Abstract
Experimental work in developmental biology has recently shown in mice that fluid flow driven by rotating cilia in the node, a structure present in the early stages of growth of vertebrate embryos, is responsible for determining the normal development of the left-right axis, with the heart on the left of the body, the liver on the right, and so on. The role of physics, in particular, of fluid dynamics, in the process is one of the important questions that remain to be answered. We show with an analysis of the fluid dynamics of the nodal flow in the developing embryo that the leftward flow that has been experimentally observed may be produced by the monocilia driving it being tilted toward the posterior. We propose a model for morphogen transport and mixing in the nodal flow and discuss how the development of left-right asymmetry might be initiated.
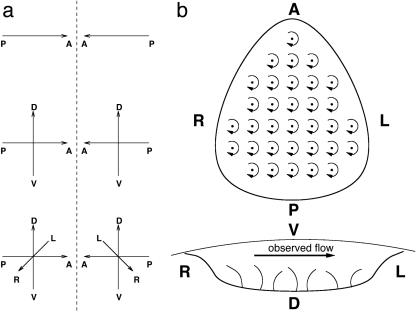
The bilaterally symmetric external appearance of vertebrates is deceptive, for beneath the skin asymmetry reigns. In the early stages of development of an organism, in the embryo are laid down the anterior-posterior, dorsal-ventral, and left-right axes of the vertebrate body plan (1). The left-right symmetry axis is decided after the anterior-posterior and dorsal-ventral axes have been laid down. The symmetry breaking involved is qualitatively different from the first two axes. In those cases, it matters only that the symmetry is broken and not in what sense, because the opposite choice in each instance, interchanging up with down or front with back, would lead to exactly the same result. The third axis is distinct because the two possible outcomes are chiral (Fig. 1a); the opposite choice would lead to an animal having all its internal structure mirror-reversed from the norm, so-called situs inversus. That nature does distinguish left from right is shown by the fact that animals normally have their hearts on the left (situs solitus), and situs inversus is a rare variation that in humans, for example, is found in only about one person in ten thousand (2, 3). This situation is curious, since an organism with complete situs inversus can function just as well as one with normal chirality.
Fig. 1.
(a) Anterior-posterior, dorsal-ventral, and left-right axes provide a coordinate system for the vertebrate body plan. When only one or two of the axes are defined, the result is achiral; the mirror image is the same as the original. But when the final, left-right axis is added, two chiral forms now exist. (b) Ventral and posterior sketch views of the node of the mouse embryo, and its rotating monocilia, showing also the experimentally observed leftward nodal flow.
Recently, elegant experiments with mice have shown that a structure on the surface of the embryo, the node, is responsible for determining left-right chirality (4-11). The node is liquid-filled and lined with cells possessing monocilia or primary cilia, hairs that are cylindrical in cross section and are seen to rotate clockwise as viewed from above (4, 10). These monocilia are sometimes termed 9 + 0 cilia in contradistinction to the 9 + 2 cilia and flagella that beat rather than rotate. The difference lies in their molecular motors; 9 + 2 refers to a ring of nine peripheral doublets of microtubules plus a central pair, whereas 9 + 0 monocilia lack this central pair (11). The monocilia are curved; hence, as they rotate, the tip of each cilium traces out a circular path. When passive tracers (submicrometer-sized spherical particles) are introduced into the nodal fluid above the cilia, they move leftward, meaning toward the left of the embryo not toward the observer's left, across the node, following the flow in the extraembryonic fluid in which they are immersed. That this movement is induced by the cilia is demonstrated by genetic abnormalities in laboratory mice and in humans which lead to the monocilia of the node being immobile. An example is Kartagener's syndrome in humans, in which the dyein arms are missing from the microtubules of the molecular motors that normally drive the cilia (12). A check with passive tracers in such mice shows no fluid flow, only Brownian motion. The same developmental error illustrates the role of this nodal flow in left-right symmetry breaking; in half of the animals with this abnormality the internal organs are mirror-reversed from the norm (5), because, without the nodal flow, symmetry is broken randomly. This finding confirms a hypothesis of Afzelius (12), who first surmised that the movement of cilia might be crucial in this symmetry breaking. Moreover, normal embryos can be made to develop situs inversus by using external forcing to artificially change the flow direction in the node from leftward to rightward (10).
How does the clockwise motion of tens of monocilia drive a leftward flow in the node? And, if the observed flow is leftward, how is the fluid recirculating within the node, as it must, since the node is a closed structure? Finally, how does the nodal flow lead to left-right symmetry breaking in the embryo? These questions are within the realm of fluid physics (13).
