With over a decade of neuroimaging research of the brain– gut axis behind us, we find ourselves at a convergence of emergent technology and pathophysiologic understanding. Although biomarkers of functional gastrointestinal disorders (FGIDs) remain elusive, we have made great progress in our understanding of the complex interrelationships of gastrointestinal sensation, motility, and immune function within the framework of the biopsychosocial model that we believe characterizes FGIDs.1 At the same time, the field of neuroimaging has grown rapidly, utilizing ever more precise and complex measures of brain structure and function. Our current environment is an enviable one for researchers in the field, although it brings with it the usual challenges of rapidly evolving technology and need for cautious interpretation. This review highlights some of the most promising avenues for brain– gut research that neuroimaging offers, emphasizing their applicability to FGIDS.
Why Image the Brain in FGIDs?
Symptom-based disorders, by definition, utilize subjective responses to determine such factors as illness severity, symptom-related distress, and response to medications or placebo. Although patient reports may be of the greatest clinical utility, less biased measures are more useful in elucidating disease pathophysiology or a medication’s mechanism of action. Pain is the signature symptom of the most prevalent FGIDS, irritable bowel syndrome (IBS) and functional dyspepsia. The study of pain without an examination of central processing is limited to the study of peripheral pain reflex pathways, autonomic responses, and patient reports. Unlike preclinical studies in organic gastrointestinal disorders, animal models of IBS, even when evaluating central factors, have largely failed to predict treatment responses of FGIDs in humans. One of the reasons for this translational failure may be the predominance of higher cortical modulation of subcortical systems involved in the generation of IBS symptoms and the difficulty in studying homologous modulatory systems in rodent models.2 Imaging the brain allows us to evaluate not only the input from the periphery via the homeostatic (visceral) afferent system in the brain, but also the role of emotional and cognitive factors in the modulation of the afferent input. Neuroimaging can provide a noninvasive way to index the central response of humans to symptoms, and to a variety of stimuli including medications, symptom-like stressors, and psychosocial stress.
Functional Imaging
Functional Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) has the advantages of being noninvasive, without any radiation, and has good spatial resolution (2–4 mm). Functional MRI (fMRI) measures changes in the proportion of oxygenated versus deoxygenated hemoglobin based on their respective magnetic properties. The blood oxygen level–dependent (BOLD) signal, is measured, with greater oxygenated blood (seen in areas of greater neural activity) leading to increased signal. Because of the physiologic delay in the hemodynamic response, fMRI has limited temporal resolution of a few seconds. Consequently, the BOLD signal represents temporally evolved, not instantaneous, neuronal activity.
Brain responses to visceral stimulation in both IBS and healthy controls have been described in a number of small studies, often with inconsistent results and interpretation. Increasingly, powerful quantitative meta-analytic approaches to cumulating information from small fMRI studies are being applied to detect consistent findings across studies. A recent meta-analysis of supraliminal rectal distension in IBS showed that regions of the brain involved in both homeostatic afferent processing and emotional arousal were differentially activated in IBS patients compared with controls.3 Further, these results are consistent with the empiric models developed years before, implicating these very regions. Such meta-analyses lay the groundwork for future studies characterizing the activity of functional neural networks.
Although the largest body of work has examined visceral pain processing networks perturbed by symptomlike stressors and pharmacologic agents, new directions for functional imaging studies include further investigation of cognitive (selective attention to threat, appraisal) and emotional processing networks that may interact with visceral processing as well as underlie the documented hypervigilance and comorbidity of anxiety in FGIDs. Functional imaging remains an important tool for identifying the brain regions comprising relevant functional brain networks and characterizing the effective connectivity of these networks.
