Summary
Objective
This study aims to investigate the regulation of expression of Cartilage oligomeric matrix protein (COMP), which is predominately expressed by chondrocytes and functions to organize the extracellular matrix. Mutations in COMP cause two skeletal dysplasias: pseudoachondroplasia and multiple epiphyseal dysplasia. The mechanism controlling COMP expression during chondrocyte differentiation is still poorly understood.
Design
Primary human bone marrow-derived stem cells were induced to differentiate into chondrocyte by pellet cultures. We then compared the temporal expression of COMP with the well-characterized cartilage-specific Type II collagen (Col2a1), and their response to transforming growth factor (TGF) β and Sox trio (Sox5, 6, and 9) stimulation.
Results
COMP and Col2a1 expression are differentially regulated by three distinct mechanisms. First, upregulation of COMP mRNA precedes Col2a1 by several days during chondrogenesis. Second, COMP expression is independent of high cell density but requires TGF-β1. Induction of COMP mRNA by TGF-β1 is detected within 2 h in the absence of protein synthesis and is blocked by specific inhibitors of the TGFβ signaling pathway; and therefore, COMP is a primary TFGβ-response gene. Lastly, while Col2a1 expression is intimately controlled by the Sox trio, overexpression of Sox trio fails to activate the COMP promoter.
Conclusion
COMP and Col2a1 expression are regulated differently during chondrogenesis. COMP is a primary response gene of TGFβ and its fast induction during chondrogenesis suggests that COMP is suitable for rapidly accessing the chondrogenic potential of stem cells.
Keywords: Chondrocyte differentiation, Chondrogenesis, TGFβ, Sox9
Introduction
The cartilage oligomeric matrix protein (COMP), also known as thrombospondin (TSP)-5, is a member of the TSP family that modulates numerous biological functions involving the organization of the extracellular matrix1. COMP is a pentameric non-collagenous matrix protein expressed predominantly by chondrocytes2,3. Some mutations in the human COMP gene cause retention of misfolded COMP within the endoplasmic reticulum, leading to premature chondrocyte death and bone growth retardation; these clinically present as two skeletal dysplasias (pseudoachondroplasia and multiple epiphyseal dysplasia)4,5.
The pentameric COMP is proposed to function as a linker protein between collagen and other extracellular matrix molecules that regulate collagen fibrilogenesis6–9. COMP knock-out mice are phenotypically normal10, suggesting that COMP’s function might be compensated by other members of the TSP family or other extracellular matrix constituents. In support of this, single deletion models of COMP and collagen IX do not result in disruption of epiphyseal growth plate architecture as seen in the double deletion mouse model11. Moreover, compound nulls involving TSP-1, -3, COMP, and collagen IX result in drastic changes in growth plate organization and reduction in length of the limbs not seen in single deletion models12. Collectively, these in vivo studies demonstrate a role of COMP in organizing the growth plate architecture.
It is not entirely clear what factors contribute to the cartilage-specific expression pattern of COMP. Posey et al. show that the first 375 nucleotides of the human COMP promoter contains cis-regulatory element that is sufficient to drive cartilage-specific expression of a reporter gene in mice13. However, the signaling pathways and transcription factors that can directly activate the expression of COMP gene are still poorly understood. The expression of COMP in articular chondrocytes is stimulated by TFG-β, implicating the involvement of the transforming growth factor (TGF) signaling14,15. COMP also appears to be a mechano-sensitive gene that is upregulated in cells subjected to mechanical stimuli16.
COMP expression changes rapidly during chondrocyte differentiation and dedifferentiation when compared to the widely used chondrocytic marker collagen type II (Col2a1)17, indicating the potential use of COMP as an early marker for accessing the chondrogenic potential. In addition, the differences in the temporal expression of COMP and Col2a1 suggest that they are regulated differently during cartilage development. Since current knowledge of regulation of COMP expression relies heavily on established cell lines, we performed a detailed comparative analysis between COMP and Col2a1 temporal expression patterns during in vitro stimulation of chondrocyte differentiation from primary human bone marrow-derived stem cells (BMSC). We also compared COMP and Col2a1’s response to regulation by the TGF-β1 signaling pathway as well as the chondrocyte-specific transcription factors L-Sox5, Sox6, and sex-determining region Y-box 9 (Sox9).
Methods
Cell culture
Human bone marrow aspirates from the femur of 10 patients undergoing total knee arthroplasty were obtained after IRB approval. Primary BMSC were isolated according to a previously described protocol18 and maintained in MSCGM media (PT-3001 from Lonza), supplemented with 1 ng/ml basic fibroblastic growth factor (Roche). The BMSC was characterized by flow cytometry to confirm the presence of characteristic mesenchymal stem cell surface antigens (CD73, CD90, CD105) and the absence of hematopoietic stem cell (CD14, CD34) and monocyte markers (CD45) (data not shown). The pluripotency of BMSC was confirmed by their in vitro differentiation into: chondrogenic lineage as indicated by positive Col2a1 expression and Alcian blue staining; osteogenic lineage as indicated by calcium mineralization; and adipogenic lineage as indicated by neutral lipid19. BMSC between passages 2 and 6 were used for the experiments throughout this study (at least two different donors per experiment). The immortalized human chondrocyte cell line C20/A4 (a generous gift from Dr. Mary Goldring at Harvard University) was maintained in DMEM (high glucose) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin (all from Invitrogen).
Plasmids
Human L-Sox5 (clone ID 9204249), Sox6 (9203962) and Sox9 (9205725) cDNA clones were purchased (Openbiosystem) and subcloned into pFLAG-CMV2 expression vector (Invitrogen) using standard PCR techniques. The human 3Kb COMP promoter (nucleotides −3026 to + 22) and 0.37Kb COMP promoter (−375 to + 22) were cloned from genomic DNA into pGL4.10 luciferase vector (Invitrogen) with or without the addition of the 48-bp Sox9-binding sequence20 immediately downstream of the transcription start site. The luciferase reporter plasmid containing 12 × 48-bp Sox9-binding site21 was a kind gift from Dr. Hiroshi Asahara, The Scripps Research Institute, La Jolla, CA.
