Abstract
Mammalian oocytes are arrested at the prophase of meiosis before induction of maturation by the preovulatory luteinizing hormone (LH) surge. LH also promotes the survival of meiotic male germ cells in the testis. Because LH binds somatic cells, the mechanism underlying its regulation of germ cell function is unclear. We found that LH stimulates Leydig insulin-like 3 (INSL3) transcripts in ovarian theca and testicular Leydig cells. INSL3, in turn, binds a G protein-coupled receptor, LGR8 (leucine-rich repeat-containing G protein-coupled receptor 8), expressed in germ cells to activate the inhibitory G protein, thus leading to decreases in cAMP production. Treatment with INSL3 initiates meiotic progression of arrested oocytes in preovulatory follicles in vitro and in vivo and suppresses male germ cell apoptosis in vivo, thus demonstrating the importance of the INSL3-LGR8 paracrine system in mediating gonadotropin actions.
Gonadotropins secreted by the anterior pituitary are essential for optimal follicle development and spermatogenesis (1, 2). The luteinizing hormone (LH) binds specific receptors in ovarian theca and mature granulosa cells as well as testicular Leydig cells. In the mammalian ovary, oocytes show a prolonged arrest at the prophase of meiosis I and undergo meiotic maturation only when exposed to the preovulatory LH surge. In testis, up to 75% of germ cells are deleted during spermatogenesis (3), and LH is a survival factor for these cells (4). Because LH acts exclusively on somatic cells in gonads, local paracrine factors are likely involved in the regulation of meiotic arrest in oocytes (5-7) and meiotic progression in male germ cells (8).
INSL3, also know as Leydig insulin-like hormone, was originally named for its exclusive expression in Leydig cells of fetal and adult testes (9). However, INSL3 is also expressed in thecal and luteal cells of the ovary (10). Male INSL3 null mice exhibit bilateral cryptorchidism (11, 12), whereas female INSL3 null mice show impaired fertility (11). Recent studies indicated that testis INSL3 acts as an endocrine factor to activate a G protein-coupled receptor, LGR8 (leucine-rich repeat-containing G protein-coupled receptor 8), in the gubernaculum with increases in cAMP production (13). Here, we demonstrate the expression of LGR8 in the germ cells of both sexes, as well as the paracrine role of INSL3 in initiating oocyte maturation and preventing male germ cell apoptosis, both of which are mediated by the inhibitory G protein (Gi) pathway.
Materials and Methods
Ovaries were obtained from immature rats at different ages or after treatment with gonadotropins, whereas testes were obtained during development and after treatment with a GnRH antagonist with or without human chorionic gonadotropin (hCG) or INSL3. Total RNA from rat gonads was extracted before Northern blotting and real-time RT-PCR. Some tissue samples were used for in situ hybridization studies.
For evaluating oocyte maturation, cumulus-enclosed oocytes (CEOs) or preovulatory follicles were obtained from pregnant mare serum gonadotropin (PMSG)-treated rats and cultured with different hormones or reagents. At the end of culture, the occurrence of germinal vesicle breakdown (GVBD) in the oocytes was examined after removing cumulus cells surrounding the CEOs.
Seminiferous tubular cells and interstitial cells were isolated after collagenase treatment before in vitro culture with different hormones. For in vivo studies, testes were obtained for weighing, Northern blotting, or apoptosis analyses. Testis DNA was isolated and labeled at 3′ ends with 32P-dideoxy-ATP by using terminal transferase. Labeled samples were fractionated through agarose gels for visualization by autoradiography and for β-counting of low-molecular-weight DNA fragments. For in situ DNA 3′-end labeling, fixed testicular tissues were embedded in paraffin before analysis.
