PRESENTATION
Strange and relatively sudden symptoms in a 27-year-old man implied evidence of a rare paraneoplastic syndrome. The patient presented to the emergency department with syncope. He had been completely healthy until 6 weeks earlier when he noticed a mild sensation of lightheadedness on standing. Over the next several weeks, this was accompanied by tunnel vision, palpitations, dyspnea, dry mouth, diaphoresis, and a sensation of flushing, all of which resolved upon recumbence. His first syncopal episode occurred 3 weeks prior to presentation. He became lightheaded and lost consciousness for several seconds. Although he awoke quickly without residual symptoms, from that point onwards, he experienced syncopal episodes with complete loss of consciousness up to 4 times a day.
In addition, he reported constipation, loss of appetite, and an unintentional 50-lb weight loss over the previous 6 months. His past medical history was significant only for a faint pruritic rash that had appeared on his lower extremities 6 months prior. This was diagnosed as cutaneous mastocytosis via skin biopsy.
ASSESSMENT
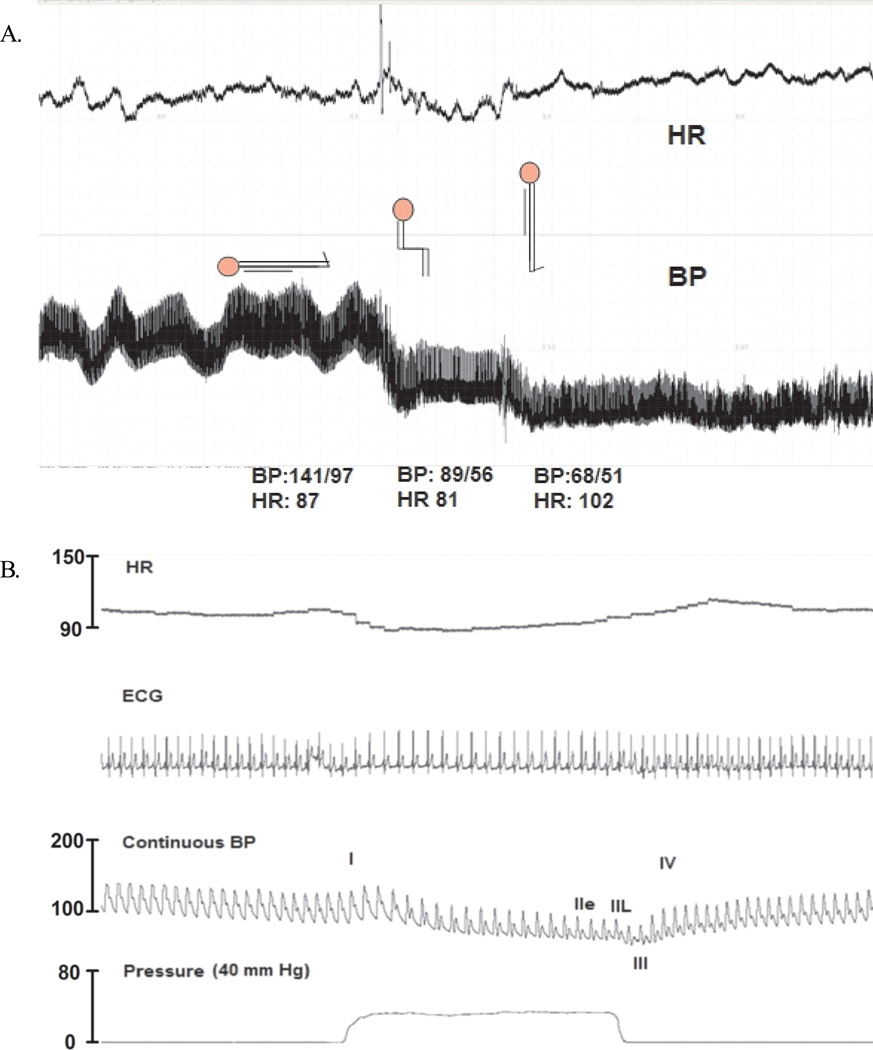
On physical examination, the patient appeared well and was in no acute distress. His temperature, respiratory rate, and oxygen saturation were normal, but he had profound orthostatic hypotension. While supine, his blood pressure was 141/82 mm Hg, and his heart rate was 112 beats per minute. After 1 minute of standing, his blood pressure dropped to 81/64 mm Hg, and his heart rate increased to 124 beats per minute (Figure 1A). Pupil reactivity and extraocular motion were normal.
