Abstract
The neural circuits underlying emotional valence and motivated behaviors are several synapses away from both defined sensory inputs and quantifiable motor outputs. Electrophysiology has provided us with a suitable means for observing neural activity during behavior, but methods for controlling activity for the purpose of studying motivated behaviors have been inadequate: electrical stimulation lacks cellular specificity and pharmacological manipulation lacks temporal resolution. The recent emergence of optogenetic tools provides a new means for establishing causal relationships between neural activity and behavior. Optogenetics, the use of genetically-encodable light-activated proteins, permits the modulation of specific neural circuit elements with millisecond precision. The ability to control individual cell types, and even projections between distal regions, allows us to investigate functional connectivity in a causal manner. The greatest consequence of controlling neural activity with finer precision has been the characterization of individual neural circuits within anatomical brain regions as defined functional units. Within the mesolimbic dopamine system, optogenetics has helped separate subsets of dopamine neurons with distinct functions for reward, aversion and salience processing, elucidated GABA neuronal effects on behavior, and characterized connectivity with forebrain and cortical structures. Within the striatum, optogenetics has confirmed the opposing relationship between direct and indirect pathway medium spiny neurons (MSNs), in addition to characterizing the inhibition of MSNs by cholinergic interneurons. Within the hypothalamus, optogenetics has helped overcome the heterogeneity in neuronal cell-type and revealed distinct circuits mediating aggression and feeding. Within the amygdala, optogenetics has allowed the study of intra-amygdala microcircuitry as well as interconnections with distal regions involved in fear and anxiety. In this review, we will present the body of optogenetic studies that has significantly enhanced our understanding of emotional valence and motivated behaviors.
Keywords: Optogenetics, Systems, Circuit dissection, Behavior, Emotion, Motivation, Brain, Projections, ChR2, NpHR, Opsin, Valence
1. Introduction
If evolution were a game, the objective would be to spread your genes to as many surviving offspring as possible (Darwin, 1909). The strategy would be straightforward: take actions that will support, and avoid actions that will threaten, the survival of you or your descendants. The challenges are (1) fierce competition and (2) an ever-changing environment overflowing with both familiar and novel sensory stimuli. To be competitive, you must filter out unimportant information, identifying whether certain cues are likely to have a positive or negative impact on your objective and subsequently select an appropriate behavioral response. To win, you must do this quickly and accurately.
Evolution has produced many winning designs, but all of these designs share the critical ability to differentiate between good and bad stimuli (Tooby and Cosmides, 1990). Emotions have been hypothesized to be a biological strategy for rapidly integrating previously recorded data (weighted for significance), assigning a motivational value to the stimulus, and orchestrating an appropriate behavioral response (Cosmides and Tooby, 1987; Barkow et al., 1995). In vertebrates, particularly mammals, the neural circuits thought to be important for this ability are remarkably well-conserved (MacLean, 1990). However, the precise neural mechanisms underlying the differentiation between positive and negative emotional valence are still poorly understood.
Despite the importance of understanding valence processing, technical, experimental, and practical obstacles have impeded progress in this field. First, for the field of behavioral neuroscience, studying sensory and motor systems (more amenable to discrete inputs and outputs) may have been a logical prerequisite. Second, regions involved in emotional valence processing are comprised of many different cell-types with heterogeneous functional roles that are not spatially segregated from each other within these “primitive” subcortical structures. Indeed, many regions such as the amygdala (LeDoux, 2000; Paton et al., 2006; Tye et al., 2008; Shabel and Janak, 2009; Pape and Pare, 2010), ventral tegmental area (Wise and Rompre, 1989; Schultz, 1998; Tan et al., 2012; van Zessen et al., 2012), and hypothalamus (Atasoy et al., 2012; Harris and Aston-Jones, 2006; Aponte et al., 2011; Lin et al., 2011) have been implicated in both positive and negative valence processing. This organization makes classical techniques that involve spatially-defined manipulations alone difficult to interpret on a mechanistic level. Third, valence processing involves a spatially distributed network and a multitude of parallel circuits, which demands projection-specific circuit-level control for functional dissection (Fig. 1). The indispensable nature of these functions may have led to the diffuse spatial distribution and system redundancy, which provide an evolutionary advantage in the face of accidents or injuries.
Fig. 1. Behavioral circuits characterized by optogenetics.

One of the greatest strengths of optogenetics is the ability to activate distinct subsets of neurons based on biochemical composition or projection target with high temporal resolution. Cell-type-specific targeting has allowed us to establish causal relationships between neural circuit elements and specific behaviors. In addition, the ability to perform projection-specific targeting has allowed us to study functionally-distinct distal projections from individual structures previously thought to be homogeneous in function. These advantages have allowed optogenetics to help build us a more comprehensive neural map of the circuits involved in emotional valence and motivated behaviors: (A) reward, aversion, salience; ((B)–(D)) aversion; (E) reward; (F) aversion; (G) reward, aversion; ((H)–(J)) reward; (K) reward, aversion, movement; (L) aggression; ((M) and (N)) feeding; ((O) and (P)) fear; (Q) anxiety; ((R)–(U)) Fear; (V) Promotes Escape-Related Behavior; (W) Suppresses Escape-Related Behavior. For all figures, structures depicted are not to scale and include a subset of optogenetic behavioral manipulations. Abbreviations: AC, auditory cortex; ARC, arcuate nucleus; BLA, basolateral amygdala; CeA, central amygdala; DG, dentate gyrus; DRN, Dorsal Raphe; EP, entopeduncular nucleus; HPC, hippocampus; LDT, laterodorsal tegmentum; LHb, lateral habenula; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; PVH, paraventricular hypothalamus; RMTg, rostromedial tegmental area; STRd, dorsal striatum; VMHvl, ventrolateral subdivision of the ventromedial hypothalamus; VTA, ventral tegmental area.
Optogenetic tools that allow for cell-type (Boyden et al., 2005; Zhang et al., 2007; Atasoy et al., 2008) and even projection-specific (Tye et al., 2011; Stuber et al., 2011) manipulation of neural activity with precise temporal control have given us the ability to overcome many of these obstacles. Although emotions are difficult to quantify, motivated behaviors provide a measurable output that summarizes many factors, including emotional state. Optogenetic tools have accelerated our understanding of a vast array of neural phenomena (Tye and Deisseroth, 2012). Here, we focus on the burst of recent insights towards understanding the neural circuits encoding emotional valence and motivated behaviors.
2. Breaking down reward: Classical views of ventral tegmental area function and new insights into the mesolimbic dopamine system
The mesolimbic dopamine system consists of the ventral tegmental area (VTA) and the regions it provides dopaminergic innervation to, including the nucleus accumbens (NAc), amygdala, hippocampus (HPC), and prefrontal cortex (PFC) (Fields et al., 2007). Dopamine neurons in the midbrain have long been thought to serve a central role in reward prediction (Romo and Schultz, 1990; Mirenowicz and Schultz, 1994). In a seminal study, Schultz et al. (1997); Schultz (1998) showed that putative dopamine neurons of the VTA are activated by presentations of unexpected rewards and inhibited by omissions of expected rewards, evidence that neuronal activity reflects a model of learning based on divergences from expectations (Rescorla and Wagner, 1972). Sugrue et al., (2005) proposed an actor-critic conceptual model for the decision-making process, in which the VTA plays a role as the critic, alongside the ventral striatum, judging valuations of higher order processes by taking in predictions about the value of a stimulus and outputting error signals comparing these predictions with the actual reward obtained.
The significant contribution of the VTA to processing motivation and hedonia makes it a region likely to be perturbed in pathological states, such as addiction and other neuropsychiatric disorders (Wise, 2002, 2005; Everitt and Robbins, 2005; Kalivas, 2005; Hyman et al., 2006; Nestler and Carlezon Jr, 2006; Fields et al., 2007; Grace et al., 2007; Schultz, 2007; Lüscher and Malenka, 2011). Although the VTA has long been implicated in addiction, reward processing, and learning (Koob, 1992; Bonci and Malenka, 1999; Di Chiara, 1999; Nestler, 2001; Picciotto and Corrigall, 2002; Wise, 2004), the advent of optogenetics has clarified the synaptic, cellular, and circuit mechanisms underlying these processes as discussed below.
2.1. Optogenetic tools aid in the identification of dopamine and GABA neurons and their functions in the ventral tegmental area
The VTA is a heterogeneous structure containing dopamine neurons (~65%), GABA neurons (~30%), and glutamate neurons (~5%) (Margolis et al., 2006; Yamaguchi et al., 2007; Nair-Roberts et al., 2008; Dobi et al., 2010). Historically, researchers have used electrophysiological criteria based on waveform characteristics and firing rates to identify dopamine neurons (Grace and Bunney, 1980,1983; Wang, 1981; Grace and Onn, 1989), but recent studies have shown that there may be non-dopaminergic neurons in the VTA that may also fit into these criteria (see Ungless and Grace, 2012 for an in-depth review). Ungless and Grace conclude in their review that using extracellular criteria is sufficient to identify dopamine neurons, but acknowledge that there is still a possibility of misidentification. However, Margolis et al. (2006) offer an opposing viewpoint, concluding that dopamine neurons cannot be distinguished from other VTA neurons by size, shape, or various electrophysiological criteria.
