Abstract
Class B scavenger receptors (SR-B3) are lipoprotein receptors which also mediate pathogen recognition, phagocytosis and clearance as well as pathogen-induced signaling. In this study we report that three members of the SR-B family namely, CLA-1, CLA-2 and CD36, mediate recognition of bacteria not only through interaction with cell wall lipopolysaccharide (LPS) but also with cytosolic chaperonin 60. HeLa cells stably transfected with any of these SR-Bs demonstrated markedly (3-5-fold) increased binding and endocytosis of E. coli, LPS and chaperonin 60 (GroEL) as revealed by both FACS analysis and confocal microscopy imaging. Increased pathogen (E. coli, LPS and GroEL) binding to SR-Bs was also associated with the dose-dependent stimulation of cytokine secretion in the order of CD36>CLA-2>CLA-1 in HEK293 cells. Pathogen-induced IL-6-secretion was reduced in macrophages from CD36- and SR-BI/II-null mice by 40-50% and 30-40%, respectively. Intravenous GroEL administration increased plasma IL-6 and CXCL1 levels in mice. The cytokine responses were 40-60% lower in CD36−/− relative to WT mice, whereas, increased cytokine responses were found in SR-BI/II−/− mice. While investigating the discrepancy of in vitro vs. in vivo data in SR-BI/II-deficiency, SR-BI/II−/− mice were found to respond to GroEL administration without increases in either plasma corticosterone or aldosterone as normally seen in WT mice. SR-BI/II−/− mice with mineralocorticoid replacement demonstrated a ~40-50% reduction in CXCL1 and IL-6 responses. These results demonstrate that, by recognizing and mediating inflammatory signaling of both bacterial cell wall LPS and cytosolic GroEL, all three SR-B family members play important roles in innate immunity and host defense.
Keywords: class B scavenger receptors, LPS, GroEL, bacteria, pro-inflammatory cytokines, signaling
Introduction
The class B scavenger receptor family is a group of integral membrane proteins which includes scavenger receptor class B type I (SR-BI), its splicing variant SR-BII (respectively, CLA-1 and CLA-2 in humans), thrombospondin receptor CD36 and lysosomal integral membrane protein II (LIMP-II). Apart from LIMP-II which is not found at the cell surface, these receptors have been demonstrated to interact with multiple ligands including those involved with atherosclerosis (1-5), inflammation (6, 7), host defense (8-10), angiogenesis (11), viral antigen presentation (12) and removal of apoptotic cells (13, 14).
SR-BI/BII and CD36 have many common features including high structural homology and localization in plasma membrane caveolae-like domains which facilitate lipid exchange and cell signaling (15). These receptors also share ligands, including native and modified lipoproteins, negatively charged phospholipids, various bacteria and viruses (12-14, 16, 17). SR-BI/BII and CD36 play a critical role in lipid metabolism where SR-BI/II mediates both selective HDL-cholesteryl ester uptake and HDL-mediated cholesterol efflux (18, 19). The SR-BII isoform also mediates holopartical uptake and internalization of HDL (20). CD36 is a well-known lipoprotein receptor and a long chain fatty acid (LCFA) transporter (21). CD36 also binds oxidized low-density lipoprotein (oxLDL) facilitating foam cell formation and development of atherosclerosis (1, 2, 22, 23). However, the role of these receptors in pathogen recognition remains to be elucidated.
The organ distribution of various SR-B receptors is different, with SR-BI and SR-BII being predominantly expressed in the liver and steroidogenic tissues, whereas CD36 expression is primarily in adipose tissue and heart (21, 24). Importantly, both SR-BI/II and CD36 are abundantly expressed in phagocytic cells such as mature monocytes, macrophages, dendritic cells and neutrophils; cells significantly involved with inflammation as well as innate and adaptive immune responses to infection.
SR-B receptors mediate binding (adhesion), internalization and phagocytosis of various bacteria (8, 9, 25, 26), in part due to their ability to interact with bacterial cell wall lipids such as lipopolysaccharides (LPS) and lipoteichoic acid (LTA) (9, 26, 27). While the involvement of CD36 in signal transduction induced by various bacteria and LPS has been demonstrated by several groups (9, 25-27), the role of LPS binding to SR-BI/II in signal transduction has been only hypothesized (10, 28) and requires further assessment. In addition to bacterial surface LTA and LPS, bacterial and eukaryotic cytosolic proteins such as chaperonin 60 (cpn 60/hsp60) have been reported as potent activators of the macrophage cytokine response (29-32). Cpn60 proteins are highly conserved cytosolic oligomers, facilitating protein folding in prokaryotic and eukaryotic cells. Under steady state, cpn60 is highly expressed with its levels being greatly increased in response to physical or chemical stress (33, 34). Importantly, cpn60 could be released due to loss of bacterial cell wall integrity upon antibiotic treatment or immunologic response.
Cpn60 from E. coli (29, 30), M. tuberculosis (31) and Chlamydia trachomatis (32) have been shown to induce pro-inflammatory cytokine production, alter the expression of cellular adhesion molecules, modulate signal transduction pathways involving ERK and p38 kinases, and induce transcription factors such as NF-kB (32, 35, 36). Moreover, the GroEL homologue Cpn60.1 of M. tuberculosis has been recently reported to be required for bacterium-induced pro-inflammatory responses in M. tuberculosis infected animals (37). Cpn/hsp60 serves as a chemoattractant for neutrophils as well as a phagocytosis enhancer of opsonized E. .coli particles (38), which could also help to activate innate and adaptive immune responses. Despite the important role of cell wall LPS and LTA as well as cytosolic cpn60 in innate immune responses, the role of scavenger receptors, especially SR-BI/II and CD36, in cpn60-induced inflammation has not yet been investigated. Recent studies have been primarily focused on the role of TLR2, TLR4 and CD14, which were initially characterized as receptors for microbial LTA (39-41) and LPS (42-44). These data suggest that the pro-inflammatory effects of cpn60/hsp60 could be the result of TLRs activation (35, 45-48). It has been also demonstrated that TLRs are not involved with hsp60 binding to mouse macrophages (49-52) and receptor-mediated signaling via TLR2 and TLR4 (53-55). Since Class B scavenger receptors are widely expressed in reticuloendothelial cells such as macrophages, dendritic and endothelial cells (56), they potentially play important roles in bacterial recognition by mediating binding, internalization and intracellular signaling of surface LPS, LTA and cytosolic cpn60.
We hypothesized that bacteria, LPS and GroEL could be the potent ligands for SR-B contributing to the overall response toward bacterial pathogens, where SR-B serve as pattern recognizing receptors complementary to the TLR system. The aim of our study was to assess the role of class B scavenger receptors in pathogen recognition and pro-inflammatory signaling induced by bacteria such as E. coli as well as by its major outer membrane component LPS and cytosolic protein - GroEL. Our findings are the first to determine that bacterial cpn60, GroEL, and LPS, could potentially contribute to bacteria-induced inflammation in vitro and vivo, acting via class B scavenger receptors.
Materials and Methods
Reagents
All media, sera, reactive fluorescent dyes and antibiotics were obtained from Invitrogen (Carlsbad, CA). Recombinant mouse macrophage colony-stimulating factor (M-CSF), Fludrocortisone acetate, LPS from Salmonella enterica (serotype Minnesota) and recombinant cpn60 (GroEL) obtained from an overproducing E. coli strain were purchased from Sigma (St. Louis, MO). Synthetic amphipathic peptides were synthesized by a solid-phase procedure (57, 58). Peptide sequences of these peptides were reported previously (28).
Animals
The National Institutes of Health (NIH) criteria for laboratory animal care were used in this study. C57BL/6 mice (24-30 weeks old) were purchased from NCI-DCT (Frederick, MD, USA). Two pairs of CD36 knockout mice were kindly provided by Dr. Moore's laboratory (Lipid Metabolism Unit, Harvard Medical School, Boston, MA) and grown into a colony at the NIH animal facility. SR-BI/II-deficient mice (strain B6; 129S2-Scarb1 tm1Kri/J) were obtained from Jackson Laboratories (Bar Harbor, ME) and a colony was established at the NIH.
Cell culture
HeLa (Tet-off) cells (Clontech, Pal Alto, CA) stably expressing CLA-1, CLA-2 and CD36 were generated, selected and cultured as previously reported (52, 53). Human embryonic (epithelial) kidney cells (HEK293) were obtained from ATCC and were stably transfected to express CLA-1 and CLA-2 (CLA-1-HEK293 and CLA-2-HEK293). The HEK293 cell line was also stably transfected with CD36 pIRES-hrGFP-2a plasmid (Stratagene, La Jolla, CA) followed by cell selection according to the highest green fluorescent protein expression. HeLa and HEK293 SR-B-expressing clones with similar expression levels of each SR-B (assessed by RT-PCR, see Suppl. Fig. 1A) were selected and used in this study. Murine SR-BI/II−/−, CD36−/− and wild type (WT) macrophages were isolated from mouse bone marrow cells (BMC) obtained from SRBI/II−/−, CD36−/− mice and control wild type strain, respectively. The macrophages were differentiated by culturing in RPMI-1640 supplemented with 10% fetal calf serum (FCS), in the presence of 10 ng/ml of mouse M-CSF and 10 ng/ml of mouse IL-4 for 7-10 days.