Figure 1.
(A) The patient had severe orthostatic hypotension, which had a dramatic effect on his heart rate (HR) and blood pressure (BP). (B) Valsalva-maneuver (VM) tracings showed a lack of blood pressure (SBP) recovery in phase II late (IIL) and an overshoot during phase IV. This indicated impaired sympathetic vasoconstrictor function. He had normal compensatory heart rate changes during the Valsalva maneuver.
The patient had sinus tachycardia with no murmurs, normal pulses in all 4 extremities, and no jugular venous distension. His lungs were clear to auscultation. Palpation indicated that his abdomen was soft, nontender, nondistended, and free of masses; normal bowel sounds were present. No clubbing, cyanosis, or edema was evident in his extremities. Aside from decreased temperature discrimination in his lower extremities, he had no neurologic abnormalities. Small hyperpigmented macules marked the skin on his feet.
Standardized autonomic-function testing was conducted with the patient in the supine position. In response to a deep-breathing test, the patient had a maximum heart rate of 112 beats per minute, a minimum heart rate of 105 beats per minute, and a ratio of maximum to minimum heart rate during sinus arrhythmia of 1.074 (normal > 1.2). Even though his sinus arrhythmia ratio was low, it was difficult to interpret because the patient had resting tachycardia.
During the Valsalva maneuver, he had an abnormal blood pressure fall during early phase II, lack of systolic blood pressure recovery in late phase II, and a lack of systolic blood pressure overshoot during phase IV (Figure 1B). These results were consistent with sympathetic vasoconstrictor failure. Yet, his cardiovagal response to the Valsalva maneuver (the ratio of maximal heart rate during phase II to minimal heart rate during phase IV), was normal, an indication of preserved vagal response. His plasma catecholamine levels were mildly elevated—epinephrine and norepinephrine levels were 92 pg/mL and 424 pg/mL, respectively, while supine and 46 pg/mL and 777 pg/mL, respectively, while standing.
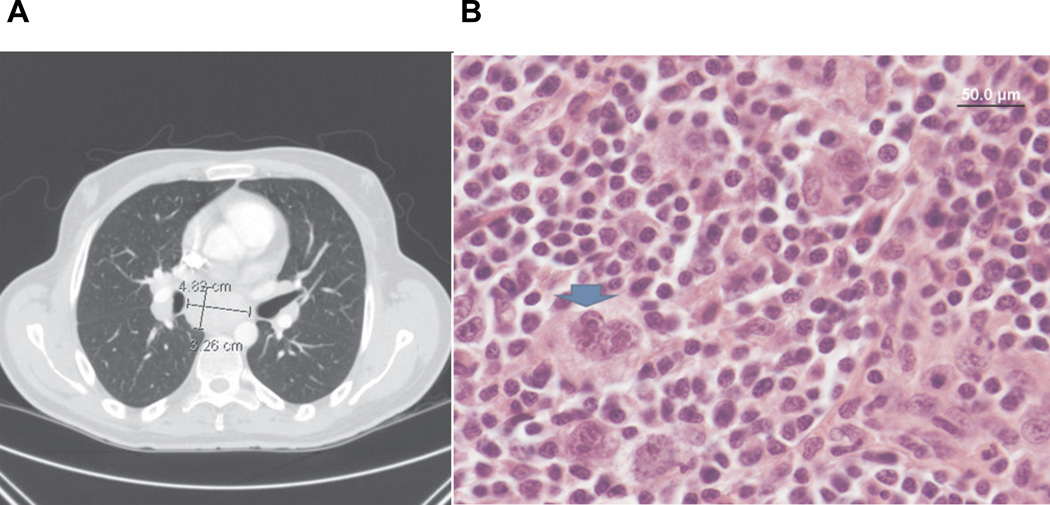
An electrocardiogram, an echocardiogram, and Holter monitoring did not reveal evidence of structural or electrical cardiac abnormality. Brain magnetic resonance imaging and electroencephalography also produced normal results. A computed tomography scan of the patient’s chest, abdomen, and pelvis demonstrated extensive lymph node enlargement in the supraclavicular, mediastinal, bilateral hilar, and celiac regions (Figure 2A). These findings were highly suggestive of lymphoma. An initial lymph node biopsy from the mediastinal region showed only reactive hyperplasia. A paraneoplastic autoantibody panel was negative. Testing for the ganglionic neuronal acetylcholine receptor autoantibody (anti-AChR), which has been previously associated with autoimmune autonomic failure, also was negative.1 Serum and urine electrophoresis were negative, as well. A fat pad biopsy was negative for amyloid protein.