The recent advent of optogenetic tools has provided a new method for characterizing neurons by cell-type, essentially eliminating the need to classify dopamine neurons by electrophysiological markers (Cohen et al., 2012). Several studies have combined the use of transgenic animals expressing Cre-recombinase with Cre-inducible viral vectors to selectively target dopamine neurons with optical stimulation (Tsai et al., 2009; Witten et al., 2011; Cohen et al., 2012). Given that dopamine neurons express tyrosine hydroxylase (TH), one strategy first employed by Tsai et al. (2009) to selectively target dopamine neurons was to deliver a Cre-inducible adeno-associated viral (AAV) vector carrying the light-activated cation channel channelrhodopsin-2 (ChR2) into the VTA of TH::Cre transgenic mice to limit expression of ChR2 to TH-positive neurons.
Using a similar cell-type specific targeting technique with dopamine transporter (DAT)::Cre mice and GABA transporter (VGAT)::Cre mice, Cohen et al. (2012) were able to selectively activate dopamine and GABA neurons in vivo. Following electrophysiological recordings during a Pavlovian conditioning task, the authors measured each neuron’s response to photostimulation. By examining which units responded in either the DAT::Cre or VGAT::Cre mice, they were able to identify recorded units as either dopaminergic or GABAergic, an approach commonly referred to as “phototagging” (Lima et al., 2009). The authors confirmed that dopamine neurons are involved in reward-prediction error (Schultz et al., 1997), increasing their firing rates during reward-predicting cues and decreasing their firing rates when expected rewards are omitted. These results fit well into the traditional views of the VTA as a critic, with dopamine neuronal activity reflecting reward prediction error.
With respect to GABA neuronal function, Cohen et al. (2012) found that GABA neurons showed persistent activity during the delay between a reward-predictive cue and the reward that reflected the value of the upcoming reward (big, small, or none). Combined with the lack of modulation by the delivery or omission of the reward, these data suggest that GABA neurons encode reward expectation, and thus are not affected by the reward itself. In addition, GABA neurons were also excited by aversive stimuli, potentially suppressing dopaminergic activity in response to aversive events. In fact, many addictive drugs are known to inhibit GABA neurons (Hyman et al., 2006; Tan et al., 2010; Lüscher and Malenka, 2011), likely leading to the sustained reinforcement of addiction (Redish, 2004; Cohen et al., 2012).
Two other studies also aimed to elucidate the function of GABA neurons in the VTA (Tan et al., 2012; van Zessen et al., 2012). Tan and colleagues selectively expressed ChR2 in GABA neurons using glutamate decarboxylase (GAD)::Cre transgenic mice. From recordings of neuronal activity in the VTA, the authors found that not only are dopamine neurons inhibited by GABA neurons, activation of GABA neurons expressing ChR2 or direct inhibition of dopamine neurons by halorhodopsin (NpHR), a light-activated chloride pump, elicited conditioned place aversion (CPA) (Tan et al., 2012) (Fig. 2). While some studies use the acronym eNpHR3.0 to refer specifically to the third variant of NpHR (Gradinaru et al., 2010), many studies use the acronym NpHR interchangeably. Taking a similar approach, van Zessen and colleagues selectively expressed ChR2 in GABA neurons of VGAT::Cre transgenic mice. Activation of VTA GABA neurons was shown to disrupt the reward consummatory behavior by inducing early termination of licking for sucrose during a cue-evoked reward-seeking task (van Zessen et al., 2012). In addition, GABA neuronal activation was shown to directly suppress the activity and excitability of dopamine neurons in the VTA and the release of dopamine in the NAc (Fig. 1A and Fig. 2). The authors also showed that while optogenetic stimulation of GABA neurons in the VTA could directly affect reward consummatory behavior, stimulating their inputs into the NAc could not (van Zessen et al., 2012), indicating that intra-VTA GABA inhibition of dopamine neurons likely mediates the interruption of reward consummatory behavior. One important caveat to note is that when illuminating axon terminals, which are more spatially dispersed than cell bodies, it can be difficult to evoke behavior due the smaller volume of illumination. This may be another possible explanation for why GABA cell-body stimulation in the VTA was able to evoke a behavioral change, while GABA terminal stimulation in the NAc was not.
Fig. 2. A new model of the mesolimbic dopamine system characterized by optogenetics.
Optogenetic studies have expanded our understanding of the distal circuit dynamics, the extended inputs and outputs, and the causal relationships underlying behavior in the mesolimbic dopamine system. Optogenetic tools have allowed us to test hypotheses about the heterogeneity of the VTA and the NAc with temporal precision in awake behaving animals, characterizing individual projections that process reward (LDT-VTA-NAc) and aversion (LHb-VTA-mPFC). Cell-type specific targeting of ChR2 has shown that GABAergic neurons in the VTA negatively regulate reward, while striatal dMSNs and iMSNs have opposing functions on valence processing. NAc MSNs receive excitatory input from vHPC, PFC, and BLA mediating reward and are modulated by cholinergic interneurons. Dotted lines represent non-optogenetic findings. Double hash marks represent uncertainty in neuronal population from projection origin. Abbreviations: ACh, acetylcholine; BLA, basolateral amygdala; DA, dopamine; dMSN, direct pathway medium spiny neuron; Glu, glutamate; HPC, hippocampus; iMSN, indirect pathway medium spiny neuron; IL, infralimbic cortex; LDT, laterodorsal tegmentum; LHb, lateral habenula; mPFC, medial prefrontal cortex; MSN, medium spiny neuron; NAc, nucleus accumbens; PL, prelimbic cortex; RMTg, rostromedial tegmental area; vHPC, ventral hippocampus; VTA, ventral tegmental area.
2.2. Functional heterogeneity of dopamine neurons—reward, aversion, and salience
If dopamine neuron activity truly mirrored reward prediction error, we would expect aversive stimuli to inhibit dopaminergic activity. While some studies show data that dopamine neurons are universally inhibited by aversive stimuli (Ungless et al., 2004), others report both inhibition and excitation (Chiodo et al., 1980; Schultz and Romo, 1987; Mantz et al., 1989; Guarraci and Kapp, 1999; Coizet et al., 2006; Matsumoto and Hikosaka, 2009; Tan et al., 2012). Matsumoto and Hikosaka (2009) suggest that neurons excited by both rewarding and aversive stimuli may reflect the salience of the stimulus. Importantly, the authors also showed that dopamine neurons inhibited and excited by aversive stimuli were anatomically distinct. Lammel et al. (2011) showed that the projection targets of dopamine neurons could also separate their function: projections to NAc medial shell responded to rewarding stimuli, projections to medial prefrontal cortex (mPFC) responded to aversive stimuli, and projections to NAc lateral shell responded to both rewarding and aversive stimuli, possibly representing salience.
In addition, afferent projections to the VTA may also target distinct subpopulations of dopamine neurons. Matsumoto and Hikosaka (2007) showed that lateral habenula (LHb) neurons responded to reward in an opposing manner to dopamine neurons and that electrical stimulation in the LHb inhibited dopamine neurons. The rostromedial tegmental area (RMTg), also known as the posterior tail of the VTA (Perrotti et al., 2005; Kaufling et al., 2009), is composed of inhibitory GABA neurons that project to dopamine neurons in the midbrain (Kaufling et al., 2009). Jhou et al. (2009) showed robust excitatory input from the LHb to the RMTg and inhibitory input from the RMTg to the VTA (Jhou et al., 2009; Matsui and Williams, 2011), suggesting a LHb-RMTg-VTA pathway that mediates responses to aversive stimuli. Stamatakis and Stuber (2012) expressed ChR2 in LHb neurons of mice and implanted optical fibers above LHb terminals in the posterior VTA and the RMTg. Behavioral experiments showed that optical stimulation of LHb terminals caused CPA, was capable of providing negative reinforcement, and also disrupted positive reinforcement (Fig. 1B and C, and Fig. 2). Shabel et al. (2012) examined projections from the entopeduncular nucleus (EP) to the LHb by expressing ChR2 in the EP and optically stimulating terminals in the LHb (Fig. 1D). The authors found that animals developed a clear aversion to optical stimulation of this pathway. In addition, serotonin, a neuromodulator that plays a major role in depression, was shown to suppress activity in this pathway, providing an insight into how serotonin may help to modulate reward processing (Shabel et al., 2012).