Generation of lentivirus
Human Sox6 and Sox9 cDNA were subcloned into the pCCL-based lentiviral vector and packaged in 293T cells as described22. BMSC were infected at five multiplicity of infection with 1 µg/ml polybrene. Lentivirus generated from the empty pCCL plasmid were used as control. Media was replaced 16 h later and the cells were harvested 2 days after infection for mRNA analysis.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA from cells grown as monolayer or pellet culture in the presence or absence of 10 ng/ml TGF-β1 (Peprotech) for various times were extracted using an RNeasy Mini Kit with DNase I (both from Qiagen) and then reverse transcribed with poly-dT primers using a Superscript first-strand kit (Invitrogen). Quantitative real-time PCR was performed as triplicate measurements using the TaqMan Gene Expression Assay and ABI Prism 7700 Sequence Detection System according to manufacturer’s instructions (Applied Biosystems) using the following primers: COL2A1 Hs01064869_m1, L-Sox5 Hs00374709_m1, Sox6 Hs00264525_m1, Sox9 Hs00165814_m1, COMPHs00164359_m1. The level of each target gene was normalized to GAPDH and expressed as fold-change relative to the levels at time 0 (Ct method; Applied Biosystems).
Luciferase assay
C20/A4 cells were seeded in 24-well plates at 70% confluence and cultured overnight. 5 ng of a Renilla Luciferase reporter (pTK-RL from Promega), together with the Sox trio plasmids (2 ng each) were co-transfected in triplicates with the 3Kb/0.37Kb COMP or 3Kb/0.37Kb COMP + 48 bp luciferase reporter constructs (50 ng), using Lipofectamine and Plus Reagent according to the manufacturer’s instructions (Invitrogen). Empty vectors were used to adjust the DNA content to a total of 0.4 µg in all transfections. After 48 h post-transfection, cells were washed with PBS and lysed in 100 µl of luciferase lysis buffer (Promega).10 µl of cell lysate were assayed for luciferase activity using the Dual Luciferase Assay System (Promega) with the Glomax 20/20 luminometer (Promega). Firefly luciferase activities were normalized to the corresponding Renilla luciferase activities to adjust for differences in transfection/expression efficiency. The value determined from the control for each construct (without Sox trio co-transfection) was set to 100%.
Western blot analysis
BMSC treated with 10 ng/ml TGF-β1 for various times were harvested and lysed with sample loading buffer (50 mM Tris–HCl, pH6.8; 100 mM dithiothreitol; 4% 2-mercaptoethanol; 2% sodium dodecyl sulfate; 10% glycerol). Lysates were resolved by 8% SDS-polyacrylamide gels, transferred onto nitrocellulose membranes (Whatman), and subjected to Western blot analysis. The membranes were blocked with 2% BSA in TBST (25 mM Tris–HCl, pH 7.5; 125 mM NaCl; 0.1% Tween 20), followed by incubation with rabbit polyclonal antiserum specific for human COMP23, Col2a1 (Santa Cruz Biotechnology) and mouse anti-GAPDH (Ambion) at 4°C overnight. Blots were then probed with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology), and reactive protein bands were visualized with Western Lightning Plus-ECL (Perkin Elmer).
In vitro chondrogenesis
5 × 105 BMSC were pelleted in 15 ml polypropylene tubes by centrifugation (500 × g, 5 min) and cultured in 5% CO2 incubator at 37°C for 1–2 weeks in BMSC Chondrogenic SingleQuots medium (Lonza) supplemented with 10 ng/ml TGF-β1 (R&D Systems). Media was changed every 3–4 days.
Statistical analysis
Results are presented as the mean, 95% confidence interval (CI), and standard deviation. Paired Student’s t-tests were used for statistical analysis.
Results
Induction of COMP begins at an early phase of chondrogenesis
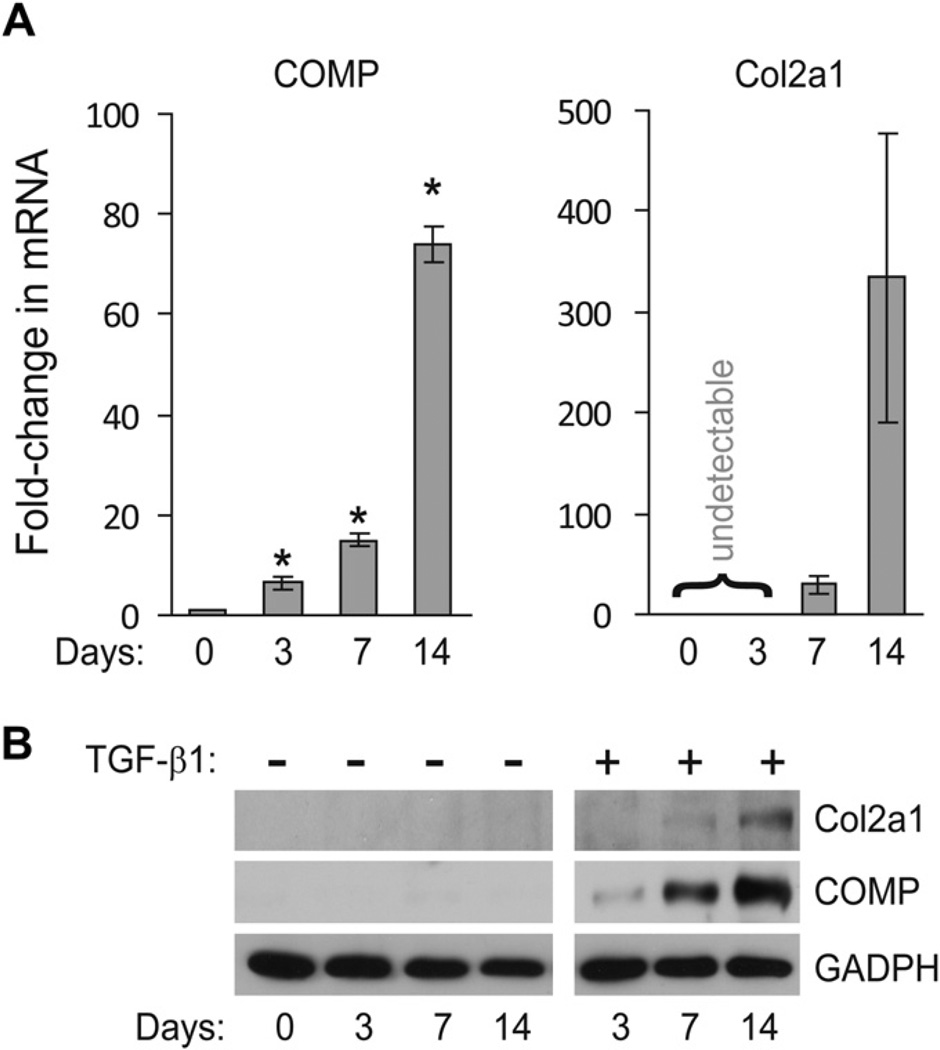
Previous studies suggested that the changes in COMP expression, during differentiation and dedifferentiation of chondrocytes occur more rapidly when compared to other widely used cartilage markers, such as Col2a117. To determine whether COMP could be used as an early and sensitive marker for chondrogenesis of primary human BMSC, we compared the temporal expression of COMP and Col2a1, during a 14-day time course of BMSC chondrogenesis induced by high-density pellet culture and TGF-β1. Quantitative real-time PCR revealed that at day 3 of chondrogenesis, the level of COMP mRNA increased ~7-fold and continued to elevate throughout the entire time course [Fig. 1(A), left panel]. In contrast, the expression of Col2a1 remained undetectable at day 3 and could only be detected at day 7 or later [Fig. 1(A), right panel]. The protein expression of COMP and Col2a1 correlated with their mRNA levels, but only in the presence of TGF-β1 [Fig. 1(B)], indicating induction of COMP and Col2a1 expression in pellet culture is largely TGF-β1-driven. These results demonstrate a delay in the expression of Col2a1 relative to COMP, and hence suggest that the expression of these two genes might be subjected to different regulators during the initial phase of chondrogenesis.