Subcellular localization of SR-Bs in CLA-1, CLA-2 and CD36-overexpressing HeLa cells visualized by confocal microscopy
The SR-Bs’ subcellular localization was analyzed by indirect immunofluorescence staining using specific SR-B antibodies and fluorescently conjugated secondary antibodies. The specific markers used for cellular compartment staining were Alexa Fluor® 594 wheat germ agglutinin (WGA) – for plasma membrane (PM) and LysoTrackerRed DND-99 – for lysosomes. Briefly, the cells were grown on glass slides (Nalge Nunc, Rochester, NY) until 20-50% confluence, subjected to a 30-min staining with fluorescently labeled organelle markers (PM or lysosomal), washed and fixed in 4% paraformaldehyde in PBS. Fixed cells were permeabilized with Triton X-100 (0.05% in PBS) for 15 min at room temperature and incubated in blocking buffer (5% BSA/PBS/0.1% Tween/5% goat serum). The SR-Bs‘ localization was visualized after incubations with CLA-1 or CLA-2 specific custom antibodies or antiCD36 mAb (JC63.1, Abcam) and secondary Alexa Fluor 488-labeled antibodies (Invitrogen).
Confocal microscopy of Alexa 488 GroEL binding and internalization in SR-B-overexpressing HeLa cells
The cells were grown on glass coverslips until 20-50% confluence and incubated with Alexa Fluor 488 GroEL (10 μg/ml/30 min) in DMEM, containing 2 mg/ml BSA and 20 mM HEPES. Cells were washed with PBS, and then either stained with a PM marker red-fluorescent Alexa Fluor® 594 WGA or chased for 1 hour in DMEM/BSA/HEPES medium and then stained with LysoTrackerRed DND-99 (50 nM/20 min, Molecular Probes); nuclei were stained with Hoechst 33342 (1 μg/ml). Cells were washed with PBS and imaged using a confocal microscope. To assess subcellular localization, images were obtained with a Zeiss 510 confocal system (Zeiss, Jena, Germany). Images were acquired sequentially by using a 488-nm laser line and emission between 505 and 580 nm for Alexa Fluor 488, a 561-nm laser line and emission between 575–615 nm for Alexa 568 and blue-fluorescent Hoechst 33342 dye (excitation/emission maxima when bound to DNA ~350/461 nm). High-resolution (100 nm per pixel) images were obtained throughout the cells with a 63x, 1.4-numerical-aperture Plan-Apochromat oil-immersion objective under conditions avoiding bleed-through.
Fluor-labeled ligand uptake
Lipoproteins, bovine serum albumin (BSA), cpn60 and E. coli were conjugated with Alexa Fluor® 488, using a protein labeling kit (Invitrogen) following the manufacturer's instructions. S. minnesota Re 595 LPS was labeled with a BODIPY FL, SE labeling kit from Molecular Probes (Eugene, OR) following the manufacturer's suggested procedure with previously reported modifications (19). HeLa cells were incubated with fluoro-labeled ligands in serum-free DMEM containing 2 mg/ml BSA and 10mM HEPES at 37°C for 1 h. The cells were then washed extensively with phosphate-buffered saline (PBS), detached with Cellstripper dissociation solution (Mediatech, Herndon, VA), fixed with 4% paraformaldehyde, and analyzed by a fluorescence-activated cell sorter (FACS, model A; Hitachi).
Dose-dependent Alexa 488 GroEL uptake and competition studies
WT, CLA-1-, CLA-2- and CD36-overexpressing HeLa cells grown in 24-well plates were incubated with increasing concentrations (0-50μg/ml) of Alexa 488 GroEL at 37°C for 2 h in the presence (non-specific uptake) or absence (total uptake) of 200 μg/ml GroEL. All incubations were performed in DMEM containing 2 mg/ml BSA and 20 mM HEPES. Specific uptake was determined as the difference between total and nonspecific uptake, and normalized for protein content. For competition studies CLA-1-, CLA-2- and CD36-overexpressing HeLa cells grown in 24-well plates were incubated with 10 μg/ml of Alexa 488 GroEL without or with increasing concentrations of unlabeled ligands at 37°C for 2 h. Cells were then washed 3 times with PBS and detached from the plate surface by incubation in 200 μl of Cellstripper solution for 30 min at room temperature with continuous rocking. Aliquots of cell suspensions were transferred into 96-well black microplates and were read using a fluorescence plate reader (Wallac Victor 1420 Multilabel Counter, Perkin Elmer, Boston, MA).
RNA Isolation and Reverse Transcription-PCR
The total RNA was isolated from wild type and SR-B-overexpressing clones of HeLa and HEK293 cells or from differentiated mouse BMC using the TRIzol reagent (Invitrogen) according to the manufacturer's recommended protocol. RNA samples were reverse-transcribed by Moloney murine leukemia virus reverse transcriptase (Superscript Reverse Transcription; Invitrogen) and oligo(dT)15 primers (Promega, Madison, WI). 5′-Fluorescein-labeled primers were obtained from Operon Biotechnologies (Huntsville, AL). cDNA was amplified using System 2400 DNA thermal cycler (PerkinElmer Life Sciences) with 25, 30 or 35 cycles for GAPDH, SRBs, and the TLRs’ primers, respectively, and annealing temperatures 50°C (for GAPDH, SRBs) and 55°C (for TLRs). Sequences for sense/antisense primer pairs were as follows: GADPH – 5- GTCTTCACCACCATGGAGAAG, 5’-GCTTCACCACCTTCTTGATGTCATC; CLA-1-CLA-2 – 5 -GAAATCTGTCGCAGGCATTGGAC, 5 - AGCTGGGAGGCTCAGGCTGTG; CD36 - 5 - GCGACATGATTAATGGTACAGATGC, 5 - GAT GGC TTG ACC AAT AGG TTG AC; TLR2 - 5’-GAGCGAGCTGGGTAAAGTAGAAA, 5’-AGCCGAGGCAAGAACAAAGA; TLR4 - 5’- CAGTGGGTCAAGGAACAGAAGC, TLR7 - 5- GGGGTCCAAAGCCAATGTGT, 5’- CGAGGGCAATTTCCACTTAGG; TLR9 - 5’- ACAGTATCGTCTCTGTGGTC, 5’-CAGAGATGGTGCAGTATAGG (59). The CLA-1-CLA-2 pair of primers was designed to generate 2 separate products for CLA-1 (480 bp) and CLA-2 (352 bp). PCR products were separated by PAGE using 6% TBE gels (Invitrogen), and the intensity of the fluorescent signal for each band was detected using a Storm 860 PhosphorImager (GE Healthcare).
Analysis of cytokine and corticosteroid production
The IL-8 secretion by HEK293 cells was analyzed in culture supernatants after a 20-h incubation in serum-free medium with or without BSA (2 mg/ml) utilizing a commercial enzyme-linked immunosorbent assay kit for human IL-8 (Invitrogen). Cytokine levels in culture supernatants of the murine macrophages and sera were measured using ELISA kits for mouse IL-6 (Invitrogen) and mouse CXCL1/KC (R&D System) following the manufacturer's instructions. Serum levels of corticosterone, cortisol and aldosterone were quantified with corresponding ELISA kits from Stressgen (Ann Arbor, MI). All samples and standards were measured in duplicate.
Limulus Amebocyte Lysate Assay (LAL)
The endotoxin activity of recombinant GroEL was determined by an automated Limulus amebocyte lysate assay (Kinetic-QCL, Lonza, Walkersville, MD) according to the manufacturer's recommendations.
Statistical analyses
All data are expressed as mean values ± standard deviation (SD). Differences between groups were analyzed by the nonparametric Mann-Whitney U-test with a P-value of <0.05 considered as significant.
Results
Co-localization of SR-Bs with a PM marker in HeLa cells overexpressing CLA-1, CLA-2 and CD36
The assess subcellular localization of SR-Bs in HeLa cells overexpressing each of these receptors, we used the fluorescent confocal imaging after co-labeling cells with an Alexa Fluor 594-conjugated PM marker and corresponding anti-SR-B antibodies. The results show considerable overlap of CLA-1 and to a lesser extent of CD36 with a PM marker at the cell surface. CLA-2 was predominantly visible intracellularly with minor localization on the cell surface (Suppl. Fig. 2). The CLA-1 and CLA-2 subcellular localizations are in a good agreement with the previously published data of Eckhardt et al. (20), demonstrating that the SR-BI isoform was primarily expressed at the cell surface, whereas 80-90% of the SR-BII protein was expressed intracellularly. With CD36, however, PM staining was observed along with the staining of various intracellular structures throughout the cells. Our observations that CD36 resides on the cell surface and to some extent within the cells are also consistent with the previously reported findings of Eyre et al. (60) indicating that CD36 could be found associated with both the PM and intracellularly located lipid rafts.