Connectivity
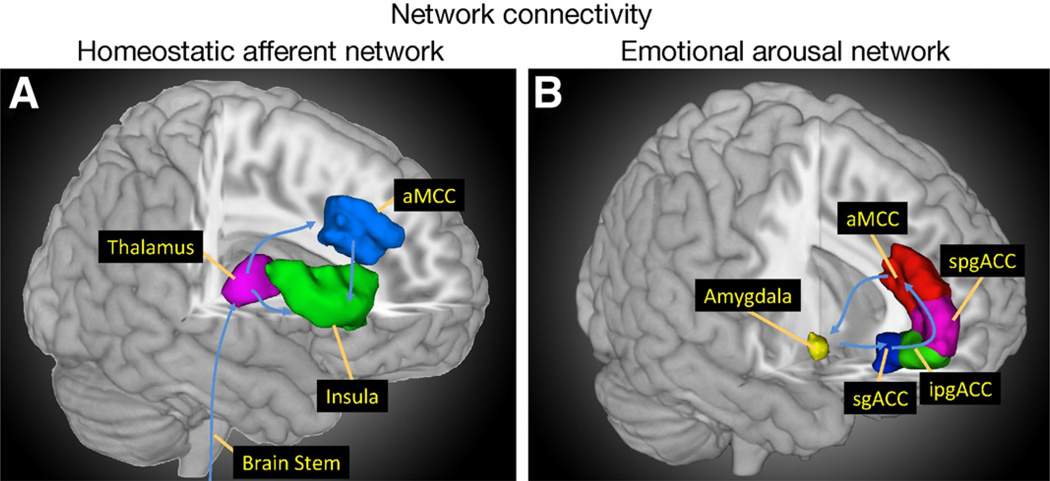
Informed by early fMRI studies that tended to test a set of a priori hypothesized regions of interest selected based on preclinical or other functional imaging studies, research has evolved toward network-driven hypotheses. Instead of determining whether an isolated region is active during a given task, entire functional networks are examined. In FGIDs, there have been 2 particular areas of focus. The first is the homeostatic afferent network (often, but incorrectly, referred to as “pain matrix”; Figure 1A). This network processes the interoceptive input to the brain via the parabrachial spinal pathway to the dorsal pons, ventromedial thalamus, posterior insula, and anterior mid cingulate cortex.4,5 The second is the emotional arousal network (Figure 1B). The core of this network is composed of the amygdala and anterior cingulate cortical subregions.6 The extended emotional arousal circuitry includes the anterior insula, hippocampus, and dorsal pons.4,7 This circuitry is expected to have increased engagement in patients with FGIDs.
Figure 1.
The core regions of the homeostatic afferent (A) and emotional arousal (B) networks are shown.
To date, effective connectivity analyses using partial least-squares and structural equation modeling have been applied to characterize and compare specific functional networks in IBS patients and healthy controls. Compared with healthy controls, IBS patients show upregulated emotional arousal circuitry and altered serotonergic modulation of this circuitry seems to play a role in centrally mediated visceral hypersensitivity in female IBS patients.8 Additionally, the importance of inhibitory corticolimbic circuitry responsible for effective descending pain inhibition has been demonstrated in IBS patients and health controls.9,10 Furthermore, network analyses indicate that gender-related differences in brain response of IBS patients are largely due to alterations in the effective connectivity of emotional arousal rather than visceral afferent processing circuits.4,11 More work is needed to define the role of these central networks in FGIDs and to explore equally relevant networks involved in attention and memory.
Resting State fMRI
In contrast with the majority of fMRI studies, which examine task-related brain activity, resting state fMRI is now being used to evaluate task-independent, spontaneous brain activity. In these studies, the subject lies quietly, usually with the eyes closed, while a 5- to 10-minute functional scan is performed. Independent components analysis or seed-based functional connectivity analyses are commonly used to allow the identification of spontaneous, low-frequency BOLD signal fluctuations in correlated brain regions, which can then be attributed to discrete, functionally connective brain networks. Early skepticism that this technique may be unduly influenced by physiologic noise unrelated to neural processes (such as respiration or heartbeat), has been overcome by the large number of studies showing consistent intrinsic brain networks. A striking example of this is seen in work by Biswal et al, which shows consistent networks across 35 centers world-wide, including pooled data for >1400 subjects.12
Although it is now accepted that reliable intrinsic brain networks exist, the significance of such networks remains an area of active investigation. It has been proposed that the intrinsic brain networks may represent endophenotypes associated with disease vulnerability. Others suggest that the presence of a chronic disease may change intrinsic brain networks over time and that this change may normalize with adequate treatment. Alterations of intrinsic brain networks in disease states has been described in neuropsychiatric disease13 and to a lesser extent in pain conditions,14 and therefore has considerable promise for FGIDs characterized by pain and comorbid mood disorders. On a practical level, this technique provides the advantages of being simple for the subjects and easier to reproduce across centers, but with the drawback that analysis and interpretation of the results are currently in evolution without clear standardization of the optimal methods of analysis.15
