Abstract
Background and Aim
The present anti-infection strategy for prosthetic joint infections (PJI) includes the use of antibiotics and surgical treatments, but the bacterial eradication rates are still low. One of the major challenges is the formation of biofilm causing poor bacterial eradication. Recently it has been reported that allicin (diallyl thiosulphinate), an antibacterial principle of garlic, can inhibit bacteria adherence and prevent biofilm formation in vitro. However, whether allicin could inhibit biofilm formation in vivo is unknown. The aim of this study was to investigate the effects of allicin on biofilm formation, and whether allicin could potentiate the bactericidal effect of vancomycin in a rabbit PJI model.
Methods
A sterile stainless-steel screw with a sterile ultra-high molecular weight polyethylene washer was inserted into the lateral femoral condyle of the right hind knee joint of rabbit, and 1 mL inoculum containing 104 colony-forming units of Staphylococcus epidermidis was inoculated into the knee joint (n = 32). Fourteen days later, rabbits randomly received one of the following 4 treatments using continuous lavages: normal saline, vancomycin (20 mcg/mL), allicin (4 mg/L), or allicin (4 mg/L) plus vancomycin (20 mcg/mL). Three days later, the washer surface biofilm formation was examined by scanning electron microscopy (SEM). The bacterial counts within the biofilm of implanted screws were determined by bacterial culture.
Results
The lowest number of viable bacterial counts of Staphylococcus epidermidis recovered from the biofilm was in the rabbits treated with allicin plus vancomycin (P<0.01 vs. all other groups). The biofilm formation was significantly reduced or undetectable by SEM in rabbits receiving allicin or allicin plus vancomycin.
Conclusion
Intra-articular allicincan inhibit biofilm formation and enhance the bactericidal effect of vancomycin on implant surface in vivo. Allicin in combination with vancomycin may be a useful anti-infection strategy for the treatment of PJI.
Introduction
Prosthetic joint infection (PJI) is a rare, but one of the most devastating complications in total joint arthroplasty [1], [2], [3], that an arthritic or dysfunctional joint is removed and replaced with an artificial joint. Approximately 1–2% of the patients who undergo prosthetic joint arthroplasty develop PJI in the first 2 years after the surgery [4]. As arthroplasty becomes more common, more patients are expected to suffer from PJI [3]. PJI causes poor function of the prosthetic joint, requires long-term use of antibiotics and even multiple surgical procedures, which impose a substantial economic burden on the individuals and the health care system [3], [5], [6]. Unfortunately, the treatment of PJI is extremely difficult. Previous studies indicated poor bacterial eradication rates [7], [8], [9]. The eradication rates were 18% to 37% for methicillin-resistant Staphylococcus aureus (MRSA) [7], [8], and 65% and 75% for streptococcal infections and all other organisms [9], respectively. Some analyses regarded staphylococcal infection as an independent predictor of failed treatment relating to irrigation and debridement with implant retention [10]. The success rates for treating PJI caused by a gram-negative pathogen, MRSA, and methicillin-sensitive gram-positive organisms were 52%, 51% and 69%, respectively, even with a two-stage procedure [11] For antibiotics to achieve satisfying results, it is important that the activity of antibiotic is preserved in the presence of biofilm [6]. There are no standard length of the initial intravenous treatment and the total treatment duration [12]. In addition, there are significant differences of practices in North America, Europe and Australia [13]. The optimal treatment protocol of PJI is still under intense investigation and open to debate [6], [14]. A majority of prosthesis-related infections are caused by Staphylococcus epidermidis (S. epidermidis) and Staphylococcus aureus [12], [15]. Polyethylene seems to be strongly attractive for S. epidermidis, while prosthetic metals are more suited for Staphylococcus aureus adherence [15]. Therefore, multiple factors are responsible for the differences in the eradication rates in the treatment of PJI. Biofilm produced by planktonic bacteria adhering to the implants makes the treatment of PJI more difficult [12]. Biofilm can resist host cellular and humoral immune responses, and bacteria within the biofilm are less susceptible to antimicrobial agents [16], [17]. Biofilm formation and development are a dynamic process that includes attachment, biofilm formation, and dispersal [18]. Bacteria within the biofilm can remain in the body for a prolonged period of time, and become a source of bacteria that disseminate to other sites of the implant surface, forming new biofilms. In conventional antibiotic therapy, some of planktonic bacteria are killed, while others quickly form new biofilms on the implant; this latter observation may explain the chronic nature of most implant device infections [19]. So inhibiting planktonic bacteria adhering to implant and biofilm formation is a potentially useful strategy to eradicate biofilm-related prosthesis infections [20].