Uptake of Alexa 488-labeled bacterial ligands is increased in HeLa cells stably overexpressing class B scavenger receptors
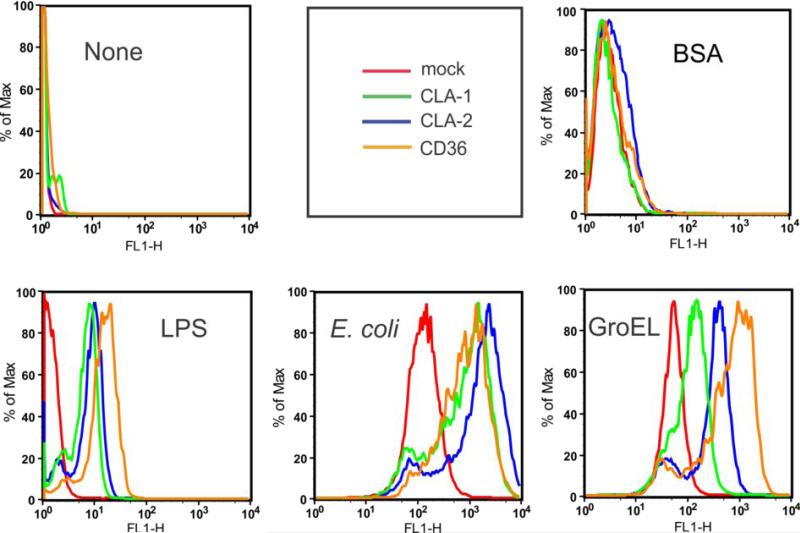
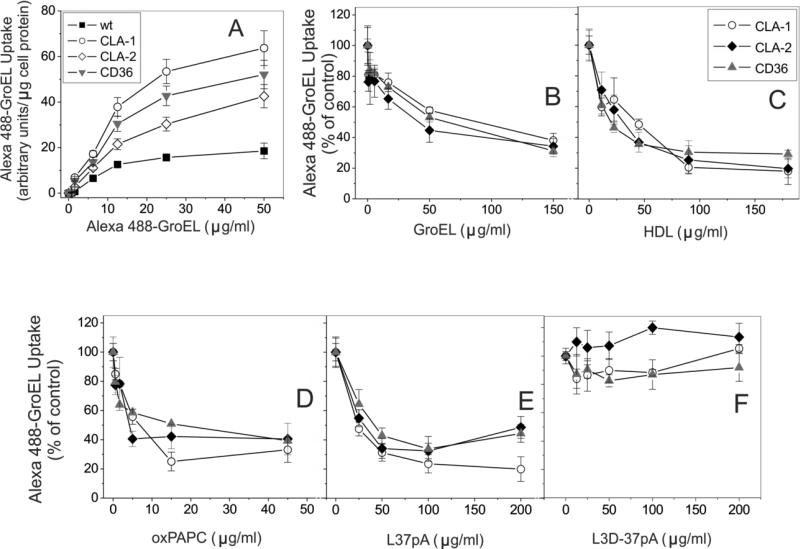
Previously we and others demonstrated that SR-BI/BII and CD36 function as endocytic receptors mediating intracellular bacterial and LPS uptake (8, 9, 25). We first evaluated the ability of SR-Bs to bind bacteria and LPS. Using FACS analyses, we demonstrated a several-fold increased uptake of fluoro-labeled E. coli (K12) bacteria and LPS by stably transfected HeLa cells expressing all three class B scavenger receptors (Fig. 1). Based on the presence of multiple amphipathic helical domains within cpn60 and the known recognition of these motifs by SR-B, we predicted that GroEL might be another candidate ligand for class B scavenger receptors. Similar to E. coli and LPS, GroEL uptake was increased in SR-B overexpressing HeLa cells. No appreciable differences were observed in Alexa 488-BSA uptake (used as negative control) between SR-B-overexpressing and control cells. In addition to the FACS data, further evidence for the affinity of SR-B receptors towards GroEL was obtained by comparing to mock transfected cells and the utilization of an alternative technique for quantifying fluoro-GroEL uptake (via use of a Victor multilabel counter, see Materials and Methods). As shown in Fig. 2A, all 3 types of SR-B-overexpressing cells demonstrated markedly (3-5-fold) increased dose-dependent specific uptake of the fluorescently labeled GroEL vs. mock-transfected control cells.
FIGURE 1.
Uptake of fluorescently labeled bacterial pathogens in mock-transfected, CLA-1-, CLA-2- and CD36 - overexpressing HeLa cells. Cells were incubated with Alexa 488-labeled K12 E. coli (2 ng/ml), 10 μg/ml ALexa 488 GroEL or 10 μg/ml of Bodipy-labeled LPS for 1h at 37°C. Cell-associated fluorescence was analyzed by FACS technique. The plots are representative of at least two separate experiments that yielded similar results.
FIGURE 2.
Dose-dependent uptake of Alexa Fluor 488 GroEL in mock-transfected and SR-B-expressing HeLa cells (A). Cells were incubated with the indicated concentrations of Alexa Fluor 488 GroEL for 2h at 37°C without (total uptake) or in the presence of 200μg/ml of unlabeled GroEL (nonspecific uptake). B-F. Competition of SR-B ligands with Alexa Fluor 488 GroEL uptake in CLA-1-, CLA-2- and CD36-overexpressing cells. Cells were incubated with 7.5 μg/ml Alexa Fluor 488 GroEL or with or without the indicated concentrations of unlabeled competitors for 2 h at 37°C. Unlabeled GroEL was used as a control. Cell-associated fluorescence was estimated using a fluorescence plate reader (see Material and Methods). The inhibition curves are expressed as percent of maximal Alexa Fluor 488 GroEL uptake in cells incubated in the absence of unlabeled competitor. The data (mean ± S.D.) represent one of three separate experiments that yielded similar results and were performed in triplicates.
Scavenger receptor class B agonists efficiently compete with Alexa Fluor 488 GroEL for uptake in CLA-1-, CLA-2- and CD36-expressing HeLa cells
Since we and others previously demonstrated SR-Bs’ role in LPS and bacteria uptake (8, 9, 25), in this study we focused on the analysis of a novel SR-B ligand, GroEL. As seen from Fig. 2B, unlabeled GroEL inhibited Alexa Fluor 488 GroEL uptake in a dose-dependent manner. HDL, oxPAPC and L-37pA peptide also potently blocked the uptake of labeled GroEL by as much as 60-80% (Figs. 2C, D, and E). In contrast, the L3D-37pA peptide, previously shown by the FACS assay as poor ligand for SR-B (25, 61), didn't compete with Alexa Fluor 488 GroEL uptake (Fig. 2F). Our experiments did not reveal a statistically significant inhibition of GroEL uptake by LPS in SR-B-overexpressing cells, indicating separate LPS and GroEL binding sites within the extracellular loop of SR-Bs (data not shown).
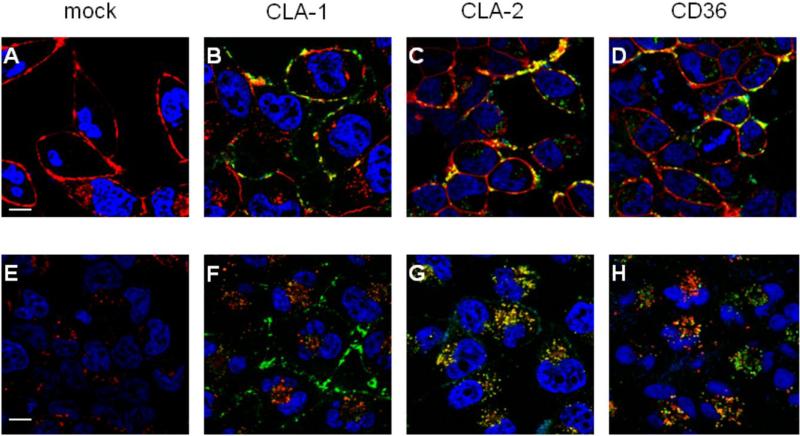
Intracellular trafficking of Alexa Fluor 488 GroEL in mock transfected and SR-B-overexpressing Hela cells
The role of each of the three SR-Bs in GroEL binding and internalization was additionally assessed via confocal microscopy by analyzing Alexa 488 GroEL co-localization with Alexa 594-conjugated organelle markers. As seen in Fig. 3 (B-D) following a 30 min incubation, Alexa 488 GroEL could be clearly observed on the cell surface of all three SR-B-overexpressing cell types, co-localizing with the PM marker. Next we analyzed Alexa 488 GroEL distribution after a 1-hour chase period, allowing for internalization and intracellular transport of bound Alexa 488 GroEL within the cells. As shown in Fig. 3F in CLA-1-overexpressing cells only a minor co-localization of Alexa 488 GroEL with the lysosomal intracellular tracker could be detected, with the major portion of the bound ligand still remaining on the cell surface. These results correlate with our (28) and others (20) data demonstrating CLA-1 as a receptor with a predominantly cell surface localization. In contrast, in CLA-2-overexpressing cells all of the Alexa 488 GroEL appeared to be internalized, co-localizing with Lysotracker (Fig. 3G), additionally indicating the important role of CLA-2 as a GroEL endocytic receptor. Analysis of the Alexa 488 GroEL distribution in CD36-overexpressing cells revealed partial co-localization of internalized ligand (Fig. 3H) and Lysotracker, possibly suggesting more than one CD36-dependent intracelluar trafficking pathway for GroEL. No visible GroEL-associated fluorescence was observed in mock transfected cells (Fig. 3, panels A and E).
