp detection (11,12). Our study showed 4.6% reduction in mean number of polyps detected for each successive hour elapsed in the day. Thus, our model showed that the last time block (1600–1700 hours) would detect, on average, 34.5% fewer polyps than the first time block (0700–0759 hours). However, contrary to previous studies on early morning cases vs. later cases by Chan et al. (11) and morning vs. afternoon colonoscopy by Sanaka et al. (12), we did not find a statistically significant difference in polyp detection between morning and afternoon colonoscopies, both in univariate and multivariate analyses. This was true despite morning colonoscopies being performed on older patients, having more colonoscopies with surveillance as an indication, having better quality bowel cleansing, and lower queue position. Despite similar polyp detection rates when the day was dichotomized to morning and afternoon, the more stringent quantification of time using a continuous time scale afforded greater sensitivity in detecting the association between elapsed time and polyp detection. The associations between polyp detection and both queue position and start time were independent of a wide range of variables, including clinical demographics, patient characteristics, and procedural characteristics (Table 1). Despite the fact that endoscopist proficiency varies and may impact polyp detection, our results remained unchanged after adjusting for endoscopist identity and for trainee involvement via multivariate modeling. Therefore, we believe our results using continuous time scales, both the hours elapsed before a given procedure (start time) and queue position, allow accurate representation of polyp detection rates, and consequently, of endoscopist fatigue.
Importantly, our results add to the growing body of evidence that fatigue may have an important role in effectiveness of colonoscopy. Screening colonoscopy by nature can be inherently repetitive and frequently prolonged, characteristics that can promote distractibility and fatigability. Fatigue resulting from repetitive and prolonged activity has already been shown to have a detrimental effect on performance in other medical specialties, such as anesthesia and surgery, as well as in industries such as commercial aviation and trucking (13–17). Further, use of 3-h shifts rather than an entire day’s endoscopy schedule is reported to maintain polyp detection rates constant throughout the day (18), and lack of fatigue with shorter shifts can be argued as a contributor to this. Others have suggested that essential elements of endoscopy (lesion detection, withdrawal times) do not seem to be impacted by fatigue, but cecal intubation rates appear to decline (19). Nevertheless, the issue of endoscopist fatigue remains controversial and difficult to accurately assess. Even with shift schedules or part day schedules, other clinical or research activities before the endoscopy shift may impact fatigue.
Conceptually, queue position may be more accurate in encapsulating the repetitive, prolonged nature of endoscopist fatigue in comparison with time of day or start time. Particularly, when compared with time of day, queue position is independent of how an endoscopist’s procedures are scheduled; colonoscopies could be scheduled exclusively in the morning or afternoon and render time of day an ineffective metric. With withdrawal time > 6 min reported as a guideline for colonoscopic quality (20), our findings raise the question of whether additional guidelines to limit colonoscopy procedure volume (and thus limit higher queue position) are warranted. Further studies and prospective investigation are needed.
Our study has a few limitations related to the retrospective nature of data collection. We could not corroborate facts in the electronic record to confirm accuracy, as we were limited by the information previously recorded. Thus, we were unable to include colonsocope withdrawal time and further elaborate on family history of colon polyps and/or colorectal cancer, known predictors for colonoscopic polyp detection (20,21). Patients were also not directly contacted to obtain information firsthand. Additionally, similar to previous studies addressing time and colonoscopic polyp detection, surrogates for fatigue were employed, as no direct metric has been established for operator fatigue; there may be unknown variables that not only impact colonoscopic polyp detection but also are covariate with time and/or queue position. Our study also did not show an association between endoscopist ratings of bowel preparation quality and polyp detection, contrary to multiple studies demonstrating decreased cleansing quality predicting decreased polyp detection and yield in colonoscopy (22,23). This is likely because of the fact that colonoscopic procedures that were aborted because of a suboptimal bowel preparation were excluded from analysis, and only procedures where the cecum was intubated were included in the study. Further, endoscopists’ perception of adequacy bowel preparation can differ broadly, and this might have impacted the reporting of raw bowel preparation scores. Thus, we employed a composite bowel preparation score encompassing multiple endoscopist-reported markers, including adequacy of bowel cleansing, need for shorter-than-standard follow-up interval, and ability to visualize polyps less than 5 mm (8). The composite bowel preparation score of “unacceptable” showed a trend toward decreased polyp detection, and this is in keeping with reports in the literature (8,22,23).
Our results and conclusions have a few other sources of bias. Although our scheduling procedures are random and on a ‘first come first served’ basis, we cannot exclude the possibility that some patients returning for scheduled surveillance colonoscopic procedures request earlier times in the day, thereby increasing morning polyp detection. It is also unknown whether endoscopists who are behind in their schedules are more susceptible to lower polyp detection rates, and our data set does not have the capability to address this point. Additionally, given the number of endoscopists involved, practice patterns in dealing with diminutive or multiple hyperplastic-appearing rectal polyps are not uniform, which could have affected the study conclusions. Procedure withdrawal times were not collected; we acknowledge that this could have added further value in the assessment of potential operator fatigue. Finally, our conclusions are hindered by the fact that we do not have accurate histopathology on each polyp removed, and that we are only able to identify patients with at least one adenoma detected. However, as definitive diagnosis of adenomatous polyps cannot be reliably made real time during the colonoscopy, and current clinical practice involves resection of all detected polyps, we feel that polyp detection rates as described in this report has clinical value (18).
