Abstract
Epidemiologic studies of medical radiation workers have found excess risks of leukemia, skin and female breast cancer in those employed before 1950, but little consistent evidence of cancer risk increases subsequently. Occupational radiation-related dose-response, risk estimates for recent years, and lifetime cancer risk data are limited for radiologists and radiologic technologists and lacking for physicians and technologists performing or assisting with fluoroscopically-guided procedures. Based on data from 80 mostly small studies of cardiologists and substantially fewer studies of physicians in other specialties, estimated effective doses to physicians per interventional procedure vary by more than an order of magnitude. There is an urgent need to expand the limited base of information on average annual occupational radiation exposures and time-trends in doses received by medical radiation workers, to assess lifetime cancer risks of radiologists and radiologic technologists in the existing cohorts, and to initiate long-term follow-up studies of cancer and other radiation-associated disease risks in physicians and technologists performing or assisting with interventional procedures. Such studies will help to optimize standardized protocols for radiologic procedures, determine if current radiation protection measures are adequate, provide guidance on cancer screening needs, and yield valuable insights on cancer risks associated with chronic radiation exposure.
Keywords: radiologists, interventional radiologists, radiologic technologists, interventional cardiologists, neoplasms, reviews
Introduction
Radiation has been used in medical practice for more than a century. Technological developments in diagnostic imaging, radiotherapy, catheters and other devices used in fluoroscopically-guided interventional and nuclear medicine procedures, and in robotics for radiological procedures have revolutionized medical practice. For close to seven decades, utilization of ionizing radiation in medicine was mostly limited to radiologists, radiation oncologists, and associated technical personnel. The advent of catheters led to exponentially increasing diagnostic and therapeutic radiologic procedures carried out by cardiologists and expanding numbers of other physician specialists in addition to radiologists.
Shortly after the discovery of x-rays and their early use in medicine, adverse biological and clinical effects, including cancer, were observed among physicians using the new technology (reviewed in [1, 2]). Cancer risks associated with a single acute or fractionated high-dose radiation exposures have been extensively studied in the Japanese atomic bomb survivors [3] and in cohorts of patients treated for benign and malignant conditions (reviewed in [4]), respectively. The most common form of exposure to the general population, however, is from chronic or fractionated low-dose radiation exposure from medical and natural background sources. Natural environmental background sources (e.g., residential radon, cosmic rays) comprised the majority of ionizing radiation exposures to the general population prior to 1980, but the dramatic increases from medical sources in the past 30 years (U.S. per capita dose rose close to 600 percent and population collective dose more than 700 percent, mostly due to computed tomography and nuclear medicine scans) have resulted in similar levels of population exposure from both environmental and medical radiation sources [5].
Occupational cohorts have been particularly important for the study of cancer risks following chronic or fractionated low-dose exposure. The largest categories of radiation-exposed workers are nuclear industry [6, 7] and medical radiation workers [8, 9]. Medical radiation workers generally experience very low radiation exposures except for those performing fluoroscopically-guided procedures and potentially those administering radionuclides for nuclear medicine procedures. Current estimates of cancer risks from low-dose-rate, moderate-dose-rate exposures are mostly based on risk coefficients derived from the Japanese atomic bomb survivors and from laboratory animal and radiobiological data from which estimates for a dose and dose-rate effectiveness factor by which the corresponding risk values for the atomic bomb survivors should be reduced [10, 11]. A recent pooled analysis of twelve epidemiological studies of populations experiencing low-dose-rate, moderate-dose-rate exposures from primarily occupational sources suggests that the cancer risk per dose for these exposures is not lower than that for the atomic bomb survivors as has commonly been assumed [12] In this review we focus on cancer and related risks in workers performing diagnostic radiology and fluoroscopically-guided procedures, but not nuclear medicine because of the paucity of clinical, epidemiologic, and dosimetry studies of the latter as well as the complexity of estimating doses from internal and external radiation exposures. We review briefly the history of key discoveries and technological developments, and clinical reports and major epidemiologic studies assessing cancer risks in medical radiation workers. Because historical reconstruction of individual dosimetry is difficult and most of the epidemiologic studies do not include individual dosimetry, we report published estimates of average annual film badge measurement survey data and summarize time trends in these measurements in medical radiation workers. We identify limitations and gaps in cancer risk estimates and occupational dosimetry of medical radiation workers, and propose future research initiatives.
Historical background
The discoveries of x-rays, radium, and radioactivity from uranium salts by Roentgen, Bequerel, and the Curies during the late 19th century heralded a new era in medical diagnosis and treatment. Their pioneering work initiated remarkable experimental, clinical and technological developments in radiologic imaging that have continued to transform medicine for more than a century, and influenced occupational as well as patient radiation exposures from radiologic procedures. Key historical discoveries and technical developments in diagnostic radiography are summarized in Table 1a [13, 14].
Table 1a.
Key discoveries and technological developments in diagnostic radiography
| Year | Discoveries and technological developments |
|---|---|
| 1895 | Röentgen: x-rays |
| 1896 | Edison: calcium tungstate |
| 1904 | Self-regulated gas tubes |
| 1908 | Snook: generator provides selectable kV and mA |
| 1913 | Coolidge: first successful roentgen-ray tube |
| 1914–16 | Patterson: fluoroscopic screen; Trendelenburg: red goggles to facilitate use of fluoroscopy |
| 1917 | Potter-Bucky diaphragm: reduce scatter by secondary radiation |
| 1917 | Kodak: Double emulsion acetate film |
| 1924 | Film changer for serial x-rays |
| 1928 | Siemans: Three-phase generators |
| 1929 | Bouwers (at Philips): rotating anode x-ray tube, shielding provided by tube housing integrated in tube assembly |
| 1934 | Ziedes des Plantes described film subtraction to aid in visualization of small blood vessels |
| 1941 | First automatic film processor |
| 1942 | X-ray phototimers |
| 1947 | Xeroradiography |
| 1948 | Coltman: Image intensifier tube for fluoroscopy |
| 1960 | Dupont: Polyester film base replaces acetate |
| 1964 | Kodak: 90 second Xomat processor |
| 1964–68 | Cormack and Hounsfield: CT scanner |
| 1973 | Buchanan: Rare earth screen phosphors |
| 1979 | Fuji Photo Film: Digital subtraction angiography |
| 1982 | Ultrafast CT scanner |
| 1984 | Computed radiography systems |
| 1989 | Heiken et al: Slip-ring helical CT volume imaging |
| 1993 | Solid state digital x-ray detectors |
Sources: Siebert JA. Health Phys 1995;69:695–720. Hall E. Radiobiology for the Radiologist, Fifth Edition. Philadelphia: Lippincott Williams & Wilkins, 2000, pp 1–4.
Within a few years of the development of the x-ray tube by Roentgen, the fluoroscope enabled real-time medical imaging without the need for processing of a film or x-ray plate [13]. Fluoroscopy competed successfully with still-film radiography for several years until technical limitations and associated serious health effects led to a decline in use. Military needs at the onset of World War I, in conjunction with important technical advances (see Table 1a) resulted in re-emergence of fluoroscopy. The value of this type of diagnostic imaging was underscored in the 1930s with recognition of the relatively low error rate associated with fluoroscopy for detection of tuberculosis. Development of the image intensifier in 1948 and other notable advances led to expansion in the use of fluoroscopy in medicine and in the 1960s the new specialty of interventional radiology. Interventional radiology is defined as manipulative procedures controlled and followed under fluoroscopic guidance that may be predominantly therapeutic or primarily diagnostic[15]. Although initially carried out mostly by radiologists, fluoroscopically-guided interventional procedures have been increasingly performed by cardiologists, with the introduction of cardiac catheters for surgical interventions [16], and by vascular surgeons, gastroenterologists, urologists, and a growing number of other specialists. Furthermore, whereas many of the developments highlighted in Table 1a have resulted in reduced occupational radiation exposures (Figure 1), this has not necessarily been the case for fluoroscopically-guided procedures [9]. Key historical discoveries and developments in interventional radiology are summarized in Table 1b [16–37]
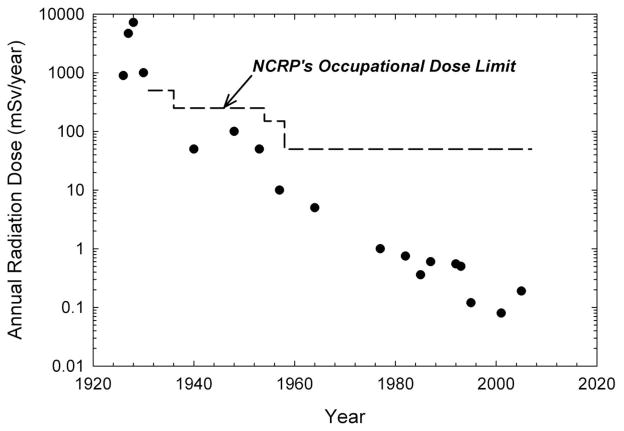
Figure 1.