FIGURE 3.
GroEL trafficking in mock-transfected, CLA-1, CLA-2 and CD36-overexpressing HeLa cells by confocal fluorescent microscopy. Following a 30-min pulse with Alexa Fluor 488 GroEL (10 μg/ml), cells were stained either immediately with the PM marker Alexa Fluor® 594 WGA (A-D) or following a 1 hr chase in Alexa Fluor488 GroEL-free medium (E-H) were stained with the lysosomal marker LysoTrackerRed DND-99. The cell type is specified at the top. Merged images are shown, where arrows indicate co-localization (yellow) of AlexaFluor 488 GroEL (green) with the corresponding (PM or lysosomal) marker (red). Nuclei (blue) were stained with Hoechst 33342. The scale bars correspond to 5μm.
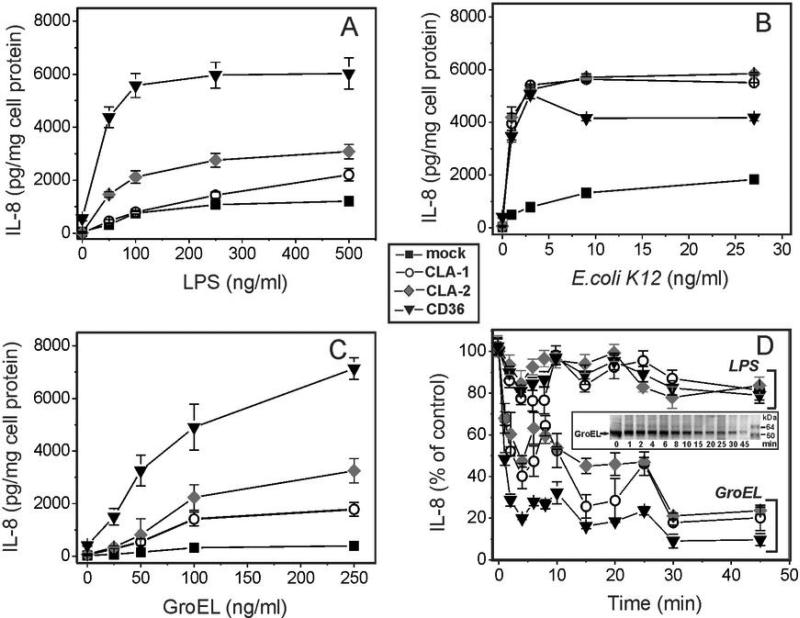
HEK293 cells overexpressing class B scavenger receptors demonstrate increased IL-8 secretion upon treatment with bacteria, LPS and GroEL
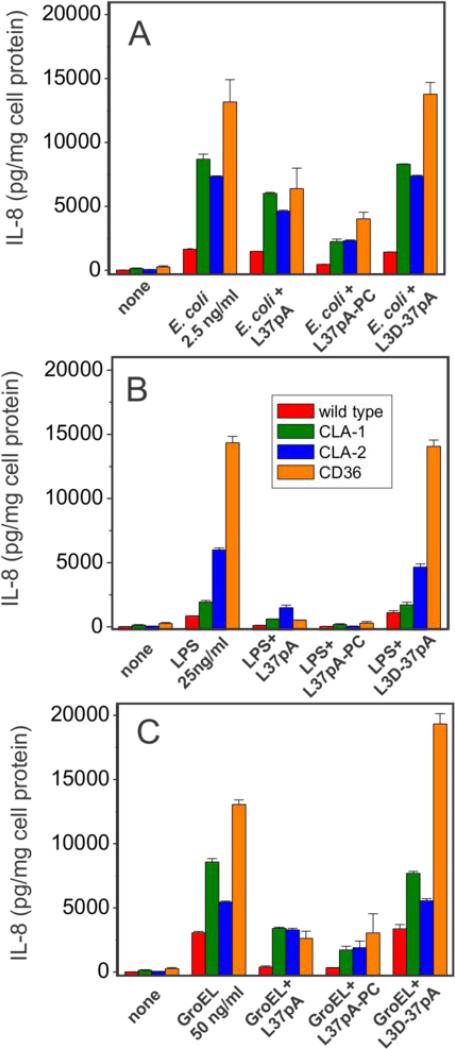
Several earlier studies provided experimental evidence for an important role of the CD36 receptor in pro-inflammatory signaling induced by various bacteria and bacterial wall-derived compounds, such as LTA and LPS (9, 25-27). The role of SR-BI and SR-BII as potential mediators of bacteria/LPS-initiated cytokine release, however, remains contradictory. In this study we observed a moderate 2-3-fold increase of IL-8 secretion in both CLA-1- and CLA-2-overexpressing vs. wild type HEK293 cells following a 20-h cell treatment with LPS (Fig. 4A). A much greater, 6-8-fold increase of IL-8 secretion was observed when cells were treated with live E. coli K12 bacteria (Fig. 4B). At the same time CD36-overexpressing cells demonstrated markedly (6-8 times) higher levels of IL-8 release induced by either LPS or bacteria when compared to wild type cells.
FIGURE 4.
Dose-dependent IL-8 secretion induced by various bacterial pathogens in WT and SR-B-overexpressing HEK293 cells. Cells were incubated with increasing concentrations of LPS (A), E. coli (B) or GroEL (C) for 20 h and IL-8 levels were determined in the cell culture supernatants. D. Effect of heat treatment duration at 100°C on the cytokine-inducing activity of LPS and GroEL in SR-B-overexpressing HEK 293 cells. The IL-8 levels were determined in cell culture supernatants after 20 h of incubation of the cells with either intact or heat treated (for the indicated time intervals) LPS or GroEL. Data represent one of three separate experiments that yielded similar results.
Bacterial cpn60 has been previously reported as a potent pro-inflammatory cytokine-inducing protein in both phagocytic and endothelial cells (29-32). In this study, we also compared GroEL effects upon IL-8 secretion in WT HEK 293 cells vs. those stably transfected with CLA-1, CLA-2 or CD36. The results presented in Fig. 4C demonstrate a marked increase in IL-8 secretion induced by bacterial cpn60 in CLA-II- (~5-6-fold) and CD36-(~7-8-fold) expressing cells and a less pronounced (2-3-fold) elevation of cytokine levels in CLA-1-overexpressing cells over those detected in WT cells. The data for the most part indicate that proinflammatory activity of LPS and GroEL can be described as CD36>CLA-2>CLA-1.
Importantly, the amphipathic helical peptide, L-37pA, a known SR-B ligand (25, 28, 62, 63), efficiently and in a dose-dependent manner blocked IL-8 release induced by E. coli (A), LPS (B) and GroEL (C) in CLA-1-, CLA-2- and CD36-expressing HEK293 cells (Fig. 5). The peptide L3D-37pA, containing three D-amino acid substitutions that are known to disturb the amphipathic α-helical motif, did not affect pathogen-induced IL-8 secretion and was used as a negative control.
FIGURE 5.
Effects of amphipathic α-helical peptides on IL-8 secretion induced by bacterial pathogens in wild type and SR-B-overexpressing HEK 293 cells. Cells were pre-incubated with or without 10 μg/ml of L-37pA, L-37pA-PC or L3D-37pA for 45 min prior to a 20-h treatment with 2 ng/ml of K12 E. coli (A), 50 ng/ml of LPS (B) or 100 ng/ml of GroEL (C) and IL-8 levels were determined in the cell culture supernatants. Data are from one of at least two separate representative experiments that were performed in duplicate.
Heat treatment significantly reduces the cytokine-inducing SR-B-dependent activity of GroEL
We have checked the potential contamination of recombinant GroEL with LPS that might contribute to the observed GroEL-induced cytokine release, using the LAL assay. The endotoxin content of GroEL was in the range 2-6 EU/μg protein (0.02-0.06%). Considering that various cells were exposed to GroEL in the range of concentrations 50-500 ng/ml, the maximal concentration of endotoxin (≤ 0.3ng/ml) potentially present in our GroEL preparations appears to be too low to induce measurable levels of cytokine release in either cell types used in this study. To further dissociate the pro-inflammatory activity of bacterial cell wall LPS and cytosolic protein GroEL, the effects of heat treatment on the cytokine-inducing activity of GroEL as well as of LPS were analyzed using HEK293 cells expressing 3 types of SR-B receptors. Heat-induced denaturation for the indicated time intervals (from 1 to 45 min) at 100°C resulted in a rapid (starting after 2 min of heating) and significant reduction (by ~ 70-80%) of the IL8-inducing activity of GroEL (Fig. 4D). In contrast, a much weaker and delayed effect of boiling upon the cytokine-inducing activity of the LPS was observed. Thus, we believe that GroEL-induced cytokine secretion observed in our study is associated mostly with the protein activity rather than LPS contamination.
