Abstract
The aim of the present study was to investigate whether the aqueous extract of Monascus-fermented grains (MFGEs) enriched with ubiquinones (Coenzyme Qs, CoQ9+CoQ10) alleviates high-fructose (60%) plus high-fat (20%) diet (HFD)-induced hyperglycemia and hepatic oxidative stress in male Sprague–Dawley rats. Animals were fed HFD for 16 weeks and orally administered with MFGEs (300 mg/kg/day) or atorvastatin (20 mg/kg/day) for the last 4 weeks of the study. HFD-fed rats exhibited hyperglycemia, hyperinsulinemia, impaired glucose tolerance, and impaired insulin sensitivity. MFGE treatment prevented the increase in glucose levels and index of insulin resistance in the HFD-induced diabetic rats. A significant decrease in hepatic lipid peroxidation and significant increases in hepatic superoxide dismutase, catalase, and glutathione peroxidase were observed in the MFGE supplemented group. The results suggest that dietary supplementation with MFGEs enriched with CoQs exerts an antidiabetic effect in type 2 diabetic rats by improving insulin resistance and hepatic antioxidant enzymes.
Key Words: : antioxidant enzymes, atorvastatin, coenzyme Qs, hyperglycemia, insulin sensitivity, Monascus-fermented grains
Introduction
Diabetes is a global health crisis affecting approximately 285 million people worldwide, and it is expected that the diabetic population will increase to 438 million by 2030.1 Although a number of pharmacological and surgical treatments have been developed for treating diabetes,2,3 several lines of evidence strongly suggest that diet and/or lifestyle modifications are the most effective therapeutic strategies for preventing the development of type 2 diabetes.2
Consumption of fructose in the form of high-fructose corn syrup is continuously increasing in most countries.4 Recent findings support that the increased consumption of fructose may be an important contributor to the type 2 diabetes, typically resulting in hyperglycemia, insulin resistance, and abnormal lipid profiles.4–6
Monascus-fermented products have been used as a functional dietary supplement to decrease cholesterol levels in humans.7,8 The valuable secondary metabolite of the Monascus species, mevinolin (natural statin), has been proven as an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase.9,10 Recently, many statin-related studies in animals and humans have revealed decreases in systemic CoQ10 levels during statin administration.11,12 CoQ10 is a fat-soluble, vitamin-like benzoquinone compound that functions as an antioxidant, a membrane stabilizer, and a cofactor in the production of adenosine triphosphate (ATP) via oxidative phosphorylation.13 Statins inhibit the activity of HMG-CoA reductase, depressing the synthesis of not only cholesterol but also isoprenoids such as CoQ10 due to their common biosynthetic pathway.11 Such effects might cause a worsening of glycemic control and increased insulin resistance.14,15 For instance, inhibition of isoprenoid biosynthesis has been shown to cause insulin resistance, and a suppressed synthesis of ubiquinone may result in delayed ATP production in beta cells and, consequently, impair insulin release.14 These undesirable effects were alleviated by co-administering CoQ10 with statins.16,17 According to a report by Kettawan,16 oral administration of simvastatin suppresses the biosynthesis of CoQs, but these undesirable effects were alleviated by co-administering CoQ10 with simvastatin. However, statin therapy is considered critical in both the primary and secondary prevention of cardiovascular disease in diabetes.15 Very recently, we demonstrated that Monascus-fermented mixed grain extracts (MFGEs) enriched with bioactive mevinolins and Coenzyme Qs (CoQ9+CoQ10) not only decrease blood lipids and lipid peroxidation but also increase levels of antioxidants such as α-tocopherol (α-Toc) and CoQs in hyperlipidemic rats.17 Thus, the present study was designed to determine whether MFGEs provide a more effective antidiabetic effect in comparison to pure statin (atorvastatin: generic medication), a positive control, in attenuating insulin resistance and hyperglycemia in rats fed a high-fructose plus high-fat diet.