Further information can be obtained in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Results
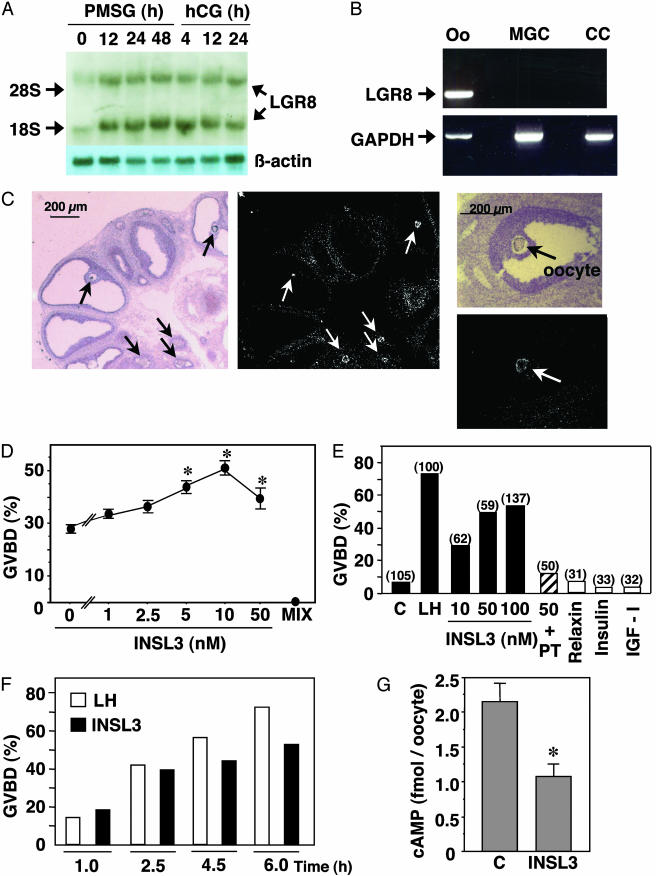
Northern blot analyses indicated the expression of LGR8 in ovaries of gonadotropin-treated rats (Fig. 1A). RT-PCR and in situ hybridization studies further demonstrated the exclusive oocyte localization of LGR8 and its absence in cumulus and mural granulosa cells (Fig. 1 B and C). The ability of INSL3 to modulate oocyte maturation was tested in vitro. Although spontaneous meiotic resumption, evidenced by the breakdown of the nuclear membrane (germinal vesicle), could be observed in cultured cumulus-enclosed oocytes obtained from preovulatory follicles (Fig. 1D), treatment with INSL3 dose-dependently augmented oocyte maturation. In contrast, incubation with a phosphodiesterase inhibitor [1-methyl-3-isobutylxanthine (MIX)] completely blocked oocyte maturation. In cultured preovulatory follicles, treatment with INSL3, like LH, induced oocyte maturation in a dose-dependent manner (Fig. 1E). In addition, treatment with Pertussis toxin (PT), a Gi inhibitor, blocked the INSL3 induction of oocyte maturation, suggesting the mediatory role of the Gi protein. In contrast, treatment with relaxin, insulin, or IGF-I was ineffective. Time course studies further demonstrated that INSL3 induced oocyte maturation as early as 1 h after treatment (Fig. 1F). Consistent with the reported association between oocyte maturation and decreases in intraoocytic cAMP content (14), treatment with INSL3 (10 nM) for 60 min suppressed cAMP levels in denuded oocytes from preovulatory follicles (Fig. 1G). Furthermore, INSL3 induction of oocyte maturation was restricted to preovulatory follicles because INSL3 treatment of antral follicles isolated before PMSG priming was ineffective (oocyte maturation: control, 13.5%; INSL3-treated (100 nM), 16.2%; n = 37 follicles). These findings suggest that functional coupling of LGR8 to oocyte maturation is induced in preovulatory follicles.
Fig. 1.
