Abstract
Astragalus polysaccharide (APS) is the most immunoreactive substance in Astragalus. APS can regulate the body's immunity and is widely used in many immune related diseases. However, till now, there is little information about its contribution to the protection of astrocytes infected by virus. Toll-like receptor 3 (TLR3) is a key component of the innate immune system and has the ability to detect virus infection and trigger host defence responses. This study was undertaken to elucidate the protective effect of APS on herpes simplex virus (HSV-1) infected astrocytes and the underlying mechanisms. The results showed that APS protected the astrocytes from HSV-1 induced proliferation inhibition along with increasing expression of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) markedly. Moreover, APS significantly promoted the expression of Toll-like receptor 3 (TLR3) and the activation of nuclear factor-κB (NF-κB) in astrocytes. In addition, while astrocytes were pretreated with TLR3 antibody before adding HSV-1 and APS, the expression of TLR3, TNF-α, and IL-6 and the activation of NF-κB decreased sharply. These results indicate that APS can protect astrocytes by promoting immunological function provoked by HSV-1 through TLR3/NF-κB pathway.
1. Introduction
Herpes simplex encephalitis (HSE) is the most common sporadic and nonepidemic viral encephalitis in both children and adults. Untreated HSE can result in prolonged neuroinflammation and compromised brain function or death [1–4]. Furthermore, the majority of treated patients would suffer various degrees of sequela despite the improvements in diagnosis and therapy [5]. HSV-1 infection of the brain results in devastating necrotizing encephalitis. Murine model of herpes simplex encephalitis showed that HSV-1 infection triggered a robust immune response including infiltration of leukocytes, production of proinflammatory mediators, activation of resident microglial cells, and focal tissue damage.
Toll-like receptor is an important class of immune recognition receptors that can recognize pathogens molecules, activate the innate immune response and invoke the releasing of cytokines, and initiate adaptive immunity [6, 7]. As an important member of the Toll-like receptor family, TLR3 can recognize double-stranded RNA (dsRNA) of viruses and induce related signal pathway to play important role in host defense against viruses [8, 9]. It is a remarkable fact that TLR3 is vital for natural immunity to HSV-1 in the central nervous system [10, 11].
Astrocytes are the most abundant cells in the central nervous system (CNS). The main function of astrocytes is to provide nutrition and support. In addition, astrocytes can secrete cytokines and neurotrophic factors to regulate the immune function [12, 13]. When mice embryos were infected by HSV-1, neuronal injury and necrosis accompanied by pathological changes of astrocytes would occur [14]. Recently, Furr et al. reported that astrocytes increased expression of DNA-dependent activator of interferon-regulatory factors (DAI) as an important innate immune mechanism underlying the rapid and potentially lethal inflammation associated with HSV-1 infection [15]. Our previous studies revealed that when astrocytes were infected by HSV-1, NF-κB was activated through Toll-like receptor 3 (TLR3) and then the activated NF-κB would translocate from the cytoplasm to the nucleus so as to promote the production of TNF-α and IL-6 to play antiviral roles [16]. These results demonstrate that astrocytes play significant roles in the inflammatory responses of resident CNS cells to HSV-1 challenge.
Astragalus is a traditional Chinese medicine which contains polysaccharides, saponins, flavonoids, amino acids, linoleic acid, alkaloids, and so forth. Astragalus polysaccharide (APS) is the most immunoreactive substance in Astragalus which can regulate the body immunity. APS has been identified as a class of macromolecules that can profoundly affect the immune system and is widely used as an immune adjuvant in China. APS can induce the expression of surface antigens on lymphocytes, promote the production of antibodies, affect the secretion of cytokines, and even stimulate cell proliferation [17, 18]. Previous studies proved the effective immunostimulatory roles of APS against various viruses [17, 19, 20].
In this paper, based on our previous research, the antiviral effect of APS on the HSV-1 infected astrocytes was investigated. Furthermore, the immunoregulatory effect and the possible immunization mechanisms of APS were evaluated.