The laterodorsal tegmentum (LDT) is another brain region that has been shown to innervate the VTA (Cornwall et al., 1990). In addition, electrical stimulation was shown to elicit dopamine release in the NAc, mediated by glutamatergic and cholinergic receptors in the VTA (Forster et al., 2002), suggesting a LDT-VTA-NAc pathway involved in processing reward. In a recent study, Lammel et al., 2012 used a combination of tracing techniques and retrograde/anterograde delivery of ChR2 to study both the projection targets of VTA dopamine neurons and afferent projections to the VTA. By infusing a retrograde rabies virus (RV) containing ChR2 into the VTA and differentially targeting two groups of animals with optical fibers in LDT or LHb, the authors induced conditioned place preference (CPP) and conditioned place aversion (CPA) by optical stimulation of these two pathways, respectively. In addition, by combining retrobead injection in the mPFC or NAc with anterograde delivery of ChR2 into the LDT or LHb, the authors could perform whole-cell recordings of retrogradely-labeled VTA neurons that could be optically stimulated ex vivo. Using this technique, LDT neurons were found to preferentially synapse onto dopamine neurons in lateral VTA projecting to the NAc lateral shell, while LHb neurons were found to preferentially synapse onto both dopamine neurons in medial VTA projecting to the mPFC and rostromedial tegmental nucleus (RMTg) GABA neurons. Moreover, optical stimulation of the LHb induced inhibitory postsynaptic currents (IPSCs) in dopamine neurons projecting to NAc lateral shell. These data indicate a distinct separation of two circuits in the VTA–while the LDT-lateral VTA-NAc lateral shell pathway encodes reward (Fig. 1A and E, and Fig. 2), the LHb-medial VTA-mPFC pathway and disynaptic inhibition of dopamine neurons projecting to NAc lateral shell (likely via RMTg neurons) encode aversion (Lammel et al., 2012) (Fig. 1B and F, and Fig. 2).
In fact, evidence of opposing functions of dopamine neurons in the VTA may also explain how addictive drugs differentially hijack neural circuits. Koo et al. (2012) found that although brain-derived neurotrophic factor (BDNF) is a positive regulator of neural plasticity in dopamine neurons to promote the actions of cocaine and other stimulants (Horger et al., 1999; Hall et al., 2003; Graham et al., 2007; Lobo et al., 2010), it plays an opposite role in the effects of morphine. BDNF knockdown in the VTA enhanced the ability of morphine to increase dopamine neuron excitability and promote reward, and optogenetic stimulation of ChR2-expressing VTA dopamine neurons in NAc reversed the suppressive effect of BDNF on morphine. These data imply that drugs of abuse may act in different ways on the VTA or recruit different subsets of VTA neurons.
Together, these studies suggest that classical approaches of organizing brain function by neurotransmitter or brain region are not sufficient. While it appears that some dopamine neurons respond as predicted by traditional models of reward processing, newer data has found parallel components involved in aversion and salience processing. Revisiting the critic role of the VTA in an actor-critic model, it is possible that the salience circuit is used to “weigh” the valuation of a stimulus rather than to simply increase or decrease it. For example, a rewarding and salient stimulus would be assigned a high value, but a rewarding and non-salient stimulus would only be assigned a moderate value. In addition, two separate streams for reward and aversion processing allows for the separation of the omission of a rewarding stimulus from the presence of an aversive stimulus. A system with these extra parallel processors would be more effective and efficient in evaluating stimuli than a system where the only processor is for reward prediction error (Fig. 3).
Fig. 3. Actor–director–critic model for decision-making.
In the actor-director-critic model for decision-making, the VTA plays the role of the critic, while the ventral and dorsal striatum play the roles of the director and actor, respectively. The VTA send dopaminergic and GABAergic signals downstream to the STRv representing the value of a cue. The STRv integrates information from the VTA with inputs from other brain regions and sends a signal through the OFC or SNc to modulate STRd activity. The STRd generates an action signal that is processed downstream to generate the appropriate motor response for the cue. However, there is also evidence that STRd encodes reward and aversion as well (See text). Abbreviations: dMSN, direct pathway medium spiny neuron; iMSN, indirect pathway medium spiny neuron; MSN, medium spiny neuron; OFC, orbitofrontal cortex; SNc, substantia nigra pars compacta; STRd, dorsal striatum; STRv, ventral striatum; VTA, ventral tegmental area.
3. From motor to motivation: Distinct circuits within the ventral and dorsal striatum
The striatum is a large subcortical area in the forebrain that is associated with a broad set of behavioral processes ranging from goal-directed learning to basic motor functions (Mogenson et al., 1980). In many ways, the striatum is the brain’s central meeting point as information from the cortex, thalamus, and dopaminergic innervation from the midbrain all converge (Smith et al., 1994; Kincaid et al., 1998; Bolam et al., 2000). The striatum has been conceptualized as the final site of information integration before the processing of motor output. The cognitive, motor, and limbic systems give rise to functionally distinct areas of the striatum (Berendse et al., 1992; Voorn et al., 2004; Schilman et al., 2008), though identifying homogenous regions has proven to be difficult. Behaviorally, differences in striatal subregions do not obey clear demarcations and instead follow various gradients (Voorn et al., 2004). However at the highest level, the striatum is commonly segregated into two halves because of their functional contributions to behavior (Voorn et al., 2004). Unlike the ventral striatum, which is anatomically connected to limbic structures and involved in reward-related learning (Kelley and Domesick, 1982; Kelley et al., 1982; McGeorge and Faull, 1989; Cardinal et al., 2002), the dorsal striatum receives inputs from the substantia nigra and cortex and is primarily involved in action selection and movement (Albin et al., 1989; Graybiel et al., 1994; Chang et al., 2002; Lauwereyns et al., 2002; Haber, 2003; Balleine et al., 2007). Here, we discuss how these striatal subregions contribute to valence processing and how optogenetics has helped advance our understanding of striatal mechanisms and circuitry.
3.1. Reward processing in the nucleus accumbens by medium spiny neurons and cholinergic interneurons
Approximately 95% of the striatum is made up of GABAergic medium spiny projection neurons (MSNs), in addition to cholinergic interneurons (~1%) (Kemp and Powell, 1971; Phelps et al., 1985; Rymar et al., 2004; Kreitzer, 2009). MSNs in the NAc are segregated into two subclasses—MSNs expressing D1 receptors that are part of the “direct” pathway (dMSN) and those expressing D2 receptors that make up the “indirect” pathway (iMSN) (Gerfen et al., 1990; Gerfen, 1992; Kawaguchi, 1997; Gerfen and Surmeier, 2011). As a result of their differential expression of dopamine receptors and projection targets, dopamine is thought to have opposing effects on MSNs, exciting dMSNs and inhibiting iMSNs (Albin et al., 1989). The existence of these two separate pathways acting in functional opposition to shape behavioral output (Alexander et al., 1986; Graybiel, 2000) has long been an accepted model, but establishing a causal relationship between activation of each subtype and its effect on behavior has proved to be challenging.
Only recently with optogenetics and Bacterial Artificial Chromosome (BAC)-transgenic mice has it been possible to isolate and selectively activate dMSNs or iMSNs (Gong et al., 2003, 2007; Shuen et al., 2008). While the NAc makes up only a portion of what is considered to be the ventral striatum, we focus solely on the NAc for the purposes of this review. Lobo et al. (2010) combined optogenetic strategies with cocaine treatment to show that activating dMSNs in the NAc enhances, whereas activating iMSNs in the NAc suppresses, the effects of cocaine reward (Fig. 1G and Fig. 2), validating the idea that the two subtypes have opposing influences on reward processing. In addition to a differentiation of cell-type, the source of excitatory input into NAc also appears to affect cocaine reward. In a study investigating various excitatory afferents to the NAc, Britt et al. (in press) found that ventral hippocampus (vHPC) input to the NAc was predominantly and selectively potentiated from cocaine exposure compared with projections from the basolateral amygdala (BLA) and the PFC. However, activation of each of these discrete pathways was able to invoke CPP and optical intracranial self-stimulation (ICSS) (Fig. 1H–J and Fig. 2). These findings suggest that cocaine may selectively activate only one of several excitatory inputs into the NAc, and future experiments should aim to determine when each of these distinct pathways are active and how they might individually shape behavior. In an earlier study, Stuber et al. (2011) also showed that stimulating BLA terminals in the NAc could evoke ICSS (Fig. 1J and Fig. 2), but that stimulating mPFC terminals could not. This contrast with data obtained from Britt and colleagues may be explained by the coordinates of the viral injection site, optical fiber placement, or illumination parameters. In addition, the authors showed that ICSS required D1, but not D2, signaling in the NAc and that inhibition of NpHR-expressing BLA projections to the NAc reduced cue-evoked intake of sucrose (Stuber et al., 2011).
Striatal MSNs also express muscarinic acetylcholine (ACh) receptors and are modulated by ACh from local cholinergic interneurons (Bolam et al., 1984; Weiner et al., 1990). Pinpointing the functional role of cholinergic interneurons has been a challenge, as they only make up ~1% of the neuronal population (Kemp and Powell, 1971; Phelps et al., 1985; Rymar et al., 2004; Kreitzer, 2009). To resolve this debate, Witten et al. (2010) found that optically inhibiting cholinergic neurons by expressing NpHR in choline acetyltransferase (ChAT)::Cre mice blocked cocaine conditioning (Fig. 2). More recently, two separate studies have also shown that cholinergic activation by optogenetic stimulation elicits dopamine release (Cachope et al., 2012; Threlfell et al., 2012), suggesting that ACh modulates MSN activity by regulating dopamine content.