Nodal Fluid Dynamics
In the mouse, the most studied case, the node is a depression on the surface of the embryo, roughly pear-shaped when viewed from above (that is, from the ventral side), some 50 μm across, and 10 μm deep(see Fig. 1b). It is covered by Reichert's membrane and filled with extraembryonic liquid. Arrayed over its base are a few tens of monocilia some 2-3 μm in length. These rotate clockwise, as viewed from above, at ≈10 Hz. The flow velocity produced by the monocilia has been measured with passive tracers to be some 20-50 μm·s-1 in normal embryos (5). The Reynolds number of the node, Ren = vL/ν, the relative importance of inertial to viscous forces in the flow, where v is the flow velocity, L the size of the node, and ν the kinematic viscosity of the extraembryonic fluid (an aqueous solution of proteins), is thus of the order of 10-3. We can obtain another Reynolds number from the cilium rotation velocity: Rec = ωa2/v, where ω is the angular velocity 2πf and a is the length of a cilium. From the cilial frequency f = 10 Hz, Rec ≈ 5·10-4 here. The two estimates are close, and, whether we take Ren or Rec as the more representative, the Reynolds number of the flow is certainly very low. The low Reynolds number means that viscosity dominates inertia; if the monocilia were to stop, the flow would instantly cease. Under this condition, known as creeping flow, the Navier-Stokes equations that describe the movement of fluid may be linearized to the Stokes equations, which are amenable to analytical solution. As the monocilia lining the floor of the node rotate, each produces a vortex about itself in the flow. A cilial vortex can then be modeled as a rotlet,
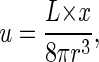 |
1 |
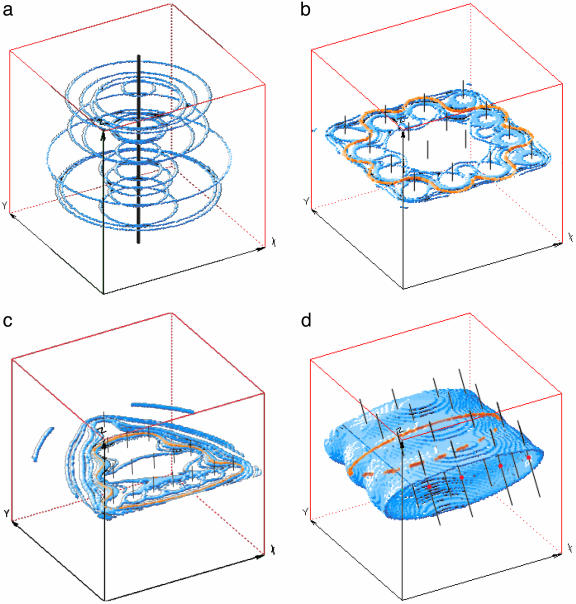
an analytical solution of the Stokes equations representing flow due to the rotation of an infinitesimal sphere in the fluid (14, 15), where u is the induced flow velocity; L is the applied torque; x = (x,y,z), the coordinate in 3D space; and 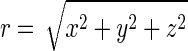 the distance from the origin. The vortical flow produced by a rotlet is shown in Fig. 2a. As the Stokes equations are linear, an array of vortices is simply a combination of rotlet solutions. An array of rotlets can be given the same topology as the array of rotating monocilia in the node, and in this way transport in the nodal flow can be investigated with this rotlet model. (For information about the model, see Supporting Text, which is published as supporting information on the PNAS web site.)
the distance from the origin. The vortical flow produced by a rotlet is shown in Fig. 2a. As the Stokes equations are linear, an array of vortices is simply a combination of rotlet solutions. An array of rotlets can be given the same topology as the array of rotating monocilia in the node, and in this way transport in the nodal flow can be investigated with this rotlet model. (For information about the model, see Supporting Text, which is published as supporting information on the PNAS web site.)
Fig. 2.
(a) Vortical flow structure produced by a single rotlet. (b) Rectangular array of rotlets with axes vertical, showing cellular structure of vortices with a general circulation only occurring at the edges. (c) Triangular array of rotlets with axes vertical, to correspond more closely with the shape of the node. As in b, a general circulation occurs only at the edges. (d) Result of tilting the rotlet axes: array of tilted rotlets with tilt angle α = 24°, showing directional flow above and below the array.