2. Materials and Methods
2.1. Laboratory Animals, Cells, and Virus
The BALB/c mice were purchased from Medicine Animal Center of Shandong University. HSV-1 SM44 strain was kept in Central Laboratory of Weifang Medical University at −80°C. The rabbit anti-mouse antibody TLR3, NF-κB, and β-actin were from Invitrogen; Nuclear and Cytoplasmic Protein Extraction Kit were from Beyotime Institute of Biotechnology; MTT kit was purchased from Sigma; 96T enzyme-linked immunosorbent assay (ELISA) kit was purchased from ADL; APS was purchased from Tianjin Sino Pharmaceutical Company; batch number is 120102.
This research was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Surgery was performed under sodium pentobarbital anesthesia; all efforts were made to minimize suffering. The protocol was approved by the Committee on the Ethics of Animal Experiments of Weifang Medical University (number 2012-056).
2.2. HSV-1 Multiplication and Tittering
The HSV-1 strain SM44 propagated in Vero cells; the supernatant was collected when cytopathic effect (CPE) was in confluence to 75%. By freezing in −80°C and thawing for three times, HSV-1 particles were released. By centrifuging for 8 min with 1200 rpm, the supernatant was collected as inoculum and stocked in freezer. All of the HSV-1 samples used in this study were of the same batch. In following research, final concentration of HSV-1 : TCID50 was 10−6 mL−1.
2.3. Astrocytes Culture and Purification
Newborn BALB/c mice in day 1 to day 3 were taken and cells culture and purification were performed according to McNaught's protocol [21]. Take the 3rd generation of astrocytes for experimental research according to our team's protocol [16].
2.4. MTT Cell Proliferation Assay, Calculating the Median Effective Concentration (EC50) of APS
Astrocytes (1 × 106 mL−1) were cultured in DMEM/F12 media in the presence of HSV-1 (final TCID50: 10−6 mL−1) with APS (0, 50, 100, 200, and 300 μg mL−1), 5 repeating groups for each concentration. 20 μL MTT solution (5 mg mL−1) was added to cell media at various time points (6, 12, 18, and 24 h) after the addition of HSV-1 and APS, incubated for 4 h, and then supernatants were gently removed and 200 μL dimethyl sulfoxide was added into each pore. After 10 minutes' vibration, the absorbance (OD value) was measured at a wavelength of 492 nm by enzyme-linked immunosorbent detector. OD value is proportional to the proliferation of living cells and is an indicator of cell proliferation.
The mean and standard deviation of OD values for each concentration of APS at every action time point were calculated. The results showed that cell proliferation is the most exuberant at 12 h time point for every concentration of APS, so 12 h is the detection time point in the following assays. EC50 of APS was 120 μg mL−1, so in the following research, the final concentration of APS was 120 μg mL−1. The formula to calculated EC50 is
| (1) |
2.5. Grouping and Detecting the Effect of APS on Astrocytes Proliferation by MTT Assay
Astrocytes were seeded at density of 1 × 106 mL−1 into 24-well plates; four groups were set up according to the different intervention conditions: HSV-1 group, HSV-1 + APS group, TLR3 antibody + HSV-1 + APS group, and blank control group. In HSV-1 groups, all flask cells were inoculated with viral suspension (final TCID50: 10−6 mL−1); in HSV-1 + APS group, all flask cells were inoculated with viral suspension (final TCID50: 10−6 mL−1) and APS (final concentration: 120 μg mL−1); in TLR3 antibody + HSV-1 + APS group, TLR3 antibody (final concentration: 10 μg mL−1) was used to pretreat cells for 30 min, and then viral suspension (final TCID50: 10− 6 mL− 1) and APS (final concentration: 120 μg mL− 1) were added to cells; in blank control group, the same volume of cell culture medium as the aforementioned groups was added into each flask cells. For each group, 3 repeating experiments were performed. The proliferation rate of cells was assayed by MTT after culturing for 12 h.