Pro-inflammatory cytokine release induced by bacterial stimuli is markedly reduced in macrophages from both SR-BI/II−/− and CD36−/− mice
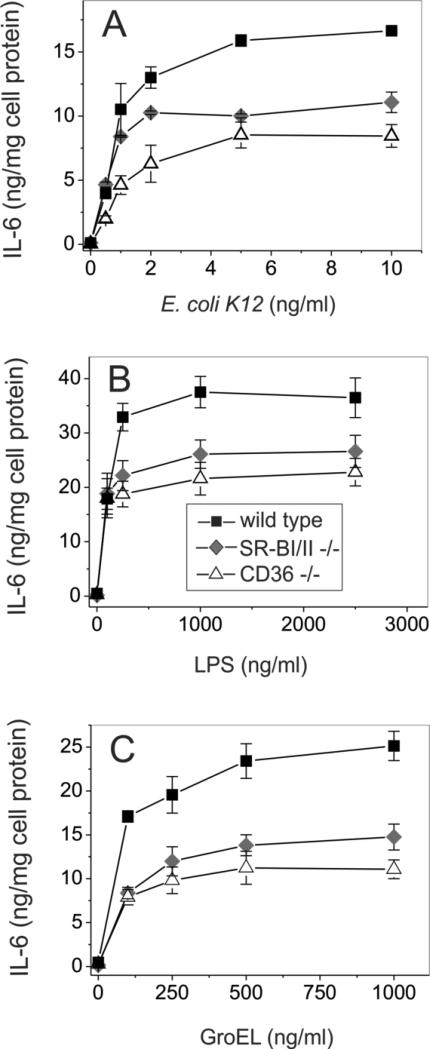
The role of SR-B in mediating pro-inflammatory bacteria-, LPS- and GroEL-induced signaling was further tested using cultured bone marrow-derived macrophages from WT, SR-BI/II−/− and CD36−/− mice. Since macrophages possess various pathogen-recognizing receptors such as TLRs, LOX, class A and class B scavenger receptors, the relative impact of SR-B was analyzed in bone marrow cells treated with murine MCSF for 5 days. Following a 20-h cell treatment with increasing concentrations of GroEL, LPS and bacteria, the levels of IL-6 secretion in normal, SR-BI/II−/− and CD36−/− macrophages were determined. Genetically modified SR-BI/II−/− and CD36−/− macrophages demonstrated ~45% and ~50% reductions, respectively, of IL-6 secretion upon GroEL stimulation when compared to macrophages from WT mice (Fig. 6A). A similar extent of cytokine production impairment was observed in the absence of functional SR-BI/II or CD36 receptors upon cell stimulation with LPS and E. coli K12, indicating that these two receptors could account for up to 50% of total IL-6 secretion in phagocytes (Figs. 6B and 6C).
FIGURE 6.
Dose-dependent LPS-, E. coli- and GroEL-induced secretion of IL-6 in wild type, SR-BI/II−/− and CD36−/− mice macrophages. Cells were treated with the indicated doses of various inflammatory stimuli for 20 h, and cytokine levels were quantified in duplicate samples of cell culture supernatants. The data shown are from one of three separate experiments that yielded similar results.
In order to rule out possible alteration of TLRs expression in macrophage cells derived from genetically modified mice, specifically SR-BI/II−/− and CD36−/−, vs. wild type cells, we performed a comparative expression assessment of 4 TLRs, known to be primarily involved in recognition of various pathogen-derived ligands, including LPS and GroEL (43-45, 48), using reverse transcription-PCR analyses. As shown in Suppl. Fig. 1B, macrophages originating from WT, SR-BI/II−/− and CD36−/− mice demonstrated similar levels of mRNA expression for all four TLRs being analyzed in our assay.
Corticosteroid hormone responses induced by GroEL in the absence of SR-BI/BII or CD36 scavenger receptors in vivo
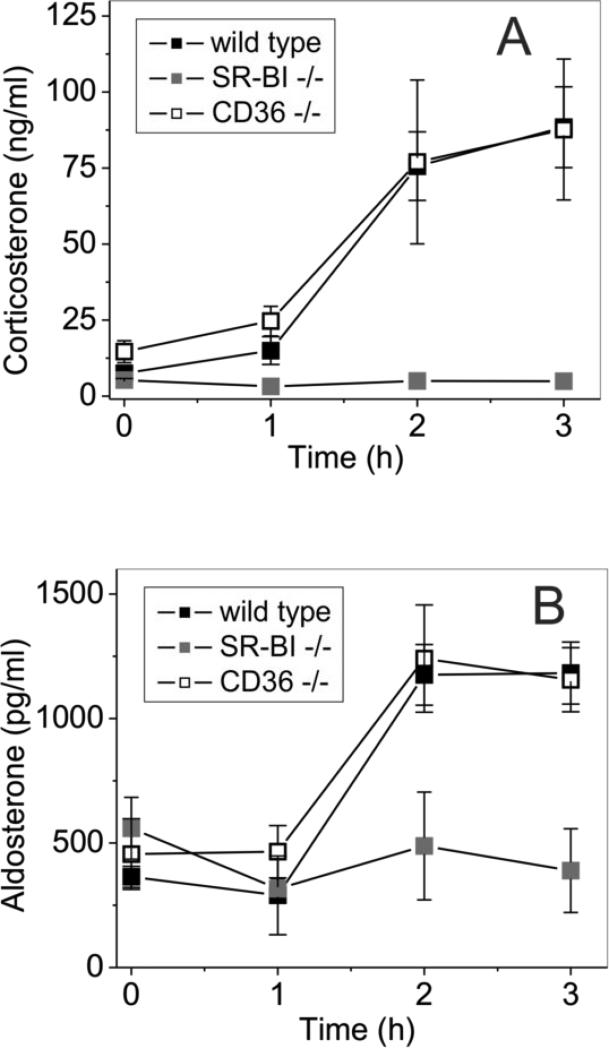
Corticosteroids are known to play an important role in inflammatory responses and sodium reabsorption, regulating blood pressure and reducing inflammation-induced hypotension. SR-BI-dependent uptake of cholesterol into adrenals is critical for depositing cholesterol for immediate adrenal steroid hormone synthesis associated with acute inflammation or stress (63). It has been demonstrated that SR-BI/II-knockout mice do not respond to acute inflammation by producing increasing amounts of corticosterone (7), the principal glucocorticoid of mice and rats (64) which has some mineralocorticoid activity. This relative adrenal insufficiency has been associated with an exaggerated inflammatory response induced by LPS administration or by sepsis (7, 65). However, corticosteroid replacement was insufficient to rescue mice from the inflammatory hyperreactivity in both cases. Since the absence of SR-BI/II could lead to aldosterone deficiency associated with poor sodium reabsorption and reduced blood pressure, we evaluated the levels of corticosteroids including corticosterone, aldosterone and cortisone in SR-BI/II-deficient mice after GroEL administration. As demonstrated in Fig. 7A, plasma levels of corticosterone (a common precursor for both cortisol and aldosterone biosynthesis) in control and CD36-deficient mice showed similar (5-10-fold) increases in response to a GroEL injection. In SR-BI/II-deficient mice, however, the serum corticosterone levels remained practically unaltered with GroEL administration. Likewise, the mineralocorticoid hormone aldosterone levels were increased (~3-fold) in GroEL-injected normal and CD36-deficient mice but were unchanged in SR-BI/II-deficient mice (Fig. 7B). In contrast, plasma levels of the glucocorticoid hormone cortisol remained practically unchanged in both normal and CD36-deficient GroEL-challenged mice, but they appeared to be even slightly elevated over the basal level in SR-BI/II-deficient mice following GroEL injection (data not shown). These results clearly indicate the impairment of the adrenal steroid production in SR-BI/II-deficient mice during GroEL-induced acute inflammation.
FIGURE 7.
GroEL-induced corticosteroid hormone responses in WT, SR-BI/II-null and CD36-null mice. Wild type, SR-BI/II−/− and CD36−/− mice (n=6 per group) were injected with E. coli cpn 60 (20 μg /mouse, i.v.) or PBS as a negative control (not shown). Blood was drawn at 1, 2 and 3 h after injection, sera samples were prepared and analyzed for corticosterone (A) and aldosterone (B) levels by ELISA. The results are shown as means ± S.D.