If the monocilia rotate about vertical axes, they create a set of vortices, one per cilium, but not a directional flow in the fluid above. Instead, as depicted in Fig. 2b, a flow consisting of a cellular network of vortices exists, in which a general circulation only occurs at the edges of the network; elsewhere, movement is vortical. This finding does not correspond with the experimental observations of a general leftward flow above the cilia. Nonaka et al. (4, 10) suggest that the key to producing such a flow is in the shape of the node; it is elongated or pear-shaped (Fig. 1b), and so the array will be not a rectangle but a triangle of vortices. As we can see in Fig. 2c, however, merely changing this aspect of the geometry does not qualitatively change the flow field; it is still vortical within the triangular array, with a general circulation only at the edges. A further possibility would be cilia shaped like oars, which, if feathered during part of the rotation, could produce a directional flow; but all observations show cylindrical cilia. To envisage how a general circulation within the node may be produced by cylindrical cilia, a useful analogy is to a kitchen blender; if this utensil is held vertically in the fluid it is mixing, so that the blades rotate in a horizontal plane, the surface flow is a vortex around the stem of the blender. But if the blender is tilted, the surface shows a general flow in the direction in which the blades are turning when they are closest to the surface. In the node, then, we should consider the possibility that the cilia are all inclined, so that they sweep out circles at an angle to the horizontal. If each one is tilted in the same direction, there will be a directional flow across the chamber above them, due to the fluid overhead being entrained in their direction of rotation; the greater the tilt, the stronger the directional flow above the vortices. It may be said that this tilt implies a prior symmetry-breaking event. This is so, but the symmetry broken is the already-defined anterior-posterior and not left-right; for, to obtain the observed leftward flow, given that they rotate clockwise, the monocilia ought to be tilted toward the posterior.
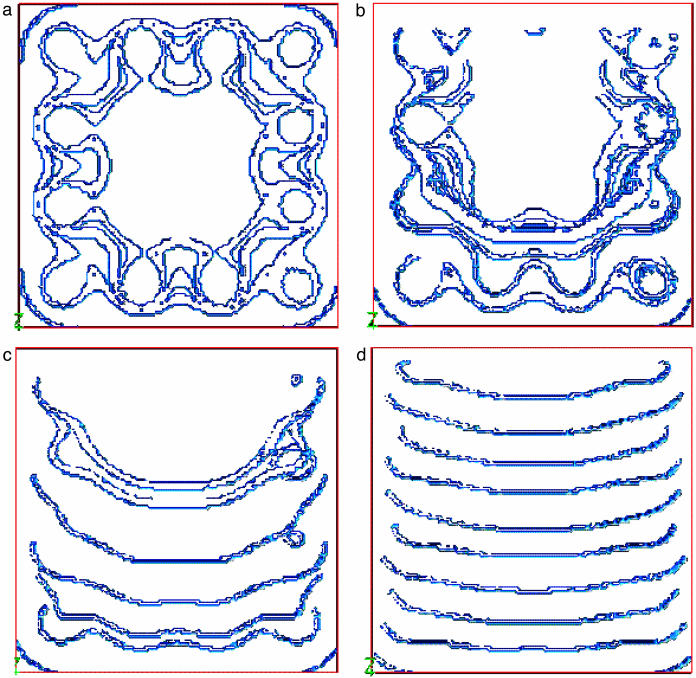
Consider a single rotlet, as in Fig. 2a, but that is now inclined at an angle α to the vertical. For a general leftward flow as is seen in the node to emerge from a cellular network of such vortices, what angle of tilt α would be necessary? We can estimate this from the observed tip velocity, V = 2πaf, of a cilium and the flow velocity v of the nodal fluid. Close to the tips of the cilia, the flow velocity will match the tip velocity. The component of the tip velocity contributing to a directional flow is V sin α, so we can estimate α = arcsin (v/2πaf). If we substitute the ranges of values for v and a mentioned earlier, plus f = 10 Hz, we find the range of angles of tilt to be between ≈5° to 25° from the vertical. We model such an array of tilted rotlets in Fig. 2d, and we see that a directional flow above the rotlets arises exactly as predicted. From the biological point of view, one can envisage two possibilities for inclined cilia: either the inclination is active, requiring the shaft to bend and straighten as it rotates, or it is passive, with the embedded bearing of the cilium inclined at an angle and no active bending of the shaft being necessary. Our rotlet model covers both these cases, because the fluid dynamics is substantially the same, and with either passive or active inclination, the cilia must be oriented toward the posterior as they rotate. As the angle of tilt varies, so does the strength of the directional flow compared with the vortical flow. An intriguing genetic abnormality occurs in mice, Inv, in which all the animals have their viscera mirror-reversed from the norm. The nodal cilia are present and rotate at the same 10 Hz and in the same direction as in normal mice. The nodal flow is present in these animals, but altered; instead of a strong leftward flow, passive-tracer experiments show a much more vortical flow structure (5). It has been speculated that this disparity may be caused by a difference in the shape of the node in Inv mice, which is appreciably narrower and rougher than normal (5). But the node remains left-right symmetric, otherwise the implication would be that left-right symmetry is broken at an earlier embryonic stage, and moreover, we have demonstrated above that the tilt of the cilia is much more influential than the precise shape of the node in producing the flow pattern. Our hypothesis is that this mutant mouse may have its monocilia less tilted than normal; a smaller tilt would make the flow more entrained in vortices, and the leftward component slower, which is just what is observed. The Inv mouse node displays flow characteristics similar to those observed in the node of normal mice in the early stages of their development, before a strong leftward flow appears (5). Thus, it may be that the tilting of the cilia occurs in normal mice as the node matures, whereas in Inv mice this tilting of the mature cilia never takes place. In Fig. 3 we plot just the xy plane for four different values of α, increasing from 0 to 24°; for small α, we see exactly the same flow behavior observed in experiments on Inv mutant mice embryos.
Fig. 3.
Views of the xy plane for α varying between 0 and 24°:0° (a), 8° (b), 16° (c), and 24° (d). For small α the flow is vortical, whereas for larger α it is increasingly linear. The small-α pictures are similar to what is seen in Inv mutant mice, whereas the larger-α cases correspond to experiments on normal mouse embryos.
Our rotlet simulations of Fig. 2 take place in an infinite medium. We can see in Fig. 2d that, corresponding to the directional flow above the rotlets, an equal and opposite flow exists below them. We should expect this counterflow in the node also, except that there it will be modified by the presence of the node walls. Moreover, in the embryo in vivo, the node is a closed structure from the point of view of advective flow, being covered by Reichert's membrane (4) (beyond the limits of the nodal chamber, diffusion but not advection may exist under this membrane), so fluid flowing leftward must return rightward. If the outward flow takes place in the center of the chamber, as experiments show, the return flow has no choice but to follow the walls, both close to the node floor around the bases of the cilia (as Fig. 2d indicates) and, in addition, close to the other walls; we sketch the complete flow pattern that would be expected with recirculatory vortices in the upper and lower halves of the node in Fig. 4a. Because flow close to a wall is slower than in the bulk of the fluid, the return flow will be slower than the primary flow, but because it necessarily transports the same volume of fluid as the outward flow, it must be more spatially extended. It has not yet been seen experimentally, probably because, first, in the embryo in vitro, to obtain access to the nodal flow, Reichert's membrane is removed, and the embryo is immersed in a much larger liquid-filled container (4). Under these experimental conditions there is an open rather than a closed flow in which the return flow above the outward flow is eliminated and that below it is diminished. Second, the passive tracers are injected into the flow above and not below the cilia, so they experience only the outward flow.
Fig. 4.
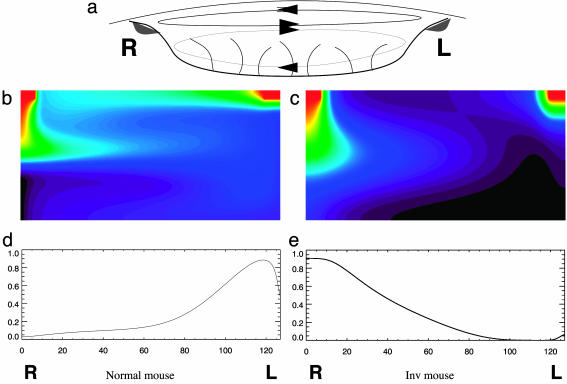
(a) Sketch of how fluid will recirculate within the node in vivo with diffuse return flows above and below the more intense outward flow in the center. Also shown is the putative placement of morphogen sources (gray areas) at the left and right sides adjacent to the upper recirculatory vortex. (b and c) Numerical simulations of our model depicting the steady-state concentration of a morphogen with a finite lifetime within the node with normal (b) and Inv (c) mice. The color scale is as for a rainbow, with red the highest concentration and violet the lowest. (d and e) Graphs of the concentration of morphogen at the floor of the node in the simulations above with normal (d) and Inv (e) mice (arbitrary units).