2.6. Determination of the Levels of TNF-α and IL-6 by ELISA
Astrocytes were seeded at density of 1 × 106 mL−1 into 12 flasks (25 cm2). Grouping and treatment were performed as previously mentioned. Supernatants were collected and filtered. TNF-α and IL-6 in the supernatant were measured by ELISA. The absorbance (OD value) was determined using a microplate reader at a wavelength of 450 nm. For each sample, the measurement was repeated 3 times and the average concentration of TNF-α and IL-6 was set as the final result.
2.7. Detection of TLR3 Protein in Cells and NF-κB Protein in Cell Nuclei by Western Blot
In brief, total protein and nuclear protein were extracted following the reagent company's instruction. Protein samples were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, immunoblotted using the appropriate primary and the HRP conjugated secondary antibodies, and visualized by using enhanced chemiluminescence reagents ECL. Anti-β-actin or LMNB1 monoclonal antibody was used as loading control. The intensities of bands in Western blots were quantified by densitometry analysis using AlphaImager HP (Alpha Innotech, USA) and NIH Image J software (Rockville, MD, USA). Western blot data shown in the paper are representatives of three independent experiments.
2.8. Statistical Analysis
All data were analyzed using the SPSS 13.0 statistical software. Values were expressed as mean ± standard deviation . The significance of differences between groups was determined using the one-way ANOVA. Statistical significance was accepted for P values <0.05.
3. Result
APS promotes the growth and proliferation of astrocytes infected by HSV-1. Observation under microscope showed that, in the blank control group, the uninfected astrocytes were in thin and flat appearance with good refraction and grew in good condition with active proliferation (Figure 1(a)); in the HSV-1 group, the proliferation of astrocytes was significantly inhibited and the infected astrocytes' bodies were gradually swollen into round and giant appearance (Figure 1(b)); the inhibited proliferation of astrocytes infected by HSV-1 could be rescued by APS apparently in HSV-1 + APS group (Figure 1(c)); when astrocytes were pretreated with TLR3 antibody and then exposed to HSV-1 and APS concurrently, the proliferation of astrocytes reduced markedly compared with the HSV-1 + APS group (Figure 1(d)).
Figure 1.