Average annual occupational effective radiation dose estimates and time trends in average annual doses in radiologists. National Council on Radiation Protection and Measurements (NCRP) recommendations for whole body doses are shown for comparison with the reported radiation doses. Sources: see detailed dose estimates and references in Table 5.
Table 1b.
Key discoveries and technological developments in interventional radiology
| Authors, Year | Discoveries and technological developments |
|---|---|
| Dotter and Judkins, 1964 | Angioplasty |
| Dotter et al., 1966 | Nonsurgical treatment of iliofemoral arteriosclerotic obstruction |
| Judkins, 1967 | Judkins technique of coronary angiography (technique currently most widely used internationally) |
|
Newton and Adams., 1968 Doppman et al., 1971 |
Non-surgical embolization of spinal cord angioma and spinal cord malformations |
| Dotter, 1969 Dotter et al., 1983 |
Catheter-delivered stents |
|
Dotter et al., 1966 Judkins et al, 1967 Judkins et al, 1968 |
Tools for interventions: heparinized (to prevent clotting) guidewires, contrast injector, disposable catheter needles |
| Leary and Parshall, 1972 | Percutaneous removal of common bile duct stones |
| Occlusive coils for blocking blood flow | |
| Rösch et al., 1972 | Selective arterial embolization for gastrointestinal bleeding: technique was adapted to treat massive arterial bleeding at other anatomic sites and to block blood supply to tumors |
| Dotter et al., 1974 | Selective arterial thrombolysis for arterial occlusions; technique was adapted to treat blood clots, stroke, deep vein thrombosis, and other forms of venous and arterial occlusions |
| Grüntzig, 1976 | first successful balloon dilatations of coronary arteries |
| Okamura J, 1983 | Chemo-embolization to treat hepatocellular cancer and disseminated liver metastases |
|
Rösch et al., 1969 Colapinto et al, 1983 Richter et al, 1989 |
Transjugular intrahepatic portosystemic shunt, experimental development and human interventions with stents |
| Palmaz et al, 1985 | Balloon-expandable peripheral stent used today |
| Cope et al, 1990 | Percutaneous extraction of gallbladder stone |
| Goldberg et al, 1997 | Radio-frequency ablation technique for liver tumors; technique was adapted to treat bone, breast, kidney, lung, and liver cancer |
| Weimar et al, 1999 | Percutaneous delivery of pancreatic islet cells transplanted to the liver to treat diabetes |
| Navarro et al, 2001 | Endovenous laser ablation procedure to treat varicose veins and venous disease |
Sources: Rösch J et al. J Vasc Interv Radiol 2003;14:841–53. Society of Interventional Radiology History of Interventional Radiology www.sirweb.org/about-us/historyIR.shtml
Initial reports of adverse biological and clinical effects in medical radiation workers
Within a few years of Roentgen’s discovery of x-rays in 1895, a growing number of short-term (e.g., dermatitis, skin ulceration, epilation, eye irritation) and longer-term (e.g., cataracts, skin carcinomas and other cancers) adverse biologic effects were identified. A summary of the initial clinical reports of cancer and other serious diseases in medical radiation workers is shown in Table 2. Clinical reports on skin cancer [38, 39], leukemia [40, 41]Henshaw, 1944 #2569;March, 1944 #2249;Ulrich, 1946 #2534;March, 1950 #2509;March, 1961 #2514;Lewis, 1963 #2508}, and aplastic anemia [42] (Table 2) motivated epidemiologists to undertake systematic follow-up studies of cohorts of radiologists and radiologic technologists in the 1950s and early 1960s [43, 44].
Table 2.
Initial reports of cancer and other adverse biologic and clinical effects in medical radiation workers
| Authors, Year | Key reports of adverse biologic and clinical effects associated with medical radiation |
|---|---|
| Frieben, 1902 | Cancer arising in skin ulcer |
| Rollins, 1901–04 | Multiple health hazards of x-rays and need for radiation protection |
| Scott, 1911 | X-ray dermatitis and fatal skin cancers |
| Von Jagic, 1911 | Leukemia in five medical radiation workers |
| Henshaw and Hawkins, 1944 | Increased occurrence of leukemia in radiologists |
| March, 1944; March, 1950; March, 1961 | Higher proportionate mortality from leukemia in radiologists |
| Ulrich, 1946 | Higher proportionate mortality from leukemia in radiologists |
| Lewis, 1963 | Excess mortality from leukemia, aplastic anemia and multiple myeloma in radiologists compared with expected death rates in U.S. while male population of same age and year of death |
| Kitabatake T et al., 1976 | Excess aplastic anemia in Japanese radiologic technologists compared with general population |
| Vañó et al., 1998 | Cataracts in interventional radiologists |
| Finkelstein, 1998 | Brain tumors in cardiologists performing interventional procedures: clinical reports |
| Wenzl, 2002 | Brain tumors in physicians performing interventional radiology procedures: literature review |
Sources: Seibert, JA. Health Phys 1995;69:695–720. Yoshinaga S et al. Radiology 2004;233:313–321. ICRP Publication 85, 2001, pp 15–23.
Radiologists and radiological technologists
Clinical and epidemiologic studies
Several epidemiologic studies have linked registries of physicians with population-based mortality or cancer incidence data, and reported data separately for radiologists [45–47]. Members of the American College of Radiology who joined this professional society during 1962–1977 and were born before 1920 experienced a significantly higher total mortality than pathologists who joined the College of American Pathologists during 1962–1977 and who were born before 1920. However, age-adjusted mortality rates were lower among both radiologists and pathologists who joined their respective professional societies after 1962 than among those joining these societies earlier [45]. Among 1,589 radiologists and radiotherapists in the United Kingdom (UK), neither total mortality nor total cancer mortality were significantly higher than in other physicians during the follow-up period of 1962–92, although deaths from respiratory diseases were significantly elevated [46]. Follow up of 1,312 physicians using radiation with physicians never monitored for radiation exposure in Finland revealed similar total cancer incidence, a slightly elevated risk of breast cancer, but no other cancer excesses or radiation dose-response relationships [47].
Eight major cohorts have been actively followed up for cancer and other serious disease occurrence, evaluated in detail, and/or characterized with group or individual dose estimates [8, 48]. Details from the epidemiologic follow-up are summarized in Table 3 and dose data are provided in Tables 4 and 5. Collectively, the eight retrospective cohort investigations have studied radiologists or radiologic technologists who first began working over a period spanning more than 80 years, including small numbers who first began working in the earliest years of the professions (e.g., between 1897 and 1926). From the eight cohort studies, notable excess leukemia mortality risks were observed in UK [49] and U.S. radiologists [50] who first worked before or during the 1920s. Smaller albeit elevated risks were seen in UK radiologists who worked in subsequent decades [51]. Significantly elevated incidence risks of leukemias other than chronic lymphocytic leukemia were seen in U.S. radiologic technologists who worked five or more years before 1950 [52]. In addition, the incidence of leukemia in Chinese x-ray workers who worked before 1970 was significantly higher than expected [53]. Estimated risks for skin [49, 50, 54, 53] and female breast cancer [54, 55, 53, 56, 57, 48]) varied by study and years of employment. Findings for other solid tumors were not as consistent in the medical radiation worker cohorts [8, 48] (Table 3).
Table 3.