Morphogen Model
How is the information on the symmetry broken by the nodal flow transmitted to the embryo? The Inv mutant mice mentioned earlier provide an important clue to the mechanism. Although the nodal flow in these animals is still leftward, albeit slower than normal, they all have situs inversus. A mechanism must be sought that would lead Inv mice to have opposite chirality. Two means have been proposed for how information may be extracted from the nodal flow: chemosensing and mechanosensing. On one hand, it has been suggested that the nodal fluid may be carrying a morphogen, a signaling molecule, probably a protein, that is released into the flow, and whose concentration is detected by chemoreceptors within the node (4). On the other hand, very recent experimental work has shown that two populations of monocilia appear to exist in the node, and it speculated that the second population may be nonrotating mechanoreceptors (16). In this section we treat the morphogen hypothesis and develop a model for morphogen transport and mixing in the node compatible with the observations and with the physics. In Discussion we examine why mechanosensing is unlikely to be the mechanism operating in the node.
The morphogen hypothesis proposes that a difference in morphogen concentration detected by receptors placed on both the left and right sides of the node should be the factor that determines the chirality of the left-right axis. This hypothesis implies that the concentration of active morphogen should reach a steady state that in normal embryos is higher on one side of the node, and in Inv embryos is higher on the other. To see how this might come about, we have to comprehend how a morphogen would be transported and mixed by the nodal flow. Mixing in the creeping flow in the node is very different from what we are more accustomed to seeing at higher Reynolds numbers; for example, when we stir milk into our tea or coffee. In particular, turbulent mixing plays no role here; for no turbulence occurs at low Reynolds numbers. We emphasize this, because some articles on nodal flow have described the slow vortical flow in the Inv mouse as turbulent; it is not. What is seen in the node is always laminar flow, and the complex particle paths observed in low-Reynolds-number flows arise not from turbulence, but from another phenomenon, chaotic advection (17). Two factors are important in mixing in creeping flows: advection by the flow and molecular diffusion. Advection can be quantified by an advective mixing time τa = L/v, where L and v, as given earlier, provide us with the estimate 1 < τa < 5 s for the time for a morphogen to be advected across the node in normal mice. On the other hand, molecular diffusion is independent of fluid flow and takes place at a rate determined by the diffusivity of the morphogen, which depends on its molecular mass. For biological macromolecules like proteins, the diffusion coefficient D is typically in the range from 10-11 to 10-10 m2·s-1. We can then estimate a diffusive mixing time τd = L2/D for a morphogen in the node, which lies in the range 25 < τd < 250 s, and compare the relative importance of advection and diffusion with the ratio τd/τa of these mixing times. This dimensionless ratio is called the Péclet number Pe = vL/D. Our estimate for the Péclet number is then in the range 5 < Pe < 250, greater than unity (if it were not, advection would be unimportant), but relatively small, so the contribution of diffusion is fundamental to mixing and cannot be ignored. In physical terms then, morphogen transport and mixing is an advection-diffusion system. The implication of this analysis for the biology is that the morphogen must be degraded and become inactive rapidly after its release, in a timescale shorter than the diffusive mixing time, otherwise it would reach a constant concentration throughout the node and be of no use for initiating symmetry breaking through concentration differences.