Fig. 1. Induction of COMP expression precedes that of Col2a1.
(A) COMP mRNA is expressed before Col2a1 during chondrogenesis of BMSC pellet culture. Total RNA was isolated from primary human BMSC cultured as high-density pellet in chondrogenic media containing TGF-β1 for 3, 7, or 14 days. The mRNA expression levels of the COMP and Col2a1 were measured using quantitative RT-PCR and normalized to GAPDH. The values shown for each gene at a given time points were fold-induction relative to un-induced BMSC at day 0 (mean with 95% CI, n = 3). A CT value of >40 PCR cycle was deemed undetectable. *Student’s t-test comparing day 0 to day 3 (P = 0.0425), day 7 (P = 0.004), and day 14 (P < 0.0001). (B) COMP protein is detected earlier than Col2a1 during chondrogenesis. Human primary BMSC grown in pellet culture were treated with or without TGF-β1 for the indicated time points. The amounts of COMP and Col2a1 proteins recovered from cells and their associated extracellular matrix were detected by Western blot.
TGF-β1 stimulates early expression of COMP mRNA independent of high cell density
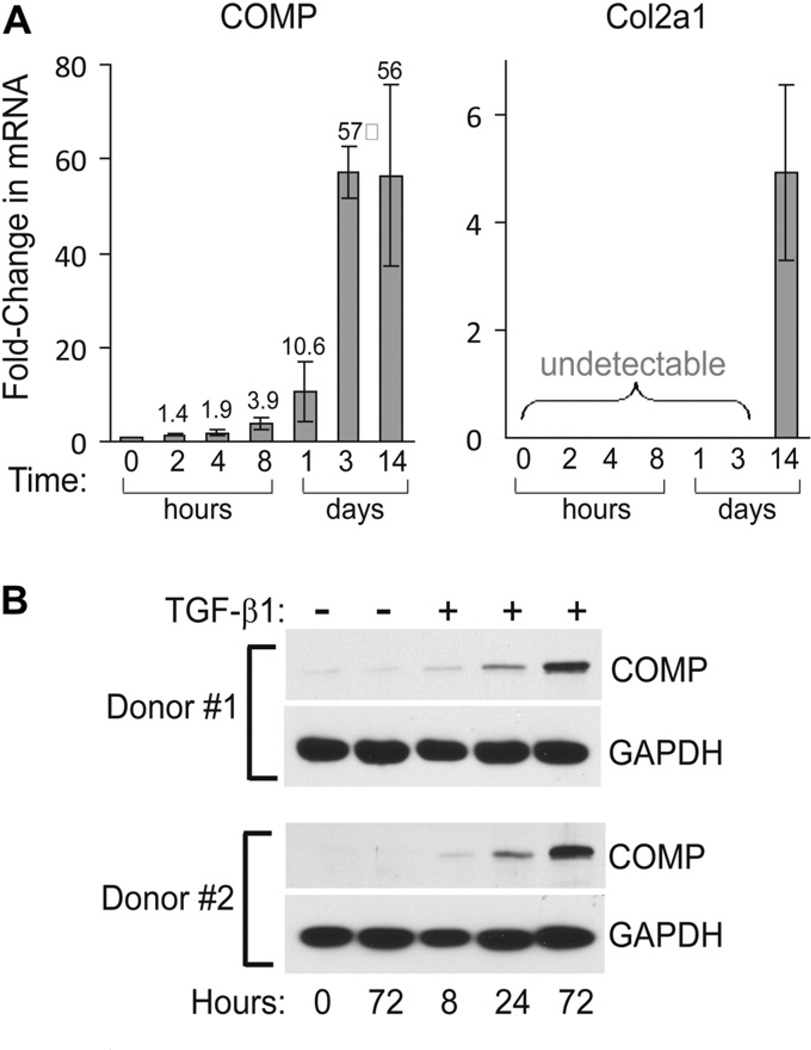
Since TGF-β1 and high cell density are two well known factors that drive stem cell chondrogenesis, we next determined whether both TGF-β1 and high cell density are required for the induction of COMP expression. BMSC cultured as monolayer to~70% confluence in basal media were treated with 10 ng/ml TGF-β1; then at various time points the expression of COMP and Col2a1 mRNA was determined. The data showed that expression of COMP mRNA in monolayer BMSC culture was induced by TGF-β1 within 2 h and continued to increase for the entire 2-week-time course [Fig. 2(A), left panel]. The magnitude of COMP’s induction was markedly higher in monolayer culture when compared to pellet culture. This can be seen by comparing the ~7-fold induction of COMP at day 3 pellet culture [Fig. 1(A), left panel], and the ~57-fold induction of COMPat day 3 monolayer culture [Fig. 2(A), left panel]. These results indicate that TGF-β1 stimulates COMP expression in BMSC independent of high cell density. In fact, high cell density appears to be inhibitory to TGF-β1-mediated induction of COMP. In contrast to COMP, Col2a1 expression in monolayer culture in the presence of TGF-β1 remained undetectable at earlier time points (2 h to 3 days) and was only elevated ~5-fold at 2 weeks [Fig. 2(A), right panel], compared to more than 300-fold induction in pellet culture at 2 weeks [Fig. 1(A), right panel]. These data indicate that both high cell density and three-dimensional culturing condition is required for robust expression of Col2a1.