Epidemiologic cohort studies of medical radiation workers
| Authors, Year | Study Population (no. workers) | Years 1st worked | Follow-up years | Total mortality SMRa (no. deaths) comparison group | Total cancer mortality SMRa (no. deaths) comparison group | Leukemia SMRa (no. deaths) comparison group | Skin cancer SMRa (no. deaths) | Other cancers SMRa (no. deaths) |
|---|---|---|---|---|---|---|---|---|
| Berrington et al., 2001 | U.K. radiologists, all males (2,700) | 1897–1979 | 1897–1997 | 0.92 (1042) MDs |
1.16 (228)b MDs |
6.15 (4) b 1897–1920 1.54 (4) 1921–54 Social class I |
7.79 (6)b 1897–1920 1.96 (2) 1921–54 |
Lung 2.18 (8)b† 1897–1920 Pancreas 3.23 (6)† 1897–1920 |
| Matanoski et al., 1987 | U.S. radiologists, all males (6,500) | 1920–1969 | 1920–1969 | NA | 1.38 (NA)b† MDs |
2.01 (NA)b 1920–1939 1.00 (NA) 1940–69 MDs |
3.38 (NA)b 1920–1939 2.41 (NA) 1940–69 |
Lung 1.22 (NA)b 1940–1969 Lymphoma 2.73 (NA)† 1920–39 |
| Mohan et al., 2003 | U.S. technologists, 106,800 females, 39,200 males (146,000) | 1926–1982 | 1926–1997 | 0.76 (5057)b Male 0.76 (7567) Female U.S. population |
0.73 (1137)b† Male 0.86 (2558) Female U.S. Population |
1.26 (16) 1926–1939 1.00 (22) 1940–49 0.71 (22) 1950–59 0.97 (43) 1960–82 |
NA | Breastc 1.53 (78) 1926–39 1.06 (97) 1940–49 0.91 (127) 1950–59 0.83 (123) 1960–82 |
| Jablon & Miller, 1978 | U.S. Army technologists, all males (6,600) | Early 1940s | 1946–1974 | 1.06 (289) lab techs |
1.05 (55) lab techs |
1.25 (8) lab techs |
NA | |
| Total Incidence SIRa | Total Cancer Incidence SIRa (no. cancers) | Leukemia SIRa (no. leukemias) | Skin Cancer SIRa (no. skin cancers) | Other Cancers SIRa | ||||
| Yoshinaga et al., 1999 | Japanese technologists, all males (12,200) | 1918–1971 | 1969–1993 | 0.88 (1097)b prof & tech workers |
0.98 (435)b prof & tech workers |
1.75 (20)b prof & tech workers |
1.58 (2) 1897–1933 0.00 (0) 1934–150 |
|
| Wang et al., 2002 | Chinese x-ray workers, 21,600 males, 5,400 females (27,000) | Before 1950–1980 | 1950–1995 | Not available | 1.24 (679)b Male 1.02 (157) Female |
2.37 (33)b before 1970 1.73 (11) 1970–80 |
4.31 (16)b before 1970 2.74 (2) 1970–80 |
Lung 1.57 (43)b 1970–80 Stomach 1.63 (36)b 1970–80 1.39 (115)b before 1970 |
| Andersson et al., 1991 | Danish RT workers, 800 males, 3,400 females (4,200) | 1954–1982 | 1968–1985 | Not available | 1.07 (163) | 0.70 (2) | NA | |
| Zielinski et al., 2009b | Canadian radiation workers, 23,600 males, 43,900 females (67,500) | 1969 – 1987 | 1969–1987 | Not available | 0.54 (213)b Male 0.57 (185) Female Canadian population |
0.66 (11)b, Male 0.43 (6)b Female Canadian population |
NA | Thyroid 1.74 (65)b 1969–87 |
| Total | 272,700 |
Modified from Yoshinaga S et al. Radiology 2004;233:313–21 & Zielinski JM et al Int J Occup Med Environ Health 2009;22:149–56.
Abbreviations: SMR=standardized mortality ratio; SIR=standardized incidence ratio; NA=not available;
Significantly increased or decreased risks p<0.05
Significantly increased p value for trend (p trend = 0.018) for breast cancer risks according to number of years worked before 1950
Table 4.
Time trends in estimated mean and median annual film badge doses (mSv) in U.S. radiologic technologists working in hospitals during 1926–84 and summary of annual uncertainty distributions of badge dose.
| Calendar perioda | Medianb | Meanb | Geometric standard deviationc |
|---|---|---|---|
| ≤ 1939 | 71 | 100 | 2.4 |
| 1940–49 | 16 | 25 | 2.5 |
| 1950–59 | 11 | 28 | 3.9 |
| 1960–76 | 2.2 | 3.6 | 2.7 |
| 1977–84 | 2.0 | 2.3 | 2.3 |
Modified from Simon et al. Radiat Res 2006;166:174–92.
Calendar year periods were chosen based on reports from the literature and availability and reporting format of dose data from the largest commercial dosimetry provider in the U.S.
Median and mean are based on model predictions (only) within each calendar year period prior to 1977. For 1977–87, mean and median include both film-badge measurements and model predictions.
Value of geometric standard deviation for all periods is based on variability of model predictions among cohort members working during the specific period. If subject had a film badge measurement, the geometric standard deviation was assumed to be 1.2.
Table 5.
History of radiation protection standards and estimated occupational doses of medical radiation workersa
| Source of information Author(s), Yr | Year(s) of recommendation, estimated doses | Radiation protection recommendation or standard | Estimated doses to medical radiation workers | Comments: historical events, organization(s) proposing protection standard, and others |
|---|---|---|---|---|
| Inkret et al., 1995 | 1902 | Standard ~ 100 mGy/dayb | ~30 Gy/yrb | Not based on biological data but based on lowest amount easily detected by fogging on photographic plate |
| Quimby, 1926 | 1926 | Mean 0.0031 skin unit dose/wk (~900 mSv/yr) | Memorial Hospital, New York City, workers carried unexposed film | |
| Fricke and Beasley, 1927 | 1927 | Estimated doses of 0.065 skin unit dose/mo (~4,5000 mSv/yr) | Cleveland and New York measurement of stray radiation | |
| Mutscheller, 1928 | 1928 | Proposed tolerance dose limit of 1/100 erythema dose/mo (~700 mSv/yr) | Estimated doses of 1/10 erythema dose/mo (~7000 mSv/yr) | Based on observations of physicians and technicians in shielded work areas |
| Brodsky et al., 1995 | 1928 | Roentgen unit (R) adopted | Second International Congress of Radiology | |
| Braestrup, 1957 | 1920s & 1930s | Estimated doses of 100 R/yr (~1000 mSv/yr) | ||
| Brodsky et al., 1995 | 1931 | Standard: 0.2 R/dy (~500 mSv/yr) | Standard proposed by U.S. Advisory Committee on X-ray and Radium Protection (ACXRP) | |
| Brodsky et al., 1995 | 1936 | Recommendation: 0.1 R/dy (~250 mSv/yr) | Recommendation by ACXRP | |
| Hall, 2000 | 1937 | Roentgen unit accepted as international dosage unit for x- and γ-radiation | Proposed by Fifth International Congress of Radiology | |
| Simon et al., 2006 | Before 1939 | Estimated mean dose of 100 mSv/yr | Annual badge dose prediction for U.S. radiologic technologists working in hospitals | |
| Cowie and Scheele, 1941 | 1940 | Mean 0.02R/dy (~50 mSv/yr) | Survey of 45 hospitals | |
| Simon et al., 2006 | 1940–49 | Estimated mean dose of 25 mSv/yr | Annual badge dose prediction for U.S. radiologic technologists working in hospitals | |
| Geist et al., 1953 | Early 1950s | Estimated dose 0.1R – 0.3R/wk (~50 – 150 mSv/yr) | ||
| Braestrup, 1957 | 1957 | Mean 1 R/yr (~10 mSv/yr) | Based on 138 radiologists in a New York hospital monitored with film badges | |
| Zielinski et al., 2009 | 1951–1987 | Mean badge doses: 1.66 and 0.54 for 1951–70 and 1971–87, respectively for diagnostic radiologists; 1.13 and 0.32 for 1951–70 and 1971–87, respectively for radiologic technologists | Data from Canadian national dose registry of radiation workers | |
| Andersson et al., 1991 | 1954–82 | Mean cumulative dose = 18 mSv (17% had zero exposure, 9% had >50 mSv | Staff members in two radiotherapy departments in Denmark | |
| Inkret et al., 1995 | 1957 | Recommended dose limit: 50 mSv/yr | Proposed by International Commission on Radiation Protection | |
| Simon et al., 2006 | 1960–76 | Mean badge dose: 3.6 mSv/yr | Annual badge dose predictions for U.S. radiologic technologists in hospitals | |
| Webb, 1974 | 1964 | Mean doses <5 mSv/yr | UK radiologists | |
| UNSCEAR, 2000 | 1975–94 | Mean dose: 1975–79=0.94 mSv/yr 1980–84=0.68 mSv/yr 1985–89=0.56 mSv/yr 1990–94=0.50 mSv/yr |
International estimates for workers in diagnostic radiology | |
| Simon et al., 2006 | 1977–84 | Mean badge dose: 2.3 mSv/yr |
Annual badge dose predictions for U.S. radiologic technologists in hospitals | |
| Canadian National Dose Registry | 1985-present | Mean badge doses: 1985=0.36 mSv/yr 1995=0.12 mSv/yr 2005=0.19 mSv/yr |
Canadian diagnostic radiologists | |
| ICRP, 1991 | 1990 | Recommended dose limit: average 20 mSv/yr, averaged over 5-year period, and not exceeding 50 mSv in any single year | Proposed by the International Commission on Radiation Protection | |
| Hughes and Riordan, 1993 | 1993 | Mean dose: 0.5 mSv/yr | UK diagnostic radiologists | |
| Watson et al., 2005 | 2001 | 0.15 mSv/yrMean dose: | UK diagnostic radiologists |
Different types of dosimetric units and quantities have been reported in the literature. To compare the reported radiation doses, the original dose units (e.g., skin unit dose, R, etc.) were roughly approximated to current dosimetry units (e.g., mSv)
The unit of absorbed dose (e.g., Gy) was not defined in 1902. The estimated dose in Gy is an interpretation in modern dose units of the measurement capability at that time based on fogging on a photographic plate
Dosimetry and radiation protection measures
Most of the epidemiologic studies do not report individual dosimetry and none have described lifetime estimated dose data. Cross-sectional studies carried out in hospitals, national surveys, radiation registries, and large film badge measurement companies during different time periods allow estimation of occupational effective dose ranges and temporal trends in effective doses. Figure 1 depicts estimated annual occupational effective radiation doses and temporal trends in average effective doses to radiologists based on literature review, which are compared with National Council on Radiation Protection and Measurements (NCRP) recommendations for occupational limits, based on effective dose [58, 59]. Estimated badge doses during calendar year periods from 1926 through 1984 are shown in Table 4 for U.S. radiologic technologists.