Many possibilities exist a priori for the positions of the sources and receptors of morphogen within the node. Okada et al. (5) and Vogan and Tabin (18) analyzed a series of models for compatibility with the genetic and biochemical evidence. We, in addition, require that any scheme be faithful to the fluid-dynamical, transport, and mixing properties of the nodal flow. We present a model that fulfills all these requirements in Fig. 4 b and c, in which we exhibit the results of numerical simulations of advection-diffusion equations in a rectangular domain representing the flow in a cross section of the node as in Fig. 4a. Morphogen is released from the perinodal regions at the left and right edges of the node and detected by receptors across the whole floor of the node. Because the sources are in the upper part of the node, the morphogen finds itself to begin with in the upper recirculatory vortex. The morphogen coming from the right side is swept leftward across the node in the central leftward current, while that from the left side is pushed rightward across the roof of the node by the upper recirculatory vortex. While it is advected by the flow, the morphogen diffuses. Diffusion allows it to cross flow streamlines and so to penetrate into the lower recirculatory vortex, where it can come into contact with the receptors on the floor of the node. Because the leftward flow is ≈5 μm above the floor of the node, the characteristic vertical diffusion time down to the floor of the node is on order of 0.25-2.5 s. If we compare this with the advection time across the node, 1-5 s for normal animals, and twice as long or more for Inv mice, it is clear that the side of the node on which the maximum concentration of morphogen reaches the node floor will depend crucially on this advection time. In a normal animal the morphogen in the central leftward current is swept to the left side of the node before it arrives by diffusion at the node floor, but with the slower nodal flow in Inv mice the advection time is longer, so the diffusing morphogen arrives at the node floor on the right side of the node and activates the right-side receptors instead of the left. Shortly thereafter, the morphogen must become degraded, so that although it will continue circulating within the node, it is now in an inactive form and unable to dock with the receptors. As a result, the steady-state active morphogen concentration pattern at the floor of the node is as shown in Fig. 4 d and e, with the maximum on the left in normal animals, but with the maximum on the right in Inv mice. This steady state, maintained for the duration of the nodal flow, for some hours (5), during the relevant stage of embryonic development, must be the signal to activate the biochemical machinery that should inform the rest of the embryo about visceral positioning (11).
Our model thus explains the symmetry breaking seen in both normal and Inv mice. In the earlier delayed-activation model proposed by Okada et al. (5), a signaling molecule is released at the left and right sides of the node in an inactive form, and receptors are present across the whole of the floor of the node. Some time after release, the molecule becomes activated and can dock with the receptors. According to this hypothesis, in a normal animal the activated morphogen would dock with more receptors on the left side, whereas in an Inv mouse the slower flow would allow the morphogen to become activated while still on the right side of the node, producing situs inversus. By taking into account the fluid dynamics, we have seen that delayed activation is not necessary, although it is perfectly compatible with our model, because the molecular diffusion time from the central nodal current to the floor ensures that a morphogen does not immediately come into contact with its receptors. Furthermore, by taking into consideration the closed nature of the node and the recirculation pattern of flow it implies, we have shown that although an activation time for the morphogen is not necessary, an inactivation time is. The morphogen must have a window of activity, between an initial time that could be zero (instant activation) or nonzero (delayed activation), and a final time <τd, the diffusive mixing time in the node. The second population of monocilia recently observed in the node (16) has been speculated to be mechanoreceptors for the flow. Monocilia possess the necessary biochemical machinery for both mechano- and chemoreception (19). We suspect that in the node they may not be mechanoreceptors but instead are chemoreceptors for the morphogen.
Discussion
Biological symmetry breaking is a problem that has long interested physical scientists. In his pioneering work on the chemical basis of morphogenesis, Turing (20) proposed the mechanism of pattern formation through the interaction of diffusing morphogens that now bears his name. He recognized the special problem for his theory posed by left-right symmetry breaking in vertebrates; how to explain that nature almost always breaks left-right symmetry in a given sense, whereas his mechanism would lead to approximately equal numbers of animals with situs inversus and situs solitus? He proposed that the input to the system must somehow be biased. Almirantis and Nicolis (21) later showed in detail how an initial gradient could seed the process. Brown and Wolpert (22) hypothesized a chiral molecule with a fixed orientation relative to the anterior-posterior and dorsal-ventral axes, which would provide the necessary information on left and right for the initial biasing. But nature, it seems, in mice at least, prefers to use not a chiral molecule, but a chiral structure, a molecular motor, to provide advection in a given direction relative to the anterior-posterior and dorsal-ventral axes to seed the symmetry breaking. The information on which side is which is then carried to the rest of the embryo by diffusion. Hamada et al. (11) propose that the subsequent phase of propagation of the broken symmetry could function by exactly the mechanism Turing imagined, with the initial small concentration difference produced by the nodal flow magnified by a nonlinear interaction between two diffusing proteins, Nodal and Lefty. A further possibility is that the interaction may involve the fluid flow itself, which would make this a biological instance of a recently proposed generalized Turing pattern-formation mechanism including fluid flow (23).
Here, we have discussed experiments on mice, but structures similar to the node with its monocilia are found in other vertebrate embryos, so it has been surmised that this left-right symmetry-breaking mechanism may be universal among vertebrates (9). On the other hand, experimental evidence has been presented for asymmetries in chick and frog embryos before the emergence of the nodal structures (24). If these observations prove to be correct, then nodal flow is not the earliest left-right symmetry-breaking event in some vertebrates. If the initial symmetry-breaking mechanism differs across species, the chiral molecule or structure, equivalent to the chiral monocilium of the node, that bootstraps the process must be sought for those cases. Moreover, the role of the nodal flow in those species would need to be clarified: Would it then be acting as a means to preserve or amplify an initial asymmetric signal from the earlier symmetry-breaking event, or would it constitute a second, independent mechanism for determining left and right? More evidence needs to be collected.