Garlic has been used to treat infections since ancient times. Its active ingredient is allicin. Allicin is an antibacterial agent with in vitro activity against biofilm formation of S. epidermidis. It has been demonstrated that sub-MIC concentration of allicin may prevent S. epidermidis adherence and biofilm formation in vitro [21], [22]. Biofilm detachment release single cells or larger cell clusters, which prompts the dispersal of bacteria to other sites and formation new biofilms [23]. Vancomycin is the treatment of choice for MRSA [24] and S. epidermidis infections [25]. However, vancomycin alone is ineffective in eradicating staphylococcal infection on orthopaedic device [12], [25]; its combination with rifampin is recommended to treat PJI caused by MRSA or S.epidermidis [12], [25]. Since allicin can prevent bacteria adhesion and aggregation, and inhibit biofilm formation in vitro, we therefore hypothesized that the vancomycin bactericidal effect can be enhanced on the implant surface by co-administration of allicin. In this study, using a rabbit model of PJI caused by S. epidermidis, we investigated the effect of intra-articular lavage of a combination of allicin and vancomycin on prevention of new biofilm formation and elimination of prosthesis surface bacteria.
Materials and Methods
Bacterial strain
Staphylococcus epidermidis strain RP62A (a biofilm-forming bacteria [26]) was obtained from ATCC (Catalog number 35984) and propagated in tryptic soy broth media.
Antimicrobial Agents
The normal saline lavage solution contained vancomycin (Eli Lilly, Kobe, Japan) and/or allicin (RYEN Pharma, Jiangsu, China). The concentration of vancomycin in the lavage fluid was 20 mcg/mL; this concentration was selected because acceptable trough concentrations of vancomycin are generally between 10 and 20 mcg/mL [24]. The concentration of allicin in the lavage fluid was 4 mg/L; this concentration was chosen as it has been previously shown to prevent S. epidermidis biofilm formation [21].
Rabbit PJI model
All animal experiments were approved by the Animal Care and Ethics Committee of Guangzhou Medical University (Permit Number: 2013-92). Thirty-two New Zealand White rabbits (weight: 2.5–3.5 kg) were used. The rabbits were housed separately in individual cages. They were exposed to a natural light-dark cycle and given free access to food and water.
The rabbit PJI model was previously described [27] with minor modifications. The amount of bacteria inoculation of S. epidermidis was pre-determined by pre-inoculation. Briefly, 32 New Zealand rabbits were randomly divided into 4 groups (8 in each group). A sterile stainless-steel screw and a sterile ultra-high molecular weight polyethylene (UHMWPE) washer were implanted in the rabbit knee joint. A titration of 103, 104, and 105 CFU S. epidermidis (strain RP62A) and 1 mL normal saline (as a negative control) were inoculated into the knee joint of each group. After two weeks, synovium and Prosthesis eluent were cultured for bacteria to determine joint infection. The rabbits with knee infection were 0 (saline group), 6 (103 CFU group), 8 (104 CFU group), 8 group (105 CFU). There were 2 rabbits with poor wound healing, and both were in the 105 CFU group. There were a large amount of biofilms on UHMWPE washer surface detected by SEM in the 104 CFU group, and the S. epidermidis grew and aggregated within the biofilms. Hence, we determined that 1 mL 104 CFU S. epidermidis was the appropriate dose to generate knee infection model.