In conclusion, our data suggest that both later colonoscopy start time and increasing number of preceding endoscopic procedures are associated with decreasing numbers of detected polyps in colonoscopy. These results suggest that endoscopist fatigue may contribute to a decline in the effectiveness of colonoscopic polyp detection. By quantifying the number of preceding endoscopic procedures with queue position, we have introduced a novel surrogate for endoscopist fatigue. Further investigation is needed to elucidate the impact of endoscopist fatigue in screening colonoscopy.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Endoscopist fatigue may impact quality of colonoscopic polyp detection.
Polyp detection rates may be a surrogate marker of endoscopist fatigue.
Afternoon colonoscopic procedures detect fewer polyps compared with morning procedures.
WHAT IS NEW HERE
Number of preceding endoscopic procedures (i.e., queue position) significantly impacts polyp detection.
Polyp detection decreases as time passes during an endoscopist’s schedule.
Queue position may be a novel surrogate marker of endoscopic fatigue.
Acknowledgments
Financial support: This investigation was partially supported by the National Institutes of Health, in the form of our fellowship-training grant, and the National Research Service Award 5-T32-DK07301-35 from the National Institute of Digestive Diseases and Kidney.
Footnotes
Presented in preliminary form at the Annual Meeting of the American Gastroenterological Association, New Orleans, 2010.
Guarantor of the article: C. Prakash Gyawali, MD, MRCP.
Specific author contributions: Project concept and design, data collection and analysis, and manuscript preparation: Alexander Lee; data collection: John M. Iskander and Nitin Gupta; database creation and critical review of data and manuscript: Brian B. Borg; project supervision and critical review of conclusions and manuscript: Gary Zuckerman and Bhaskar Banerjee; project concept, project design and direction, analysis and review of data, and manuscript preparation and critical review: C. Prakash Gyawali.
Potential competing interests: None.
References
- 1.Rex DK, Sledge G, Harper P, et al. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: influence of age, gender, and family history. Am J Gastroenterol. 1993;88:825–31. [PubMed] [Google Scholar]
- 2.Lynch KL, Ahnen DJ, Byers T, et al. First-degree relatives of patients with advanced colorectal adenomas have an increased prevalence of colorectal cancer. Clin Gastroenterol Hepatol. 2003;1:96–102. doi: 10.1053/cgh.2003.50018. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 5.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–9. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 6.Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–84. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 7.Harris JK, Froehlich F, Wietlisbach V, et al. Factors associated with the technical performance of colonoscopy: an EGAPE Study. Dig Liver Dis. 2007;39:678–89. doi: 10.1016/j.dld.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Borg BB, Gupta NK, Zuckerman GR, et al. Impact of obesity on bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2009;7:670–5. doi: 10.1016/j.cgh.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc. 2000;51:33–6. doi: 10.1016/s0016-5107(00)70383-x. [DOI] [PubMed] [Google Scholar]
- 10.Van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 11.Chan MY, Cohen H, Spiegel BMR. Fewer polyps detected by colonoscopy as the day progresses at a Veteran’s Administration teaching hospital. 2009;7:1217–23. doi: 10.1016/j.cgh.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Sanaka MR, Deepinder F, Thota PN, et al. Adenomas are detected more often in morning than in afternoon colonoscopy. Am J Gastroenterol. 2009;104:1659–64. doi: 10.1038/ajg.2009.249. [DOI] [PubMed] [Google Scholar]
- 13.Howard SK, Rosekind MR, Katz JD, et al. Fatigue in anesthesia: implications and strategies for patient and provider safety. Anesthesiology. 2002;97:1281–94. doi: 10.1097/00000542-200211000-00035. [DOI] [PubMed] [Google Scholar]
- 14.Taffinder NJ, McManus IC, Gul Y, et al. Effect of sleep deprivation on surgeons’ dexterity on laparoscopic simulator. Lancet. 1998;352:1191. doi: 10.1016/s0140-6736(98)00034-8. [DOI] [PubMed] [Google Scholar]
- 15.Eastridge BJ, Hamilton EC, O’Keefe GE, et al. Effect of sleep deprivation on the performance of simulated laparoscopic surgical skill. Am J Surg. 2003;186:169–74. doi: 10.1016/s0002-9610(03)00183-1. [DOI] [PubMed] [Google Scholar]
- 16.Philip P, Taillard J, Moore N, et al. The effects of coffee and napping on nighttime highway driving: a randomized trial. Ann Intern Med. 2006;144:785–91. doi: 10.7326/0003-4819-144-11-200606060-00004. [DOI] [PubMed] [Google Scholar]
- 17.Evaluation of US Department of Transportation efforts in the 1990s to address operator fatigue. Safety Report NTSB/SR-99-01. National Transportation Safety Board; Washington, DC: 1999. [last accessed 3.16.2011]. www.ntsb.gov/publictn/1999/sr9901.pdf. [Google Scholar]
- 18.Munson GW, Harewood GC, Francis DL. Time of day variation in polyp detection rate for colonoscopies performed on a 3-h shift schedule. Gastrointest Endosc. 2010;73:467–75. doi: 10.1016/j.gie.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Harewood GC, Chrysostomou K, Himy N, et al. Impact of operator fatigue on endoscopy performance: implications for procedure scheduling. Dig Dis Sci. 2009;54:1656–61. doi: 10.1007/s10620-008-0549-7. [DOI] [PubMed] [Google Scholar]
- 20.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman DA, Prindiville S, Weiss DG, et al. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959–67. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 22.Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single centre study of preparation on colonoscopy. Colorectal Dis. 2007;9:745–8. doi: 10.1111/j.1463-1318.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 23.Kazarian ES, Carreira FS, Toribara NW, et al. Colonoscopy completion in a large safety net health care system. Clin Gastroenterol Hepatol. 2008;6:438–42. doi: 10.1016/j.cgh.2007.12.003. [DOI] [PubMed] [Google Scholar]