Diffusion is ubiquitous in biology. Nature also often uses advection to achieve its ends, for example, in the cardiovascular system, and it has recently been found to be fundamental in the development of the heart (25). The use of cilia to move fluid is also common, for example, in the lung. Microbes use cilia and flagella for propulsion, just as the node uses them to advect fluid, and the similarity of scale implies a similarity of environment. This finding highlights the resemblance of the situation to that of microbial swimming. In both cases, we are talking of life at low Reynolds numbers (26). The problems of moving fluids at the microscale, with their associated low Reynolds numbers, are also now interesting engineers who design fluid flow microsystems; so-called microfluidics devices (27). We humans inhabit a world of much higher Reynolds numbers, and our intuition on how fluids behave is not straightforwardly transferrable into this alien inertialess environment, which is why some ideas put forward for producing a directional flow from rotating cilia cannot work. In creeping flow, algorithms, like those varying the angular velocity of the cilia in different parts of the rotation cycle, do not obtain the desired effect, because the fluid has no inertia. Similarly, the idea of the mechanical sensing of the shear stress on the node walls, or the velocities of the flow at the side walls, rather than a morphogen, being responsible for the symmetry breaking is not tenable, since at low Reynolds numbers the magnitudes of the shear stresses and flow velocities are symmetric across the node; it is only the direction that breaks the symmetry. Mechanosensing of the flow by cilia (28, 19) could then provide the signal for symmetry breaking only if the sensors could detect not just the magnitude but also the direction of the flow; however, no reports have been published that monocilia possess this vectorial capability. [Such a signal is produced, for example, by the hair cells of the ear (29), but these are cells possessing bundles of cilia.] Producing the nodal flow is not like waving your arms about in a swimming pool (30), but more akin to finding oneself “in a swimming pool that is full of molasses, and... forbid[den]... to move any part of [the] body faster than 1 cm/min” (26). In the nodal environment, nine orders of magnitude lower in Reynolds number than the person above in a swimming pool, the lack of inertia constrains the fluid physics that the biology can exploit, leaving our proposed mechanisms of a posterior tilt of the cilia and the chemosensing of the flow as the best hypotheses, compatible with the facts, for producing the observations reported in experiments.
The direction of rotation of a monocilium is determined by the motor proteins that power its molecular motor. These, like the vast majority of naturally occurring proteins, are made up of chiral amino acids in just one of their two possible forms: they are all laevo. An equivalent molecular motor using the same amino acids in their opposite, dextro, configuration would rotate in the reverse direction. In this way left-right symmetry breaking here is ultimately determined by the chirality of natural amino acids, not directly through a chiral molecule, but by setting the direction of the nodal flow. Several schools of thought exist as to how this natural chirality has arisen. One supposes that it is a frozen accident and that life could equally well have chosen to use dextro amino acids, but a competing idea points out that laevo amino acids are slightly more stable than the dextro forms owing to the broken parity of the weak nuclear force (31). This hypothesis provides us with the fascinating idea that we may have our hearts on the left because, as Wolfgang Pauli famously put it, God is weakly left-handed (3). But, whether or not there turns out to be a causal link between parity violation and the asymmetry of the vertebrate body plan, after answering the how part of the symmetry-breaking problem, the most intriguing question that remains may be: Why does nature take care to break the symmetry in a given direction, rather than leaving things to chance and allowing half the population to have situs inversus?
Supplementary Material
Acknowledgments
We thank Chris McManus and Yukio Saijoh for useful correspondence. O.P. and I.T. received financial support from the Spanish Ministerio de Ciencia y Tecnología Projects conoce and imagen.
References
- 1.Beddington, R. S. P. & Robertson, E. J. (1999) Cell 96 195-209. [DOI] [PubMed] [Google Scholar]
- 2.Torgersen, J. (1950) Am. J. Hum. Genet. 2 361-370. [PMC free article] [PubMed] [Google Scholar]
- 3.McManus, C. (2002) Right Hand, Left Hand (Weidenfeld & Nicholson, London).