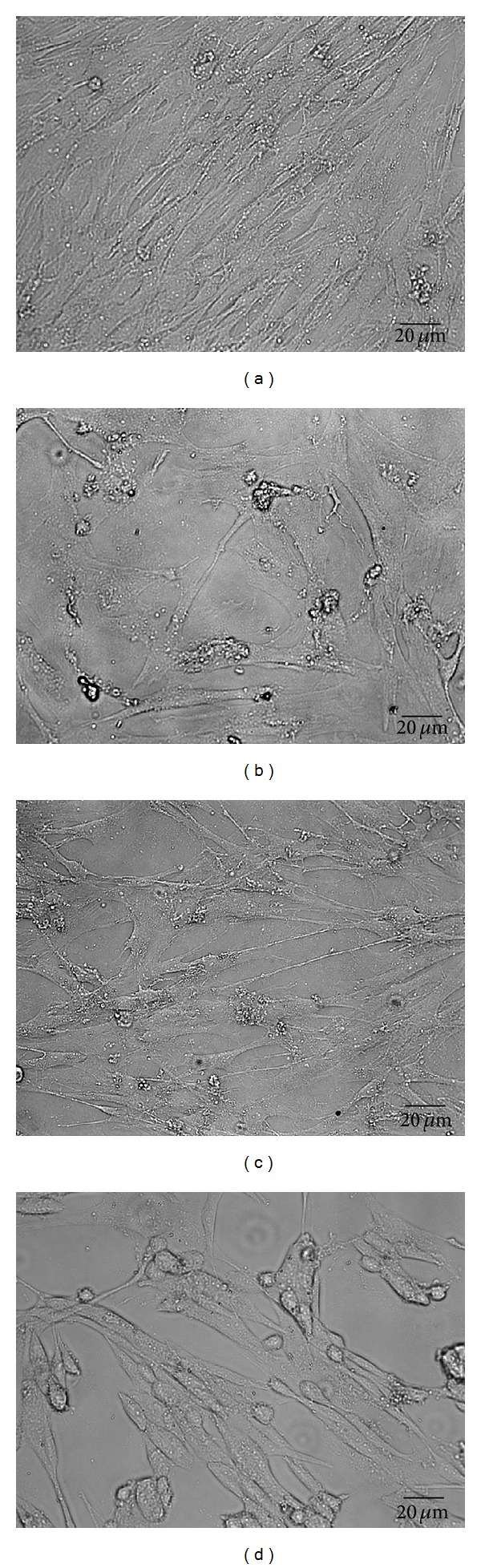
Effect of APS on the growth and proliferation of astrocytes. (a) Blank control group: the astrocytes grew in good condition with active proliferation. (b) HSV-1 group: 12 h after infection with HSV-1, the proliferation of astrocytes was significantly inhibited and the infected astrocytes' bodies were gradually swollen into round and giant appearance. (c) HSV-1 + APS group: the proliferation inhibitory effect of HSV-1 could be suppressed by APS, and most astrocytes grew in good conditiosn. (d) TLR3 antibody + HSV-1 + APS group: when astrocytes were pretreated with TLR3 antibody, the proliferation of astrocytes reduced markedly, and some infected astrocytes' bodies were swollen into round appearance. Scale bar: 20 μm.
MTT analysis (Figure 2) showed that when astrocytes were exposed to HSV-1, the proliferation of astrocytes was significantly inhibited compared to the blank control group. The inhibited proliferation of astrocytes infected by HSV-1 could be rescued by APS apparently in the HSV-1 + APS group. In the presence of APS, the proliferation of astrocytes increased to some extent and the OD value of HSV-1 + APS group was greater than that of the HSV-1 group (P < 0.01), which suggests that APS can protect astrocytes from HSV-1 induced proliferation inhibition. Interestingly, when astrocytes were pretreated with TLR3 antibody before adding HSV-1 and APS, the proliferation of astrocytes decreased markedly when compared to the HSV-1 + APS group (P < 0.05). This result indicates that the protective effect of APS against HSV-1 infection may be associated with TLR3 pathway.
Figure 2.
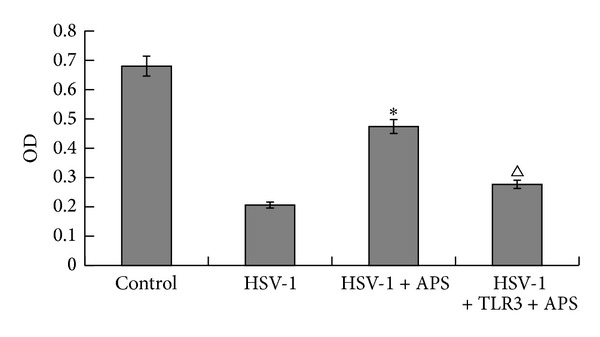
Astrocytes proliferation detected by MTT. *P < 0.01 versus the HSV-1 group. Δ P < 0.05 versus the TLR3 antibody + HSV-1 + APS group. n = 3.
Secretion levels of TNF-α and IL-6 in culture supernatant were detected by ELISA (Figure 3). The concentrations of TNF-α and IL-6 were very low in culture supernatant of the blank control group, whereas in culture supernatant of the HSV-1 group, the concentrations of both TNF-α and IL-6 increased obviously (P < 0.01). In the presence of APS, HSV-1 infected astrocytes expressed higher amount of TNF-α and IL-6 than that of the HSV-1 group. Remarkably, pretreatment of astrocytes with TLR3 antibody decreased the expression of TNF-α and IL-6 in the TLR3 antibody + HSV-1 + APS group (P < 0.05).