Radiologists
Only limited measurements and estimated doses to radiologists were available before 1940. Estimated effective doses ranged from 900 to 7,000 mSv/year during the 1920s [60–62], were generally in the range of 50 to 100 mSv/year before 1950 [63, 49], and were approximately 50 mSv/year in the early 1950s, although some institutions described a broader range of radiation doses with higher levels characterizing those handling radium [64]. Annual effective doses to medical radiation workers decreased notably from less than 5 mSv in the early 1960s to 1 mSv in the 1970s, 0.75-0.34 mSv in the 1980s, 0.55-0.12 mSv in the 1990s, and 0.23-0.08 mSv after 2000 (see Figure 1) [65–69]. Although the early hospital-based dose data are from the U.S., after 1957 most of the data shown in Figure 1 are from the UK, Canada or other countries.
U.S. radiologic technologists
Using 350,000 film badge measurements for individual radiologic technologists, work history and protection-related data from questionnaires, and measurement and other data derived from the literature, estimated average annual uncertainty distributions of badge doses assigned to members of the U.S. radiologic technologist cohort who worked in hospitals during 1926–84 are shown in Table 4. During the five calendar years periods, average annual estimated badge doses declined notably from an estimated 100 mSv before 1939 to 2.3 mSv during 1977–84 [70]
History of radiation protection
Increasing recognition of serious morbidity and mortality associated external and internal sources of radiation [2] led to formation of international and national Advisory Committees on X-ray and Radium Protection in 1928 and 1929, respectively [71–74] (see Table 5). Initial radiation protection efforts were largely directed at limiting exposures of medical radiation workers [75, 76], whereas subsequent recommendations and regulations were developed for both occupational and non-occupationally-exposed populations. Radiation protection efforts were complicated by difficulties in measuring exposures, in determining ‘safe levels’ due to the delayed nature of most serious health effects, and in developing a systematic approach to standardizing radiation protection limits [77]. Overall, however, the time trend effective dose data for radiologists and radiologic technologists are generally reassuring in demonstrating that occupational exposures of these workers have declined dramatically over time (Figure 1 and Table 4) despite the substantial increase in medical radiation procedures over time, particularly during the past 30 years [78].
Limitations and current status
Follow-up for the U.S. radiologist and U.S. Army technologist cohorts ended in the mid-1970s, [79, 50], for the Danish cohort in the mid-1980s [54] and for the UK radiologists and the Japanese and Chinese cohorts in the mid-1990s [80, 51, 53]. There is currently no active follow-up for the major medical radiation worker cohorts except for the Canadian medical radiation workers [81, 82, 48] and the U.S. radiologic technologists (http://radtechstudy.nci.nih.gov). The substantial latency period for cancer development has, at this time, precluded assessment of cancer risks for medical radiation workers performing or assisting with the newer, higher-dose radiologic procedures including fluoroscopically-guided interventional procedures. Another important limitation is that follow-up of workers ended before the majority of medical radiation workers attained ages at which cancer risks are the highest. Additionally, there has been extremely limited study of female radiologists and their risk for breast, thyroid and other cancers. Also, some of the epidemiologic studies lacked internal comparison of risks and most lack individual dose data
Physicians and radiologic technologists performing fluoroscopically-guided procedures
Clinical and epidemiologic studies
Although the decline in estimated annual average effective dose data for radiologists and radiologic technologists has been dramatic, this may not be the case for physicians performing fluoroscopically-guided procedures [9]. A rapidly expanding number of physicians in other specialties, often with little or no training in radiation sciences, have been conducting fluoroscopically-guided interventional procedures in both developed and developing countries[83–85]. Increasingly complex fluoroscopically-guided interventional procedures have been undertaken on growing numbers of patients, thus requiring longer fluoroscopy time[86, 87]. Despite clinical reports of cataracts [88] and brain tumors [89, 90] among physicians performing fluoroscopically-guided procedures, there has been no long-term epidemiologic cohort study although projections of cancer risk have been carried out based on dosimetry data in small numbers of workers [91]. A relatively short-term mortality follow-up through 2003 was undertaken to evaluate risks in U.S. radiologic technologists assisting with fluoroscopically-guided interventional procedures [92]. Work history (including the frequency of performing or assisting with interventional procedures before 1980, during 1980–1989 or in 1990 or later) was ascertained among 88,766 technologists using a self-administered questionnaire completed during 1994–98 and the subjects were followed up for mortality through 2003. Compared with radiologic technologists who rarely or never performed or assisted with fluoroscopically-guided interventional procedures, risks among those who regularly or frequently performed or assisted with such procedures were not significantly increased for all-cause mortality and risk of mortality from all cancers, breast cancer, or all circulatory system diseases, but there were non-significant increases in mortality from cerebrovascular disease that were observed among technologists who daily performed or assisted with these procedures.
Dosimetry and radiation protection recommendations
Occupational radiation exposures to physicians performing fluoroscopically-guided interventional procedures have been studied for more than 30 years [93], but mostly focusing on dose per procedure rather than cumulative or annual doses. The estimates from these studies have been difficult to compare due to a number of factors, and doses have been observed to vary by more than an order of magnitude among studies [94]. Some of the difficulties in comparing dose estimates can be attributed to differences in placement of dosimeters, and difficulties in identifying and quantifying the predictive value of multiple factors influencing doses such as fluoroscopy system operation and radiation protection measures [9]. Assessment of dose trends is also complicated by lack of systematic placement of film badges on the operator’s body and/or failure to wear film badges [95] as confirmed by biological dosimetry [96].