- 4.Nonaka, S., Tanaka, Y., Okada, Y., Takeda, S., Harada, A., Kanai, Y., Kido, M. & Hirokawa, N. (1998) Cell 95 829-837, and [DOI] [PubMed] [Google Scholar]; erratum (1999) 99 117.
- 5.Okada, Y., Nonaka, S., Tanaka, Y., Saijoh, Y., Hamada, H. & Hirokawa, N. (1999) Mol. Cell 4 459-468. [DOI] [PubMed] [Google Scholar]
- 6.Afzelius, B. A. (1999) Int. J. Dev. Biol. 43 283-286. [PubMed] [Google Scholar]
- 7.Capdevila, J., Vogan, K. J., Tabin, C. J. & Izpisúa Belmonte, J. C. (2000) Cell 101 9-21. [DOI] [PubMed] [Google Scholar]
- 8.Supp, D. M., Potter, S. S. & Brueckner, M. (2000) Trends Cell Biol. 10 41-45. [DOI] [PubMed] [Google Scholar]
- 9.Essner, J. J., Vogan, K. J., Wagner, M. K., Tabin, C. J., Yost, H. J. & Brueckner, M. (2002) Nature 418 37-38. [DOI] [PubMed] [Google Scholar]
- 10.Nonaka, S., Shiratori, H., Saijoh, Y. & Hirokawa, N. (2002) Nature 418 96-99. [DOI] [PubMed] [Google Scholar]
- 11.Hamada, H., Meno, C., Watanabe, D. & Saijoh, Y. (2002) Nature Rev. Genet. 3 103-113. [DOI] [PubMed] [Google Scholar]
- 12.Afzelius, B. A. (1976) Science 193 317-319. [DOI] [PubMed] [Google Scholar]
- 13.Stern, C. D. (2002) Nature 418 29-30. [DOI] [PubMed] [Google Scholar]
- 14.Batchelor, G. K. (1970) J. Fluid Mech. 41 545-570. [Google Scholar]
- 15.Currie, I. G. (1993) Fundamental Mechanics of Fluids (McGraw-Hill, New York), 2nd Ed.
- 16.McGrath, J., Somlo, S., Makova, S., Tian, X. & Brueckner, M. (2003) Cell 114 61-73. [DOI] [PubMed] [Google Scholar]
- 17.Ottino, J. M. (1989) The Kinematics of Mixing: Stretching, Chaos, and Transport (Cambridge Univ. Press, Cambridge, U.K.).
- 18.Vogan, K. J. & Tabin, C. J. (1999) Nature 397 295-298. [DOI] [PubMed] [Google Scholar]
- 19.Pazour, G. J. & Witman, G. B. (2003) Curr. Opin. Cell Biol. 15 105-110. [DOI] [PubMed] [Google Scholar]
- 20.Turing, A. M. (1952) Philos. Trans. R. Soc. London B 237 37-72. [Google Scholar]
- 21.Almirantis, Y. & Nicolis, G. (1987) Bull. Math. Biol. 49 519-538. [DOI] [PubMed] [Google Scholar]
- 22.Brown, N. A. & Wolpert, L. (1990) Development (Cambridge, U.K.) 109 1-9. [DOI] [PubMed] [Google Scholar]
- 23.Satnoianu, R. A., Maini, P. K. & Menzinger, M. (2001) Physica D 160 79-102. [Google Scholar]
- 24.Mercola, M. (2003) J. Cell Sci. 116 3251-3257. [DOI] [PubMed] [Google Scholar]
- 25.Hove, J. R., Köster, R. W., Forouhar, A. S., Acevedo-Bolton, G., Fraser, S. E. & Gharib, M. (2003) Nature 421 172-177. [DOI] [PubMed] [Google Scholar]
- 26.Purcell, E. M. (1977) Am. J. Phys. 45 3-11. [Google Scholar]
- 27.Gad-el-Hak, M. (1999) J. Fluids Eng. 121 5-33. [Google Scholar]
- 28.Tabin, C. J. & Vogan, K. J. (2003) Genes Dev. 17 1-6. [DOI] [PubMed] [Google Scholar]
- 29.Roberts, W., Howard, M. J. & Hudspeth, A. J. (1988) Annu. Rev. Cell Biol. 4 63-92. [DOI] [PubMed] [Google Scholar]
- 30.Whitfield, J. (July 4, 2002) Nat. Sci. Update, www.nature.com/nsu/020701/020701-7.html.
- 31.Mason, S. F. (1984) Nature 311 19-23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.