3.2. Cocaine-induced synaptic plasticity in the nucleus accumbens
In addition to using optogenetic tools to study circuit activity, optogenetics can also be used to investigate the mechanisms of drug-induced synaptic plasticity. Pascoli and colleagues were able to examine the effects of cocaine at the synaptic level by expressing ChR2 in infralimbic cortical cells and optically stimulating terminals in the NAc (Fig. 1H and Fig. 2). The authors optogenetically depotentiated cortical inputs to the NAc to abolish cocaine-induced locomotor sensitization, demonstrating a causal link between cocaine-evoked plasticity and behavioral adaptation (Pascoli et al., 2012). Their findings also demonstrated that cocaine-evoked potentiation relies on extracellular signal-regulated kinase (ERK)-dependent long-term potentiation (LTP) in dMSNs, but not iMSNs (Pascoli et al., 2012).
Optogenetic tools provide new techniques for “remote control” of neural activity, without rupturing the cell membrane, thus allowing a more physiologically relevant LTP induction protocol (Zhang et al., 2008). Extracellular control of neural activity offers the added advantage of manipulating a cell for longer time periods compared to whole-cell patch-clamp, making it possible to study homeostatic plasticity mechanisms that operate over longer time scales (Goold and Nicoll, 2010). One potential technique for studying spike timing dependant plasticity (STDP), is to use ChR2, in combination with the red-shifted ChR1/VChR1 chimaera opsin variant (C1V1) (Yizhar et al., 2011) to allow remote induction of STDP by millisecond-scale manipulation of activity of both the presynaptic and postsynaptic neurons. This can be achieved by selectively stimulating axon terminals of the presynaptic neuron expressing ChR2 with blue light and stimulating the postsynaptic neuron expressing C1V1 with yellow light (Fig. 4).
Fig. 4. Optogenetic strategies for in vivo manipulation of synaptic plasticity.
(A) When a presynaptic terminal is expressing ChR2, the synapse can be manipulated by LTP and LTD induction protocols by stimulating the axon terminals using light delivered by a fiber optic. (B) A synapse with the presynaptic axon terminal expressing ChR2 and postsynaptic neuron expressing C1V1 can be manipulated by spike timing dependent plasticity protocols. (C) A postsynaptic cell expressing C1V1 (or ChR2) can be manipulated by homeostatic plasticity induction protocols. (D) Optogenetics allows selective manipulation of specific synapses, and synapses that do not express either opsin will not be directly manipulated by any of the plasticity induction protocols. Abbreviations: C1V1, ChR1/VChR1 chimaera opsin variant; ChR2, channelrhodopsin-2; LTD, long-term depression; LTP, long-term potentiation.
3.3. Integrating the actor-critic model with the ventral striatum
Looking back at the actor-critic model of decision-making (Sugrue et al., 2005), it is important to note that the ventral striatum may also play a significant role as a critic (Montague et al., 2004; O’Doherty et al., 2004; van der Meer and Redish, 2009), as human functional magnetic resonance imaging (fMRI) data have shown that ventral striatum activity correlates with prediction error during both Pavlovian and instrumental conditioning (O’Doherty et al., 2004). In an extension of the model, Atallah et al. (2007) suggest that the ventral striatum may actually serve as a “director” in an actor–director–critic model of decision-making (Fig. 3). While VTA dopaminergic inputs to the NAc serve as input from the critic, the ventral striatum learns the relative task demands and directs the dorsal striatum to perform an action. Thus, the role of ventral striatum may actually be to facilitate the effect of the critic on the actor, possibly through modulating activity in the orbitofrontal cortex, which provides top-down control of the dorsal striatum (Joel and Weiner, 1994; Frank and Claus, 2006), or through projections to the substantia nigra, which sends dopaminergic projections back to the dorsal striatum (Haber et al., 2000; Joel and Weiner, 2000).
3.4. Diverse functions in the dorsal striatum
Since the dorsal striatum receives its dopamine inputs from the substantia nigra pars compacta (SNc) (Heimer and Wilson, 1975; Grace and Bunney, 1983; Graybiel et al., 1994; Hikosaka et al., 2000), evidence that degeneration of SNc dopamine neurons causes motor deficits in both Parkinson’s disease (PD) and Huntington’s disease (Graybiel, 2000; DeLong, 2007) supports the motor-centric role of dorsal striatum. By expressing ChR2 separately in dMSNs and iMSNs in the dorsal striatum, Kravitz et al. (2010) showed that activation of dMSNs reduced freezing and increased locomotion, while activation of iMSNs induced a Parkinsonian state with increased freezing, bradykinesia, and decreased locomotor initiations (Fig. 1K). These data suggest that degeneration of SNc dopamine neurons likely acts by creating an imbalance in dMSN and iMSN activity.
However, it is important to note that PD patients not only suffer from severe motor impairments, but also display a learning bias, showing enhanced learning from negative feedback and impaired learning from positive feedback. Following dopamine medication (L-Dopa, D2 receptor agonists, and/or monoamine activity enhancers), this disparity was reversed and PD patients became more responsive to positive outcomes than negative ones (Frank et al., 2004). These data would suggest that motor and reinforcement may not be so easily separable.
In a more recent study, Kravitz et al. (2012) again expressed ChR2 in dMSNs and iMSNs and revealed that dorsal striatal MSNs also show similar opposing influences on reinforcement that were shown in the NAc (Lobo et al., 2010). The authors showed that illuminating ChR2-expressing dMSNs in the dorsomedial striatum was sufficient to induce ICSS, whereas activating iMSNs punished the same behavior (Kravitz et al., 2012) (Fig. 1K). This discovery that neurons in dorsal striatum may have similar function to those in ventral striatum shows that our model of striatal function and the dorsal/ventral separation may need to be revisited. In another surprising study, Tritsch et al. (2012) found that dopamine neurons projecting from SNc to dorsal striatum co-release GABA, providing an inhibitory influence on striatal activity. In combination with studies showing glutamate co-release from dopamine neurons projecting to the NAc (Stuber et al., 2010; Tecuapetla et al., 2010), these findings show an interesting dichotomy between dopamine neurons that innervate dorsal versus ventral striatum.
4. Fighting and feeding: Investigating behavioral circuits in the hypothalamus
The hypothalamus is a prime example of functional, structural, and cellular heterogeneity, making it an ideal target for optogenetic approaches to gather new insights linking cell- or projection-specific circuits with behavior. Indeed, some of the seminal in vivo optogenetic studies have been performed in the hypothalamus, providing novel insights into the remarkably diverse set of behaviors central to survival, including aggression (Lin et al., 2011), feeding (Aponte et al., 2011; Domingos et al., 2011; Atasoy et al., 2012), and sleep (Adamantidis et al., 2007, 2010; Carter et al., 2009). Keeping with the theme of emotional valence and motivated behaviors in this review, we will discuss the body of literature encompassing aggression and feeding, and respectfully note that there are significant optogenetic studies on sleep and arousal that we are unable to review here.
4.1. The role of the ventromedial hypothalamus in aggression
More than four decades ago, electrical stimulation studies in cats provided evidence that there may be a specific hypothalamic subregion that mediates aggression (Siegel and Skog, 1970; Chi and Flynn, 1971). This work, replicated in rats, also supported the existence of a hypothalamic attack area (HAA) (Kruk et al., 1983; Lammers et al., 1988; Kruk, 1991; Siegel et al., 1999; Hrabovszky et al., 2005) that serves as the aggression locus in the rodent brain. However, Lin et al. (2011) reported that electrical stimulation in this same region failed to elicit attack behaviors in mice. This circuit is further complicated by evidence that aggression and mating may recruit neurons from the same neural structures, or neurons in close spatial proximity (Kollack-Walker and Newman, 1995; Veening et al., 2005).
By delivering ChR2 into the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) using an AAV that infects neurons preferentially without retrogradely infecting cells from uptake at axons terminals, Lin et al. (2011) could selectively activate cell bodies at the injection site, while sparing axons of passage. The authors found that optical stimulation of the VMHvl was able to acutely evoke aggressive behavior in mice (Lin et al., 2011) (Fig. 1L and Fig. 5). In addition, pharmacological silencing of the VMHvl was able to inhibit aggression. Lin and colleagues explain that failure to evoke the same behavior with electrical stimulation is likely due to overlapping populations of neurons controlling aggression and mating. Distinct neurons, such as those showing increased firing rates during aggression, were inhibited during mating, suggesting opposing inhibition. Electrical stimulation could have stimulated both aggression and mating neurons simultaneously, leading to a zero-sum effect on behavior.
Fig. 5. Optogenetic tools elucidate functional circuits in the hypothalamus related to feeding and aggression.