Expression of LGR8 in the ovary, and INSL3 stimulation of oocyte maturation. (A) Northern blot analysis of ovarian LGR8 transcripts in gonadotropin-treated rats. Immature rats were treated with PMSG and, 2 days later, with an ovulatory dose of hCG. (B) RT-PCR amplification of LGR8 transcripts in oocyte (Oo) but not mural granulosa cells (MGC) or cumulus cells (CC) from preovulatory follicles at 48 h after PMSG treatment. GAPDH serves as an internal control. (C) In situ hybridization localization of LGR8 in the oocyte (arrows) of preovulatory follicles. (D) INSL3 augmentation of GVBD of cultured cumulus-enclosed oocytes obtained from preovulatory follicles. *, P < 0.01 vs. controls without INSL3. (E) INSL3 stimulation of oocyte maturation in cultured preovulatory follicles, and the inhibitory effects of Pertussis toxin (PT) (1μg/ml). C, controls. Numbers in parentheses indicate the number of oocytes examined. (F) Time-dependent stimulation of oocyte maturation by INSL3 in cultured preovulatory follicles. Oocytes from 80-140 follicles per group were evaluated. (G) Suppression of intraoocyte cAMP levels by INSL3. C: control. *, P < 0.05.
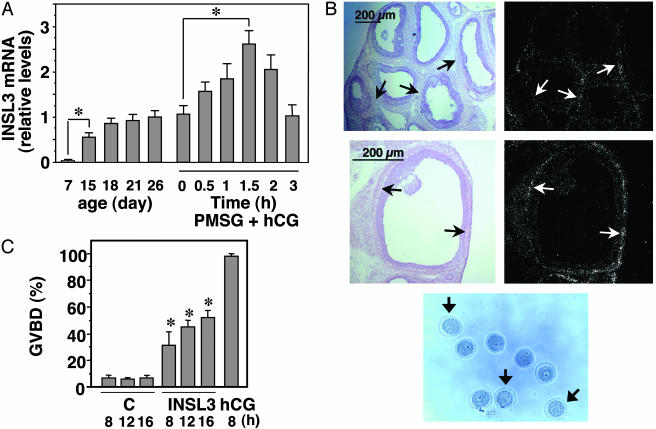
To assess developmental changes in INSL3 expression, real-time RT-PCR was performed. Ovarian INSL3 transcripts increased with the first wave of follicle development in immature rats showing an increase between days 7 and 18 of age (Fig. 2A). These data suggest increases in INSL3 expression during early antral follicle development, consistent with an earlier study in mice (15). Although treatment of 26-day-old rats with PMSG did not alter ovarian INSL3 levels (day 26 vs. PMSG + hCG 0 h), subsequent administration of an ovulatory dose of hCG, an agonist of LH, stimulated INSL3 expression with peak increases at 1.5 h of treatment (Fig. 2 A). In situ hybridization analyses further confirmed the expression of INSL3 in theca cells surrounding preovulatory follicles (Fig. 2B). To test INSL3 actions in vivo, local intrabursal administration of INSL3 was performed in PMSG-primed rats. As shown in Fig. 2C, a time-dependent induction of oocyte maturation was evident based on germinal vesicle breakdown found in treated ovaries (Fig. 2D). Unlike LH/hCG, treatment with INSL3 alone did not lead to follicle rupture.
Fig. 2.
Regulation of thecal INSL3 expression and INSL3 induction of oocyte maturation in vivo.(A) Increases in ovarian INSL3 mRNA levels during development and after gonadotropin treatment. Quantitative RT-PCR was performed by using ovarian samples from rats at different ages (postnatal day). Ovaries were also collected before (day 26) and after treatment with 15 units of PMSG for 2 days (0 h of the PMSG + hCG group), followed by administration of 10 units of hCG for different intervals. The ratio of INSL3/β-actin transcript levels at 26 days of age was set as 1. *, P < 0.01. (B) In situ hybridization analysis of INSL3 expression in theca cells (arrows) of preovulatory follicles at 1.5 h after hCG treatment of PMSG-primed rats. (C) In vivo induction of oocyte maturation after INSL3 treatment. Immature rats at 2 days after PMSG priming were treated with INSL3 (2 μg per 0.1 ml of PBS) via intrabursal injections or hCG via s.c. injections. Oocytes were retrieved at different intervals after puncture of the ovary to release cumulus-oocyte complexes for assessing morphology. C, controls. *, P < 0.01 between control and INSL3-treated groups at the same time point. (D) Morphology of oocytes after INSL3 treatment (8 h) in vivo. Arrows indicate mature oocytes.