Figure 3.
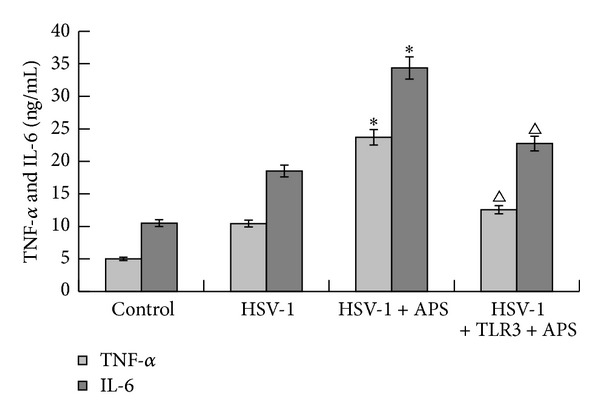
Concentration of TNF-α and IL-6 (ng mL−1) in cell culture medium detected by ELISA. *P < 0.01 versus the HSV-1 group. Δ P < 0.05 versus the TLR3 antibody + HSV-1 + APS group. n = 3.
Western blot analysis showed that the expression level of TLR3 and the activation of NF-κB were low in the control group (Figure 4). In the HSV-1 group, after being exposed to HSV-1 for 12 h, astrocytes expressed higher level of TLR3 than the control group. At the same time, HSV-1 infection induced the activation of NF-κB significantly. Moreover, in the presence of APS, the expression level of TLR3 and the activation level of NF-κB were obviously higher than those of the HSV-1 group. However, when astrocytes were pretreated with TLR3 antibody before addition of HSV-1 and APS, astrocytes reduced the expression of TLR3 and the activation of NF-κB significantly. Considering the different secretion levels of TNF-α and IL-6 in different groups, these results indicate that APS may activate NF-κB which leads to the production of TNF-α and IL-6 through TLR3 pathway (P < 0.05).
Figure 4.
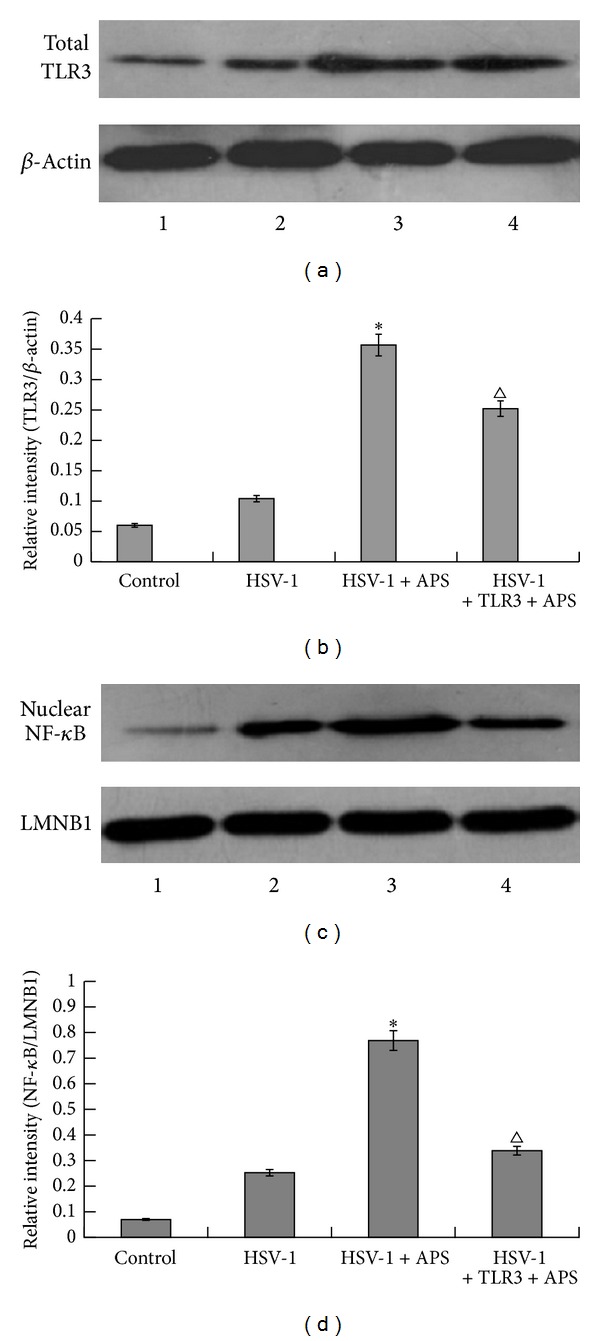
Western blot analysis of expression levels of total TLR3 and nuclear NF-κB (activated NF-κB). (a) Expression levels of total TLR3 protein in blank control group, HSV-1 group, HSV-1 + APS group, and TLR3 antibody + HSV-1 + APS group. β-Actin was used as a loading control. (b) The relative intensity of total TLR3 protein as analyzed by Western blot. (c) Expression levels of nuclear NF-κB protein (activated NF-κB) in blank control group, HSV-1 group, HSV-1 + APS group, and TLR3 antibody + HSV-1 + APS group. LMNB1 was used as a loading control. (d) The relative intensity of nuclear NF-κB protein (activated NF-κB) as analyzed by Western blot. *P < 0.01 versus the HSV-1 group. Δ P < 0.05 versus the TLR3 antibody + HSV-1 + APS group. The results of Western blot were from a representative of at least three repeated experiments. n = 3.
4. Discussion
Recent studies have confirmed that many herbs have effects of modulating immunity and inhibiting and killing pathogenic microorganisms [22, 23]. As one of the bioactive ingredients from the natural traditional Chinese medicinal herb Astragalus membranaceus, APS is used widely as an immunomodulator in China. Evidences indicate that APS can enhance lymphocyte blastogenesis and stimulate macrophage activation without cytotoxic effects [7, 8]. APS cannot inhibit virus directly but it can