Materials and Methods
Sample preparation
MFGEs were produced as described elsewhere.17 A subsample was extracted with 70% ethanol and lyophilized. The MFGEs contained 2.32±0.14 mg mevinolin, 0.49±0.07 mg CoQs, and 109.7±1.6 μg α-Toc per gram of dry-weight samples.17
Animals and treatment
Five-week-old laboratory-bred male Sprague–Dawley rats weighing 150–200 g were selected for study and were acclimatized for 1 week before being randomly assigned into experimental groups. All the experimental procedures were carried out in accordance with the guidelines of the Care Use of Laboratory Animals established by Korea Food Research Institute (KFRI, Seongnam, Korea). The rats were divided into four groups, each containing 10 rats: Group 1 rats were fed a regular diet (RD) for 16 weeks; Group 2 rats were fed a high-fructose (60%, w/w) plus high-fat (20%, w/w) diet (HFD) for 16 weeks as the negative control; Group 3 rats were fed a HFD for 12 weeks and orally administered MFGEs (300 mg/kg body weight) daily (HF+MD) for the last 4 weeks; and Group 4 rats were fed a HFD for 12 weeks and administered atorvastatin (20 mg/kg body weight) daily as the positive control (HF+SD) for the last 4 weeks of this 16-week period. The RD group was fed Purina rat chow diet (Ralston Purina, St. Louis, MO, USA), while the other 30 rats were fed a high fructose plus fat diet (Purina rat chow diet [38.8% w/w] supplemented with fructose, lard, cholesterol [1% w/w], and bile salt [0.2% w/w]). RD and HFD were given in the form of pellets, whereas MFGEs and drugs were given to the animals orally.
Sample collection
The animals in all groups were sacrificed at the end of the 16-week treatment. The livers were isolated, and blood samples were collected from the orbital venous plexus, which were stored at −80°C before use.
Oral glucose tolerance test
The oral glucose tolerance test (OGTT) was performed in overnight-fasted rats from all experimental groups at week 16. All animals received a load of 1.5 g of glucose/kg body weight. Blood glucose level was determined by using ACCU-CHEK glucometer (Roche Diagnostics, Mannheim, Germany) from the tail vessels of conscious animals after glucose administration.
Insulin level and insulin sensitivity index
Insulin level was determined using a rat insulin enzyme-linked immunosorbant assay (ELISA) kit (Alpco Diagnostics, Salem, NH, USA). The insulin resistance index (IR) was estimated by the homeostatic model assessment (HOMA) according to the following formula: HOMA-IR=fasting insulin (μU/mL)×fasting glucose (mM/L)/22.51.18
Hepatic markers of oxidative stress
Liver homogenates were used for the estimation of thiobarbituric acid reactive substances (TBARS) and expressed as malondialdehyde equivalents using a TBARS assay kit (Cayman Chemical Company, Ann Arbor, MI, USA). Supernatant protein concentration was determined using a BCA protein assay kit (Novagen, Madison, WI, USA). Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were measured in liver homogenates using a SOD, CAT, and GPx assay kit respectively (Cayman Chemical Company).
Statistics
Data were expressed as mean±standard deviation from three independent parallel experiments. Statistical analysis was performed by one-way analysis of variance (ANOVA). Unpaired Student's t-test was used for statistical analysis of significant difference between the HFD group and the HF+MD group. Significance was set at P<.05 at the 95% confidence level.