Pro-inflammatory cytokine responses induced by GroEL in the absence of SR-BI/BII or CD36 scavenger receptors in vivo
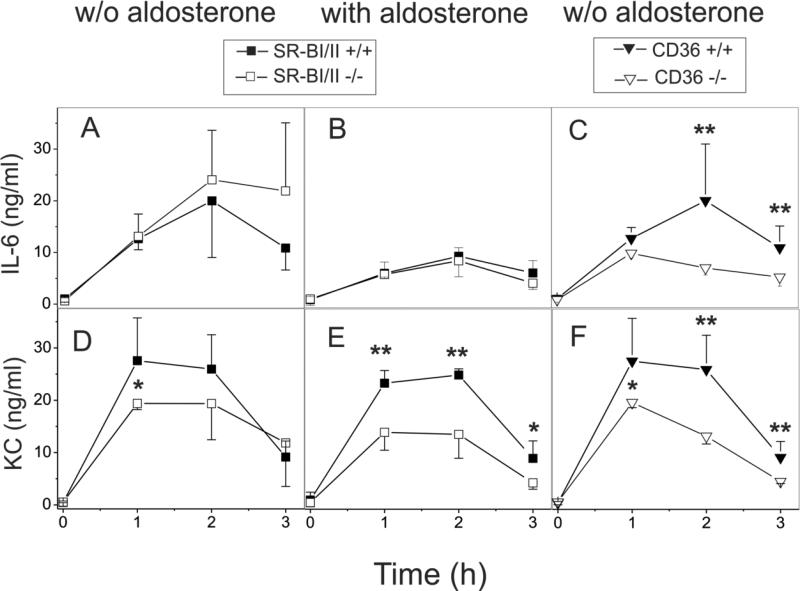
In order to determine the influence of SR-BI/II and CD36 scavenger receptors on GroEL-induced inflammation in vivo, WT, SRBI/II−/− and CD36−/− mice were injected i.v. with GroEL (20 μg per mouse) and serum levels of IL-6 and the chemokine CXCL1 (KC) were determined by ELISA at various times following injection. As shown in Fig. 8, GroEL injection resulted in a strong induction of both IL-6 and KC secretion that peaked after 1 h in CD36-deficient mice and in 2 h in normal and SRBI/II-deficient mice. CD36−/− mice demonstrated ~50% and ~55% reduction of IL-6 and KC serum levels, respectively, when compared to normal mice (Fig. 8C and 8F). The IL-6 concentrations were slightly (1.3 times) higher, while the KC levels were slightly lower, though not statistically significant, in SR-BI/II-deficient vs. WT mice (Fig. 8A and 8D). When fludrocortisone acetate (a corticosteroid with moderate glucocorticoid and much greater mineralocorticoid activity) was provided through drinking water for 24 h prior to GroEL injection, the levels of both cytokines, IL-6 and KC, were markedly reduced in SR-BI/II–deficient mice compared to untreated SR-BI/II–deficient mice. The peak plasma levels of IL-6 measured in SR-BI/II-deficient mice treated with fludrocortisone acetate were similar to those in normal mice (Fig. 8B), whereas a 30-55% reduction was observed in KC concentrations of SR-BI/II−/− mice vs. WT mice (Fig. 8E). A reduced cytokine response in CD36-deficient mice compared to control mice was evident at each time point tested, while no cytokine response was observed in mice injected with PBS alone (data not shown). These results are in agreement with the findings of several other studies demonstrating an important role for CD36 as a mediator of the pro-inflammatory signaling induced by various pathogenic agents, including LPS, LTA and bacteria (9, 25-27). In contrast, SR-BI/II-deficient mice were shown to be more sensitive to LPS-induced septic shock (7, 64) and CLP-induced sepsis (65). Previous studies demonstrated that corticosterone replacement alone was insufficient to completely correct an abnormal inflammatory state due to adrenal insufficiency and to protect SR-BI/II-knockout mice against septic death (65). In our experiments mineralocorticoid supplementation alone rescued SR-BI/II-deficient animals from the GroEL-induced hyperinflammatory response.
FIGURE 8.
GroEL-induced pro-inflammatory responses in normal, SR-BI/II- and CD36-deficient mice. A, C, D, F. Levels of IL-6 (A, C) and KC (D, F) in the same serum samples obtained as described in the legend for Fig. 7 were analyzed by ELISA for IL-6 (A-C) and KC (D-E) levels by ELISA. B, E. Experiments were conducted as described above, but after wild type (n=7) and SR-BI/II-deficient mice (n=9) were given drinking water supplemented with fludrocortisone acetate (2×10−5M) for 24 h prior to GroEL injection. The results are shown as the mean ± S.D.,* - p< 0.05, ** - p< 0.01 vs. wild type.
Discussion
Increasingly there has been a greater interest in class B scavenger receptors, primarily known as key mediators in lipoprotein metabolism, due to their newly discovered role as pattern recognition receptors involved with innate immune responses to bacteria, viruses and other pathogens (9, 12, 25, 66, 67). Class B scavenger receptors - CLA-1 (a human orthologue of rodent SR-BI), its splicing homolog CLA-2/SR-BII and CD36 have been recently demonstrated to mediate uptake and endocytosis of a broad range of pathogens including gram-positive and gram-negative bacteria (8, 9, 26, 66). We and others have previously demonstrated that uptake of gram-negative bacteria through CLA-1/2 is mediated in part through an interaction of bacterial cell wall LPS with these receptors (66). LPS is a well-known inducer of endotoxic shock and inflammation and is a potent ligand for CLA-1 expressed in HeLa cells (10). Bacterial recognition and innate immune responses could also be induced by intracellular proteins which are released during bacterial death due to antibiotic treatment and/or complement-dependent bacterial targeting. Several studies have demonstrated bacterial cpn60 as a potent stimulator of inflammatory cytokine synthesis in both human and murine myeloid cells (29-32). By analogy with the LPS, bacterial cpn60 has been reported to activate TLRs such as TLR4 and/or TLR2 (35, 44, 45). Since other unknown receptors potentially bind cpn60 (49, 52), we envisioned that GroEL, known to contain amphipathic helices, a structural motif recognized by class B scavenger receptors, could be a ligand for CLA-1/2 and CD36. Moreover, our earlier data showed that GroEL- as well as LPS- and LTA-induced inflammatory effects in phagocytes could be blocked by SR-B antagonists such as the peptide L-37pA (61). However, until this report, only CD36 has been identified as the single class B receptor mediating pro-inflammatory responses to LTA and LPS, functioning either as a TLR co-receptor or as an independent signaling molecule (6, 9, 25, 27, 68). No direct data have been reported regarding the role of CLA-1 and CLA-2 in bacterial recognition and inflammatory signaling.
In this article, the uptake studies are the first to demonstrate a markedly increased uptake of E. coli, LPS and GroEL in HeLa cells due to overexpression of CLA-1, CLA-2- and CD36 receptors. Further analyses of GroEL uptake demonstrated that SR-B ligands, including various lipoproteins and amphipathic helical peptides, efficiently compete for Alexa 488 GroEL uptake in all 3 types of SR-B-overexpressing cells. Similar to other studies, the role of SR-B proteins in bacteria-, LPS- and GroEL-induced pro-inflammatory signaling was analyzed in HEK293 cells, characterized by low inflammatory interleukin secretion due to low or absent expression of TLRs (25, 59, 69). These cells demonstrated greatly elevated IL-8 secretion in response to a challenge with E. coli, LPS and GroEL upon transfection with SR-B expressing plasmids. While responses to E. coli bacteria were comparable between HEK293 cells stably expressing CLA-1, CLA-2 and CD36, IL-8 secretion was increased in the order CD36>CLA-2>CLA-1> when cells were treated with LPS or GroEL. Importantly, our results demonstrate that SR-B-dependent inflammation induced by the bacterial pathogens could be inhibited by SR-B antagonists such as the amphipathic helical peptide L-37pA, and even more efficiently (by 90-95%) when used in association with phosphatidylcholine.