The hypothalamus is a largely heterogeneous structure that has been difficult to functionally dissect, but the ability of optogenetics to precisely target specific cell-types and projections has allowed for the characterization of circuits mediating feeding and aggression. POMC and AGRP neurons in the ARC play opposing roles on feeding through AGRP inhibition of ARC-POMC and PVH-OT neurons to evoke feeding. In addition, activating neurons in the VMHvl acutely and reversibly induces aggression. Abbreviations: AGRP, agouti-related protein; ARC, arcuate nucleus; OT, oxytocin; POMC, pro-opiomelanocortin; PVH, paraventricular hypothalamus; VMHvl, ventrolateral subdivision of the ventromedial hypothalamus.
4.2. AGRP, POMC, and leptin control of feeding behaviors
Agouti-related protein (AGRP) neurons and proopiomelanocortin (POMC) neurons are two cell populations with competing functions found in the arcuate nucleus (ARC) of the mediobasal hypothalamus. While AGRP neurons promote feeding behaviors (Ollmann et al., 1997), POMC neurons suppress them (Yaswen et al., 1999). Evidence has also been shown that AGRP neurons inhibit POMC neurons (Cowley et al., 2001; Roseberry et al., 2004) and that AGRP directly blocks melanocortin receptors (Ollmann et al., 1997). This has led to a proposal that AGRP neurons might serve as a modulatory signal, blocking the melanocortin pathway to promote feeding (Cowley et al., 2003). However, evidence also exists showing that AGRP’s effect on feeding may be independent of the melanocortin signaling pathway (Wu et al., 2008).
Aponte et al. (2011) were able to express ChR2 into AGRP and POMC neurons separately in AGRP::Cre and POMC::Cre transgenic mice. AGRP stimulation alone was able to induce feeding within minutes, and as expected, POMC stimulation reduced feeding and led to weight loss (Fig. 1M and Fig. 5). In mice expressing an agouti protein that blocks melanocortin receptors, stimulation of POMC neurons was unable to reduce feeding, indicating that POMC-mediated hypophagia required melanocortin signaling. In contrast, AGRP stimulation in these mice evoked similar feeding behaviors as in the AGRP-ChR2 mice, suggesting that melanocortin receptors are not necessary for AGRP function, evidence that AGRP neurons have direct access to the feeding pathway, in addition to modulating feeding through inhibition of POMC neural activity. In a more recent study, Atasoy et al. (2012) found evidence that AGRP suppression of POMC activity instead modulates food intake on the order of hours, rather than acute feeding. In addition, by expressing ChR2 in AGRP neurons and targeting an optic fiber over axons in paraventricular hypothalamus (PVH), the authors also showed that feeding could be evoked by AGRP terminal stimulation in the PVH through the suppression of PVH oxytocin neurons (Fig. 1N and Fig. 5).
Leptin is a peripheral hormone secreted by adipocytes heavily connected in the feeding circuit with POMC and AGRP neurons that signals the status of energy reserves to the brain in order to modulate appetite and feeding behavior (Elias et al., 1999; Meister, 2000; Leinninger et al., 2009). Evidence suggests that leptin may modulate food intake by suppressing the reward value of food (Fulton et al., 2000; Figlewicz et al., 2007). In humans, fMRI studies have shown that patients with congenital leptin deficiency give higher ratings to food images and that ratings could be lowered with leptin treatments (Farooqi et al., 2007). Domingos et al. (2011) developed an assay where animals were given choices between a combination of sweeteners (sucrose, sucralose, and water) and optical stimulation of ChR2-expressing VTA dopamine neurons. Mice were found to prefer optogenetic stimulation to sucralose, but not sucrose, and sucralose with stimulation over sucrose. From here, the authors showed that when the animals were food restricted, they increased their preference for sucrose over stimulation with sucralose. However, when treated with leptin, animals experienced a decrease in preference for sucrose, showing that leptin and food restriction elicit opposite effects on the value of sucrose (Domingos et al., 2011).
5. The many facets of fear: Dissection and control of fear and anxiety
The circuits mediating fear and anxiety are remarkably well-conserved between humans and rodents (Adolphs et al., 1995; Morris et al., 1996; LaBar et al., 1998; De Bellis et al., 2000; Thomas et al., 2001; Chen et al., 2006; Kienast et al., 2008; Etkin et al., 2009). Anxiety disorders, which comprise the most common class of psychiatric disorders, with a lifetime prevalence of ~28%, include more specific disease states such as generalized anxiety disorder, social anxiety, panic disorder, post-traumatic stress disorder (PTSD), and specific phobias (Kessler et al., 2005). Although related, fear and anxiety are distinctly different—fear is an emotional reaction triggered by an immediate threat, while anxiety is a state of heightened apprehension in the absence of an immediate threat (Davis et al., 2010).
Decades of studies have focused on the amygdala and established its central role in the processing of fear and anxiety (LeDoux, 2000; Maren and Quirk, 2004; Pape and Pare, 2010). Among the many subnuclei of the amygdala, most researchers have focused on the lateral nucleus of the amygdala (LA), which is an early site of convergence for sensory information about both conditioned and unconditioned stimuli (LeDoux et al., 1990). The LA is thought to send projections to the central amygdala (CeA), which in turn activates output neurons to trigger the expression of fear or anxiety (LeDoux, 2000; Maren and Quirk, 2004; Paré et al., 2004). Studies employing focal lesions, pharmacological manipulations, and electrophysiological recordings, have established a fundamental model of amygdala microcircuitry (LeDoux, 2000; Maren and Quirk, 2004; Ehrlich et al., 2009; Pape and Pare, 2010; Johansen et al., 2011). However, the causal role of specific circuit elements has, due to technical limitations, largely remained elusive. With the advent of optogenetics, it has become possible to manipulate specific elements of fear or anxiety circuitry and probe the neural mechanisms underlying these behaviors.
5.1. Plastic changes in the lateral amygdala mediate fear conditioning
Pavlovian fear conditioning is the most widely used behavioral paradigm to study fear in rodents. In this paradigm, an initially neutral cue, such as a tone or a context (conditioned stimulus; CS), and an aversive stimulus, such as a footshock (unconditioned stimulus; US), are presented to the subject, usually paired with a brief US co-terminating with a long CS (Maren, 2001). After pairing the CS with the US, the CS acquires the aversive property of the US and can alone evoke fear responses, such as freezing. The association of CS and US signals are thought to recruit Hebbian plasticity in LA neurons, where the synaptic inputs that convey weak sensory CS become strengthened if they coincide with a US that strongly depolarizes LA neurons (Blair et al., 2001; Maren and Quirk, 2004).
However, the causal role of this postsynaptic depolarization had never been directly tested until Johansen et al. (2010) substituted the shock-evoked US with direct optogenetic depolarization of LA neurons. The authors expressed ChR2 in the pyramidal neurons of the LA and found that optical stimulation alone was sufficient to evoke a small amount of unconditioned freezing (~10% increase from the baseline) (Fig. 1O and Fig. 6). After sixteen paired stimulations with a CS, the CS alone could evoke the conditioned freezing response, which indicates that optogenetic depolarization of LA neurons could replace the shock and therefore serve as a “teaching signal” for the CS-US association. However, the authors also noted that LA stimulation was only able to elicit very low levels of freezing behavior, suggesting that nonspecific depolarization of LA neurons is not the only mechanism by which fear conditioning occurs.
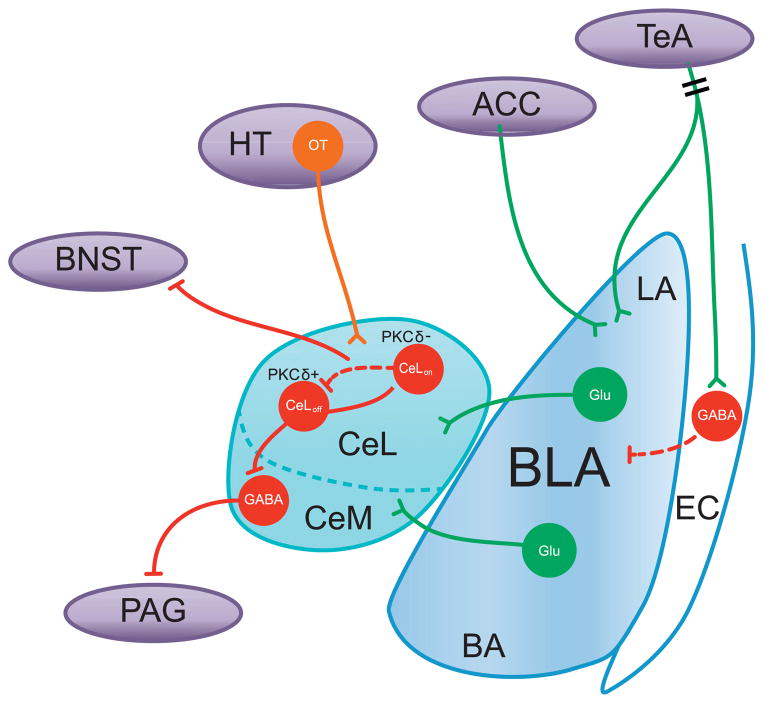
Fig. 6. Amygdala circuitry mediates fear and anxiety.