Results and Discussion
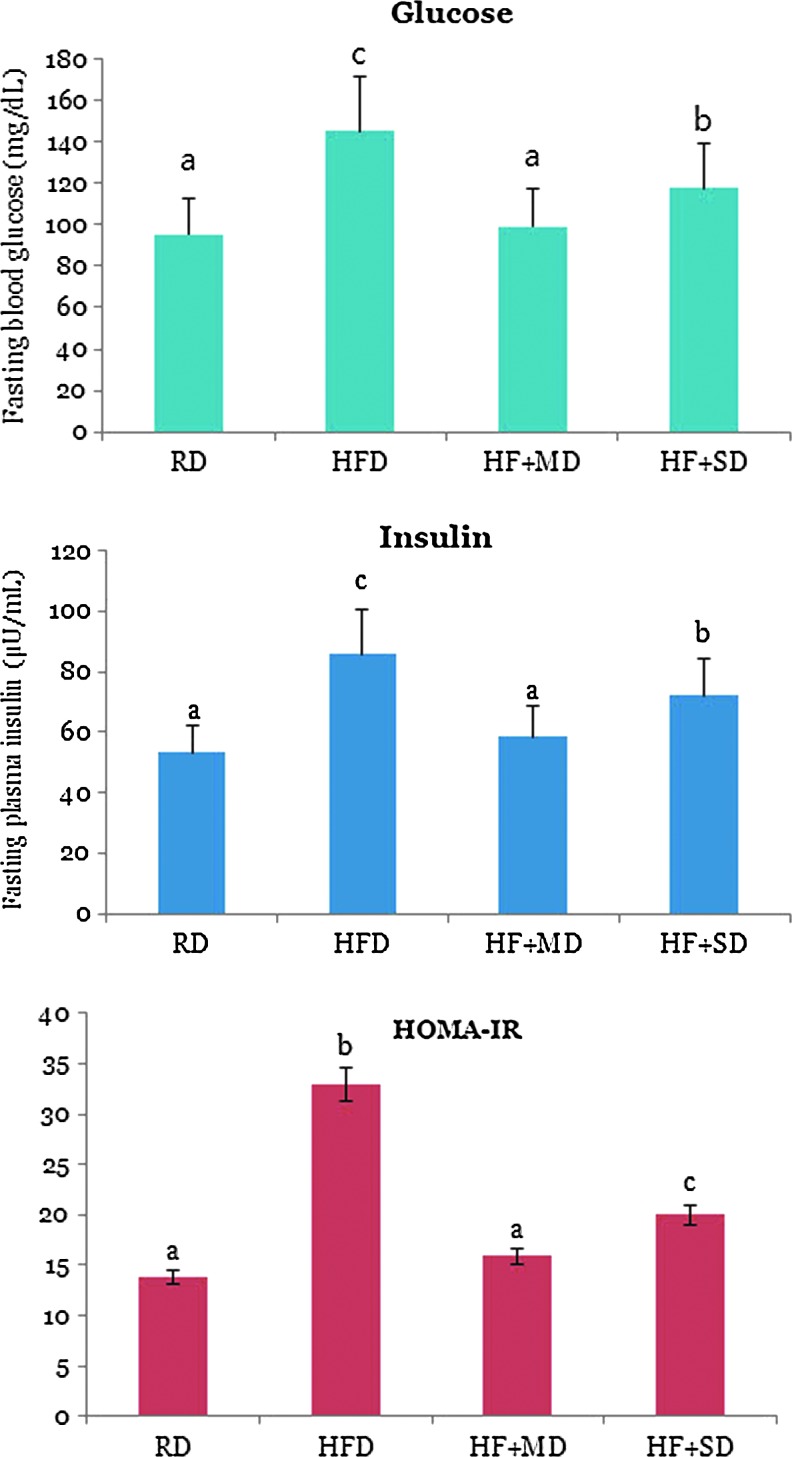
Administration of a high-fructose/high-fat diet induces the development of metabolic syndrome characterized by obesity, insulin resistance, and oxidative stress.19 As shown in Figure 1, the HFD group had up to a 1.52-fold increase in plasma glucose, and a 1.61-fold increase in insulin concentrations compared to the RD group. However, the plasma glucose and insulin concentrations of the HFD group supplemented with MFGEs were significantly lower than the HFD group by 31.7% and 31.6% respectively. Interestingly, the reduction of blood glucose of the MD group was much higher than the SD group and was similar to the RD group. The degree of insulin resistance was estimated at the baseline by an insulin score (HOMA-IR). Low HOMA-IR values indicate high insulin sensitivity, whereas high HOMA-IR values indicated low insulin sensitivity.18 As shown in Figure 1, HOMA-IR was significantly higher in the HFD group than others. The beneficial action on HOMA-IR was also greater in the MD group than the SD group. It is presumed that a more potent antidiabetic effect of MFGEs might account for the combined effect of mevinolin and other bioactive compounds such as CoQs and α-Toc in MFGEs. CoQs has been considered useful for improving glycemic control through various mechanisms, including a decrease in oxidative stress.16 It has been reported that oral administration of atorvastatin suppresses the biosynthesis of CoQs, thereby compromising the physiological function of reduced CoQs, which possesses antioxidant activity.15 In particular, lipophilic statins such as atorvastatin and lovastatin enter extrahepatocytic cells more easily, thereby inhibiting isoprenoid synthesis.19
FIG. 1.