Surgery was performed under general anesthesia with intramuscular injection of ketamine (35 mg/kg) and diazepam (5 mg/kg) as a single dose. After cutting the hairs in the area, a 2 cm longitudinal skin incision was made along the lateral aspect of the right hind knee joint. The fascia and joint capsule were then incised, exposing the lateral femoral condyle and the lateral collateral ligament (LCL). A hole was drilled (with a 3.5 mm sterile drill bit) into the lateral femoral condyle, anterior to the LCL and in the direction of the coronal axis. A sterile stainless-steel screw (3.5 mm diameter, 15 mm length) with a sterile UHMWPE washer (3.5 mm inner diameter, 8 mm external diameter, 1.5 mm thickness) was inserted into the hole. The joint capsule, deep fascia and skin were closed with 4–0 nylon suture. Immediately after surgery, 1 mL inoculum containing 104 colony-forming units (CFUs) of S. epidermidis was inoculated into the knee joint, near the implants. Each rabbit received acetaminophen 30 mg/kg/day for analgesia for 3 days following surgery. We have recorded the temperature and weight of the animal, and not found the rabbits to have fever or weight loss.
Study groups and intra-articular lavage
Fourteen days after bacteria inoculation, rabbits were randomly assigned to four groups of eight rabbits each, and were given continuous intra-articular lavage of the following solutions: normal saline (Group A); vancomycin 20 mcg/mL (Group B); allicin 4 mg/L (Group C); and vancomycin 20 mcg/mL plus allicin 4 mg/L (Group D).
The intra-articular lavage device was installed as follows. Under general anesthesia as mentioned above, a 2.5 cm skin incision was made in the midline of the knee. A 12-gauge needle was inserted into the joint cavity at the lateral suprapatellar port and then withdrawn. An intravenous infusion tubing was inserted through the channel formed by the 12-gauge needle until the tip was 1.5–2 cm into the knee joint cavity. It was secured in this position by suturing to the skin with 4-0 nylon suture. This tubing acted as the inflow conduit for the lavage. The outflow for the lavage was another intravenous infusion tubing, which was placed 1.5–2 cm into the knee joint at the medial infrapatellar area, using the same method as for the inflow tubing. This tubing was likewise fixed to the skin with 4-0 nylon suture. A plaster cast was applied to immobilize the knee joint. After recovery from anesthesia, the rabbits were housed in narrow cages that limited movement to prevent kinking (and thus blockage) of the lavage tubing. A 500 mL bag of lavage liquid was placed 1 meter above the knee and connected to the inflow tubing. Lavage fluids were administered continuously at 1–1.5mL/min for 3 days.
Determination of biofilm formation and bacterial burden
The rabbits were euthanized with intravenous injection of phenobarbital 100 mg/kg 12 hours after completion of the lavage. The implanted screws and washers were harvested from the surgical site using sterile technique. The screws were rinsed three times with 5 mL phosphate-buffered saline (PBS) to remove surface planktonic bacteria, and then placed in a glass beaker, to which 5 mL sterile saline was added. The saline in the beaker was subsequently sonicated in an ultrasonic cleaner (Kunshan Hechuang Ultrasonic Co. Ltd, Jiangsu, China) using the procedure previously described [28]. One milliliter of the solution after sonication was used for bacterial culture. Samples were serially diluted in TSB and inoculated on TSB agar plates. After incubation at 37°C for 24 h, the final bacterial counts were calculated, and expressed as log10 colony forming unit (CFU)/mL. The experiments were repeated three times.
The washers were also rinsed three times with 5 mL PBS. Each washer was then preserved in fixative (2.5% glutaraldehyde) for 24 h, as described in previous study [29]. After dehydration and coating with gold–palladium, an observation area was randomly selected and marked on the washer. And then, the washers were analyzed with a scanning electron microscope (SEM) (JEOL Ltd., Tokyo, Japan). Because of their irregular shape, the screws cannot be scanned by SEM.