The amygdala plays a major role in fear and anxiety responses through its complex microcircuits and its connectivity with distal regions. Information about environmental stimuli converge in the LA, which is gated by GABAergic inputs from the external capsule. The BLA projects to the CeL to modulate anxiety-related behavior, where CeLon and CeLoff neurons form a reciprocal inhibitory feedback loop. CeLoff neurons inhibit CeM output neurons, which innervate downstream effector structures, such as the PAG. Furthermore, hypothalamic projections to the CeL modulate activity via OT, and CeL projections inhibit BNST activity. Dotted lines represent non-optogenetic findings. Double hash marks represent uncertainty in neuronal population from projection origin. Abbreviations: ACC, anterior cingulate cortex; BA, basal amygdala; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeL, lateral division of the central amygdala; CeM, medial division of the central amygdala; EC, external capsule; Glu, glutamate; HT, hypothalamus; LA, lateral amygdala; OT, oxytocin; PAG, periaqueductal gray; PKC-δ, protein kinase C-delta; TeA, temporal association cortex.
CS information can reach the LA via many routes, but in the case of auditory fear conditioning, tone information is conveyed to the LA from both the thalamus and the cortex, including the posterior intralaminar nucleus (PIN), the medial sector of the medial geniculate nucleus (MGm), and the temporal association cortex (TeA) (LeDoux et al., 1984; Romanski and LeDoux, 1992; Campeau and Davis, 1995; Antunes and Moita, 2010). Morozov et al. (2011) directly examined the inhibitory gating in the cortico-amygdala pathway using optogenetics (Fig. 6). The authors expressed ChR2 in the TeA and recorded from GABAergic neurons in the external capsule (EC), where excitatory responses were elicited by laser pulses. Then they recorded from LA neurons, where cutting off the EC or applying picrotoxin (a GABAA receptor antagonist) enabled the induction of LTP by a STDP protocol (Fig. 6). Therefore, to induce LTP in the TeA-LA pathway, feed-forward inhibition from the EC needs to be blocked, which suggests that the TeA-LA CS input pathway is under the control of this inhibition from the EC. In contrast, when the authors expressed ChR2 in the anterior cingulate cortex (ACC), LTP in LA neurons did not require inhibition of GABAA receptor-mediated transmission (Morozov et al., 2011).
5.2. Disinhibitory microcircuits in the central amygdala regulate the expression of conditioned fear
LA neurons project to the central amygdala (CeA), either directly or indirectly via the basal nucleus (BA) or the intercalated cells of the amygdala (ITC) (Paré et al., 2004; Pape and Pare, 2010). The CeA is mostly composed of GABAergic medium spiny neurons and is further subdivided into the lateral division (CeL) and the medial division (CeM) (McDonald, 1982; Ehrlich et al., 2009). Emerging evidence suggests that the CeL is critical for the acquisition of conditioned fear, whereas the CeM is the main output structure of the amygdala (Wilensky et al., 2006; Ciocchi et al., 2010). Ciocchi and colleagues expressed ChR2 in the CeM and found that optical stimulation evoked unconditioned freezing (Fig. 1P and Fig. 6). Notably, a larger freezing response was evoked (40–60% increase from the baseline) than the freezing response obtained from stimulation of the LA, consistent with the idea that the CeM is the main output of the amygdala. Given the GABAergic content of the CeM (McDonald, 1982) however, it remained unclear how GABAergic output from the CeA affects downstream structures to trigger conditioned motor and autonomic responses.
Using in vivo single unit recording in awake, behaving mice, Ciocchi et al. (2010) also discovered two functionally distinct types of units in the CeL that acquired opposite responses to the CS, namely CeLon and CeLoff units. In the companion paper, Haubensak et al. (2010) found that a subpopulation of GABAergic neurons within the CeL, marked by the expression of protein kinase C-delta (PKC-δ), corresponded to CeLoff units. Optogenetic stimulation of PKC-δ(+) CeL neurons directly inhibited CeM neurons projecting to the periaqueductal gray (PAG)–a region implicated in freezing behavior (LeDoux et al., 1988; Kim et al., 1993; De Oca et al., 1998)) and also inhibited PKC-δ(−) CeL neurons (Fig. 6). Haubensak and colleagues further provided anatomical evidence that PKC-δ(−) GABAergic neurons also project to PKC-δ(+) neurons. Based on these results, the authors proposed a model where a CS presentation activates PKC-δ(−) CeLon neurons, which in turn inhibits PKC-δ(+) CeLoff neurons, thereby disinhibiting CeM output neurons that trigger conditioned fear responses.
The amygdala is heavily influenced by neuropeptidergic inputs (Veinante and Freund-Mercier, 1997; Asan, 1998; Cassell et al., 1999; Grace and Rosenkranz, 2002; Fuxe et al., 2003; Muller et al., 2007; Jüngling et al., 2008; Pinard et al., 2008; Ehrlich et al., 2009), and one such neuropeptide is oxytocin (OT), which has been implicated in social behaviors, attachment, fear, and anxiety (Lee et al., 2009). Huber et al. (2005) observed that OT activates a subpopulation of CeL neurons expressing OT receptors (OTRs) and inhibits CeM neurons. More recently, Knobloch et al. (2012) expressed ChR2 in OT-labeled regions of the hypothalamus and found that stimulating OT terminals in CeL decreased contextual freezing after fear conditioning (Fig. 6). Stimulation was also shown to increase action potential frequencies in CeL neurons and IPSC frequencies in CeM neurons that were abolished by OTR antagonists. These data demonstrate that OTR-expressing CeL neurons inhibit CeM neurons when activated by OT released from hypothalamic terminals.
5.3. Amygdala circuits for anxiety and their outputs to the bed nucleus of the stria terminalis
The functional processing of anxiety was also recently discovered in amygdala circuitry (Tye et al., 2011). Based on prior lesion and pharmacology studies, it was proposed that the expression of acute cued fear and sustained anxiety-like responses are mediated by the CeA and the bed nucleus of the stria terminalis (BNST), respectively (Davis et al., 2010). The most common and well-established assays for anxiety include the elevated plus maze test and the open field test (Carola et al., 2002), which were used by Tye and colleagues. The authors expressed ChR2 in the pyramidal neurons of the BLA, which collectively refers to the LA and the BA, and implanted an optical fiber over the CeL. Photostimulation of BLA terminals in the CeL demonstrated an acute and reversible decrease in anxiety-like behavior, whereas the opposite behavioral change was induced by inhibiting the BLA-CeL projection using NpHR (Tye et al., 2011) (Fig. 1Q and Fig. 6). By restricting light illumination to the CeL, the authors also showed that selectively stimulating excitatory BLA inputs to the CeL exerts feedforward inhibition on the CeM, supported by findings of Knobloch et al. (2012) showing that OTR-expressing CeL neurons inhibit CeM neurons and decreases fear responses.
Another major output of the amygdala is the BNST, which has also been implicated in the expression of anxiety and contextual fear in mice (Sullivan et al., 2004; Walker et al., 2009; Davis et al., 2010) as well as in the pathophysiology of anxiety disorders in humans (Straube et al., 2007; Yassa et al., 2012). The BNST is extensively interconnected with the amygdala–both the BLA and the CeA heavily project to the BNST, which in turn projects back to the CeA (Dong et al., 2001a, 2001b; Alheid, 2003; Dong and Swanson, 2004). Notably, the BNST and the CeA share many of the downstream structures that are known to mediate the expression of fear or anxiety (Alheid et al., 1995). Their shared connectivity, along with their similarities in cell types and neurochemical makeup, have led to the concept of the “extended amygdala”, which refers to a continuum of structures from the CeA to the BNST (Johnston, 1923; Alheid and Heimer, 1988; Alheid et al., 1995). Recently, Li et al. (2012) optogenetically targeted ChR2-expressing GABAergic CeA projections to the BNST. Stimulating CeA terminals in the BNST evoked inhibitory postsynaptic currents (IPSCs) in BNST neurons, and the amplitudes of these IPSCs were reduced by a kappa opioid receptor (KOR) agonist. This suggests that KOR reduces inhibitory synaptic transmission in the CeA-BNST pathway.
6. Distributed fear circuitry: Looking at the cortex and hippocampus
Given the critical importance of fear and anxiety in survival, a distributed network mediating negative valence may have provided an evolutionary advantage in the mammalian brain. Indeed, both the prefrontal cortex and the hippocampus have been heavily implicated in mediating several different facets of fear acquisition, expression, and extinction (Morgan and LeDoux, 1995; Maren et al., 1997; Fanselow, 2000; Milad and Quirk, 2002; Sotres-Bayon and Quirk, 2010). While both the prefrontal cortex and the hippocampus are reciprocally connected with the amygdala, they have been noted to have distinct functions (Kim et al., 1993; Garcia et al., 1999, 1999; Frankland et al., 2004; McHugh et al., 2004; Frankland and Bontempi, 2005; Herry et al., 2008).