activate the immune system and induce the production of cytokines to initiate an antiviral response [24, 25]. Consistent with these previous reports, the current study showed that HSV-1 infection induced the secretion of TNF-α and IL-6 in astrocytes and the secretion notably increased in response to APS. These results indicate that APS can promote immunomodulatory effects of astrocytes.
In addition, we also found that HSV-1 infection induced a higher expression of TLR3 and decreased the proliferation of astrocytes; these results are in accordance with previous reports which showed that high expression of TLR3 impaired cell proliferation and inhibited cell cycle progress [26, 27]. Furthermore, we found that APS was capable of promoting the proliferation of astrocytes infected by HSV-1. This result squares with what we already know about the role of APS on cell proliferation [18, 28, 29]. Although the potential mechanism is still to be clarified, regarding the current understanding of TLR3 signaling function, we infer that the effect of APS on proliferation of astrocytes may not be associated with its effect on TLR3.
Antiviral innate immunity depends on different sensor systems that recognize viral-pathogen-associated molecular patterns (PAMPs) and affect specific signaling pathways, including those leading to the activation of NF-κB. Recent studies demonstrate that TLR3 is also present in cells as a sensor to recognize the structure of the pathogens and participate in the adaptive immune response directly [30]. TLR3 can recognize the dsRNA of viruses, initiates intracellular signal transduction pathway, and then induces the activation of NF-κB [30, 31]. Activated NF-κB translocates from cytoplasm to the nucleus, combines with target genes to trigger gene transcriptions, and increases the production of certain antiapoptotic proteins and proinflammatory cytokines [26–28]. Our previous study revealed that when astrocytes were infected by HSV-1, the NF-κB was activated through TLR3 and the generation of TNF-α and IL-6 increased obviously [5]. In this paper, we further confirmed that TLR3 and NF-κB positively contribute to the immune response to HSV-1 infection. Moreover, we showed that, for HSV-1 infected astrocytes, APS could promote the expression of TLR3 and the activation of NF-κB which elicits the secretion of inflammatory cytokines TNF-α and IL-6, indicating that APS can protect astrocytes by promoting immunological function provoked by HSV-1 through TLR3/NF-κB pathway.