Effect of Monascus-fermented grains (MFGEs) on fasting blood glucose, plasma insulin levels, and homeostatic model assessment as an index of insulin resistance (HOMA-IR) in rats at the end of week 16. RD, regular diet; HFD, high-fructose (60%) plus high-fat (20%) diet; HF+MD, HF+MFGEs (300 mg/kg body weight); HF+SD, HF+atorvastatin (20 mg/kg body weight). a–cWithin a bar, data with different letters are significantly different (P<.05). Results are expressed as mean values±standard deviation (SD). Color images available online at www.liebertpub.com/jmf
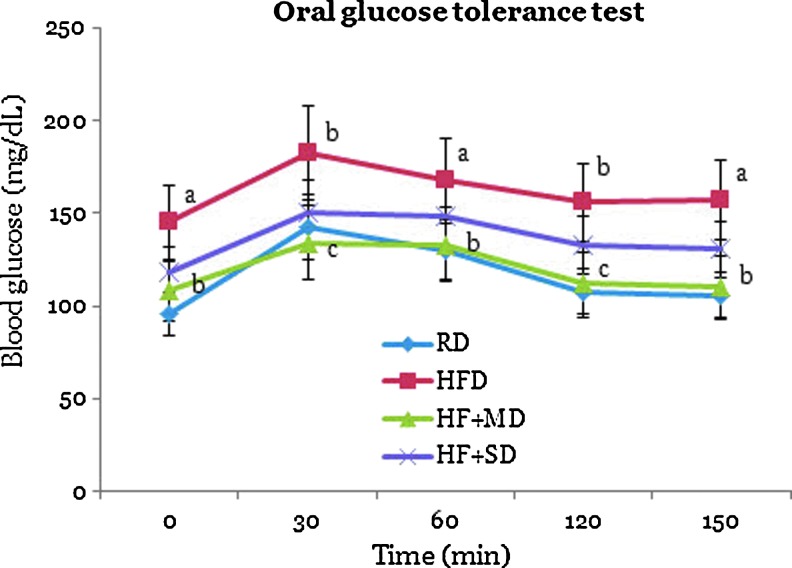
The OGTT is a screening method for acute antihyperglycemic activity because the results give the overall effect of the tested sample on the handling of elevated blood glucose by the organism.20 Figure 2 shows the incremental changes in plasma glucose concentrations of rats following an oral glucose challenge. The blood glucose levels during OGTT were significantly increased in the HFD rats compared to those fed a normal diet (Fig. 2), indicating the development of insulin resistance.3,20 In contrast, MFGE administration markedly prevented hyperglycemia and reduced insulin resistance in HFD rats. The possibility exists that MFGEs exert activities by various mechanism responsible for their hypoglycemic effects and which may help to reduce insulin resistance and impair glucose intolerance. Hyperglycemia plays an important role in the development of type 2 diabetes and complications associated with the disease such as microvascular and macrovascular diseases.3,21 Therefore, the effective control of blood glucose levels is the key for preventing or reversing diabetic complications and improving the quality of the life in diabetic patients.21 The current study showed that supplementing with MFGEs can improve blood glucose levels and impaired glucose tolerance in type 2 diabetic rats.