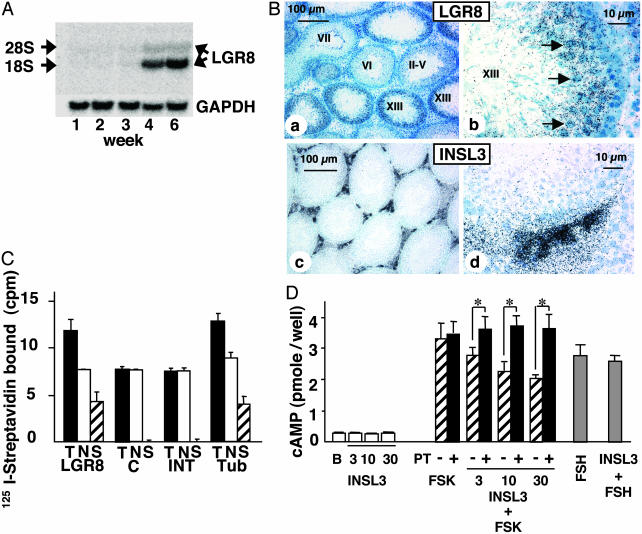
We further tested the paracrine roles of the INSL3-LGR8 system in testis. Northern blot analyses indicated that LGR8 transcripts were present in testes of rats at 4 and 6 weeks of age but not before (Fig. 3A). In situ hybridization analyses further demonstrated that LGR8 is expressed in seminiferous tubules but not in the interstitial cells of adult rats (Fig. 3B). LGR8 signals were found in select germ cells (stage XIII, Figs. 3Bb and 5, which is published as supporting information on the PNAS web site). In contrast, INSL3 expression was restricted to interstitial cells. By using biotinylated INSL3 as a tracer, INSL3 binding was found in seminiferous tubular cells but not in interstitial cells (Fig. 3C). Tubular cells were further treated with INSL3 to activate LGR8. Although basal cAMP production was not affected, INSL3 treatment dose-dependently prevented cAMP increases induced by forskolin (Fig. 3D). Also, the suppressive effect of INSL3 was blocked by pretreatment with Pertussis toxin. In contrast, although FSH stimulated cAMP production by Sertoli cells in the tubular preparation, cotreatment with INSL3 did not alter cAMP levels (Fig. 3D).
Fig. 3.
Expression of LGR8 in male germ cells, and INSL3 activation of the Gi protein in seminiferous tubules. (A) Northern blot analyses of LGR8 expression in testes of developing rats. (B) In situ hybridization localization of LGR8 and INSL3 transcripts in testes from adult rats. Hybridization signals for LGR8 and INSL3 were present in seminiferous tubules and interstitial cells, respectively. Two magnifications are shown. Roman numerals indicate the stage of the seminiferous epithelial cycle. The strongest LGR8 expression was found in tubule stage XIII with prominent signals in selective germ cells (b, arrows). (C) Direct binding of biotinylated INSL3 to different testicular compartments. T, total binding; N, nonspecific binding; S, specific binding; LGR8, 293T cells expressing recombinant LGR8; C, nontransfected 293T cells; INT, interstitial cells; Tub, tubular cells. Biotinylated INSL3 signals were detected by using labeled streptavidin. (D) INSL3 suppression of forskolin (FSK)-induced cAMP production and blockage by Pertussis toxin (PT) pretreatment. Tubular cells were treated with INSL3 (nM), forskolin (50 nM), or FSH (100 ng/ml) for 12 h before cAMP determination. Some cells also were pretreated for 6 h with Pertussis toxin (100 ng/ml) to suppress Gi activity. B, basal. *, P < 0.05.