Since bacterial recognition could be also associated with the binding of bacterial cytosolic proteins to SR-B, GroEL-induced pro-inflammatory responses were further analyzed using murine bone marrow derived macrophages. Since cultured phagocytes express various pattern recognizing receptors including the Fc receptors, TLRs, COX, SR-A and SR-B, it was important to establish the contribution of SR-B deficiency on the overall phagocyte response toward bacteria and their products. Our findings indicate that the pro-inflammatory effects of bacteria, LPS or GroEL were reduced by 30-40% and 40-60% in SR-BI/II- and CD36-deficient cells, respectively. These data suggest that in cultured macrophages bacteria-induced inflammation is partially CD36- and SR-BI/II-dependent, with other receptors such as TLR2 and TLR4 also contributing to cytokine secretion induced by bacterial cpn60 or LPS. The macrophage response in vitro is known to be critically dependent on cultivation conditions. Likely reflecting cultivation conditions, our results, in contrast to Guo et al. (65), demonstrate that SR-BI/II receptors potently contribute to bacteria-mediated inflammatory responses in cultured BMC-derived phagocytes (Fig. 5). To distinguish between culture conditions and pathophysiologically important responses, we further investigated pathogen-induced inflammation in vivo. Because the effects of both LPS and bacteria have been previously investigated, we studied the effect of bacterial GroEL on SR-B-mediated inflammation. The results of in vivo experiments revealed that the pro-inflammatory cytokine/chemokine responses induced by bacterial cpn60 were 30-50% lower in CD36-deficient mice than in control animals, fully consistent with the in vitro observations indicating a pro-inflammatory role of CD36. At the same time, our in vivo data was quite similar to Cai et al. (7), demonstrating no difference or mildly increased plasma cytokine levels in SR-BI/II-null mice (Fig. 8A). However, there was an inconsistency between our in vivo and vitro results observed in SR-BI/II-deficient macrophages. In our opinion, this discrepancy could be easily explained considering earlier reported results revealing that adrenal glucocorticoid deficiency occurring in SR-BI/II-deficient mice could greatly affect immune cell response to LPS or other pro-inflammatory stimuli in vivo. As demonstrated previously by Cai et al. (7), SR-BI-deficient mice show an abnormally high inflammatory cytokine response to LPS. However, without appropriately compensating for adrenal corticosteroid deficiency, the true role of SR-BI during LPS-induced inflammation might be misinterpreted. In view of the well-recognized relative adrenal insufficiency and abnormal stress-induced glucocorticoid/corticosteroid response in SR-BI/II-null mice, as well as the known clinical inefficiency of glucocorticoid therapy (70) we investigated the GroEL-induced mineralocorticoid response in SR-BI/II−/− vs. WT mice (Fig. 7). In contrast to responsive WT mice, a GroEL challenge was associated with no aldosterone release in SR-BI/II-null mice (Fig. 7B). Consequently, the effect of mineralocorticoid replacement given prior to cpn60 injection was evaluated. The synthetic fludrocortisone reduced IL-6 plasma levels in both normal and SR-BI/II−/− mice treated with GroEL, whereas CXCL1/KC levels were reduced (to 40-50% of normal mice levels) only in SR-BI/II-deficient mice. Previous studies demonstrated that administration of the exogenous corticosterone, although improving the survival rate of SR-BI−/− mice during LPS-induced endotoxic shock (7), failed to completely reverse mortality of the CLP-treated SR-BI-deficient mice (65), indicating that glucocorticoid therapy alone is insufficient to correct the aberrant inflammatory response. Additionally, clinical trials have demonstrated that combined hydrocortisone/fludrocortisone treatment markedly reduced the risk of death in patients with septic shock and relative adrenal insufficiency (70). Our findings demonstrate that supplementation with the mineralocorticoid fludrocortisone could significantly normalize the acute inflammation response in mice with SR-BI/II-deficiency. These results provide experimental evidence that mineralocorticoid replacement therapy, known to regulate blood pressure, may improve the outcomes of clinical sepsis in patients with complete or partial adrenal insufficiency.
In conclusion, our study indicates that SR-B receptors play an important role in bacterial recognition and mediate bacteria-associated inflammation and signaling. In addition, SR-B receptors function as TLR-independent mediators of bacteria-induced signaling triggered by the bacterial wall compound LPS, as well as by cytosolic proteins such as bacterial cpn60 – GroEL.
Supplementary Material
Acknowledgements
We thank Dr. B. L. Vaisman and Dr. A. T. Remaley (NHLBI, National Institutes of Health) for the development, maintenance, and genotyping of SRBI/II-null and CD36-null mouse colonies used in this study. We also thank Dr. J. Philip McCoy, Jr., Director of the Flow Cytometry Core, (NHLBI, National Institutes of Health) for conducting cell sorting.
Footnotes
This research was supported by the Intramural Research Program of the Clinical Center, National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations used in this paper: SR-B, class B scavenger receptors; DMEM, Dulbecco's Modified Eagle's medium; LPS, lipopolysaccharide; LTA, lipoteichoic acid; cpn60, chaperonin 60; PC, phosphatidylcholine; HDL, high-density lipoprotein; oxLDL, oxidized low-density lipoprotein; oxPAPC, oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine; TLRs, Toll-like receptors; WT, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 2.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, Picard MH, Vasile E, Zhu Y, Raffai RL, Weisgraber KH, Krieger M. Diet-induced occlusive coronary atherosclerosis, myocardial infarction, cardiac dysfunction, and premature death in scavenger receptor class B type I-deficient, hypomorphic apolipoprotein ER61 mice. Circulation. 2005;111:3457–3464. doi: 10.1161/CIRCULATIONAHA.104.523563. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Yancey PG, Su YR, Babaev VR, Zhang Y, Fazio S, Linton MF. Inactivation of macrophage scavenger receptor class B type I promotes atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2003;108:2258–2263. doi: 10.1161/01.CIR.0000093189.97429.9D. [DOI] [PubMed] [Google Scholar]
- 5.Van Eck M, Bos IS, Hildebrand RB, Van Rij BT, Van Berkel TJ. Dual role for scavenger receptor class B, type I on bone marrow-derived cells in atherosclerotic lesion development. Am. J. Pathol. 2004;165:785–794. doi: 10.1016/S0002-9440(10)63341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunnol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai L, Ji A, de Beer FC, Tannock LR, van der Westhuyzen DR. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J. Clin. Invest. 2008;118:364–375. doi: 10.1172/JCI31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- 9.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennesy EJ, Ezekowitz RAB, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic omain. J. Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vishnyakova TG, Bocharov AV, Baranova IN, Chen Z, Remaley AT, Csako G, Eggerman TL, Patterson AP. Binding and internalization of lipopolysaccharide by Cla-1, a human orthologue of rodent scavenger receptor B1. J. Biol. Chem. 2003;278:22771–22780. doi: 10.1074/jbc.M211032200. [DOI] [PubMed] [Google Scholar]
- 11.Dawson DW, Pearce SFA, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the inhibitory effects of thrombospondin-1 on endothelial cells. J. Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barth H, Schnober EK, Neumann-Haefelin C, Thumann C, Zeisel MB, Diepolder HM, Hu Z, Liang TJ, Blum HE, Thimme R, Lambotin M, Baumert TF. Scavenger Receptor Class B Is Required for Hepatitis C Virus Uptake and Cross-Presentation by Human Dendritic Cells. J. Virol. 2008;82:3466–3479. doi: 10.1128/JVI.02478-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bird DA, Gillotte KL, Hörkkö S, Friedman P, Dennis EA, Witztum JL, Steinberg D. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells. Proc. Natl. Acad. Sci USA. 1999;96:6347–6352. doi: 10.1073/pnas.96.11.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J. Exp. Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel H, Murray H,F, Insel PA. Caveolae as Organizers of Pharmacologically Relevant Signal Transduction Molecules. Annu. Rev. Pharmacol. Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J. Biol. Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 17.Murao K, Terpstra V, Green SR, Kondratenko N, Steinberg D, Quehenberger O. Characterization of CLA-1, a human homologue of rodent scavenger receptor BI, as a receptor for high density lipoprotein and apoptotic thymocytes. J. Biol. Chem. 1997;272:17551–17557. doi: 10.1074/jbc.272.28.17551. [DOI] [PubMed] [Google Scholar]
- 18.Webb NR, Connell PM, Graf GA, Smart EJ, de Villiers WJ, de Beer FC, van der Westhuyzen DR. SR-BII, an isoform of the scavenger receptor BI containing an alternate cytoplasmic tail, mediates lipid transfer between high density lipoprotein and cells. J. Biol. Chem. 1998;273:15241–15248. doi: 10.1074/jbc.273.24.15241. [DOI] [PubMed] [Google Scholar]
- 19.Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 20.Eckhardt ERM, Cai L, Sun B, Webb NR, van der Westhuyzen DR. High Density Lipoprotein Uptake by Scavenger Receptor SR-BII. J. Biol. Chem. 2004;279:14372–14381. doi: 10.1074/jbc.M313793200. [DOI] [PubMed] [Google Scholar]
- 21.Abumrad NA, El-Maghrabi MR, Amri EZ, Lopez E, Grimaldi P. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 22.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II . Cardiovasc. Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverstein RL. Inflammation, atherosclerosis, and arterial thrombosis: Role of the scavenger receptor CD36. Cleve Clin. J. Med. 76 Suppl. 2009;2:S27–30. doi: 10.3949/ccjm.76.s2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Fitzsimmons RL, Cleland LG, Ey PL, Zannettino AC, Farmer EA, Sincock P, Mayrhofer G. CD36/fatty acid translocase in rats: distribution, isolation from hepatocytes, and comparison with the scavenger receptor SR-B1. Lab. Invest. 2003;83:317–332. doi: 10.1097/01.lab.0000059923.67198.ba. [DOI] [PubMed] [Google Scholar]