FIG. 2.
Effect of MFGEs on blood glucose levels of rats at selected time intervals. a–cA significant difference (P<.05) compared with the HFD group and the HF+MD group at the same time point. Results are expressed as mean values±SD. Color images available online at www.liebertpub.com/jmf
Fructose-induced hyperglycemia is one of the important factors that increases reactive oxygen species (ROS) and lipid peroxidation, causing the depletion of the antioxidant defense status in various tissues.6 ROS can themselves reduce the activity of antioxidant enzymes such as CAT and GPx.21 As shown in Table 1, the HFD rats experienced oxidative stress as evidenced by elevation of TBARS and reduction of SOD (−46.9%), CAT (−27.8%), and GPx (−38.2%) activities in comparison to the RD group. However, supplementation with MFGEs or atorvastatin significantly lowered concentrations of hepatic TBARS by 56.5% and 39.9% respectively when compared to the HFD group. Moreover, MFGE administration increased hepatic antioxidant enzyme activities, whereas the SD group only increased catalase activity (Table 1). These results indicate that MFGEs were more effective than statins in reducing oxidative stress in diabetic liver. The superior improvements in the MD group may be a consequence of CoQs and α-Toc in MFGEs. Antioxidants may improve insulin action by reducing lipid peroxidation in hepatic cell membranes, which would enhance the ability of insulin to bind to its receptor.22,23 In addition, antioxidant treatment has been reported to protect β-cells against glucose toxicity in diabetic mice,22 and CoQ10 has been reported to stimulate insulin secretion from pancreatic islets.22,24 Thus, the current study indicates that supplementation with MFGEs can prevent the development of hyperglycemia and insulin resistance, as well as decrease hepatic oxidative stress in type 2 diabetic rats.
Table 1.
Effect of Monascus-Fermented Grains on Hepatic Oxidative Markers of Rats at the End of Week 16
| RD | HFD | HF+MD | HF+SD | |
|---|---|---|---|---|
| TBARS (μM/mg protein) | 0.72±0.06a | 2.78±0.45c | 1.21±0.08b | 1.67±0.09b |
| CAT (nmol/min/mg protein) | 6.55±0.23a | 4.73±0.21a | 6.13±0.56a | 5.78±0.17a |
| SOD (U/mg protein) | 0.64±0.05a | 0.34±0.04b | 0.48±0.03b | 0.36±0.07b |
| GPx (nmol/min/mg protein) | 9.72±1.02a | 6.01±0.35b | 8.07±0.68c | 6.11±0.17b |
Within a row, data with different letters are significantly different (P<.05). Results are expressed as mean values±standard deviation.
RD, regular diet; HFD, high fructose (60%) plus fat (20%) diet; HF+MD, HF+MFGEs (300 mg/kg body weight); HF+SD, HF+atorvastatin (20 mg/kg body weight); MFGEs, Monascus-fermented grains.