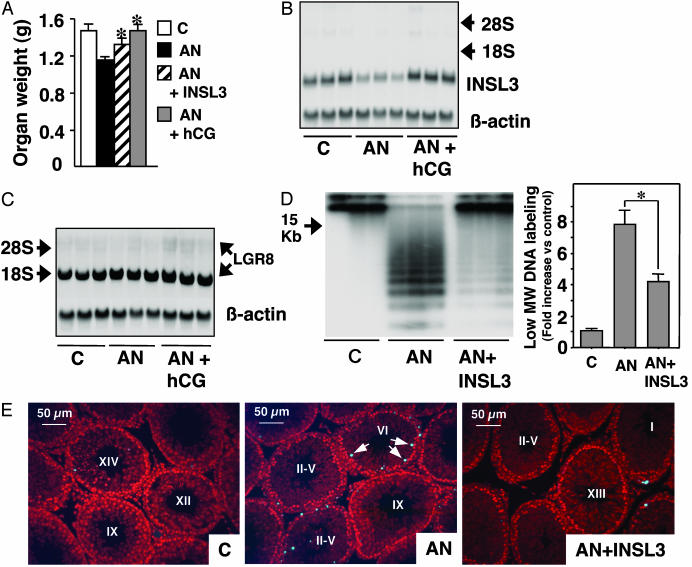
The role of INSL3 in testis was tested in vivo by using a gonadotropin deprivation model (4). Immature rats were treated for 5 days with a GnRH antagonist with or without INSL3 or hCG. GnRH antagonist treatment decreased testis weight; however, this inhibitory effect was partially blocked by cotreatment with INSL3 or hCG (Fig. 4A). In contrast, no weight change in kidney or heart was detected (Fig. 6, which is published as supporting information on the PNAS web site). Northern blot analyses indicated that treatment with the GnRH antagonist decreased testicular INSL3 transcripts, whereas cotreatment with hCG increased INSL3 expression (Fig. 4B). However, these treatments did not alter testicular LGR8 expression (Fig. 4C). Analysis of internucleosomal DNA fragmentation further indicated that treatment with the GnRH antagonist increased ladder-like DNA fragmentation characteristic of apoptosis but that cotreatment with INSL3 blocked cell death (Fig. 4D Left). Quantification of labeled DNA fragments confirmed the antiapoptotic effects of INSL3 (Fig. 4D Right). In situ staining of DNA fragments (Figs. 4E and 7, which is published as supporting information on the PNAS web site) further indicated that GnRH antagonist treatment induced apoptosis in select germ cells from epithelial cycle stages VI through VIII. In control and INSL3-treated groups, basal apoptotic signals could be seen in stages XIII through I.
Fig. 4.
Treatment with hCG increased INSL3 expression, whereas treatment with INSL3 suppressed testis germ cell apoptosis induced by a GnRH antagonist (AN). (A) Changes in testis weight after hormonal treatment. Immature rats at 28 days of age were treated daily with a GnRH antagonist (25 μg/day) with or without hCG (75 units/day) or INSL3 (2 μg twice daily) for 5 days. *, Different from treatment with GnRH antagonist alone. (B) Northern blot analyses of testicular INSL3 transcripts. C, controls. (C) Northern blot analyses of testicular LGR8 transcripts. (D) Analysis of apoptotic DNA fragmentation in testes by using 3′-end DNA labeling with the terminal transferase, followed by gel fractionation and autoradiography (Left) or by β-counting of low-molecular-weight (<15 kb) DNA fragments (Right). *, P < 0.05. (E) In situ staining of apoptotic DNA fragmentation in seminiferous tubules. Cellular nucleic acids were stained (red) by using propidium iodide. Positive apoptosis signals (blue fluorescence) are evident in the GnRH antagonist-treated group showing apoptosis in selective germ cells (arrows) in tubule stage VI. Testis from control animals (C) and those treated with both INSL3 and the GnRH antagonist showed basal apoptotic staining in selective germ cells in stages XIV to I of the seminiferous epithelial cycle. Because of random sectioning, only selected stages are shown. Some tubules were labeled as II-V because these stages could not be discerned by using propidium iodide staining.