All individuals involved in the microbial experiments and SEM study were blinded to the identities of the samples.
Statistical analyses
SPSS 17.0 (SPSS, Inc., Chicago, IL) software was used for the statistical analyses. Data are expressed as mean ± standard deviation (SD). The differences in bacterial burden of the biofilm among the groups were assessed by one-way analysis of variance (ANOVA) with the least significant difference (LSD) method. P<0.05 was considered statistically significant.
Results
In the rabbit knee PJI model, we evaluated the effects of the antibiotics intra-articular lavage on biofilm formation and bacterial counts (S. epidermidis) on the prosthesis joints. SEM was used to directly visualize the biofilm formation and bacteria accumulation on the prosthesis washers in randomly selected areas (Figure 1). In the saline group (Figure 1 a), large amount of biofilms were seen and bacteria were densely packed. In the vancomycin group, biofilms were abundant and some bacteria were seen inlaid in the biofilms (Figure 1 b). In the allicin group, the biofilm formation was significant reduced and bacteria were still detectable (Figure 1 c). However, there were minimal or no biofilms visualized and only sporadic bacteria were detected on the washers in the group treated with both vancomycin and allicin intra-articular lavage (Figure 1 d), which were clearly fewer than those observed for the other groups.
Figure 1. SEM images of biofilms and attached Staphylococcus epidermidis on the implant washers.
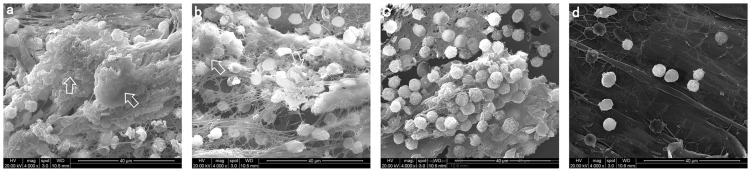
Arrows indicate Staphylococcus epidermidis biofilm on the washers. Representative SEM images from animals received intra-articular lavage with normal saline (a), vancomycin (b), allicin (c) and allicin plus vancomycin (d) are shown. The magnifications are X1000for all images.
The bacteria detected by SEM was further verified and quantified by bacterial cultures. The bacterial counts of the sonicated eluates from the screws for each group are shown in Figure 2. There was a significant overall difference among the groups by ANOVA (F = 71.14, P<0.001). The bacterial counts (as expressed as log10 CFU/mL) of sonicated eluates of saline (group A), vancomycin (group B), allicin (group C), and vancomycin and allicin group (group D) were 5.25±0.53, 4.75±0.61, 3.56±0.66, and 1.71±0.19, respectively. The lowest bacterial count was observed in vancomycin plus allicin group which was significantly lower than all other groups (P<0.01). The bacterial count of sonicated eluate from the allicin group was slightly lower than the saline and vancomycin groups (P<0.01). The bacterial count difference between the vancomycin and saline group was not significant (P>0.05).
Figure 2. Bacterial counts of eluates from the knee joint implants (screw).
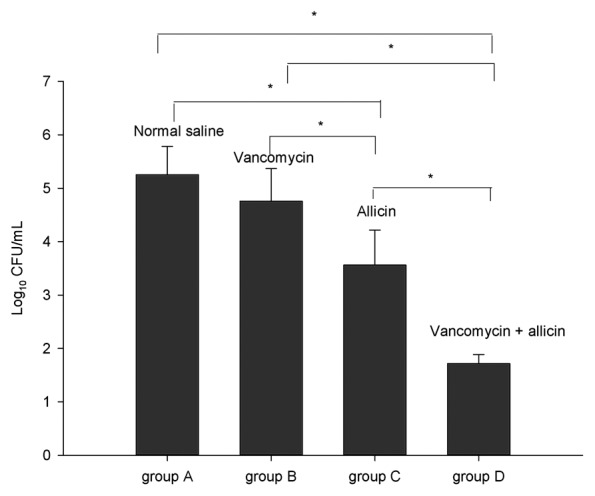
Thirty two rabbits were randomly assigned to four groups of eight rabbits each. The viable organisms recovered from the screw eluate of the indicated groups are expressed as log10CFU/mL. The experiments of bacterial counts were repeated three times. The data are expressed as the mean and the error bars represent the standard deviation. Asterisks denote statistically significant differences: * P<0.01.