Fig. 2. TGF-β1 stimulates COMP expression independent of high cell density.
(A) Induction of COMP mRNA precedes that of Col2a1 in monolayer BMSC. Primary human BMSC grown as monolayer culture to ~70% confluence was treated with TGF-β1 for the indicated time points. The mRNA levels of COMP and Col2a1 were measured as described above (mean with 95% CI, n = 3). P-values from Student’s t-test comparing COMP mRNA at time 0 to: 2 h (P = 0.0639), 4 h (P = 0.0051), 8 h (P = 0.0069), day 1 (P = 0.0213), day 3 (P = 0.0004), and day 14 (P = 0.0062). (B) TGF-β1 stimulates synthesis of COMP protein. Human primary BMSC grown in monolayer were treated with TGF-β1 for the indicated time points. The amounts of COMP protein recovered from cells and their associated extracellular matrix were detected by Western blot using a rabbit polyclonal anti-COMP antibody.
Upregulation of COMP protein by TGF-β1
Given the induction of COMP mRNA occurred within hours of TGF-β1 treatment, we next determined whether the protein level of COMP is also elevated. BMSC from two different donors grown in monolayer were treated with TGF-β1 for the indicated times. Cells and their associated extracellular matrix were collected and the amounts of COMP protein were determined by Western blot. The results showed that induction of COMP protein could be detected as early as 8 h and was markedly increased at 72 h [Fig. 2(B)], thus correlated well with the mRNA expression patterns. Note that the increase in COMP protein is strictly dependent on TGF-β1 as no COMP can be detected at 72 h in the absence of TGF-β1.
COMP is directly activated by the TGF-β signaling pathway
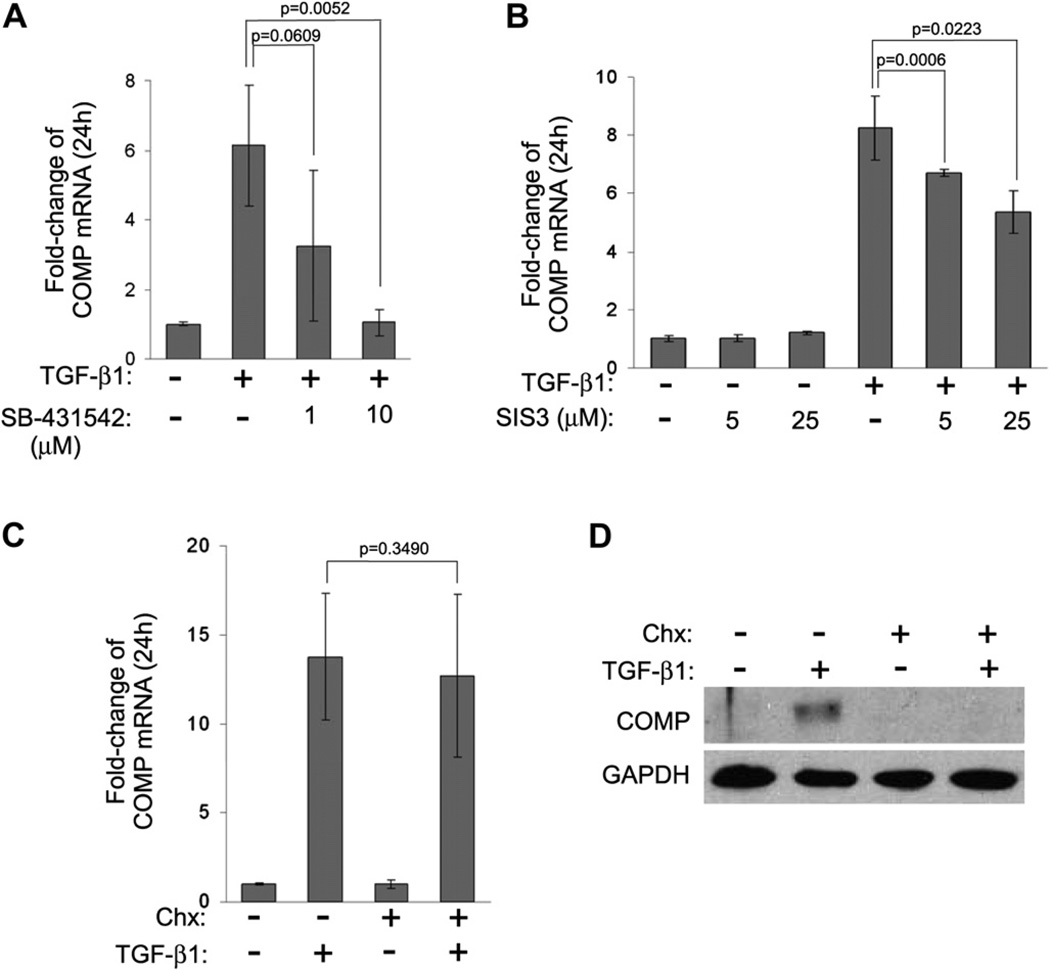
In order to determine if the TGF-β1-mediated signaling pathway is responsible for the upregulation of COMP expression in BMSC, we pre-treated BMSC in monolayer culture with the specific TGF type I receptor kinase inhibitor SB-43154224, followed by stimulation with TGF-β1 for 24 h. The results demonstrated that SB-431542 significantly inhibited TGF-β1-induced COMP expression at a concentration of 10 µM, but not at 1 µM [Fig. 3(A)]. Next, we tested the ability of another pharmacological inhibitor, SIS3 (specific inhibitor of Smad3)25, to suppress the basal and TGF-β1-induced transcription of COMP. The results showed that while the basal transcription of COMP was not affected, the TGF-β1-mediated activation of COMP was suppressed by SIS3 in a dose-dependent manner [Fig. 3(B)]. Taken together, these data demonstrate the direct involvement of TGF-β signaling pathway in activating COMP mRNA expression in BMSC.