Figure 2.
(A) Computed tomography of the chest showed mediastinal lymphadenopathy. (B) Supraclavicular lymph node histopathology identified Hodgkin’s lymphoma. The blue arrow highlights a classic Reid-Steinberg (RS) cell, confirming the diagnosis.
DIAGNOSIS
The patient was diagnosed with subacute autonomic failure. At the time he presented with severe orthostatic hypotension, autonomic function tests showed isolated impairment of sympathetic-mediated vasoconstriction (Figure 1A) and the absence of a pressor response during phase II of the Valsalva maneuver (Figure 1B). Of note, the parasympathetic nervous system seemed to be preserved; the patient had a normal compensatory increase in heart rate on standing and a normal cardiovagal response during the Valsalva test.
After he had a prolonged course with multiple hospitalizations, concern remained for an underlying malignancy. A repeat excisional biopsy of the supraclavicular lymph nodes, obtained 5 months after the first biopsy, showed classic Hodgkin’s lymphoma of the nodular sclerosis type; stage IVA (Figure 2B). Previous studies have reported the presence of subclinical autonomic failure in patients with lymphoma.2–4 Yet, our patient’s presentation of acute autonomic failure, affecting primarily the sympathetic nervous system with preservation of parasympathetic pathways, is unusual. A 1986 report detailed the case of a patient whose initial presentation of severe orthostatic hypotension with intact vagal heart-rate control preceded a diagnosis of Hodgkin’s lymphoma.2 These 2 very similar occurrences suggest that this tumor type might rarely present with a unique paraneoplastic autonomic syndrome involving only the sympathetic fibers.
Classic onconeural antibodies, particularly Anti-Hu and anti-CRMP-5 autoantibodies, have been found in patients with paraneoplastic syndromes affecting the autonomic nervous system. Subacute and acute isolated autonomic failure also have been associated with anti-AChR antibodies, which irreversibly bind to acetylcholine receptors in the autonomic ganglia, producing sympathetic and parasympathetic failure.5–7 Patients develop severe orthostatic hypotension, often with significant concurrent gastrointestinal symptoms, such as delayed gastric emptying, chronic nausea, vomiting, and weight loss.
Our patient retained parasympathetic function and had a negative anti-AChR antibody test, factors that suggest a considerably different pathogenesis. Possibly, novel autoantibodies produced against the developing tumor cause direct damage to postganglionic sympathetic nerve fibers or work as a norepinephrine antagonist at the level of postsynaptic adrenergic receptors.8 Of note, a recent report proposed a novel mechanism in which antibodies serve as agonists to the β2-adrenergic and M3 muscarinic acetylcholine receptors, inducing vasodilation and exacerbation of orthostatic hypotension in patients with diabetes autonomic neuropathy.9 However, the significance of these findings remains under investigation.
MANAGEMENT
Because of the high suspicion that the patient’s autonomic impairment was due to an autoimmune paraneoplastic etiology, we first treated him empirically with plasmapheresis, and 4 months later, with intravenous immune globulin (IVIG). Plasmapheresis has been used to treat patients who have autoimmune autonomic ganglionopathy and positive anti-AChR antibodies.10 The removal of pathological antibodies has been shown to transiently resolve orthostatic hypotension, improve autonomic reflexes, and restore baroreflex sensitivity. Anecdotal reports indicate that treatment with IVIG also has resulted in dramatic clinical improvement.
Our patient, however, did not experience any improvement in autonomic function. His orthostatic hypotension persisted, and he experienced only temporary relief for the presyncopal symptoms that occurred upon standing. This brief respite could have been related to an increase in plasma volume.