Positron Emission Tomography
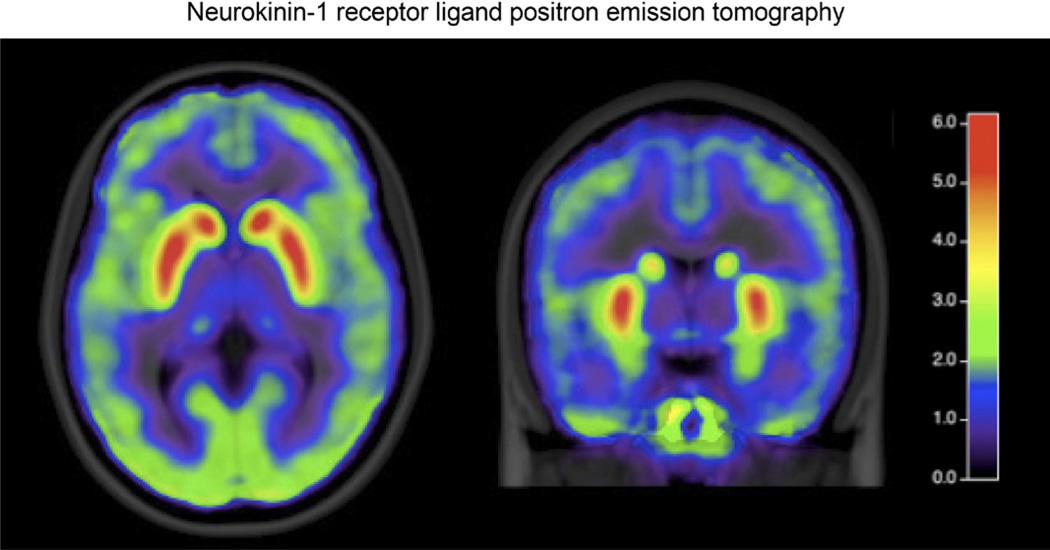
Utilization of positron emission tomography (PET) imaging has decreased owing to the ready availability, noninvasive nature, and better temporal resolution of fMRI. However, despite its drawbacks, PET continues to have some advantages. The ability to use radiolabeled ligands to probe specific receptor systems continues to make PET imaging an attractive tool. Two aspects of PET ligand research are of particular interest. The first is the study of receptor activity during tasks and to assess differences between patients and healthy subjects. In the study of pain and placebo, these techniques have been used to describe the endogenous opioid and dopamine reward systems in healthy controls.16,17 Less work has been done in FGIDs, although early studies have targeted receptors in dopamine and neurokinin-1 receptor systems (Figure 2).18 The second important use of PET in FGIDs is the study of labeled pharmaceuticals to determine distribution of binding as well as to identify patient subgroups with potentially differential benefit from a given compound. The use of PET has been limited by cost, the difficulty in generating relevant ligands, and the radiation exposure, which limits the number of studies that can be performed on an individual. Despite the shortcomings, PET will play a crucial role in drug development for agents targeting the brain– gut axis.
Figure 2.
The distribution of [18f]SPA-RQ binding to neurokinin-1 receptors in IBS patients is shown. (Image courtesy of Johanna Jarcho.)
Structural Imaging
Cortical Thickness and Gray Matter Density
High-resolution structural MRI (sMRI) can be used to produce structural imaging datasets comprised of global (whole-brain), regional, and voxel-level indices of local concentrations of gray and white matter as well as cortical thickness (neuronal density in the cortical mantle). Gray and white matter density and cortical thickness can be statistically analyzed by applying the general linear model then performing between-group or longitudinal within-group comparisons. sMRI datasets provide the means to assess baseline differences between groups and the central nervous system effects of treatments (novel drugs, cognitive behavioral therapy, hypnosis, meditation) targeted at improving the symptom burden on FGID patients.
Increasingly, sMRI is being used to uncover disease or gene-related characteristics. Studies of depression and chronic pain, as well as IBS, have shown disease-related differences in gray matter.19–22 What is unknown in most cases is whether these changes are preexisting risk factors for disease or whether they are secondary changes. Also, the biological substrate underlying these gray matter changes are unknown at the moment; they may involve changes in glial number or volume, changes in dendritic spines or synapses or less likely, neural degeneration. Contrary to the notion that anatomic structures of the brain are fixed in adulthood, dynamic alterations in brain structure have been observed in as few as 5 days. Research utilizing sMRI has demonstrated replicable changes in gray and white matter structure under environmental demands including learning, aging, and illness.23 Early life stressors experienced by patients with persistent pain disorders such as IBS may even impact brain structures early in development, potentially creating a vulnerability for further brain changes, induced and maintained by conditioned fear of gastrointestinal sensations, symptoms, and the context in which these visceral sensations and symptoms occur.