In the frontal lobe, the mPFC has been implicated in fear expression and extinction (Sotres-Bayon and Quirk, 2010; Milad and Quirk, 2012). Milad and Quirk (2002) showed that infralimbic neurons fire to the CS only when rats are recalling extinction on the following day, suggesting that extinction potentiates infralimbic activity, congruent with data showing that mPFC inputs selectively target extinction neurons in the BA (Herry et al., 2008). Yizhar et al. (2011) showed that sustained depolarization of mPFC pyramidal neurons using the stable step-function opsin (SSFO), a variant of ChR2 that enables prolonged depolarization induced by a pulse of light, impaired the acquisition of both auditory and context-conditioned fear (Fig. 1R), confirming the importance of mPFC in fear learning.
The auditory cortex has been thought to convey information about auditory cues to the LA during auditory fear conditioning (Campeau and Davis, 1995; Boatman and Kim, 2006), and is shown to undergo plastic changes with fear conditioning (Quirk et al., 1997; Suga and Ma, 2003; Weinberger, 2007). More recently, a study using optogenetics dissected the local inhibitory circuitry and demonstrated its necessity for fear learning (Letzkus et al., 2011) (Fig. 1S). Letzkus and colleagues found that footshock activated layer 1 interneurons in the auditory cortex. Layer 1 interneurons inhibit layer 2/3 parvalbumin (PV)-expressing interneurons, which in turn disinhibit layer 2/3 pyramidal neurons. The authors showed that disinhibition of layer 2/3 neurons is critical for fear learning, as optogenetic stimulation of PV+ interneurons during footshock impaired the acquisition of conditioned fear (Letzkus et al., 2011).
Just ventral to the cortex is the hippocampus, which is well-known for its critical role in the processing of spatial memory and the formation of contextual fear memory (Squire, 1992; LeDoux, 2000). The prevalent view, based on lesion and inactivation studies, was that the CA1 region of the hippocampus is required only for the short-term memory, but not for long-term memory (Kim and Fanselow, 1992; Squire and Alvarez, 1995; Maviel et al., 2004; Squire and Bayley, 2007). Goshen and colleagues demonstrated that the CA1 actually contributes to remote memory recall, using fast optogenetic intervention (Fig. 1T). The authors further showed that fast inhibition of the CA1 and prolonged inhibition of the CA1 (for 30 min) differentially influenced network activity during fear recall (Goshen et al., 2011).
The dentate gyrus (DG) of the hippocampus is thought to play an essential role in the discrimination between contexts (McHugh et al., 2007; Nakashiba et al., 2012). In a unique study, Liu et al. (2012) injected an AAV-ChR2 virus with a tetracycline-responsive-element (TRE) into the DG of c-fos-tetracycline transactivator (tTA) transgenic mice. This unique tool allowed for selective labeling of c-Fos-expressing DG neurons with ChR2 following training-induced neuronal activity in the absence of doxycycline (DOX), and importantly prevented labelling in the presence of DOX. Using this technique, the authors were able to show that optogenetic reactivation of distinct DG neurons activated during a fear conditioning task in a different context was able to induce fear-evoked freezing (Fig. 1U). Importantly, activating a subset of DG neurons not active during fear conditioning did not induce freezing. These data demonstrate that reactivating a distinct subset of task-dependent neurons involved in the formation of a memory is sufficient to induce a behavioral response to that memory (Liu et al., 2012).
7. Cautionary note
Despite all the advantages that optogenetic tools offer, we should exercise caution and carefully characterize each new tool, device, and variant to inform our data interpretation. For example, ChR2 is a non-specific cation channel and hence, opening these channels will allow Ca2+ influx into the cell. If ChR2 is used to achieve pathway-specific stimulation of presynaptic axon terminals, then neurotransmitter release probability can be changed from normal physiological levels due to Ca2+ influx (Schoenenberger et al., 2011). Thorough characterization of the effects of ChR2 mediated Ca2+ influx into cells is required prior to interpreting mechanisms of synaptic plasticity that will be uncovered using ChR2.
Furthermore, hyperpolarizing opsins must also continue to be characterized with care under each preparation. Two primary classes of hyperpolarizing opsins including a light-activated proton pump, Archaerhodopsin-3 from Halorubrum sodomense (Arch) (Chow et al., 2010; Mattis et al., 2011), and a light-activated chloride pump, Natronomonas pharaonis halorhodopsin (eNpHR3.0 or NpHR), (Zhang et al., 2007; Gradinaru et al., 2010; Mattis et al., 2011), along with their variants, have been commonly used in numerous model organisms and preparations. Since the development of eNpHR3.0, a NpHR variant with reduced toxicity and improved trafficking to the cell membrane (Gradinaru et al., 2010), many groups have adopted this new opsin and use the acronyms of NpHR and eNpHR3.0 interchangeably. One potential caveat of using light-sensitive proton pumps (including variants in the archaerhotopsin and bacteriorhodopsin families) under continuous illumination parameters is the alterations in intracellular and extracellular pH, due to the efflux of protons (Zhang et al., 2011). A potential caveat for light-sensitive chloride pumps was raised in a recent report regarding the post-photoinhibition spiking with NpHR, likely attributable to the alteration in the GABAA receptor reversal potential (Raimondo et al., 2012). While optogenetic tools offer a new level of precision and control in neuroscience research, they present new artifacts that must be considered with care.
8. The future of optogenetics: new targets for potential therapies
While the early years of optogenetic research were aimed at developing new opsins, filling the optogenetic toolbox, and conducting proof-of-principle experiments, recent years have seen a maturation of optogenetics, leading to studies utilizing it to produce novel insights about neural circuit, cellular, and synaptic function. Researchers now have a wide selection of well-characterized viruses, promoters, optical equipment, and surgical protocols that facilitate the adoption of optogenetic techniques without significant barriers to entry. We anticipate that one major area of growth for future applications of optogenetics will be in its integration with existing techniques and its use to functionally dissect circuits with causal relationships to behavior.
The body of research we have presented here, detailing how neural circuits are involved in reward, motivation, aggression, feeding, fear, and anxiety in animal models are significant insights that will be applicable to understanding our own processing of emotional valence in health and disease. The recent proliferation of studies applying optogenetic manipulations to the investigation of disease-relevant behaviors has highlighted the complexity of these behaviors, as well as the subtleties of optogenetic manipulations. Beginning with a study from Covington et al. (2010) showing that nonspecific optical stimulation of ChR2-expressing neurons in the mPFC has anti-depressant-like effects, several optogenetic studies have been published recently on depression. Warden et al. (in press) recently demonstrated that optogenetic stimulation of glutamatergic pyramidal neurons in the mPFC had no effect on depression-like behavior, but specifically stimulating projections to the dorsal raphe (DRN) elicited escape-related behavior in depression assays, while stimulating projections to the LHb had the opposite effect (Fig. 1V and W).
In a pair of recent experiments on the modulation of depression-related behaviors by the mesolimbic dopamine system, Tye et al. (in press); Chaudhury et al. (in press) examined how VTA dopamine neurons respond in distinct mouse models of depression and how optogenetic modulation can reverse these depression-like phenotypes. Although these studies highlight the complexity of depression-related behaviors and the need for deeper investigation, they both demonstrate that optogenetic manipulations of the VTA-NAc pathway are bidirectionally and causally linked to depression-related behaviors. These emerging studies have the potential to reinvigorate systems-level research in the neural underpinnings of psychiatric disease. Optogenetic approaches have opened the floodgates to understanding the distributed, yet evolutionarily well-conserved, neural circuits mediating emotional valence and motivated behaviors in both adaptive and pathological states.
Acknowledgments
We want to acknowledge Lynne D. Tye and Jonathan Leung for their scholarly input and helpful discussion. K.M.T. was supported by the Whitehall Foundation (2012-08-45), the Picower Neurological Disorder Research Fund, the Picower Institute Innovation Fund, and the Wade Award. E.H.N. was supported by the Integrative Neuronal Systems Center Grant (6926328), the Brain and Cognitive Sciences Special Award (1497200), and the National Science Foundation Graduate Research Fellowship. S.-Y.K. was supported by the Samsung Scholarship. P.N. was supported by the Marcus Fellowship to Honor Leventhal (3891441) and the Brain and Cognitive Sciences Special Award (1497200).