5. Conclusion
In conclusion, APS can protect the astrocytes against HSV-1 induced proliferation inhibition and enhance the immunological function of astrocytes by upregulating the TLR3/NF-κB signaling pathway along with increasing expression of TNF-α and IL-6. The study suggests that APS has potential in the treatment of HSV-1 infectious diseases in central nervous system.
Acknowledgments
This work was supported by a Project of Shandong Province High Educational Science and Technology Program (no. J12LK04), Traditional Chinese Medicine Science and Technology Research Projects of Shandong Province (nos. 2013-140, 2013-236, and 2013-240), the Natural Science Foundation of Shandong Province (nos. ZR2011HL047 and ZR2011HL066), and Natural Science Foundation of China (81341137).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. Journal of Immunology. 2005;175(7):4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 2.Armien AG, Hu S, Little MR, et al. Chronic cortical and subcortical pathology with associated neurological deficits ensuing experimental herpes encephalitis. Brain Pathology. 2010;20(4):738–750. doi: 10.1111/j.1750-3639.2009.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marques CP, Cheeran M-C, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. The Journal of Immunology. 2008;181(9):6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavouras J, Prandovszky E, Valyi-Nagy K, et al. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. Journal of NeuroVirology. 2007;13(5):416–425. doi: 10.1080/13550280701460573. [DOI] [PubMed] [Google Scholar]
- 5.Whitley RJ, Gnann JW. Viral encephalitis: familiar infections and emerging pathogens. The Lancet. 2002;359(9305):507–513. doi: 10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JM, Iwasaki A. Toll-like receptors regulation of viral infection and disease. Advanced Drug Delivery Reviews. 2008;60(7):786–794. doi: 10.1016/j.addr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabeta K, Georgel P, Janssen E, et al. Toll-like receptors 9 and 3 as essential complonents of innate immune defense against mouse cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(10):3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda K, Tsujita T, Matsumoto M, et al. Analysis of the interaction between human TLR3 ectodomain and nucleic acids. Nucleic Acids Symposium Series. 2006;(50):249–250. doi: 10.1093/nass/nrl124. [DOI] [PubMed] [Google Scholar]
- 9.Lu Z-Y, Yu SP, Wei J-F, Wei L. Age-related neural degeneration in nuclear-factor κB p50 knockout mice. Neuroscience. 2006;139(3):965–978. doi: 10.1016/j.neuroscience.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Jouanguy E, Ugolini S, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317(5844):1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 11.Tabiasco J, Devêvre E, Rufer N, et al. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. The Journal of Immunology. 2006;177(12):8708–8713. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 12.Jack CS, Arbour N, Manusow J, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. The Journal of Immunology. 2005;175(7):4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 13.Jessen KR. Glial cells. International Journal of Biochemistry and Cell Biology. 2004;36(10):1861–1867. doi: 10.1016/j.biocel.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Schäbitz W, Berger C, Kollmar R, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35(4):992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 15.Furr SR, Chauhan VS, Moerdyk-Schauwecker MJ, Marriott I. A role for DNA-dependent activator of interferon regulatory factor in the recognition of herpes simplex virus type 1 by glial cells. Journal of Neuroinflammation. 2011;8, article no. 99 doi: 10.1186/1742-2094-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Guan Y, Sun X, et al. HSV-1 activates NF-kappaB in mouse astrocytes and increases TNF-alpha and IL-6 expression via Toll-like receptor 3. Neurological Research. 2013;35(7):755–762. doi: 10.1179/016164113X13703372991516. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Zhong Y, Li H, et al. Enhancement of Astragalus polysaccharide on the immune responses in pigs inoculated with foot-and-mouth disease virus vaccine. International Journal of Biological Macromolecules. 2011;49(3):362–368. doi: 10.1016/j.ijbiomac.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Kong X, Hu Y, Rui R, Wang D, Li X. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. International Immunopharmacology. 2004;4(7):975–982. doi: 10.1016/j.intimp.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Lim K, Jazayeri SD, Yeap SK, et al. Co-administration of avian influenza virus H5 plasmid DNA with chicken IL-15 and IL-18 enhanced chickens immune responses. BMC Veterinary Research. 2012;8, article 132 doi: 10.1186/1746-6148-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Shang Y, Yao H, et al. Immune responses of chickens inoculated with a recombinant fowlpox vaccine coexpressing HA of H9N2 avain influenza virus and chicken IL-18. Antiviral Research. 2011;91(1):50–56. doi: 10.1016/j.antiviral.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 21.McNaught KS, Jenner P. Extracellular accumulation of nitric oxide, hydrogen peroxide, and glutamate in astrocytic cultures following glutathione depletion, complex I inhibition, and/or lipopolysaccharide-induced activation. Biochemical Pharmacology. 2000;60(7):979–988. doi: 10.1016/s0006-2952(00)00415-9. [DOI] [PubMed] [Google Scholar]
- 22.Dorman SE, Holland SM. Interferon-γ and interleukin-12 pathway defects and human disease. Cytokine and Growth Factor Reviews. 2000;11(4):321–333. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhuge Z, Zhu Y, Liu P, et al. Effects of astragalus polysaccharide on immune responses of porcine PBMC stimulated with PRRSV or CSFV. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0029320.e29320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du X, Chen X, Zhao B, et al. Astragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting the expression of transforming growth factor β and the frequency of regulatory T cells. FEMS Immunology and Medical Microbiology. 2011;63(2):228–235. doi: 10.1111/j.1574-695X.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 25.Makino S, Ikegami S, Kano H, et al. Immunomodulatory effects of polysaccharides produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Journal of Dairy Science. 2006;89(8):2873–2881. doi: 10.3168/jds.S0022-0302(06)72560-7. [DOI] [PubMed] [Google Scholar]
- 26.Yang M, Xiao Z, Lv Q, et al. The functional expression of TLR3 in EPCs impairs cell proliferation by induction of cell apoptosis and cell cycle progress inhibition. International Immunopharmacology. 2011;11(12):2118–2124. doi: 10.1016/j.intimp.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Meyer C, Pries R, Wollenberg B. Established and novel NF-κB inhibitors lead to downregulation of TLR3 and the proliferation and cytokine secretion in HNSCC. Oral Oncology. 2011;47(9):818–826. doi: 10.1016/j.oraloncology.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Xu CJ, Jian XC, Guo F, Gao QP, Peng JY, Xu XP. Effect of astragalus polysaccharides on the proliferation and ultrastructure of dog bone marrow stem cells induced into osteoblasts in vitro. Hua Xi Kou Qiang Yi Xue Za Zhi. 2007;25(5):432–436. [PubMed] [Google Scholar]
- 29.Liu Y, Wang WJ, Chen WH, Yin J. Effects of Astragalus polysaccharides on proliferation and differentiation of 3T3-L1 preadipocytes. Zhong Xi Yi Jie He Xue Bao. 2007;5(4):421–426. doi: 10.3736/jcim20070412. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald KA, McWhirter SM, Faia KL, et al. IKKE and TBKI are essential components of the IRF3 signalling pathway. Nature Immunology. 2003;4(5):491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 31.Wang YY, Li L, Han KJ, Zhai Z, Shu HB. A20 is a potent inhibitor of TLR3- and Sendai virus-induced activation of NF-κB and ISRE and IFN-β promoter. FEBS Letters. 2004;576(1-2):86–90. doi: 10.1016/j.febslet.2004.08.071. [DOI] [PubMed] [Google Scholar]