Discussion
Mammalian oocytes exhibit prolonged arrest in the meiotic prophase (G2/M transition). In response to the midcycle LH surge, oocytes of preovulatory follicles resume meiosis, proceeding to the metaphase of the second meiotic division. Dissolution of the nuclear membrane is followed by extrusion of the first polar body. The prolonged arrest of oocytes in the meiotic prophase (G2/M transition) and subsequent resumption of meiosis is correlated with changes in cAMP levels in the oocyte. Meiotic arrest of the oocyte is most likely maintained by follicular purines that increase oocyte cAMP levels (16); however, changes in hypoxanthine levels could not be found before oocyte maturation was induced by gonadotropin. Although LH stimulates cAMP production in follicular somatic cells, a decrease in intraoocyte cAMP level is required for meiotic resumption (6). Indeed, meiotic arrest is released after injection of an antibody for the stimulatory G protein (17). Although resumption of meiosis is induced by INSL3 in mammals and by progesterone and insulin in amphibians, a decrease in the intraoocyte cAMP level is an evolutionarily conserved mechanism for regulating meiotic progression (18). Our findings of transient stimulation of INSL3 expression in theca cells by LH/hCG, INSL3 suppression of intraoocyte cAMP levels, and INSL3 induction of oocyte maturation suggest a paracrine role of the INSL3-LGR8 system in mediating preovulatory LH actions. Although meiosis-activating sterols (MAS) also induce oocyte maturation in culture (7), the exact role of MAS in mediating LH-stimulated oocyte maturation remains controversial (19). Because FSH treatment also stimulates a putative paracrine factor from cumulus cells to promote oocyte maturation (20), INSL3 likely is not the only paracrine factor mediating the gonadotropin induction of oocyte maturation. The ovulatory process consists of oocyte maturation, follicle rupture, and luteinization. Earlier studies indicated that cycling rats treated with inhibitors for the oocyte-specific phosphodiesterase 3 enzyme maintained normal cycling and follicle rupture, but ovulated oocytes were immature and not fertilizable (21). The present study further confirms the possibility of separating oocyte maturation and follicle rupture, thus providing a basis for fertility regulation with LGR8 modulators.
Most male germ cells undergo apoptosis, perhaps as a mechanism to delete superfluous or defective germ cells (3). INSL3, like LH (4), is a survival factor for male germ cells. The observed stimulation of INSL3 transcripts in the testis by hCG is consistent with earlier studies in hypogonadal mice (22). Our findings further demonstrate that INSL3 binds to seminiferous tubules by interacting with the LGR8 receptor expressed in meiotic germ cells to activate the Gi protein. In contrast to the stimulatory effects of INSL3 on cAMP production by gubernacular cells (13), LGR8 expressed in germ cells is coupled to the Gi protein. It appears that G protein coupling of LGR8 depends on the cell type from which it is expressed.
In both female and male germ cells, a complete GPCR/G protein/adenylyl cyclase/phosphodiesterase system exists. Oocyte maturation is regulated by antibodies for Gs (17) and phosphodiesterase 3 inhibitors (23). Rat oocytes also express adenylyl cyclase AC3 (24) and the inhibitory G proteins (25). Although LGR8 is the only known functional GPCR found in the oocyte, the adenosine A3 receptor (26) and several odorant receptors are present in male germ cells and are implicated in germ cell maturation and chemotaxis (27). Male germ cells also express an olfactory type Gα subunit protein and adenylyl cyclase III (28, 29). Our data indicate that a low level of cAMP maintained by INSL3-LGR8 may be important for meiotic progression in both female and male germ cells.
Female INSL3 null mice exhibit impaired fertility associated with increases in follicular atresia and premature luteolysis (11, 30). In contrast, female LGR8 null mice are fertile (31), suggesting the existence of additional intraovarian pathways that participate in the regulation of oocyte maturation (32). For males, deletion of either the INSL3 or LGR8 gene led to bilateral cryptorchidism and impaired spermatogenesis (11, 12, 31) due to defective gubernaculum development during embryogenesis. Defective spermatogenesis was attributed to the secondary effects of undescended testes, and surgical correction of cryptorchid testes in INSL3 null mice partially corrected male infertility (33). Because paralogous genes for both INSL3 and LGR8 are expressed in testis (34, 35), survival of male germ cells could be regulated by redundant signaling systems.