References
- Adamantidis A, Carter MC, de Lecea L. Optogenetic deconstruction of sleep–wake circuitry in the brain. Front Mol Neurosci. 2010:2. doi: 10.3389/neuro.02.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Fear and the human amygdala. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alheid G, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. Academic Press; New York: 1995. pp. 495–578. [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Antunes R, Moita MA. Discriminative auditory fear learning requires both tuned and nontuned auditory pathways to the amygdala. J Neurosci. 2010;30:9782–9787. doi: 10.1523/JNEUROSCI.1037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Lopez-Paniagua D, Rudy JW, O’Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat Neurosci. 2007;10:126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkow JH, Cosmides L, Tooby J. The Adapted Mind: Evolutionary Psychology and the Generation of Culture. Oxford University Press; USA: 1995. [Google Scholar]
- Berendse HW, Graaf YGD, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur J Neurosci. 2006;24:894–900. doi: 10.1111/j.1460-9568.2006.04965.x. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PAC, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgiimpregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. doi: 10.1016/j.neuron.2012.09.040. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Chang JY, Chen L, Luo F, Shi LH, Woodward DJ. Neuronal responses in the frontal cortico-basal ganglia system during delayed matching-to-sample task: ensemble recording in freely moving rats. Exp Brain Res. 2002;142:67–80. doi: 10.1007/s00221-001-0918-3. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai H-C, Pomeranz L, Christoffel DJ, et al. Rapid control of depressive behaviors by firing pattern- and projection-specific optogenetic regulation of midbrain dopamine neurons. Nature. In Press. [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi CC, Flynn JP. Neuroanatomic projections related to biting attack elicited from hypothalamus in cats. Brain Res. 1971;35:49–66. doi: 10.1016/0006-8993(71)90594-4. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Antelman SM, Caggiula AR, Lineberry CG. Sensory stimuli alter the discharge rate of dopamine (DA) neurons: evidence for two functional types of DA cells in the substantia nigra. Brain Res. 1980;189:544–549. doi: 10.1016/0006-8993(80)90366-2. [DOI] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Cosmides L, Tooby J. From Evolution to Behavior: Evolutionary Psychology as the Missing Link. 1987. [Google Scholar]
- Covington HE, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann N Y Acad Sci. 2003;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Darwin C. The Origin of Species. P.F. Collier & son; 1909. [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, Deisseroth K, de Araujo IE, Friedman J. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbæk C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355–1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2007;91:473–478. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Falcon AJ, Miller AD, Heruc GA, Blaha CD. Effects of laterodorsal tegmentum excitotoxic lesions on behavioral and dopamine responses evoked by morphine and D-amphetamine. Neuroscience. 2002;114:817–823. doi: 10.1016/s0306-4522(02)00365-2. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: striatoorbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Jacobsen KX, Höistad M, Tinner B, Jansson A, Staines WA, Agnati LF. The dopamine D1 receptor-rich main and paracapsular intercalated nerve cell groups of the rat amygdala: relationship to the dopamine innervation. Neuroscience. 2003;119:733–746. doi: 10.1016/s0306-4522(03)00148-9. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Goold CP, Nicoll Ra. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Nigral dopamine neurons: intracellular recording and identification with L-Dopa injection and histofluorescence. Science. 1980;210:654–656. doi: 10.1126/science.7433992. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiol Behav. 2002;77:489–493. doi: 10.1016/s0031-9384(02)00909-5. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–R511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav Brain Res. 1999;99:169–179. doi: 10.1016/s0166-4328(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Wilson RD. The Subcortical Projections of the Allocortex: Similarities in the Neural Associations of the Hippocampus, the Piriform Cortex, and the Neocortex. Golgi Centennial Symposium Proceedings; New York: Raven Press; 1975. pp. 177–193. [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Halász J, Meelis W, Kruk MR, Liposits Z, Haller J. Neurochemical characterization of hypothalamic neurons involved in attack behavior: glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience. 2005;133:657–666. doi: 10.1016/j.neuroscience.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, DeGarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal gangliathalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Nat Acad Sci USA. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB. Further contributions to the study of the evolution of the forebrain. J Comp Neurol. 1923;35:337–481. [Google Scholar]
- Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, et al. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJH. The amygdalostriatal projection in the rat—an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TPS. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc London, Ser B. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, Smolka MN, Gr|[uuml]|nder G, Cumming P, Kumakura Y, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Zheng T, Wilson CJ. Connectivity and convergence of single corticostriatal axons. J Neurosci. 1998;18:4722–4731. doi: 10.1523/JNEUROSCI.18-12-04722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, et al. BDNF Is a Negative Modulator of Morphine Action. Science. 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Dopamine, addiction and reward. Sem Neurosci. 1992:139–148. [Google Scholar]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Kruk MR. Ethology and pharmacology of hypothalamic aggression in the rat. Neurosci Biobehav Rev. 1991;15:527–538. doi: 10.1016/s0149-7634(05)80144-7. [DOI] [PubMed] [Google Scholar]
- Kruk MR, Van der Poel AM, Meelis W, Hermans J, Mostert PG, Mos J, Lohman AH. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res. 1983;260:61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012 doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers JH, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988;449:311–327. doi: 10.1016/0006-8993(88)91046-3. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, Lüthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, Stuber GD, Kash TL. Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biol Psychiatry. 2012;71:725–732. doi: 10.1016/j.biopsych.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromádka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012 doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. The Triune Brain in Evolution: Role in Paleocerebral Functions. Springer; 1990. [Google Scholar]
- Mantz J, Thierry AM, Glowinski J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res. 1989;476:377–381. doi: 10.1016/0006-8993(89)91263-8. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci. 2011;31:17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2011;9:159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of Neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RLM. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RMJ, Rawlins JNP, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- van der Meer MAA, Redish AD. Covert expectation-of-reward in rat ventral striatum at decision points. Front Integr Neurosci. 2009:3. doi: 10.3389/neuro.07.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B. Control of food intake via leptin receptors in the hypothalamus. Vitam Horm. 2000;59:265–304. doi: 10.1016/s0083-6729(00)59010-4. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol. 1994;72:1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morozov A, Sukato D, Ito W. Selective suppression of plasticity in amygdala inputs from temporal association cortex by the external capsule. J Neurosci. 2011;31:339–345. doi: 10.1523/JNEUROSCI.5537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A Differential Neural Response in the Human Amygdala to Fearful and Happy Facial Expressions. 1996;383:812–815. doi: 10.1038/383812a0. Published Online: 31 October 1996. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;505:314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Barrera VR, Chittajallu R, Iwamoto KS, McBain CJ, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Total recall—the memory of addiction. Science. 2001;292:2266–2267. doi: 10.1126/science.1063024. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- De Oca BM, DeCola JP, Maren S, Fanselow MS. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci. 1998;18:3426–3432. doi: 10.1523/JNEUROSCI.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2012;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Bolaños CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. ΔFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Houser CR, Vaughn JE. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J Comp Neurol. 1985;238:286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard CR, Muller JF, Mascagni F, McDonald AJ. Dopaminergic innervation of interneurons in the rat basolateral amygdala. Neuroscience. 2008;157:850–863. doi: 10.1016/j.neuroscience.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neurosci. 2012 doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Rescorla R, Wagner A. Classical Conditioning II: Current Research and Theory. Appleton-Century-Crofts; 1972. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement; pp. 64–99. [Google Scholar]
- Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Schultz W. Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol. 1990;63:592–606. doi: 10.1152/jn.1990.63.3.592. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004;41:711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- Rymar VV, Sasseville R, Luk KC, Sadikot AF. Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J Comp Neurol. 2004;469:325–339. doi: 10.1002/cne.11008. [DOI] [PubMed] [Google Scholar]
- Schilman EA, Uylings H, Graaf YG, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Schoenenberger P, Schärer YPZ, Oertner TG. Channelrhodopsin as a tool to investigate synaptic transmission and plasticity. Exp Physiol. 2011;96:34–39. doi: 10.1113/expphysiol.2009.051219. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R. Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J Neurophysiol. 1987;57:201–217. doi: 10.1152/jn.1987.57.1.201. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. PNAS. 2009;106:15031–15036. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Siegel A, Skog D. Effects of electrical stimulation of the septum upon attack behavior elicited from the hypothalamus in the cat. Brain Res. 1970;23:371–380. doi: 10.1016/0006-8993(70)90063-6. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344:1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Bayley PJ. The neuroscience of remote memory. Curr Opin Neurobiol. 2007;17:185–196. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WHR. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: neural currencies for valuation and decision making. Nat Rev Neurosci. 2005;6:363–375. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tooby J, Cosmides L. The past explains the present:: emotional adaptations and the structure of ancestral environments. Ethol Sociobiol. 1990;11:375–424. [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, De Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Tsai H-C, Finkelstein J, Kim S-Y, Ferenczi E, Adhikari A, Thompson KR, Andalman AS. Dopamine neurons modulate the neural encoding and expression of depression-related behavior. Nature. doi: 10.1038/nature11740. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012 doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Veening JG, Coolen LM, de Jong TR, Joosten HW, de Boer SF, Koolhaas JM, Olivier B. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur J Pharmacol. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin-and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJM, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY. Dopaminergic neurons in the rat ventral tegmental area. I identification and characterization. Brain Res Rev. 1981;3:123–140. [Google Scholar]
- Warden MR, Selimbeyoglu A, Tye KM, Mirzabekov JJ, Lo M, Thompson KR, Kim S-Y, Adhikari A, Frank LM, Deisseroth K. Rising to challenge: role of a prefrontal cortex to brainstem neural projection in motivated behavioral states. Nature. doi: 10.1038/nature11617. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Nat Acad Sci. 1990;87:7050. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Nat Acad Sci USA. 2008;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Euro J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CEL, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res. 2012 doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, et al. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Holbro N, Oertner TG. Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Nat Acad Sci. 2008;105:12039–12044. doi: 10.1073/pnas.0802940105. [DOI] [PMC free article] [PubMed] [Google Scholar]