Acknowledgment
This work was supported by a Korea Research Foundation Grant funded by the Korean Government (2011-0013015).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA: Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999;131:281–303 [DOI] [PubMed] [Google Scholar]
- 3.Ademiluyi A, Oboh G: Aqueous extracts of roselle (Hibiscus sabdariffa Linn.) varieties inhibit α-amylase and α-glucosidase activities in vitro. J Med Food 2013;16:88–93 [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Nielsen SJ, Popkin BM: Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–543 [DOI] [PubMed] [Google Scholar]
- 5.Samuel VT. Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metabol 2011;22:60–65 [DOI] [PubMed] [Google Scholar]
- 6.Reddy SS, Ramatholisamma P, Karuna R, Saralakumari D: Preventive effect of Tinospora cordifolia against high-fructose diet-induced insulin resistance and oxidative stress in male Wistar rats. Food Chem Toxicol 2009;47:2224–2229 [DOI] [PubMed] [Google Scholar]
- 7.Journoud M, Jones PJ: Red yeast rice: a new hypolipidemic drug. Life Sci 2004;74:2675–2683 [DOI] [PubMed] [Google Scholar]
- 8.Li CL, Zhu Y, Wang YY, Zhu JS, Chang J: Monascus purpureus-fermented rice (red yeast rice): a natural food product that lowers blood cholesterol in animal models of hypercholesterolemia. Nutr Res 1998;18:71–81 [Google Scholar]
- 9.Subhagar S, Aravindan R, Viruthagiri T: Statistical optimization of anticholesterolemic drug lovastatin production by the red mold Monascus purpureus. Food Bioprod Proc 2010;88:266–276 [Google Scholar]
- 10.Pyo YH, Seong KS: Hypolipidemic effects of Monascus-fermented soybean extracts in rats fed a high-fat and -cholesterol diet. J Agric Food Chem 2009;57:8617–862 [DOI] [PubMed] [Google Scholar]
- 11.Mortensen SA, Leth A, Agner E, Rohde M: Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol Asp Med 1997;18:137–144 [DOI] [PubMed] [Google Scholar]
- 12.Yang HT, Lin SH, Huang SH, Chou HJ: Acute administration of red yeast rice (Monascus purpureus) depletes tissue coenzyme Q10 levels in ICR mice. Br J Nutr 2005;93:131–135 [DOI] [PubMed] [Google Scholar]
- 13.Ernster L, Dallner G: Biochemical, physiological, and medical aspects of ubiquinone function. Biochim Biophys Acta 1995;1271:195–207 [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain LH: Inhibition of isoprenoid biosynthesis causes insulin resistance in 3T3-L1 adipocytes. FEBS Lett 2001;507:357–361 [DOI] [PubMed] [Google Scholar]
- 15.Koh K, Quon M, Ha S: Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol 2010;55:1209–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kettawan A, Takahashi T, Kongkachuichai R, Charoenkiatkul S, Okamoto T: Protective effects of coenzyme Q10 on decreased oxidative stress resistance induced by simvastatin. J Clin Biochem Nutr 2007;40:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pyo YH, Seong KS: Effects of Monascus-fermented grain extracts on plasma antioxidant status and tissue levels of ubiquinones and α-tocopherol in hyperlipidemic rats. Food Chem 2013;141:428–435 [DOI] [PubMed] [Google Scholar]
- 18.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB: Homeostasis model assessment closely mirror the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 2000;23:57–63 [DOI] [PubMed] [Google Scholar]
- 19.Colbert JD, Stone JA: Statin use and the risk of incident diabetes mellitus: a review of the literature. Can J Cardiol 2012;28:581–589 [DOI] [PubMed] [Google Scholar]
- 20.Chidambaram J, Carani Venkatraman A: Cissus quadrangularis stem alleviates insulin resistance, oxidative injury and fatty liver disease in rats fed high fat plus fructose diet. Food Chem Toxicol 2010;48:2021–2029 [DOI] [PubMed] [Google Scholar]
- 21.King GL, Locken MR: Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol 2004;122:333–338 [DOI] [PubMed] [Google Scholar]
- 22.Maritim AC, Sanders RA, Watkins JB: Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 2003;17:24–38 [DOI] [PubMed] [Google Scholar]
- 23.Bentinger M, Brismar K, Dallner G: The antioxidant role of coenzyme Q. Mitochondrion 2007;7:S41–S50 [DOI] [PubMed] [Google Scholar]
- 24.Palomaki A, Malminiemi K, Metsa KT: Enhanced oxidizability of ubiquinol and alpha-tocopherol during lovastatin treatment. FEBS Lett 1997;410:254–258 [DOI] [PubMed] [Google Scholar]