Fig. 3. COMP is an early response gene regulated by TGF-β1.
(A) TGF-β1 signaling pathway is involved in the induction of COMP expression. BMSC grown in monolayer were pretreated with the indicated amount of the specific TGF receptor type I inhibitor SB-431542 for 2 h, followed by stimulation with TGF-β1 for 24 h. The mRNA level of COMP was measured as described in Fig. 1(A). (B) BMSC grown in monolayer were pre-treated with the indicated amount of SIS3 for 2 h, followed by treatment with or without TGF-β1 for 24 h. The mRNA level of COMP was measured as described in Fig. 1(A). (C) Induction of COMP mRNA by TGF-β1 does not involve new protein synthesis. BMSC grown in monolayer were pre-treated with 20 mM Chx for 2 h, followed by the addition of TGF-β1 for 24 h. The mRNA level of COMP was measured as described above (all data shown were means with 95% CIs, n = 3). (D) Parallel BMSC samples from C were lysed in sample buffer and the level of COMP protein was determined by Western blot analysis.
COMP is an early response gene targeted by TGF-β
Given the fast response (within 2 h) of COMP induction by TGF-β1, we next asked whether COMP is induced directly by TGF-β1 or if additional protein synthesis is required to produce secondary factors that in turn stimulate COMP expression. Monolayer BMSC culture was pre-treated with the protein synthesis inhibitor cycloheximide (Chx), followed by stimulation with TGF-β1. The results showed that despite the presence of Chx, TGF-β1 was still able to stimulate COMP mRNA expression to the same extent as the untreated control [Fig. 3(C)]. The effectiveness of Chx treatment was confirmed by Western analysis, which showed no newly synthesized COMP protein by BMSC in the presence of TGF-β1 [Fig. 3(D)]. These results indicate that additional protein synthesis is not required for TGF-β1-mediated stimulation of COMP expression. Hence, COMP can be classified as a primary response gene of the TGF-β signaling pathway.
Expression of Sox trio remains unchanged in BMSC after 24 h TGF-β1 treatment
Both COMP and Col2a1 are predominately expressed in chondrocytes. As noted above, the induction of COMP mRNA by TGF-β1 occurred days before the expression of Col2a1 was detected, suggesting that although expressed in the same cell types, the transcription of these two genes might be activated by different mechanisms or transcription factors. It is well documented that Col2a1 expression is activated by the coordinated action of the transcription factors Sox9, L-Sox5, and Sox6 (the so called Sox trio)26,27. In contrast, conflicting data exist regarding whether Sox9 can activate COMP expression13,28,29. Therefore, we sought to determine whether TGF-β1-mediated induction of COMP in primary human BMSC corresponds to a concomitant elevation in the Sox trio’s expression. The changes in mRNA expression of the Sox trio in the presence of TGF-β1 within 24 h were determined. The results showed that TGF-β1 markedly stimulated COMP expression as before, however, the mRNA levels of Sox9, Sox5, and Sox6 did not change significantly (<2-fold), and the expression of Col2a1 remained undetectable (Table I). These data suggest that the upregulation of COMP by TGF-β1 is not due to an increase in Sox trio expression.
Table I.
Changes in mRNA levels of Sox 5, 6, 9 and COMP after 24 h TGF-β1 treatment (n = 3)
| Untreated | TGF-β1-treated | P-value | |
|---|---|---|---|
| Mean fold-change in mRNA (95% CI) | |||
| Sox5 | 1.08 (0.65–1.62) | 0.29 (0.12–0.49) | 0.1794 |
| Sox6 | 1.12 (0.59–1.84) | 1.08 (0.83–1.55) | 0.9538 |
| Sox9 | 1.19 (0.59–2.22) | 1.81 (1.53–2.02) | 0.2688 |
| COMP | 1.14 (0.67–2.01) | 10.54 (8.40–13.18) | 0.0251 |
| Col2a1 | Undetectable | Undetectable | – |
The human 3Kb COMP promoter is not activated by Sox trio
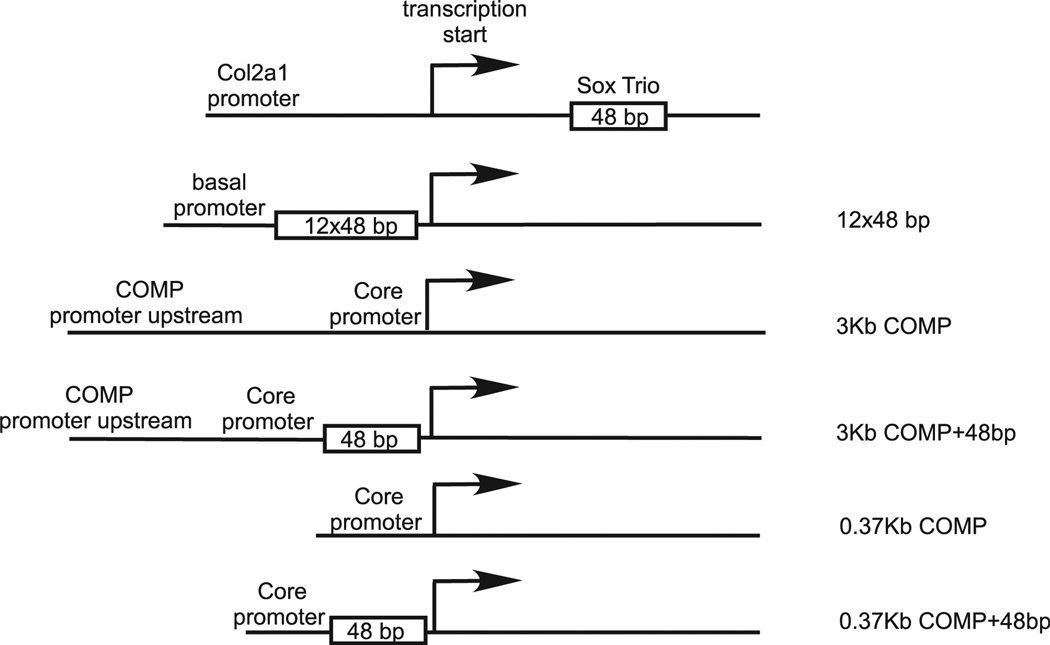
The core human COMP promoter (+ 1 to −375 nt) can direct cartilage-specific expression of a reporter gene in mouse13, and a 3Kb region upstream to the core promoter contains chondrocyte-specific enhancer elements13 (and our unpublished data). Previously, a putative 13-bp Sox9-binding site was identified in the mouse COMP promoter29, and this sequence, located at −80 nt upstream of the transcription start site, is also conserved in the human COMP promoter. To further confirm whether Sox trio activates the human COMP promoter, we performed luciferase reporter assays using constructs driven by various promoters (Fig. 4); these include a positive control (12 × 48 bp) with a basal promoter linked to 12 copies of a 48-bp Sox9-binding element in the first intron of the Col2a1 gene21, the human COMP promoter (3Kb COMP) encompassing a 3Kb region upstream of the transcription start site (nucleotide −3026 to + 22), and the