Research indicates that IBS is associated with decreased gray matter density in widespread areas of the brain, including the medial prefrontal and ventrolateral prefrontal cortex, posterior parietal cortex, ventral striatum, and thalamus. IBS patients also have greater gray matter density in the pregenual anterior cingulate and orbitofrontal cortex. Controlling for anxiety and depression, several of the regions involved in affective processing no longer differed between patients with IBS and controls, whereas the differences in prefrontal and posterior parietal cortices remained.21 This combination of overlapping and disease-specific structural findings is consistent with the close relationship of IBS with mood disorders.
Diffusion Tensor Imaging
Another mode of investigating changes in brain structure is diffusion tensor imaging (DTI). This technique allows the evaluation of white matter integrity and anatomy. As our approach to neuroimaging relies increasingly on functional networks, the connectivity between gray matter regions via white matter tracts is of great interest. One mode of white matter imaging is the assessment of tract integrity, most commonly expressed as fractional anisotropy (FA). This technique assesses the diffusivity of water. Water molecules that are unconstrained by cellular architecture, such as in the cerebrospinal fluid, are isotropic and thus have a FA value of 0. Because they are constrained to move in the direction of axons, water molecules in dense, parallel white matter tracts have high FA values. Decreases in the FA of white matter tracts can occur because of decreased axonal number, myelin integrity, or axonal cytoskeleton integrity. Because multiple factors can impact white matter, examination of patient groups should be performed in subjects who are otherwise healthy, have no significant history of neurologic illness or injury, and are matched for age. In a pilot study, differences in FA between IBS and healthy control subjects have been reported, with IBS subjects showing differential FA in the brainstem (corticospinal tract), regions of the corpus callosum, and frontal tracts.24
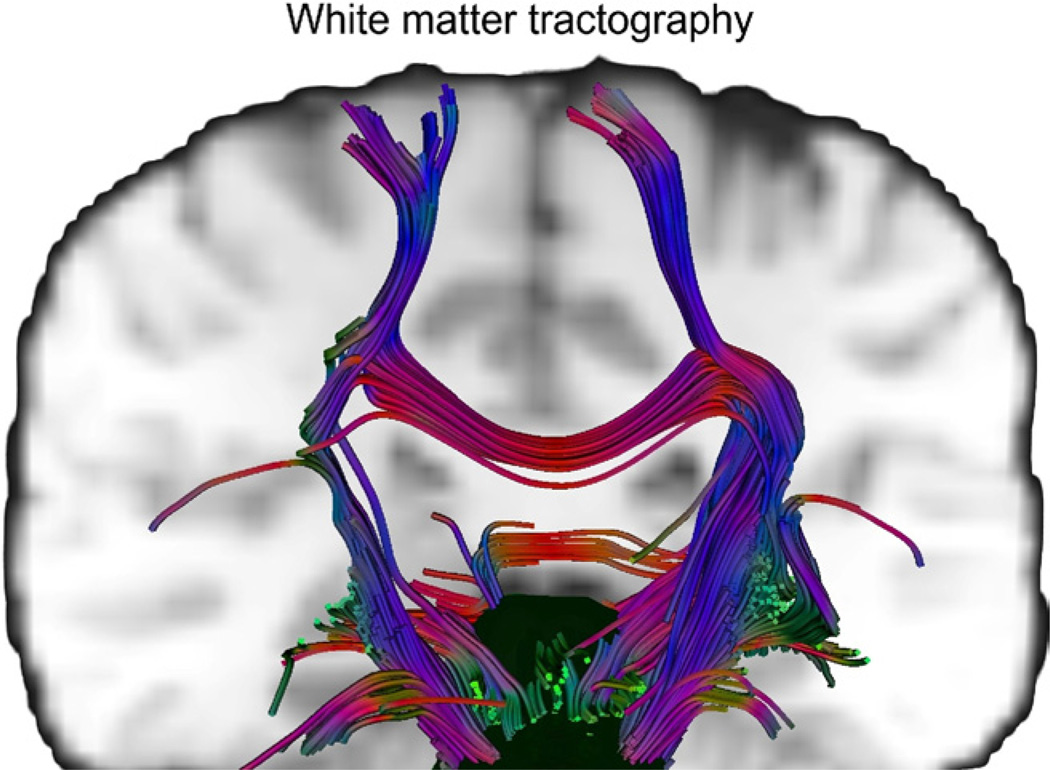
Another DTI method—quantitative fiber tracking or probabilistic tractography—allows identification of specific fiber tracts between brain regions, and can be useful for confirming anatomic connectivity between regions determined to be functionally connected based on fMRI studies. An example of white matter tracts originating in the brainstem is shown in Figure 3. Although fMRI results are often interpreted in the context of neuroanatomic studies in primates, the use of tractography can assess the anatomic connectivity of functionally determined regions within individual subjects or groups. One such study established connectivity between regions associated with response to visceral distension in healthy volunteers.25 Although white matter tracts have not yet been shown to be as plastic as gray matter, DTI studies in stroke patients do show dynamic remodeling of white matter tracts, suggesting that longitudinal studies in FGIDs before and after therapeutic intervention could be of interest.