Discussion
Biofilm infection is a potentially catastrophic complication of prosthetic joint replacement. Biofilm-embedded bacteria are 100–1000 times more resistant to antibiotics than their planktonic counterparts [16]. To improve the bactericidal effect of antimicrobial agents on biofilm-embedded bacteria, inhibition of bacteria adherence and biofilm formation are useful strategies. Recently, studies have shown that allicin can inhibit bacterial adhesion and aggregation, and then prevent the biofilm formation in vitro [21], [22]. In addition, the effect of allicin plus vancomycin on biofilm-related PJI in vivo has not been studied. In the present study, we evaluated the efficacy of intra-articular lavage using a combination of allicin and vancomycin for the treatment of PJI in a rabbit model. Our results showed that allicin inhibited the formation of biofilm on prosthetic joint surfaces in vivo; and combined use of allicin and vancomycin effectively killed bacteria within the biofilm.
Compared with normal saline, lavage with vancomycin plus allicin had strong bactericidal effects on biofilm-embedded bacteria of the screw surface. The rabbits received combination lavage had lower bacterial counts in cultures of the eluates of the screws implanted and no biofilm visualized by SEM on the washers. There are several possible explanations for this result, which are not mutually exclusive. Firstly, biofilm formation and development are dynamic processes [18], [30]. Bacteria released from biofilm dispersal adhere to the surface of implants and form new biofilms. In the presence of allicin, the biofilm-released bacteria (i.e. S. epidermidis in our study) are prevented to adhere and aggregate on the implanted materials surface in the articular cavity [21], and new biofilm formation is inhibited. The bacteria dissociate in the articular cavity and are more easily killed by antibiotics (vancomycin in our study). Secondly, the antimicrobials are only able to diffuse through the biofilm shallowly and kill bacteria located shallow in the biofilms [31]. In the presence of allicin, the new biofilm cannot form, and bacteria that are previously deep in biofilm and inaccessible to antibiotics are now exposed and rendered sensitive to antibiotics. In the SEM images, biofilm was significantly reduced or eliminated in the allicin-containing lavage groups (Figure 1 c, d). This provides in vivo evidence to support in vitro findings that allicin inhibits biofilm formation [21], [22]. The bacterial count in the sonicated eluates in the allicin group was slightly lower than that in the saline and vancomycin group (Figure 2). This is likely due to allicin preventing bacteria to form new biofilms, which leads to the decrease of residual biofilm-embedded bacteria on the prosthesis. The bacterial counts of the sonicated eluates in the vancomycin group were only slightly lower than that in the saline group (P>0.05). This suggests that vancomycin has poor bactericidal effect on biofilm-embedded bacteria. The shorter course of vancomycin treatment (3 days) might also be one of the contributing factors.
The mechanism(s) of biofilm formation is complex and the mechanism by which allicin inhibits biofilm formation is still poorly understood. Nevertheless, our results have shown, for the first time, that allicin inhibits S. epidermidis biofilm formation in vivo, an effect that was previously demonstrated only in vitro.