human 3Kb COMP promoter linked to a single copy of the 48-bp Sox9-binding site (3Kb COMP + 48 bp). As shown in Table II, co-transfection with Sox9-expressing plasmid markedly activated the Sox9-responsive promoter (12×48 bp) in C20/A4 cells as expected; however, Sox9 alone or in combination with Sox5 or Sox6 failed to activate the human 3Kb COMP promoter, indicating the absence of a Sox9-responsive element within this 3Kb region. In contrast, insertion of a 48-bp Sox9-binding element into the human COMP promoter immediately upstream of the transcription start site allowed the COMP promoter to be markedly activated by Sox9 and Sox5/6 co-transfection. These data suggest that a Sox9-binding site is absent in the 3Kb human COMP promoter. The 3Kb COMP promoter construct contains extra upstream sequence in addition to the 375 bp core promoter. To rule out the possibility of this extra region acting as a repressor for Sox trio activation of the core promoter, luciferase constructs driven by only the core 0.37Kb COMP promoter with or without insertion of the 48 bp Sox9-binding sequence were generated (see Fig. 4). These constructs were then tested for Sox trio activation. The results showed that similar to the 3Kb COMP, the core 0.37Kb COMP promoter alone cannot be activated by Sox trio (Table II). Whereas the addition of the 48 bp Sox-binding sequence again allowed the 0.37Kb core promoter to be activated by Sox trio. This result indicates that the 3Kb upstream region of the transcription start site does not act as a repressor for Sox trio activation.
Fig. 4.
COMP promoter-driven luciferase constructs used for Sox trio activation study. The 12 × 48 bp construct is a luciferase reporter plasmid containing 12 copies of the 48-bp Sox9-binding sequence (from the first intron of Col2a1 gene) cloned next to a basal promoter. The 3Kb COMP + 48 bp and 0.37Kb COMP + 48 bp construct were obtained by inserting a single copy of the 48-bp Sox9-binding site immediately downstream to the transcription start site of the respective COMP promoter constructs.
Table II.
Activation of different COMP promoter-driven luciferase constructs by Sox trio (n = 3)
| Construct | Sox9 | Sox5 | Sox6 | Relative luciferase unit (Mean fold of control, 95% CI) |
P-value |
|---|---|---|---|---|---|
| 12 × 48 bp | |||||
| − | − | − | 100.0 (85.1–111.4) | – | |
| + | − | − | 1,808.2 (1,666.8–1,907.4) | 0.0018 | |
| 3Kb COMP | |||||
| − | − | − | 100.1 (96.5–104.5) | – | |
| + | − | − | 62.5 (59.3–64.6) | 0.0010 | |
| − | + | − | 115.0 (113.6–116.6) | 0.0147 | |
| − | − | + | 132.1 (122.0–142.5) | 0.0298 | |
| + | + | − | 114.9 (97.9–141.1) | 0.3096 | |
| + | − | + | 67.0 (62.6–70.1) | 0.0098 | |
| + | + | + | 158.7 (138.2–177.1) | 0.0229 | |
| 3Kb COMP +48 bp | |||||
| − | − | − | 101.4 (88.0–111.5) | – | |
| + | − | − | 173.1 (146.8–188.2) | 0.0503 | |
| − | + | − | 137.3 (115.7–149.6) | 0.0190 | |
| − | − | + | 296.5 (274.5–330.1) | 0.0037 | |
| + | + | − | 1,522.5 (1,370.3–1,630.8) | 0.0033 | |
| + | − | + | 760.4 (718.9–793.7) | 0.0012 | |
| + | + | + | 2,331.7 (2,091.2–2,600.6) | 0.0047 | |
| 0.37Kb COMP | |||||
| − | − | − | 99.8 (97.8–101.1) | – | |
| + | − | − | 73.4 (71.2–74.9) | 0.0046 | |
| + | + | + | 152.6 (149.1–156.9) | 0.0028 | |
| 0.37Kb COMP + 48 bp | |||||
| − | − | − | 100.2 (98.4–102.5) | – | |
| + | − | − | 134.6 (121.0–147.6) | 0.0506 | |
| + | + | + | 758.1 (729.7–802.2) | 0.0010 | |
Ectopic expression of Sox trio activates endogenous Col2a1, but not COMP expression
The above results did not rule out the presence of a Sox trio-responsive element outside of the 3Kb COMP promoter; and therefore, we next tested whether ectopic expression of Sox9 alone or with Sox5/6 could activate the endogenous human COMP promoter. Plasmids encoding Sox5, 6, & 9 (100 ng each) were transfected into the immortalized human chondrocyte cell line C20/A4 grown in 6-well plates in triplicates, and the expression of endogenous COMP and Col2a1 mRNAs were determined by quantitative RT-PCR. The results showed that the Sox trio together induced ~100-fold transcription of the endogenous Col2a1mRNAs in C20/A4 cells (Table III). On the other hand, neither Sox9 alone, nor in combination with Sox5 and 6, could significantly activate endogenous COMP mRNA expression in C20/A4 (Table III). Next, Sox6 and Sox9 were ectopically expressed in primary BMSC by lentiviral transduction and the results again showed that only endogenous Col2a1, but not COMP mRNA was activated by Sox6 and/or Sox9 (Table III). The fact that only endogenous Col2a1, but not COMP expression, in an established chondrocyte cell line and in primary BMSC is successfully activated by Sox trio strongly implicates that COMP is not subjected to the Sox trio regulation.