Figure 3.
Representative brain stem white matter projections to the cortex from a healthy subject are shown. Horizontal paths are shown in red, anterior–posterior paths are in green, and vertical tracts are blue.
Moving Neuroimaging of the Brain–Gut Axis Into the Future
Rapid advances in imaging technologies and statistical analyses have continued to broaden the inferences that can be made from neuroimaging studies by increasing the scope of hypotheses from fairly simple tests of specific regional activation toward evaluation of complex structural and functional interactions in the brain– gut axis, which can advance our understanding of FGID pathophysiology. As the field of neuroimaging marches onward, emerging technological innovation in the field has initiated a paradigm shift from analyzing data from single imaging modalities toward a multimodal approach. Multimodal imaging is based on combining data from different structural (DTI, sMRI) or functional modalities (connectivity, resting state fMRI, receptor ligands), improving knowledge of structure–function relationships. Further, combination of fMRI with electroencephalography to obtain high temporal and spatial resolution will allow more accurate characterization of functional and intrinsic brain networks. The successful integration of these technologies requires increasing attention to issues such as study design, patient characterization, gender differences, and analytic technique. In addition, to reduce the expense and burden of executing fMRI studies while increasing the statistical power, the practice of large scale multisite collaborations and shared imaging data sets is becoming widespread in human neuroimaging.26–28 The incorporation of these practices is essential for examination of topics of particular clinical interest in FGIDs, such as treatment-related longitudinal neural changes, gene– brain interactions, and definition of patient subgroups.
Acknowledgments
Funding
Grant Support: K23 DK073451 (KT); K08 DK 071626 (JSL).
Footnotes
Supplementary Material
Note: The first 5 references associated with this article are available below in print. The remaining references accompanying this article are available online only with the electronic version of the article. To access the remaining references, as well as additional online-only data, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi:10.1053/j.gastro.2010.12.014.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Drossman DA, Corazziari E, Delvaux M, et al. ROME III: the functional gastrointestinal disorders. Degnon Associates. 2006 [Google Scholar]
- 2.Bradesi S, Mayer EA. Experimental models of stress and pain: do they help to develop new therapies? Dig Dis. 2009;27(Suppl 1):55–67. doi: 10.1159/000268122. [DOI] [PubMed] [Google Scholar]
- 3.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. Aug 7; doi: 10.1053/j.gastro.2010.07.053. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
References
- 6.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 7.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Labus JSME, Jarcho J, Kilpatrick L, et al. Acute tryptophan depletion affects brain-gut responses in irritable bowel syndrome patients and controls. Gut. 2010 doi: 10.1136/gut.2004.041657. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Craggs JG, Price DD, Verne GN, et al. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2010;38:720–729. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labus JS, Naliboff BD, Berman SM, et al. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage. 2009;47:952–960. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 14.Baliki MN, Geha PY, Apkarian AV, et al. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott DJ, Heitzeg MM, Koeppe RA, et al. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarcho JMMM, Ebrat B, Smith SR, et al. Reduced neurokinin-1 (substance P) receptor binding in patients with irritable bowel syndrome: A positron emission tomography study with [18f]SPA-RQ. Gastroenterology. 2010;138:s-372. [Google Scholar]
- 19.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 20.Blankstein U, Chen J, Diamant NE, et al. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 21.Seminowicz DA, Labus JS, Bueller JA, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 139:48–57. e2. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taubert M, Draganski B, Anwander A, et al. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labus JSVE, Jarcho JM, Tillisch K, et al. Reduced structural connectivity between amygdala and prefrontal cortex in patients with irritable bowel syndrome: A diffuse tensor imaging study. Gastroenterology. 2010;138:S-118. [Google Scholar]
- 25.Moisset X, Bouhassira D, Denis D, et al. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur J Pain. 2010;14:142–148. doi: 10.1016/j.ejpain.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Dinov I, Lozev K, Petrosyan P, et al. Neuroimaging study designs, computational analyses and data provenance using the LONI pipeline. PLoS One. 2010:5. doi: 10.1371/journal.pone.0013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]