Intra-articular lavage with allicin plus vancomycin may be a useful anti-infection strategy for the treatment of PJI. Biofilm plays an important role in PJI and the successful treatment of PJI must eradicate the biofilm dwelling microorganisms [6], [13]. Debridement with retention of device is the most conservative surgical approach. The success rate was approximately 80% of appropriately selected cases [32], which was less than the single-stage and two-stage exchange procedures [12]. In implant-associated infection, the cure rate was extremely low by antibiotics alone, because even therapy with susceptible antibiotics cannot eliminate the biofilm from the surface of an implanted device [12]. The intra-articular minimal inhibitory concentration (MIC) is difficult to achieve by systemic antibiotics delivery [33]. By using of lavage, the intra-articular antibiotics concentrations can be insured. We speculate that allicin plus vancomycin lavage therapy in addition to the treatment of retained prosthesis could improve the cure rate of PJI.
In theory, longer lavage time could eliminate the residuals of biofilm-embedded bacteria and biofilms on the surface of the implant. However, longer lavage time could potentially lead to greater risk of infection. A balance of efficacy and risk needs to be sought. It has been demonstrated that the irrigation-drainage system was recommended for a period from 2 to 6 days [34]. Based on the study, 3 days was used as the lavage time in our study.
Joint lavage can wash out microscopic and macroscopic intra-articular debris from inside the joint space. It is commonly used in the treatment of knee osteoarthritis, septic arthritis, and temporomandibular joint disorders [35], [36], [37], [38], [39]. It has also been explored to treat PJI using different drugs in the lavage [40], [41], [42], [43], [44]. The continuous lavage can effectively reduce the bacterial load on various surfaces [45] and maintain the concentration of drug in the joint cavity. The results of our study support this notion.
A low dose of 104 CUF S. epidermidis was inoculated into rabbit articular cavity, the SEM results presented a large of biofilm on prosthesis in the saline lavage group. We believe the real clinical situation of PJI was reflected by inoculating this amount of bacteria. If a higher dose of bacteria was inoculated, it might cause wound disunion, sinus formation, and other complications.
The animal model presented in this study replicated the intra-articular implantation of biomaterials commonly employed in joint prosthesis, and not the mechanics of human prosthetic joint replacement. This animal model is suitable for studying the effect of drugs on PJI, but not suitable for other artificial joint research, such as joint prosthesis loosening. Nevertheless, developing artificial joints animal models that more closely mimicking human prosthesis is necessary.
Further studies are needed to determine whether allicin can inhibit the biofilm produced by other bacteria. In this study, the biofilm on the implant surface was difficult to quantify. Because of the irregular shape, screws cannot be scanned by SEM. Further experiments should improve the metal implant shape for optimal observation by SEM. Future work should also include systemic administration of allicin combined with vancomycin to verify their synergistic effect in the treatment of PJI. In fact, the systemic administration has less risk regarding to exogenous infection and can be used for longer time than lavage.
In summary, our results showed allicin can inhibit S. epidermidis biofilm formation in vivo that has been previously demonstrated only in vitro. Combined with allicin, the bactericidal effect of vancomycin is enhanced. Allicin combined with vancomycin may thus be a useful anti-infection strategy for the retention prosthesis treatment of PJI.
Acknowledgments
We thank Hanyin Liu for providing help on animal breeding and management.
Funding Statement
The authors have no support or funding to report.