Table III.
Differential activation of endogenous COMP and Col2a1 mRNA expression by Sox trio (n = 3)
| Mean folds of control (95% CI) | ||||
|---|---|---|---|---|
| COMP | P-value | Col2a1 | P-value | |
| C20/A4 | ||||
| Control | 1.01 (0.87–1.19) | – | 1.00 (0.99–1.01) | – |
| Sox9 | 0.76 (0.62–1.03) | 0.3696 | 1.23 (1.19–1.26) | 0.0065 |
| Sox5 & 6 | 1.64 (1.29–2.11) | 0.0521 | 1.25 (1.19–1.30) | 0.0129 |
| Sox5, 6, & 9 | 0.67 (0.44–0.81) | 0.0625 | 95.27 (85.63–114.56) | 0.0103 |
| BMSC | ||||
| Control | 1.00 (0.91–1.06) | – | 1.00 (0.95–1.06) | – |
| Sox9 | 1.15 (1.05–1.25) | 0.0168 | 28.73 (20.95–36.02) | 0.0236 |
| Sox6 | 1.30 (1.27–1.35) | 0.0149 | 14.14 (11.21–16.19) | 0.0123 |
| Sox6 & 9 | 1.59 (1.39–1.80) | 0.0468 | 7,169 (6,293–7,856) | 0.0041 |
Discussion
Our current knowledge on the regulation of COMP expression relies heavily on data from established cell lines. In this study we examined the temporal expression of COMP, with respect to the most widely used chondrocytic marker Col2a1, during chondrogenesis of primary human BMSC. Our data clearly demonstrated that COMP expression during chondrogenesis precedes that of Col2a1, and this may indicate a role of COMP in early chondrogenesis. In support of this, Kipnes et al. demonstrate that overexpression of COMP enhances chondrogenesis of mesenchymal stem cells30.
The expression of COMP mRNA is specifically and rapidly induced (within 2 h) by TGF-β1 in the absence of new protein synthesis. Therefore, COMP can be classified as a primary TGF-β-response gene. Given that TGF-β is commonly used in cartilage engineering and repair to stimulate chondrogenesis of stem cells, COMP could proved be a valuable early marker for rapidly accessing the chondrogenic potential of stem cells; this is in sharp contrast to the common marker Col2a1, which is usually detectable only after several days of chondrogenesis in a pellet culture system.
Although both COMP and Col2a1 are predominantly expressed by chondrocytes, these two proteins differ in their temporal expression during chondrogenesis. In addition, we also show that COMP and Col2a1 differ in their responses to regulation by the Sox trio. It is well established that Col2a1 is activated by the coordinated action of the Sox trio. In contrast, despites several potential Sox9-binding sites being identified in human and mouse COMP promoters29,31, contradictory data exist regarding whether Sox trio directly regulates COMP expression. Two independent studies show that overexpression of Sox9 failed to activate either the human COMP promoter in mouse 3T3 fibroblast13, or the mouse COMP promoter in rat chondrosarcoma and mouse C3H10T1/2 mesenchyme cell lines31. In contrast, Liu et al. demonstrated that Sox9 alone activates the mouse COMP promoter in rat chondrosarcoma cell line, and L-Sox5/Sox6 work synergistically on this activation29.
The discrepancy in the above studies might arise from the different promoter expression systems and cell lines/species being employed in these studies. Whereas in the present study, our data clearly demonstrate the inability of Sox9 alone or with L-Sox5 and Sox6 to activate the COMP promoter-driven luciferase construct, as well as the endogenous human COMP promoter. Furthermore, using primary human BMSC, we show that endogenous COMP mRNA is rapidly induced by TGF-β1 treatment while at the same time, Col2a1 expression is undetectable and the Sox trio expression remains unchanged. Collectively, these data point to the same conclusion and strongly suggest that TGF-β1-mediated induction of COMP is independent of the Sox trio regulation. Since two of the three analyzed observations were provided by the same donor, and the statistical evaluation of the results is based on an assumption of three different donors, the uncertainty of the results is greater than indicated by their P-values. However, the fact that COMP expression has also been reported in cell types other than chondrocytes such as skin fibroblasts32 and vascular smooth muscle cells33, in which Sox9 expression is not detected, strongly argues against the involvement of Sox9 in COMP expression.
Interestingly, TSP-1, a close relative of COMP within the TSP protein family, is also an early response gene of the TGF-β signaling pathway34.However, in addition to the induction of its mRNA by TGF-β1, the TSP-1 protein can also specifically bind to the latent form of TGF-β1 and mediate its activation in endothelial cells, which in turn regulates cell adhesion and migration that are important in postnatal development of epithelial structure and during wound healing34,35. Therefore, the induction of TSP-1 by TGF-β1 appears to form a positive feed-back loop to augment TGF-β signaling in endothelial cells. However, the TGF-β1-binding domain (Type I repeat domain) in TSP-1 is not present in COMP; and therefore, whether other domains of COMP can directly or indirectly interact with TGF-β1 remains to be determined.
In summary, we have characterized COMP as a primary response gene of the TGF-β signaling pathway and provide new insight into the regulation of COMP expression during chondrogenesis of human BMSC. These results suggest that COMP might be a suitable early marker for rapidly accessing the chondrogenic potential of stem cells. The possibility of manipulating the expression of COMP through controlling the TGF-β pathway might also lead to novel therapeutic intervention in the management of pseudoachondroplasia and multiple epiphyseal dysplasia.
Acknowledgments
We thank Dr Fernando Fierro for help with the characterization of BMSC surface markers. This work was supported by the Chapman Endowed Fund from UC Davis Medical Center to PD.