A unified picture of the gonadotropin regulation of germ cell function is emerging. LH, in addition to promoting androgen production by ovarian thecal cells and testis Leydig cells, stimulates INSL3 biosynthesis. INSL3, in turn, activates Gi-coupled LGR8 to initiate oocyte maturation and suppress male germ cell apoptosis. Although INSL3 is secreted into general circulation during fetal life, the present data highlight its paracrine role in female and male gonads.
Supplementary Material
Acknowledgments
We thank C. Spencer for editorial assistance. This work was supported by National Institutes of Health Grant HD44130 (to A.J.W.H.) and National Health and Medical Research Council (NHMRC) Institute Block Grant 983001. R.A.D.B. is the recipient of a NHMRC Fellowship.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: hCG, human chorionic gonadotropin; INSL3, Leydig insulin-like 3; LGR8, leucine-rich repeat-containing G protein-coupled receptor 8; LH, luteinizing hormone; PMSG, pregnant mare serum gonadotropin.
References
- 1.Richards, J. S., Russell, D. L., Ochsner, S. & Espey, L. L. (2002) Annu. Rev. Physiol. 64, 69-92. [DOI] [PubMed] [Google Scholar]
- 2.Themmen, A. P. N. & Huhtaniemi, I. T. (2000) Endocr. Rev. 21, 551-583. [DOI] [PubMed] [Google Scholar]
- 3.Huckins, C. (1978) Anat. Rec. 190, 905-926. [DOI] [PubMed] [Google Scholar]
- 4.Tapanainen, J. S., Tilly, J. L., Vihko, K. K. & Hsueh, A. J. (1993) Mol. Endocrinol. 7, 643-650. [DOI] [PubMed] [Google Scholar]
- 5.Eppig, J. J. & Downs, S. M. (1987) Dev. Biol. 119, 313-321. [DOI] [PubMed] [Google Scholar]
- 6.Tsafriri, A. & Pomerantz, S. H. (1986) Clin. Endocrinol. Metab. 15, 157-170. [DOI] [PubMed] [Google Scholar]
- 7.Byskov, A. G., Andersen, C. Y., Nordholm, L., Thogersen, H., Xia, G., Wassmann, O., Andersen, J. V., Guddal, E. & Roed, T. (1995) Nature 374, 559-562. [DOI] [PubMed] [Google Scholar]
- 8.Griswold, M. D. (1995) Biol. Reprod. 52, 211-216. [DOI] [PubMed] [Google Scholar]
- 9.Ivell, R. & Einspanier, A. (2002) Trends Endocrinol. Metab. 13, 343-348. [DOI] [PubMed] [Google Scholar]
- 10.Bathgate, R., Balvers, M., Hunt, N. & Ivell, R. (1996) Biol. Reprod. 55, 1452-1457. [DOI] [PubMed] [Google Scholar]
- 11.Nef, S. & Parada, L. F. (1999) Nat. Genet. 22, 295-299. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann, S., Steding, G., Emmen, J. M., Brinkmann, A. O., Nayernia, K., Holstein, A. F., Engel, W. & Adham, I. M. (1999) Mol. Endocrinol. 13, 681-691. [DOI] [PubMed] [Google Scholar]
- 13.Kumagai, J., Hsu, S. Y., Matsumi, H., Roh, J. S., Fu, P., Wade, J. D., Bathgate, R. A. & Hsueh, A. J. (2002) J. Biol. Chem. 277, 31283-31286. [DOI] [PubMed] [Google Scholar]
- 14.Schultz, R. M., Montgomery, R. R. & Belanoff, J. R. (1983) Dev. Biol. 97, 264-273. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann, S., Schottler, P., Engel, W. & Adham, I. M. (1997) Mol. Reprod. Dev. 47, 30-38. [DOI] [PubMed] [Google Scholar]