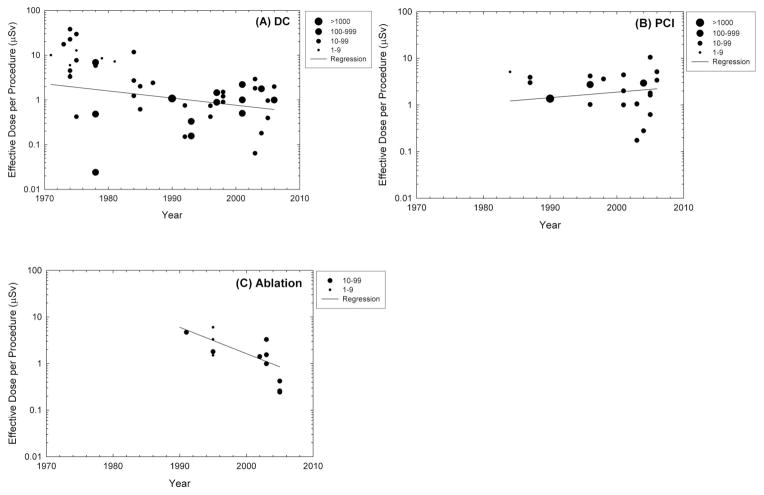
A recent comprehensive and systematic summary of the reported occupational radiation doses received by operators performing diagnostic or interventional fluoroscopically-guided cardiac catheterization procedures found effective doses ranging from 0.02–38.0 μSv for diagnostic catheterization, from 0.17–31.2 μSv for percutaneous coronary interventions, 0.24–9.6 μSv for ablations, and 0.29–17.4 μSv for pacemaker or intracardiac defibrillator implantations [9] (Figure 2). Mean dose per procedure measured over personal protective devices placed at eye, thyroid, or trunk sites (the latter over the apron) or on the hands ranged from 0.4–1,100 μSv at eye level, 1.2–580 μSv at thyroid level, 3.5–750 μSv at trunk level, and 0.4–790 μSv at hand level, whereas measurements under the apron at the trunk level ranged from 0–16 μSv [9]. There were modest albeit significant reductions in average dose for diagnostic catheterization and ablation. For percutaneous coronary interventions, there was some evidence of increasing operator dose over time. Changes over time in radiation doses to patients or operators may be due to changes in procedure protocols and technology. Improvements in procedure protocols and the technology of x-ray equipment, catheters, and other devices generally decrease the time required for procedures (due to decreased fluoroscopy or cineradiography time) and may result in lower radiation doses for procedures with similar complexity. On the other hand, improved protocols and technologies make it possible to carry out more complex procedures, which can require longer fluoroscopy or cineradiography time or both. The increase in radiation associated with more complex procedures may negate the effect of technological improvements that might otherwise result in reduction of operator dose. [86, 87]. Although the numbers of dosimetry studies are substantially less for fluoroscopically-guided interventional procedures carried out by specialists other than cardiologists, in general the same degree of variation in dose per procedure is apparent for nephrostomy and stone removal [97, 98], vertebroplasty [99, 100], transjugular intrahepatic portosystemic shunt [101, 102], and others.
Figure 2.
Average occupational effective radiation doses per procedure and trends in effective doses per procedure to interventional cardiologists for three types of interventional procedures: (a) diagnostic catheterization (DC), (b) percutaneous coronary interventions (PCI), and (c) ablation. Each data point represents the mean value from one published study under similar exposure conditions where the year of publication is used as a surrogate for the year when the procedures were conducted. The size of the data points represents the number of procedures reported. Source: Kim K et al. Health Phys 2008;94:211-227.
The wide variation in operator dose suggests that reduction in operator dose is possible [9]. Since doses to operators performing fluoroscopically-guided interventional procedures correlate highly with patient doses [103, 104], measures that reduce patient dose will also reduce worker dose. These include technical improvements in dose reduction technology for fluorscopes and radiation management training for all operators. At present, operators in some medical specialties receive relatively little training in radiation management.
There is also opportunity for improvement in occupational dosimetry. There is no standardized national or international method of determining occupational dose for operators who perform fluoroscopically-guided interventions. NCRP Report No. 122 [78] provides recommended methods which could be adopted. Methods to improve compliance with monitoring are also needed as reliable dosimetry is not possible unless monitors are worn regularly and consistently.
Limitations and current status of epidemiologic and dosimetric research
The single epidemiologic cohort study of radiologic technologists reported to date involved relatively short follow-up, did not include incidence data, and lacked individual technologist dose information [92]. A comprehensive literature review of occupational dose data reveals substantial numbers of studies reporting dose per procedure to cardiologists, but more limited assessment of dose per procedure for interventional radiologists and physicians other than cardiologists who perform fluoroscopically-guided procedures. The data reveal enormous variation in the dose received by the operator per procedure and suggest that operator dose depends on numerous factors, but the contribution of each specific determinant has not been well quantified. Most dosimetry investigations have studied a relatively small number of operators and hospitals, and thus provide little information about geographic variation on a national or international basis. Data are limited on the numbers of procedures performed annually per specialist or on the variability in the workload according to type of procedure for the different categories of physicians who carry out fluoroscopically-guided procedures. Cumulative occupational dose data are lacking, a problem which has been complicated by the substantial proportions of operators rarely or never wear their dosimeters [105, 96].
Future Directions
Growing concern about the effects of chronic radiation exposure [106], the dramatic increases in per capita dose from medical sources of radiation exposure [107, 5], the increasing numbers of radiologists [108] and other specialties of physicians and other medical workers who perform or assist with fluoroscopically-guided interventional procedures [10] [109], the growing workload of radiologists [110] and likely also that of physicians performing fluoroscopically-guided procedures, and the limited numbers or lack of long-term epidemiologic follow-up studies to assess cancer risks in radiologists and radiologic technologists or physicians performing fluoroscopically-guided procedures, respectively, underscores the need for additional research to clarify occupational radiation doses and cancer and other serious disease risks of medical radiation workers. To this end, nationwide monitoring surveys should be regularly carried out in all major categories of medical radiation workers to ascertain average annual occupational radiation doses. The currently active cohort follow-up studies of Canadian medical radiation workers and U.S. radiologic technologists should be extended to assess lifetime risks of cancer and other serious diseases. It will be important to apply the recently available individual cumulative occupational radiation dose estimates [70] to assess dose-response in relation to cancer risks and other serious disease outcomes in the U.S. radiologic technologists.
New large epidemiologic cohort studies are urgently needed to evaluate cancer and other serious disease risks in interventional radiologists, cardiologists and physicians in other specialties who perform fluoroscopically-guided procedures. We have undertaken a new cohort mortality study comparing cancer and other serious disease outcomes in 44,000 physicians performing fluoroscopically-guided procedures (including interventional radiologists, cardiologists, neuro-radiologists and others) and in 42,000 non-interventional radiologists with risks in 101,000 physicians who are unlikely to be occupationally exposed to radiation (e.g., family physicians and psychiatrists). Because doses to operators performing fluoroscopically-guided interventional procedures correlate highly with patient doses [111, 85, 104] cohort studies that evaluate dose-related cancer risks in interventional physicians may provide data that can be extrapolated to patient risks as well as mechanistic insights on cancer risks associated with chronic, fractionated, low-to-moderate-dose radiation exposures.
Table 6.