Footnotes
Author contributions
HL, DH, PD, KP, JH, and JY participated in the conception and design of the study, data analysis and interpretation, and drafting and revision of the manuscript. DH and JY supervised the study, and JY, HL, DH performed the experiments and acquired data.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Oldberg A, Antonsson P, Lindblom K, Heinegard D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992;267:22346–22350. [PubMed] [Google Scholar]
- 2.Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267:6132–6136. [PubMed] [Google Scholar]
- 3.Newton G, Weremowicz S, Morton CC, Copeland NG, Gilbert DJ, Jenkins NA, et al. Characterization of human and mouse cartilage oligomeric matrix protein. Genomics. 1994;24:435–439. doi: 10.1006/geno.1994.1649. [DOI] [PubMed] [Google Scholar]
- 4.Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10:330–336. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- 5.Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FF, Harrison WR, et al. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10:325–329. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- 6.Blumbach K, Bastiaansen-Jenniskens YM, DeGroot J, Paulsson M, van Osch GJ, Zaucke F. Combined role of type IX collagen and cartilage oligomeric matrix protein in cartilage matrix assembly: cartilage oligomeric matrix protein counteracts type IX collagen-induced limitation of cartilage collagen fibril growth in mouse chondrocyte cultures. Arthritis Rheum. 2009;60:3676–3685. doi: 10.1002/art.24979. [DOI] [PubMed] [Google Scholar]
- 7.Halasz K, Kassner A, Morgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282:31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 8.Hauser N, Paulsson M, Heinegard D, Morgelin M. Interaction of cartilage matrix protein with aggrecan. Increased covalent cross-linking with tissue maturation. J Biol Chem. 1996;271:32247–32252. doi: 10.1074/jbc.271.50.32247. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg K, Olsson H, Morgelin M, Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- 10.Svensson L, Aszodi A, Heinegard D, Hunziker EB, Reinholt FP, Fassler R, et al. Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol Cell Biol. 2002;22:4366–4371. doi: 10.1128/MCB.22.12.4366-4371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumbach K, Niehoff A, Paulsson M, Zaucke F. Ablation of collagen IX and COMP disrupts epiphyseal cartilage architecture. Matrix Biol. 2008;27:306–318. doi: 10.1016/j.matbio.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Posey KL, Hankenson K, Veerisetty AC, Bornstein P, Lawler J, Hecht JT. Skeletal abnormalities in mice lacking extracellular matrix proteins, thrombospondin-1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am J Pathol. 2008;172:1664–1674. doi: 10.2353/ajpath.2008.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posey KL, Davies S, Bales ES, Haynes R, Sandell LJ, Hecht JT. In vivo human cartilage oligomeric matrix protein (COMP) promoter activity. Matrix Biol. 2005;24:539–549. doi: 10.1016/j.matbio.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 15.Recklies AD, Baillargeon L, White C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998;41:997–1006. doi: 10.1002/1529-0131(199806)41:6<997::AID-ART6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Giannoni P, Siegrist M, Hunziker EB, Wong M. The mechano-sensitivity of cartilage oligomeric matrix protein (COMP) Biorheology. 2003;40:101–109. [PubMed] [Google Scholar]
- 17.Zaucke F, Dinser R, Maurer P, Paulsson M. Cartilage oligomeric matrix protein (COMP) and collagen IX are sensitive markers for the differentiation state of articular primary chondrocytes. Biochem J. 2001;358:17–24. doi: 10.1042/0264-6021:3580017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grogan SP, Olee T, Hiraoka K, Lotz MK. Repression of chondrogenesis through binding of notch signaling proteins HES-1 and HEY-1 to N-box domains in the COL2A1 enhancer site. Arthritis Rheum. 2008;58:2754–2763. doi: 10.1002/art.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furumatsu T, Tsuda M, Yoshida K, Taniguchi N, Ito T, Hashimoto M, et al. Sox9 and p300 cooperatively regulate chromatin-mediated transcription. J Biol Chem. 2005;280:35203–35208. doi: 10.1074/jbc.M502409200. [DOI] [PubMed] [Google Scholar]
- 22.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu CJ, Prazak L, Fajardo M, Yu S, Tyagi N, Di Cesare PE. Leukemia/lymphoma-related factor, a POZ domain-containing transcriptional repressor, interacts with histone deacetylase-1 and inhibits cartilage oligomeric matrix protein gene expression and chondrogenesis. J Biol Chem. 2004;279:47081–47091. doi: 10.1074/jbc.M405288200. [DOI] [PubMed] [Google Scholar]
- 24.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 25.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9(Suppl A):S69–S75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- 27.Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han F, Kipnes JR, Li Y, Tuan RS, Hall DJ. The murine COMP (cartilage oligomeric matrix protein) promoter contains a potent transcriptional repressor region. Osteoarthritis Cartilage. 2002;10:638–645. doi: 10.1053/joca.2002.0532. [DOI] [PubMed] [Google Scholar]
- 29.Liu CJ, Zhang Y, Xu K, Parsons D, Alfonso D, Di Cesare PE. Transcriptional activation of cartilage oligomeric matrix protein by Sox9, Sox5, and Sox6 transcription factors and CBP/p300 coactivators. Front Biosci. 2007;12:3899–3910. doi: 10.2741/2359. [DOI] [PubMed] [Google Scholar]
- 30.Kipnes J, Carlberg AL, Loredo GA, Lawler J, Tuan RS, Hall DJ. Effect of cartilage oligomeric matrix protein on mesenchymal chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11:442–454. doi: 10.1016/s1063-4584(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 31.Deere M, Rhoades Hall C, Gunning KB, LeFebvre V, Ridall AL, Hecht JT. Analysis of the promoter region of human cartilage oligomeric matrix protein (COMP) Matrix Biol. 2001;19:783–792. doi: 10.1016/s0945-053x(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 32.Farina G, Lemaire R, Pancari P, Bayle J, Widom RL, Lafyatis R. Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann Rheum Dis. 2009;68:435–441. doi: 10.1136/ard.2007.086850. [DOI] [PubMed] [Google Scholar]
- 33.Riessen R, Fenchel M, Chen H, Axel DI, Karsch KR, Lawler J. Cartilage oligomeric matrix protein (thrombospondin-5) is expressed by human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:47–54. doi: 10.1161/01.atv.21.1.47. [DOI] [PubMed] [Google Scholar]
- 34.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 35.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]