- 16.Eppig, J. J., Ward-Bailey, P. F. & Coleman, D. L. (1985) Biol. Reprod. 33, 1041-1049. [DOI] [PubMed] [Google Scholar]
- 17.Mehlmann, L. M., Jones, T. L. & Jaffe, L. A. (2002) Science 297, 1343-1345. [DOI] [PubMed] [Google Scholar]
- 18.Maller, J. L. (1985) Cell Differ. 16, 211-221. [DOI] [PubMed] [Google Scholar]
- 19.Tsafriri, A., Popliker, M., Nahum, R. & Beyth, Y. (1998) Mol. Hum. Reprod. 4, 483-489. [DOI] [PubMed] [Google Scholar]
- 20.Downs, S. M., Daniel, S. A. & Eppig, J. J. (1988) J. Exp. Zool. 245, 86-96. [DOI] [PubMed] [Google Scholar]
- 21.Wiersma, A., Hirsch, B., Tsafriri, A., Hanssen, R. G., Van de Kant, M., Kloosterboer, H. J., Conti, M. & Hsueh, A. J. (1998) J. Clin. Invest. 102, 532-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balvers, M., Spiess, A. N., Domagalski, R., Hunt, N., Kilic, E., Mukhopadhyay, A. K., Hanks, E., Charlton, H. M. & Ivell, R. (1998) Endocrinology 139, 2960-2970. [DOI] [PubMed] [Google Scholar]
- 23.Tsafriri, A., Chun, S. Y., Zhang, R., Hsueh, A. J. & Conti, M. (1996) Dev. Biol. 178, 393-402. [DOI] [PubMed] [Google Scholar]
- 24.Horner, K., Livera, G., Hinckley, M., Trinh, K., Storm, D. & Conti, M. (2003) Dev. Biol. 258, 385-396. [DOI] [PubMed] [Google Scholar]
- 25.Williams, C. J., Schultz, R. M. & Kopf, G. S. (1996) Mol. Reprod. Dev. 44, 315-323. [DOI] [PubMed] [Google Scholar]
- 26.Zhou, Q. Y., Li, C., Olah, M. E., Johnson, R. A., Stiles, G. L. & Civelli, O. (1992) Proc. Natl. Acad. Sci. USA 89, 7432-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spehr, M., Gisselmann, G., Poplawski, A., Riffell, J. A., Wetzel, C. H., Zimmer, R. K. & Hatt, H. (2003) Science 299, 2054-2058. [DOI] [PubMed] [Google Scholar]
- 28.Gautier-Courteille, C., Salanova, M. & Conti, M. (1998) Endocrinology 139, 2588-2599. [DOI] [PubMed] [Google Scholar]
- 29.Defer, N., Marinx, O., Poyard, M., Lienard, M. O., Jegou, B. & Hanoune, J. (1998) FEBS Lett. 424, 216-220. [DOI] [PubMed] [Google Scholar]
- 30.Spanel-Borowski, K., Schafer, I., Zimmermann, S., Engel, W. & Adham, I. M. (2001) Mol. Reprod. Dev. 58, 281-286. [DOI] [PubMed] [Google Scholar]
- 31.Gorlov, I. P., Kamat, A., Bogatcheva, N. V., Jones, E., Lamb, D. J., Truong, A., Bishop, C. E., McElreavey, K. & Agoulnik, A. I. (2002) Hum. Mol. Genet. 11, 2309-2318. [DOI] [PubMed] [Google Scholar]
- 32.Park, J. Y., Su, Y. Q., Ariga, M., Law, E., Jin, S. L. & Conti, M. (2004) Science 303, 682-684. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen, M. T., Showalter, P. R., Timmons, C. F., Nef, S., Parada, L. F. & Baker, L. A. (2002) J. Urol. 168, 1779-1783. [DOI] [PubMed] [Google Scholar]
- 34.Hsu, S. Y. (1999) Mol. Endocrinol. 13, 2163-2174. [DOI] [PubMed] [Google Scholar]
- 35.Hsu, S. Y., Nakabayashi, K., Nishi, S., Kumagai, J., Kudo, M., Sherwood, O. D. & Hsueh, A. J. (2002) Science 295, 671-674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.