Limitations of existing epidemiologic studies of cancer risks in radiologists and radiologic technologists
| Study characteristic | Limitations | Comment |
|---|---|---|
| Population size | Some are relatively small |
|
| Source of population | Not population-based |
|
| Nature of study populations | Some are heterogeneous |
|
| Recency of follow-up, assessment of risk for workers performing newer procedures | Limited numbers of person-years of follow- up in recent decades ; little evaluation of cancer risks associated with newer radiologic procedures |
|
| Length of follow-up in relation to lifetime | Limited assessment of lifetime cancer risks |
|
| Quality and extent of dosimetric information | Limited dosimetry data |
|
Acknowledgments
We are grateful to Dr. Alice Sigurdson and Ms. Michele Doody for their helpful suggestions. This review was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Footnotes
Presented at: Late Health Effects of Ionizing Radiation: Bridging the Experimental and Epidemiologic Divide, Georgetown University, Washington, DC, May 4-6, 2009
References
- 1.DiSantis DJ. Early American Radiology: the pioneer years. AJR Am J Roentgenol. 1986;147:850–3. doi: 10.2214/ajr.147.4.850. [DOI] [PubMed] [Google Scholar]
- 2.Walker JS. The controversy over radiation safety. A historical overview. Jama. 1989;262:664–8. [PubMed] [Google Scholar]
- 3.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 4.Boice JD., Jr . Ionizing radiation. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3. New York, New York: Oxford University Press; 2006. [Google Scholar]
- 5.Mettler FA, Jr, Thomadsen BR, Bhargavan M, et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008;95:502–7. doi: 10.1097/01.HP.0000326333.42287.a2. [DOI] [PubMed] [Google Scholar]
- 6.Cardis E, Vrijheid M, Blettner M, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. Bmj. 2005;331:77. doi: 10.1136/bmj.38499.599861.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardis E, Vrijheid M, Blettner M, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 8.Yoshinaga S, Mabuchi K, Sigurdson AJ, et al. Cancer risks among radiologists and radiologic technologists: review of epidemiologic studies. Radiology. 2004;233:313–21. doi: 10.1148/radiol.2332031119. [DOI] [PubMed] [Google Scholar]
- 9.Kim KP, Miller DL, Balter S, et al. Occupational radiation doses to operators performing cardiac catheterization procedures. Health Phys. 2008;94:211–27. doi: 10.1097/01.HP.0000290614.76386.35. [DOI] [PubMed] [Google Scholar]
- 10.ICRP. ICRP Publication 85; Ann ICRP. Oxford; UK: 2000. (International Commission on Radiation Protection). Avoidance of radiation injuries from medical interventional procedures. [Google Scholar]
- 11.BEIR VII. Beir VII. Washington, DC: National Research Counsil; 2006. Committee to assess health risks from exposure to low levels of ionizing radiation. Health risks from exposure to low levels of ionizing radiation. [Google Scholar]
- 12.Jacob P, Ruhm W, Walsh L, et al. Cancer risk of radiation workers larger than expected? Occup Environ Med. 2009 doi: 10.1136/oem.2008.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seibert JA. One hundred years of medical diagnostic imaging technology. Health Phys. 1995;69:695–720. doi: 10.1097/00004032-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Hall E. Radiobiology for the Radiologist. 5. Philadelphia: Lipincott Williams and Wilkins; 2000. Milestones in the radiation sciences. [Google Scholar]
- 15.Margulis AR. Some new approaches to the examination of the gastrointestinal tract. Am J Roentgenol Radium Ther Nucl Med. 1967;101:265–86. doi: 10.2214/ajr.101.2.viii. [DOI] [PubMed] [Google Scholar]
- 16.Dotter CT, Judkins MP. Transluminal Treatment of Arteriosclerotic Obstruction. Description of a New Technic and a Preliminary Report of Its Application. Circulation. 1964;30:654–70. doi: 10.1161/01.cir.30.5.654. [DOI] [PubMed] [Google Scholar]
- 17.Dotter CT, Judkins MP, Frische LH. Safety guidespring for percutaneous cardiovascular catheterization. Am J Roentgenol Radium Ther Nucl Med. 1966;98:957–60. doi: 10.2214/ajr.98.4.957. [DOI] [PubMed] [Google Scholar]
- 18.Judkins MP. Selective coronary arteriography. I. A percutaneous transfemoral technic. Radiology. 1967;89:815–24. doi: 10.1148/89.5.815. [DOI] [PubMed] [Google Scholar]
- 19.Judkins MP, Kidd HJ, Frische LH, Dotter CT. Lumen-following safety J-guide for catheterization of tortuous vessels. Radiology. 1967;88:1127–30. doi: 10.1148/88.6.1127. [DOI] [PubMed] [Google Scholar]
- 20.Judkins MP, Hinck VC, Dotter CT. Teflon-coated safety guides. An adjunct to the use of polyurethane catheters. Am J Roentgenol Radium Ther Nucl Med. 1968;104:223–4. [PubMed] [Google Scholar]
- 21.Newton TH, Adams JE. Angiographic demonstration and nonsurgical embolization of spinal cord angioma. Radiology. 1968;91:873–6. doi: 10.1148/91.5.873. passim. [DOI] [PubMed] [Google Scholar]
- 22.Rösch J, Hanafee WN, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology. 1969;92:1112–4. doi: 10.1148/92.5.1112. [DOI] [PubMed] [Google Scholar]
- 23.Doppman JL, Di Chiro G, Ommaya AK. Percutaneous embolization of spinal cord arteriovenous malformations. J Neurosurg. 1971;34:48–55. doi: 10.3171/jns.1971.34.1.0048. [DOI] [PubMed] [Google Scholar]
- 24.Dotter CT, Rosch J, Lakin PC, et al. Injectable flow-guided coaxial catheters for selective angiography and controlled vascular occlusion. Radiology. 1972;104:421–3. doi: 10.1148/104.2.421. [DOI] [PubMed] [Google Scholar]
- 25.Leary JB, Parshall WA. Percutaneous common-duct stone extraction. Radiology. 1972;105:452–4. doi: 10.1148/105.2.452. [DOI] [PubMed] [Google Scholar]
- 26.Rösch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303–6. doi: 10.1148/102.2.303. [DOI] [PubMed] [Google Scholar]
- 27.Dotter CT, Rosch J, Seaman AJ. Selective clot lysis with low-dose streptokinase. Radiology. 1974;111:31–7. doi: 10.1148/111.1.31. [DOI] [PubMed] [Google Scholar]
- 28.Grüntzig A. Percutane dilatation von koronarstenoses. Beschreibunt eines neuen kathetersystsem. Klin Wochenshr. 1976;54:543–545. doi: 10.1007/BF01468977. [DOI] [PubMed] [Google Scholar]
- 29.Colapinto RF, Stronell RD, Gildiner M, et al. Formation of intrahepatic portosystemic shunts using a balloon dilatation catheter: preliminary clinical experience. AJR Am J Roentgenol. 1983;140:709–14. doi: 10.2214/ajr.140.4.709. [DOI] [PubMed] [Google Scholar]
- 30.Dotter CT, Buschmann RW, McKinney MK, Rosch J. Transluminal expandable nitinol coil stent grafting: preliminary report. Radiology. 1983;147:259–60. doi: 10.1148/radiology.147.1.6828741. [DOI] [PubMed] [Google Scholar]
- 31.Okamura J. Chemo-embolization in hepatic cancer--the mechanism of necrosis induction and subsequent surgical treatment. Gan To Kagaku Ryoho. 1983;10:340–50. [PubMed] [Google Scholar]
- 32.Palmaz JC, Sibbitt RR, Reuter SR, et al. Expandable intraluminal graft: a preliminary study. Work in progress. Radiology. 1985;156:73–7. doi: 10.1148/radiology.156.1.3159043. [DOI] [PubMed] [Google Scholar]
- 33.Richter GM, Palmaz JC, Noldge G, et al. The transjugular intrahepatic portosystemic stent-shunt. A new nonsurgical percutaneous method. Radiologe. 1989;29 :406–11. [PubMed] [Google Scholar]
- 34.Cope C, Burke DR, Meranze SG. Percutaneous extraction of gallstones in 20 patients. Radiology. 1990;176:19–24. doi: 10.1148/radiology.176.1.2353089. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg SN, Ryan TP, Hahn PF, et al. Transluminal radiofrequency tissue ablation with use of metallic stents. J Vasc Interv Radiol. 1997;8:835–43. doi: 10.1016/s1051-0443(97)70669-9. [DOI] [PubMed] [Google Scholar]
- 36.Weimar B, Rauber K, Brendel MD, et al. Percutaneous transhepatic catheterization of the portal vein: A combined CT- and fluoroscopy-guided technique. Cardiovasc Intervent Radiol. 1999;22:342–4. doi: 10.1007/s002709900403. [DOI] [PubMed] [Google Scholar]
- 37.Navarro L, Min RJ, Bone C. Endovenous laser: a new minimally invasive method of treatment for varicose veins--preliminary observations using an 810 nm diode laser. Dermatol Surg. 2001;27:117–22. doi: 10.1046/j.1524-4725.2001.00134.x. [DOI] [PubMed] [Google Scholar]
- 38.Frieben A. Demonstration eines cancroids des rechten handruckens, das sich nach langdaurernder einwirkung von roentenstrahlen enwickelt hatte. Fortschr Roentgenstr. 1902;6:106–111. [Google Scholar]
- 39.Rollins W Notes on X-light. Boston, self published, 1904. Contains the following articles: Rollins W. X-light kills. Boston Med Surg J. 1901;144:173.Rollins W. Non-radiable cases for x-light tubes. Elec Rev. 1902;40:795–99.Rollins W. Notes on X-light: the effect of X-light on the crystalline lens. Boston Med Surg J. 1903;148:364.Cited in Siebert JA. One hundred years of medical diagnostic imaging technology. Health Phys. 1995;69:695–20. doi: 10.1097/00004032-199511000-00006.