We highlight this rare case as a possible autoimmune autonomic failure caused by Hodgkin’s lymphoma. The underlying autoantibodies in this case are uncharacterized. After 1 course of plasmapheresis and IVIG therapy, the patient was treated with 6 cycles of doxorubicin, bleomycin, vincristine, and dacarbazine. He then went into remission from the malignancy. Nonetheless, autonomic impairment persisted. Possibly, the neoplasm elicited an immune response, causing resistance to therapy for autonomic failure. In such cases, plasmapheresis is only effective when combined with immunosuppressant medications like We highlight this rare case as a possible autoimmune autonomic failure caused by Hodgkin’s lymphoma. The underlying autoantibodies in this case are uncharacterized. After 1 course of plasmapheresis and IVIG therapy, the patient was treated with 6 cycles of doxorubicin, bleomycin, vincristine, and dacarbazine. He then went into remission from the malignancy. Nonetheless, autonomic impairment persisted. Possibly, the neoplasm elicited an immune response, causing resistance to therapy for autonomic failure. In such cases, plasmapheresis is only effective when combined with immunosuppressant medications like
He underwent repeat autonomic function tests 1 year after therapy. These demonstrated a supine blood pressure of 121/83 mm Hg with a heart rate of 78 beats per minute. After 1 minute of standing, his blood pressure dropped to 81/65 mm Hg, and his heart rate increased to 103 beats per minute. This confirms persistent isolated sympathetic failure despite remission of Hodgkin’s lymphoma and resolution of constitutional symptoms.
Acknowledgments
Funding: None.
CS receives funding from the American Heart Association, Clinical Research Program 10CRP4310026, National Institute of Health (NIH) grant K23 HL103976-02, and by a Pharmaceutical Research and Manufacturers of America Foundation Career Development Award. The study was supported in part by the Vanderbilt Clinical and Translational Science Award grant 5UL1 RR024975-03 from the National Center for Research Resources/NIH.
Footnotes
Conflict of Interest: None.
Authorship: All authors had access to the data. All the authors declared that they met criteria for authorship including acceptance of responsibility for the scientific content of the manuscript.
References
- 1.Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146:3–7. doi: 10.1016/j.autneu.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Lieshout JJ, Wieling W, van Montfrans GA, et al. Acute dysautonomia associated with Hodgkin’s disease. J Neurol Neurosurg Psychiatry. 1986;49:830–832. doi: 10.1136/jnnp.49.7.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crespo M, Correia L, Ferreira F, Ducla-Soares JL. Acute disautonomia associated to Hodgkin lymphoma. Acta Med Port. 2002;15:153–154. [PubMed] [Google Scholar]
- 4.Lavi S, Aharon-Peretz J, Haim N, Vlodavsky E, Jacob G. Unusual cause of partially reversible severe cardiovascular autonomic failure. Am J Med Sci. 2003;326:159–163. doi: 10.1097/00000441-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 6.Manganelli F, Dubbioso R, Nolano M, et al. Autoimmune autonomic ganglionopathy: a possible postganglionic neuropathy. Arch Neurol. 2011;68:504–507. doi: 10.1001/archneurol.2011.60. [DOI] [PubMed] [Google Scholar]
- 7.Parize P, Gaultier JB, Badet F, et al. Autoimmune autonomic ganglionopathy: a case series of six patients and literature review. Rev Med Interne. 2010;31:476–480. doi: 10.1016/j.revmed.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto LE, Shibao C, Gamboa A, et al. Synergistic effect of norepinephrine transporter blockade and α-2 antagonism on blood pressure in autonomic failure. Hypertension. 2012;59:650–656. doi: 10.1161/HYPERTENSIONAHA.111.184812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Kem DC, Reim S, et al. Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension. 2012;59:402–408. doi: 10.1161/HYPERTENSIONAHA.111.184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroeder C, Vernino S, Birkenfeld AL, et al. Plasma exchange for primary autoimmune autonomic failure. N Engl J Med. 2005;353:1585–1590. doi: 10.1056/NEJMoa051719. [DOI] [PubMed] [Google Scholar]
- 11.Hollenbeck R, Black BK, Peltier AC, et al. Long-term treatment with rituximab of autoimmune autonomic ganglionopathy in a patient with lymphoma. Arch Neurol. 2011;68:372–375. doi: 10.1001/archneurol.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]