References
- 1. Barrett J, Losina E, Baron JA, Mahomed NN, Wright J, et al. (2005) Survival following total hip replacement. J Bone Joint Surg Am 87: 1965–1971. [DOI] [PubMed] [Google Scholar]
- 2. Del PJL, Patel R (2009) Clinical practice. Infection associated with prosthetic joints. N Engl J Med 361: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, et al. (2009) Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res 467: 2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, et al. (2010) Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 468: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iarikov D, Demian H, Rubin D, Alexander J, Nambiar S (2012) Choice and doses of antibacterial agents for cement spacers in treatment of prosthetic joint infections: review of published studies. Clin Infect Dis 55: 1474–1480. [DOI] [PubMed] [Google Scholar]
- 6. Peel TN, Buising KL, Choong PF (2012) Diagnosis and management of prosthetic joint infection. Curr Opin Infect Dis 25: 670–676. [DOI] [PubMed] [Google Scholar]
- 7. Bradbury T, Fehring TK, Taunton M, Hanssen A, Azzam K, et al. (2009) The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty 24: 101–104. [DOI] [PubMed] [Google Scholar]
- 8. Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH (2009) Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res 467: 1732–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Odum SM, Fehring TK, Lombardi AV, Zmistowski BM, Brown NM, et al. (2011) Irrigation and debridement for periprosthetic infections: does the organism matter. J Arthroplasty 26: 114–118. [DOI] [PubMed] [Google Scholar]
- 10. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS (2012) Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am 94: e104. [DOI] [PubMed] [Google Scholar]
- 11. Zmistowski B, Fedorka CJ, Sheehan E, Deirmengian G, Austin MS, et al. (2011) Prosthetic joint infection caused by gram-negative organisms. J Arthroplasty 26: 104–108. [DOI] [PubMed] [Google Scholar]
- 12. Zimmerli W, Moser C (2012) Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 65: 158–168. [DOI] [PubMed] [Google Scholar]
- 13. Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351: 1645–1654. [DOI] [PubMed] [Google Scholar]
- 14. Beswick AD, Elvers KT, Smith AJ, Gooberman-Hill R, Lovering A, et al. (2012) What is the evidence base to guide surgical treatment of infected hip prostheses? systematic review of longitudinal studies in unselected patients. BMC Med 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gallo J, Kolar M, Novotny R, Rihakova P, Ticha V (2003) Pathogenesis of prosthesis-related infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 147: 27–35. [DOI] [PubMed] [Google Scholar]
- 16. Widmer AF (2001) New developments in diagnosis and treatment of infection in orthopedic implants. Clin Infect Dis 33 Suppl 2 S94–106. [DOI] [PubMed] [Google Scholar]
- 17. del PJL, Patel R (2007) The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther 82: 204–209. [DOI] [PubMed] [Google Scholar]
- 18. Yang L, Hu Y, Liu Y, Zhang J, Ulstrup J, et al. (2011) Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ Microbiol 13: 1705–1717. [DOI] [PubMed] [Google Scholar]
- 19. Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 20. Arciola CR, Montanaro L, Costerton JW (2011) New trends in diagnosis and control strategies for implant infections. Int J Artif Organs 34: 727–736. [DOI] [PubMed] [Google Scholar]
- 21. Perez-Giraldo C, Cruz-Villalon G, Sanchez-Silos R, Martinez-Rubio R, Blanco MT, et al. (2003) In vitro activity of allicin against Staphylococcus epidermidis and influence of subinhibitory concentrations on biofilm formation. J Appl Microbiol 95: 709–711. [DOI] [PubMed] [Google Scholar]
- 22. Cruz-Villalon G, Perez-Giraldo C (2011) Effect of allicin on the production of polysaccharide intercellular adhesin in Staphylococcus epidermidis . J Appl Microbiol 110: 723–728. [DOI] [PubMed] [Google Scholar]
- 23. Otto M (2008) Staphylococcal biofilms. Curr Top Microbiol Immunol 322: 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malloy KM, Davis GA (2009) Summary of ASHP/IDSA/SIDP vancomycin monitoring recommendations: a focus on osteomyelitis. Orthopedics 32: 499. [DOI] [PubMed] [Google Scholar]