- 40.Scott SG. Notes on a case of x-ray dermatitis with fatal termination. Arch Roentgen Ray. 1911;15:443–444. [Google Scholar]
- 41.Von Jagic N, Schwartz G, Siebenrock L. Blutkefunde bei Rontgenologen. Berl Klin Wchnschr. 1911;48:1220. [Google Scholar]
- 42.Kitabatake T, Watanabe T, Saito A, Nakamura M. Aplastic anemia in Japanese radiological technicians. Strahlentherapie [Sonderb] 1976;152:187–90. [PubMed] [Google Scholar]
- 43.Court-Brown WM, Doll R. Expectation of life and mortality from cancer among British radiologists. Br Med J. 1958:181–187. doi: 10.1136/bmj.2.5090.181. ii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seltser R, Sartwell PE. The influence of occupational exposure to radiation on the mortality of american radiologists and other medical specialists. Am J Epidemiol. 1965;81:2–22. doi: 10.1093/oxfordjournals.aje.a120493. [DOI] [PubMed] [Google Scholar]
- 45.Logue JN, Barrick MK, Jessup GL., Jr Mortality of radiologists and pathologists in the Radiation Registry of Physicians. J Occup Med. 1986;28:91–9. [PubMed] [Google Scholar]
- 46.Carpenter LM, Swerdlow AJ, Fear NT. Mortality of doctors in different specialties: findings from a cohort of 20000 NHS hospital consultants. Occup Environ Med. 1997;54:388–95. doi: 10.1136/oem.54.6.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jartti P, Pukkala E, Uitti J, Auvinen A. Cancer incidence among physicians occupationally exposed to ionizing radiation in Finland. Scand J Work Environ Health. 2006;32:368–73. doi: 10.5271/sjweh.1032. [DOI] [PubMed] [Google Scholar]
- 48.Zielinski JM, Garner MJ, Band PR, et al. Health outcomes of low-dose ionizing radiation exposure among medical workers: a cohort study of the Canadian national dose registry of radiation workers. Int J Occup Med Environ Health. 2009;22:149–56. doi: 10.2478/v10001-009-0010-y. [DOI] [PubMed] [Google Scholar]
- 49.Smith PG, Doll R. Mortality from cancer and all causes among British radiologists. Br J Radiol. 1981;54:187–94. doi: 10.1259/0007-1285-54-639-187. [DOI] [PubMed] [Google Scholar]
- 50.Matanoski GM, Sartwell P, Elliott E, et al. Cancer risks in radiologists and radiation workers. In: Boice JD Jr, Fraumeni JF Jr, editors. Radiation carcinogenesis: epidemiology and biological significance. New York: Raven Press; 1984. [Google Scholar]
- 51.Berrington A, Darby SC, Weiss HA, Doll R. 100 years of observation on British radiologists: mortality from cancer and other causes 1897–1997. Br J Radiol. 2001;74:507–19. doi: 10.1259/bjr.74.882.740507. [DOI] [PubMed] [Google Scholar]
- 52.Linet MS, Freedman DM, Mohan AK, et al. Incidence of haematopoietic malignancies in US radiologic technologists. Occup Environ Med. 2005;62:861–7. doi: 10.1136/oem.2005.020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang JX, Zhang LA, Li BX, et al. Cancer incidence and risk estimation among medical x-ray workers in China, 1950–1995. Health Phys. 2002;82:455–66. doi: 10.1097/00004032-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Andersson M, Engholm G, Ennow K, et al. Cancer risk among staff at two radiotherapy departments in Denmark. Br J Radiol. 1991;64:455–60. doi: 10.1259/0007-1285-64-761-455. [DOI] [PubMed] [Google Scholar]
- 55.Mohan AK, Hauptmann M, Linet MS, et al. Breast cancer mortality among female radiologic technologists in the United States. J Natl Cancer Inst. 2002;94:943–8. doi: 10.1093/jnci/94.12.943. [DOI] [PubMed] [Google Scholar]
- 56.Mohan AK, Hauptmann M, Freedman DM, et al. Cancer and other causes of mortality among radiologic technologists in the United States. Int J Cancer. 2003;103:259–67. doi: 10.1002/ijc.10811. [DOI] [PubMed] [Google Scholar]
- 57.Doody MM, Freedman DM, Alexander BH, et al. Breast cancer incidence in U.S. radiologic technologists. Cancer. 2006;106:2707–15. doi: 10.1002/cncr.21876. [DOI] [PubMed] [Google Scholar]
- 58.NCRP. NCRP Report No 93. Bethesda, MD: 1987. (National Council on Radiation Protection and Measurements). Ionizing radiation exposure of the population of the United States. [Google Scholar]
- 59.NCRP. NCRP Report No 116. Bethesda, MD: 1993. (National Council on Radiation Protection and Measurements). Limiation of exposure to ionisizing radiation. [Google Scholar]
- 60.Quimby A. Method for the study of scattered and secondary radiation in x-ray and radium laboratories. Radiology. 1926;VII:211–217. [Google Scholar]
- 61.Fricke H, Beasley IE. Measurement of the stray radiation in roentgen ray clinics. Am J Roentgenol Radium Ther Nucl Med. 1927;XVIII:146–148. [Google Scholar]
- 62.Mutscheller A. Safety standards of protection against x-ray dangers. Radiology. 1928;IX:468–476. [Google Scholar]
- 63.Cowie DB, Scheele IA. Survey of radiation protection in hospital. J Natl Cancer Inst. 1941:767–787. [Google Scholar]
- 64.Braestrup CB. Past and present radiation exposure to radiologists from the point of view of life expectancy. Am J Roentgenol Radium Ther Nucl Med. 1957;78:988–92. [PubMed] [Google Scholar]
- 65.Webb GAM. HMSO. 1974. Radiation exposure of the public-current levels in the United Kingdom. [Google Scholar]
- 66.NRPB. (National Radiological Protection Board). Radiation exposure of the UK population -1993 review. Chilton, UK: 1993. [Google Scholar]
- 67.ORHD. (Occupational Radiation Hazards Division), Radiation Protection Bureau, Health Canada Occupational Radiation exposures in canada 1994. Ontario, Canada: 1995. [Google Scholar]
- 68.UNSCEAR. (United Nations Scientific Committee on Effects of Atomic Radiation). Sources and effects of ionizing radiation. Vol. 1. New York, United Nations: 2000. Sources. (Available online at: http://www.unscear.org/unscear/en/publications/2000_1.html) [Google Scholar]
- 69.HPA Ionising radiation exposure of the UK population - 2005 review. 2005. [Google Scholar]
- 70.Simon SL, Weinstock RM, Doody MM, et al. Estimating historical radiation doses to a cohort of U.S. radiologic technologists. Radiat Res. 2006;166:174–92. doi: 10.1667/RR3433.1. [DOI] [PubMed] [Google Scholar]
- 71.Taylor LS. Brief history of the National committee of Radiation Protection and Measurements (NCRP) covering the period 1929–1946. Health Phys. 1958;1:3–10. doi: 10.1097/00004032-195801000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Taylor LS. Radiation protection trends in the United States. Health Phys. 1971;20:499–504. doi: 10.1097/00004032-197105000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Taylor LS. The development of radiation protection standards (1925–1940) Health Phys. 1981;41:227–32. [PubMed] [Google Scholar]
- 74.Inkret WC, Meinhold CB, Taschner JC. Radiation and risk: a hard look at the data protection standards. Los Alamos. Science. 1995:23. [Google Scholar]
- 75.Taylor LS. Will radiation control be by reason or regulation? Health Phys. 1988;55:133–8. doi: 10.1097/00004032-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 76.Kocher DC. Perspective on the historical development of radiation standards. Health Phys. 1991;61:519–27. doi: 10.1097/00004032-199110000-00007. [DOI] [PubMed] [Google Scholar]
- 77.Jones CG. A review of the history of U.S. radiation protection regulations, recommendations, and standards. Health Phys. 2005;88:697–716. [PubMed] [Google Scholar]