- 25.Baranova IN, Kurlander R, Bocharov AV, Vishnyakova TG, Chen Z, Remaley AT, Csako G, Patterson AP, Eggerman TL. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J. Immunol. 2008;181:7147–7156. doi: 10.4049/jimmunol.181.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 27.Triantafilou M, Gamper FG, Lepper PM, Mouratis MA, Schumann C, Harokopakis E, Schifferle RE, Hajishengallis G, Triantafilou K. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell Microbiol. 2007;9:2030–2039. doi: 10.1111/j.1462-5822.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 28.Bocharov AV, Baranova IN, Vishnyakova TG, Remaley AT, Csako G, Thomas F, Patterson AP, Eggerman TL. Targeting of scavenger receptor class B type I by synthetic amphipathic α-helical-containing peptides blocks lipopolysaccharide (LPS) uptake and LPS-induced pro-inflammatory cytokine responses in THP-1 monocyte cells. J. Biol. Chem. 2004;279:36072–36082. doi: 10.1074/jbc.M314264200. [DOI] [PubMed] [Google Scholar]
- 29.Galdiero M, de l'Ero GC, Marcatili A. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infect. Immun. 1997;65:699–707. doi: 10.1128/iai.65.2.699-707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabona P, Reddi K, Khan S, Nair SP, Crean SJ, Meghji S, Wilson M, Preuss M, Miller AD, Poole S, Carne S, Henderson B. Homogeneous Escherichia coli chaperonin 60 induces IL-1β and IL-6 gene expression in human monocytes by a mechanism independent of protein conformation. J. Immunol. 1998;161:1414–21. [PubMed] [Google Scholar]
- 31.Friedland JS, Shattock R, Remick DG, Griffin GE. Mycobacterial 65-kD heat shock protein induces release of proinflammatory cytokines from human monocytic cells. Clin. Exp. Immunol. 1993;91:58–62. doi: 10.1111/j.1365-2249.1993.tb03354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J. Clin. Invest. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner M, Hermanns I, Bittinger F, Kirkpatrick CJ. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am. J. Physiol. 1999;277:L1026–L1033. doi: 10.1152/ajplung.1999.277.5.L1026. [DOI] [PubMed] [Google Scholar]
- 34.Trautinger F, Kindås-Mügge I, Knobler RM, Hönigsmann H. Stress proteins in the cellular response to ultraviolet radiation. J. Photochem. Photobiol. B. 1996;35:141–148. doi: 10.1016/s1011-1344(96)07344-7. [DOI] [PubMed] [Google Scholar]
- 35.Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- 36.Bethke K, Staib F, Distler M, Schmitt U, Jonuleit H, Enk AH, Galle PR, Heike M. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J. Immunol. 2002;169:6141–6148. doi: 10.4049/jimmunol.169.11.6141. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Henderson B, Lund PA, Tormay P, Ahmed MT, Gurcha SS, Besra GS, Coates AR. A Mycobacterium tuberculosis mutant lacking the groEL homologue cpn60.1 Is viable but fails to induce an inflammatory response in animal models of infection. Infect. Immun. 2008;76:1535–1546. doi: 10.1128/IAI.01078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osterloh A, Geisinger F, Piédavent M, Fleischer B, Brattig N, Breloer M. Heat shock protein 60 (HSP60) stimulates neutrophil effector functions. J. Leukoc. Biol. 2009;86:423–434. doi: 10.1189/jlb.0109011. [DOI] [PubMed] [Google Scholar]
- 39.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and Lipoteichoic Acid-induced Cell Activation Is Mediated by Toll-like Receptor 2. J. Biol. Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of grampositive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2. J. Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 41.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 42.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431 –1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 43.Beutler B, Du X, Poltorak A. Identification of Toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies. J. Endotoxin Res. 2001;7:277–280. [PubMed] [Google Scholar]
- 44.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Häcker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway ininnate immune cells. J. Biol. Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 45.Ohashi K, Burkart V, Flohé S, Kolb H. Cutting Edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 46.Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ. Res. 2001;89:244–250. doi: 10.1161/hh1501.094184. [DOI] [PubMed] [Google Scholar]
- 47.Lewthwaite JC, Coates AR, Tormay P, Singh M, Mascagni P, Poole S, Roberts M, Sharp L, Henderson B. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (Hsp 65) and contains a CD14-binding domain. Infect. Immun. 2001;69:7349–7355. doi: 10.1128/IAI.69.12.7349-7355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takenaka R, Yokota K, Ayada K, Mizuno M, Zhao Y, Fujinami Y, Lin S-N, Toyokawa T, Okada H, Shiratori Y, Oguma K. Helicobacter pylori heat-shock protein 60 induces inflammatory responses through the Toll-like receptor-triggered pathway in cultured human gastric epithelial cells. Microbiology. 2004;150:3913–3922. doi: 10.1099/mic.0.27527-0. [DOI] [PubMed] [Google Scholar]
- 49.Habich C, Baumgart K, Kolb H, Burkart V. The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins. J. Immunol. 2002;168:569–576. doi: 10.4049/jimmunol.168.2.569. [DOI] [PubMed] [Google Scholar]
- 50.Habich C, Burkart V. Heat shock protein 60: regulatory role on innate immune cells. Cell. Mol. Life Sci. 2007;64:742–751. doi: 10.1007/s00018-007-6413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osterloh A, Veit A, Gessner A, Fleischer B, Breloer M. Hsp60-mediated T cell stimulation is independent of TLR4 and IL-12. Int. Immunol. 2008;20:433–443. doi: 10.1093/intimm/dxn003. [DOI] [PubMed] [Google Scholar]
- 52.Henderson B, Mesher J. The search for the chaperonin 60 receptors. Methods. 2007;43:223–228. doi: 10.1016/j.ymeth.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 54.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 55.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee HG, Wagner H, Schild H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the tolllike receptor 2/4 pathway. J. Biol. Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 56.Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182:1–15. doi: 10.1016/j.atherosclerosis.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 57.Merrifield RB. The synthesis of biologically active peptides and proteins. J. Am. Med. Assoc. 1969;210:1247–1254. [PubMed] [Google Scholar]
- 58.Fairwell T, Hospattankar AV, Brewer HB, Jr, Khan SA. Human plasma apolipoprotein C-II: total solid-phase synthesis and chemical and biological characterization. Proc. Natl. Acad. Sci. USA. 1987;84:4796–4800. doi: 10.1073/pnas.84.14.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurt-Jones EA, Sandor F, Ortiz Y, Bowen GN, Counter SL, Wang TC, Finberg RW. Use of murine embryonic fibroblasts to define Toll-like receptor activation and specificity. J. Endotoxin Res. 2004;10:419–424. doi: 10.1179/096805104225006516. [DOI] [PubMed] [Google Scholar]
- 60.Eyre NS, Cleland LG, Tandon NN, Mayrhofer G. Importance of the carboxyl terminus of FAT/CD36 for plasma membrane localization and function in long chain fatty acid uptake. J. Lipid Res. 2007;48:528–542. doi: 10.1194/jlr.M600255-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Baranova IN, Bocharov AV, Vishnyakova TG, Kurlander R, Chen Z, Fu D, Arias IM, Csako G, Patterson AP, Eggerman TL. CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAA-induced signaling in human and rodent cells. J. Biol. Chem. 2010;285:8492–8506. doi: 10.1074/jbc.M109.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams DL, de La Llera-Moya M, Thuahnai ST, Lund-Katz S, Connelly MA, Azhar S, Anantharamaiah GM, Phillips MC. Binding and cross-linking studies show that scavenger receptor BI interacts with multiple sites in apolipoprotein A-I and identify the class A amphipathic alpha-helix as a recognition motif. J. Biol. Chem. 2000;275:18897–18904. doi: 10.1074/jbc.M002411200. [DOI] [PubMed] [Google Scholar]
- 63.Hoekstra M, Ye D, Hildebrand RB, Zhao Y, Lammers B, Stitzinger M, Kuiper J, Van Berkel TJ, Van Eck M. Scavenger receptor class B type I-mediated uptake of serum cholesterol is essential for optimal adrenal glucocorticoid production. J. Lipid Res. 2009;50:1039–1046. doi: 10.1194/jlr.M800410-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bentley PJ. Endocrine pharmacology: physiological basis and therapeutic applications. Cambridge University Press; 1980. 1980. p. 154. [Google Scholar]
- 65.Guo L, Song Z, Li M, Wu Q, Wang D, Feng H, Bernard P, Daugherty A, Huang B, Li XA. Scavenger Receptor BI protects against septic death through its role in modulating inflammatory response. J. Biol. Chem. 2009;284:19826–19834. doi: 10.1074/jbc.M109.020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vishnyakova TG, Kurlander R, Bocharov AV, Baranova IN, Chen Z, Abu-Asab MS, Tsokos M, Malide D, Basso F, Remaley AT, Csako G, Eggerman TL, Patterson AP. CLA-1 and its splicing variant CLA-2 mediate bacterial adhesion and cytosolic bacterial invasion in mammalian cells. Proc. Natl. Acad. Sci. USA. 2006;45:16888–16893. doi: 10.1073/pnas.0602126103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J. Exp. Med. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Triantafilou M, Gamper FGJ, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 69.Gon Y, Asai Y, Hashimoto S, Mizumura K, Jibiki I, Machino T, Ra C, Horie T. A20 inhibits Toll-like receptor 2- and 4-mediated interleukin-8 synthesis in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2004;31:330–336. doi: 10.1165/rcmb.2003-0438OC. [DOI] [PubMed] [Google Scholar]
- 70.Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troché G, Chaumet-Riffaut P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.