- 25.Isiklar ZU, Darouiche RO, Landon GC, Beck T (1996) Efficacy of antibiotics alone for orthopaedic device related infections. Clin Orthop Relat Res : 184–189. [DOI] [PubMed]
- 26. Patel JD, Ebert M, Ward R, Anderson JM (2007) Staphylococcus epidermidis biofilm formation: effects of biomaterial surface chemistry and serum proteins. J Biomed Mater Res A 80: 742–751. [DOI] [PubMed] [Google Scholar]
- 27. Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, et al. (2005) A novel total knee arthroplasty infection model in rabbits. J Orthop Res 23: 1100–1104. [DOI] [PubMed] [Google Scholar]
- 28. Sampedro MF, Huddleston PM, Piper KE, Karau MJ, Dekutoski MB, et al. (2010) A biofilm approach to detect bacteria on removed spinal implants. Spine (Phila Pa 1976) 35: 1218–1224. [DOI] [PubMed] [Google Scholar]
- 29. Fujimura S, Sato T, Kikuchi T, Zaini J, Gomi K, et al. (2009) Efficacy of clarithromycin plus vancomycin in mice with implant-related infection caused by biofilm-forming Staphylococcus aureus . J Orthop Sci 14: 658–661. [DOI] [PubMed] [Google Scholar]
- 30. Monds RD, O’Toole GA (2009) The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol 17: 73–87. [DOI] [PubMed] [Google Scholar]
- 31. Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shuman EK, Urquhart A, Malani PN (2012) Management and prevention of prosthetic joint infection. Infect Dis Clin North Am 26: 29–39. [DOI] [PubMed] [Google Scholar]
- 33. Haerdi-Landerer MC, Habermacher J, Wenger B, Suter MM, et al. (2010) Slow release antibiotics for treatment of septic arthritis in large animals. Vet J 184: 14–20. [DOI] [PubMed] [Google Scholar]
- 34.Parisien JS, Shaffer B (1992) Arthroscopic management of pyarthrosis. Clin Orthop Relat Res : 243–247. [PubMed]
- 35. Chaudhry H, Madden K, Bhandari M (2014) Cochrane in CORR (R): Joint lavage for osteoarthritis of the knee. Clin Orthop Relat Res 472: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meijer MC, van Weeren PR, Rijkenhuizen AB (2000) Clinical experiences of treating septic arthritis in the equine by repeated joint lavage: a series of 39 cases. J Vet Med A Physiol Pathol Clin Med 47: 351–365. [DOI] [PubMed] [Google Scholar]
- 37. Manadan AM, Block JA (2004) Daily needle aspiration versus surgical lavage for the treatment of bacterial septic arthritis in adults. Am J Ther 11: 412–415. [DOI] [PubMed] [Google Scholar]
- 38. Dodwell ER (2013) Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr 25: 58–63. [DOI] [PubMed] [Google Scholar]
- 39. Vos LM, Huddleston SJJ, Stegenga B (2013) Lavage therapy versus nonsurgical therapy for the treatment of arthralgia of the temporomandibular joint: a systematic review of randomized controlled trials. J Orofac Pain 27: 171–179. [DOI] [PubMed] [Google Scholar]
- 40. Lettin AW, Neil MJ, Citron ND, August A (1990) Excision arthroplasty for infected constrained total knee replacements. J Bone Joint Surg Br 72: 220–224. [DOI] [PubMed] [Google Scholar]
- 41. Mella-Schmidt C, Steinbrink K (1989) Value of irrigation-suction drainage in the treatment of early infection of joint implants. Chirurg 60: 791–794. [PubMed] [Google Scholar]
- 42. Tsumura H, Ikeda S, Ono T, Itonaga I, Taira H, et al. (2005) Synovectomy, debridement, and continuous irrigation for infected total knee arthroplasty. Int Orthop 29: 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirr R, Wiberg J, Hertlein H (2006) Clinical experience and results of using the V.A.C. instill therapy in infected hip- and knee prosthetics. Zentralbl Chir 131 Suppl 1 S79–82. [DOI] [PubMed] [Google Scholar]
- 44. Brown NM, Cipriano CA, Moric M, Sporer SM, Della VCJ (2012) Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty 27: 27–30. [DOI] [PubMed] [Google Scholar]
- 45. Bahrs C, Schnabel M, Frank T, Zapf C, Mutters R, et al. (2003) Lavage of contaminated surfaces: an in vitro evaluation of the effectiveness of different systems. J Surg Res 112: 26–30. [DOI] [PubMed] [Google Scholar]