- 78.NCRP. NCRP Report No. 160. Bethesda, MD: 2009. (National Council on Radiation Protection and Measurements). Ionizing radiation exposure of the population of the United States. [Google Scholar]
- 79.Jablon S, Miller RW. Army technologists: 29-year follow up for cause of death. Radiology. 1978;126:677–9. doi: 10.1148/126.3.677. [DOI] [PubMed] [Google Scholar]
- 80.Yoshinaga S, Aoyama T, Yoshimoto Y, Sugahara T. Cancer mortality among radiological technologists in Japan: updated analysis of follow-up data from 1969 to 1993. J Epidemiol. 1999;9:61–72. doi: 10.2188/jea.9.61. [DOI] [PubMed] [Google Scholar]
- 81.Ashmore JP, Krewski D, Zielinski JM, et al. First analysis of mortality and occupational radiation exposure based on the National Dose Registry of Canada. Am J Epidemiol. 1998;148:564–74. doi: 10.1093/oxfordjournals.aje.a009682. [DOI] [PubMed] [Google Scholar]
- 82.Sont WN, Zielinski JM, Ashmore JP, et al. First analysis of cancer incidence and occupational radiation exposure based on the National Dose Registry of Canada. Am J Epidemiol. 2001;153:309–18. doi: 10.1093/aje/153.4.309. [DOI] [PubMed] [Google Scholar]
- 83.Rösch J, Keller FS, Kaufman JA. The birth, early years, and future of interventional radiology. J Vasc Interv Radiol. 2003;14:841–53. doi: 10.1097/01.rvi.0000083840.97061.5b. [DOI] [PubMed] [Google Scholar]
- 84.Miller DL. Overview of contemporary interventional fluoroscopy procedures. Health Phys. 2008;95:638–44. doi: 10.1097/01.HP.0000326341.86359.0b. [DOI] [PubMed] [Google Scholar]
- 85.Tsapaki V, Ahmed NA, AlSuwaidi JS, et al. Radiation exposure to patients during interventional procedures in 20 countries: initial IAEA project results. AJR Am J Roentgenol. 2009;193:559–69. doi: 10.2214/AJR.08.2115. [DOI] [PubMed] [Google Scholar]
- 86.Strzelczyk JJ, Damilakis J, Marx MV, Macura KJ. Facts and controversies about radiation exposure, part 1: controlling unnecessary radiation exposures. J Am Coll Radiol. 2006;3:924–31. doi: 10.1016/j.jacr.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 87.Dendy PP. Radiation risks in interventional radiology. Br J Radiol. 2008;81:1–7. doi: 10.1259/bjr/15413265. [DOI] [PubMed] [Google Scholar]
- 88.Vañó E, Gonzalez L, Beneytez F, Moreno F. Lens injuries induced by occupational exposure in non-optimized interventional radiology laboratories. Br J Radiol. 1998;71:728–33. doi: 10.1259/bjr.71.847.9771383. [DOI] [PubMed] [Google Scholar]
- 89.Finkelstein MM. Is brain cancer an occupational disease of cardiologists? Can J Cardiol. 1998;14:1385–8. [PubMed] [Google Scholar]
- 90.Wenzl T, McDonald JC. Is there an elevated risk of brain cancer among physicians performing interventional radiology procedures? Radiat Prot Dosimetry. 2002;102:99–100. doi: 10.1093/oxfordjournals.rpd.a006088. [DOI] [PubMed] [Google Scholar]
- 91.Venneri L, Rossi F, Botto N, et al. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am Heart J. 2009;157:118–24. doi: 10.1016/j.ahj.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 92.Linet MS, Hauptmann M, Freedman DM, et al. Interventional radiography and mortality risks in U.S.radiologic technologists. Pediatr Radiol. 2006;36 (Suppl 2):113–20. doi: 10.1007/s00247-006-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balter S, Sones FM, Jr, Brancato R. Radiation exposure to the operator performing cardiac angiography with U-arm systems. Circulation. 1978;58:925–32. doi: 10.1161/01.cir.58.5.925. [DOI] [PubMed] [Google Scholar]
- 94.Padovani R, Rodella CA. Staff dosimetry in interventional cardiology. Radiat Prot Dosimetry. 2001;94:99–103. doi: 10.1093/oxfordjournals.rpd.a006490. [DOI] [PubMed] [Google Scholar]
- 95.Marx MV, Niklason L, Mauger EA. Occupational radiation exposure to interventional radiologists: a prospective study. J Vasc Interv Radiol. 1992;3:597–606. doi: 10.1016/s1051-0443(92)72903-0. [DOI] [PubMed] [Google Scholar]
- 96.Montoro A, Rodriguez P, Almonacid M, et al. Biological dosimetry in a group of radiologists by the analysis of dicentrics and translocations. Radiat Res. 2005;164:612–7. doi: 10.1667/rr3444.1. [DOI] [PubMed] [Google Scholar]
- 97.Geterud K, Larsson A, Mattsson S. Radiation dose to patients and personnel during fluoroscopy at percutaneous renal stone extraction. Acta Radiol. 1989;30:201–5. [PubMed] [Google Scholar]
- 98.Kumari G, Kumar P, Wadhwa P, et al. Radiation exposure to the patient and operating room personnel during percutaneous nephrolithotomy. Int Urol Nephrol. 2006;38:207–10. doi: 10.1007/s11255-005-4972-9. [DOI] [PubMed] [Google Scholar]
- 99.Komemushi A, Tanigawa N, Kariya S, et al. Radiation exposure to operators during vertebroplasty. J Vasc Interv Radiol. 2005;16:1327–32. doi: 10.1097/01.RVI.0000179794.65662.01. [DOI] [PubMed] [Google Scholar]
- 100.Ortiz AO, Natarajan V, Gregorius DR, Pollack S. Significantly reduced radiation exposure to operators during kyphoplasty and vertebroplasty procedures: methods and techniques. AJNR Am J Neuroradiol. 2006;27:989–94. [PMC free article] [PubMed] [Google Scholar]
- 101.Williams JR. The interdependence of staff and patient doses in interventional radiology. Br J Radiol. 1997;70:498–503. doi: 10.1259/bjr.70.833.9227232. [DOI] [PubMed] [Google Scholar]
- 102.Hidajat N, Wust P, Kreuschner M, et al. Radiation risks for the radiologist performing transjugular intrahepatic portosystemic shunt (TIPS) Br J Radiol. 2006;79:483–6. doi: 10.1259/bjr/67632946. [DOI] [PubMed] [Google Scholar]
- 103.Tsapaki V, Kottou S, Vano E, et al. Correlation of patient and staff doses in interventional cardiology. Radiat Prot Dosimetry. 2005;117:26–9. doi: 10.1093/rpd/nci705. [DOI] [PubMed] [Google Scholar]
- 104.Vañó E, Ubeda C, Leyton F, et al. Staff radiation doses in interventional cardiology: correlation with patient exposure. Pediatr Cardiol. 2009;30:409–13. doi: 10.1007/s00246-008-9375-0. [DOI] [PubMed] [Google Scholar]
- 105.Niklason LT, Marx MV, Chan HP. Interventional radiologists: occupational radiation doses and risks. Radiology. 1993;187:729–33. doi: 10.1148/radiology.187.3.8497622. [DOI] [PubMed] [Google Scholar]
- 106.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100:13761–6. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shannoun F, Zeeb H, Back C, Blettner M. Medical exposure of the population from diagnostic use of ionizing radiation in luxembourg between 1994 and 2002. Health Phys. 2006;91:154–62. doi: 10.1097/01.HP.0000205237.00357.58. [DOI] [PubMed] [Google Scholar]
- 108.Sunshine JH, Lewis RS, Bhargavan M. A portrait of interventional radiologists in the United States. AJR Am J Roentgenol. 2005;185:1103–12. doi: 10.2214/AJR.05.0237. [DOI] [PubMed] [Google Scholar]
- 109.Bhargavan M. Trends in the utilization of medical procedures that use ionizing radiation. Health Phys. 2008;95:612–27. doi: 10.1097/01.HP.0000327659.42618.c1. [DOI] [PubMed] [Google Scholar]
- 110.Bhargavan M, Sunshine JH. Workload of radiologists in the United States in 2002–2003 and trends since 1991–1992. Radiology. 2005;236:920–31. doi: 10.1148/radiol.2363041316. [DOI] [PubMed] [Google Scholar]
- 111.Vañó E, Gonzalez L, Fernandez JM, et al. Occupational radiation doses in interventional cardiology: a 15-year follow-up. Br J Radiol. 2006;79:383–8. doi: 10.1259/bjr/26829723. [DOI] [PubMed] [Google Scholar]