Abstract
Background
Tumor characteristics was sought to be related to axillary lymph node metastasis (ALNM), the paramount prognostic factor in patients with invasive breast cancer. This study was aimed to identify the ALNM-associated tumor characteristics and to determine the predictive clinical pathway.
Material/Methods
Data from 1325 patients diagnosed with invasive breast cancer between January 2004 and January 2010 were retrospectively reviewed. The structure equation model (SEM) was used to build the predictive clinical pathway.
Results
Among the factors found in the final model, the status of human epidermal growth factor receptor 2 is the primary influence on ALNM through histology grade (β=0.18), followed by tumor size (β=0.16). Tumor size was highly relevant to lymphovascular invasion (LVI) and influenced ALNM through LVI (β=0.26), the strongest predictor of ALNM in the final model (β=0.46) and the highest risk of ALNM (odds ratio=9.282; 95% confidence interval: 7.218–11.936).
Conclusions
The structure equation model presented the relation of these important predictors, and might help physicians to assess axillary nodal condition and appropriate surgical procedures.
MeSH Keywords: Breast Neoplasms, Lymph Nodes, Lymphatic Metastasis - diagnosis, Neoplasm Grading, Neoplasm Invasiveness, Receptor, erbB-2
Background
Breast cancer is the most common female cancer in Western countries [1]. In Taiwan, breast cancer is the leading cause of cancer and is the fourth-ranked cause of cancer death. In Taiwan in 2010, more than 7500 breast cancers were diagnosed, causing 1600 deaths. The Health Promotion Administration, Ministry of Health and Welfare (Taiwan), provides biennial breast cancer mammogram screening for women aged 45–69 years and to women older than 40 with high risk.
Axillary lymph node metastasis (ALNM) is the most important prognostic factor in patients with invasive breast cancer [2–6]. Patients without axillary nodal involvement have a more favorable prognosis. Metastases to more than 6 nodes demonstrated the risk of distant metastasis. In recent years, the concept of sentinel lymph node sampling has greatly change surgical procedure in breast cancer. With the advance of sentinel lymph node sampling, it has rapidly replaced standard axillary lymph node dissection in patients who are clinically axillary node metastasis negative. Pre-operative assessment of axillary condition has become an essential issue in surgical planning. Researchers began to search for tumor characteristics related to axillary nodal metastasis. The aim of this study was to identify tumor characteristics associated with axillary lymph node metastasis. We studied consecutive patients with invasive breast cancer who underwent surgical breast and axillary procedures in our hospital and determined the predictive factor of axillary nodal disease by structure equation model (SEM). We hope to improve cancer counseling and provide optimal surgical planning in high-risk patients pre-operatively.
Material and Methods
Patient population
We conducted a review analysis of retrospective databases of patients diagnosed with invasive breast cancer at Changhua Christian Hospital between January 2004 and January 2010. This study was approved by the institutional review board and the ethics committee of the Changhua Christian Hospital. A population of 1325 patients was identified. Their medical charts and treatments were retrospectively reviewed for information on tumor characteristics. The inclusion criteria were: (1) female breast cancers diagnosed and treated at our hospital, and (2) patients with complete medical information. Patients were excluded from this study if they had ductal carcinoma in situ or if they were treated with neoadjuvant therapy, had bilateral breast cancers, or if clinical data were not complete. All patients underwent breast-conserving surgery with axillary lymph node dissection, sentinel lymph node sampling, or modified radical mastectomy.
Data collection
The clinical characteristics included age at diagnosis, tumor size, lymph node metastasis, histology grade, hormonal receptor (estrogen receptor and progesterone receptor) status, human epidermal growth factor receptor 2 (HER2) status, and the presence of lymphovascular invasion (LVI). The quality of the cancer registry database was reviewed and approved by a committee of radiologists, oncologists, pathologists, and surgeons, as well as an epidemiologist with special expertise in breast cancer. The hormonal receptor status was tested based on standard immunostaining. For determination of HER2 expression, immunohistochemical staining assay and semiquantitative scoring were used. No or weak incomplete membrane staining (0 to 1+) was considered to be a negative result; 2+ staining with complete membrane was considered as equivocal over-expression; and 3+ staining was considered over-expression. Patients with equivocal HER2 over-expression were further assessed by fluorescence in situ hybridization method.
Statistics analysis
Data analysis was conducted with SPSS (Statistical Package for Social Science) 16.0 version. SEM was used to examine the proposed model. Continuous variables were expressed as the mean ± standard deviation (SD). Categorical data were tested by the chi-square test. All p values were two-tailed. A p value less than 0.05 was considered as statistically significant. AMOS (Analysis of Moment Structure) 16.0 version was applied to perform SEM. Model fitting: chi-squared values were significant (p<0.05) and the fit indices had value more than 0.9, which indicated good model fit.
Results
There were 1325 female patients include in this analysis, with an average age of 51.3 years old (SD=11.2). Mean tumor size was 2.4 cm (SD=1.5). Patients were divided into 2 groups: ALNM-positive and ALNM-negative. A total of 583 axillary lymph node involvements were ascertained. Table 1 showed patient clinical features and tumor characteristics of 1325 cases. Clinical features and tumor characteristics among women with breast cancer were analyzed according to the axillary lymph node status. There was no significant difference between 2 groups with regard to age or estrogen receptor. The distributions of tumor size, histology grade, progesterone receptor, and HER2 within the 2 groups were significantly different.
Table 1.
Patients’ characteristics and tumor features.
| Variables | Axillary lymph node metastases | |||
|---|---|---|---|---|
| Negative (n=742) | Positive (n=583) | p* | Total (N=1,325) | |
| Age (mean ±SD) | 51.12 (11.06) | 51.43 (11.38) | 0.6154 | 51.25 (11.20) |
| Clinical factors | ||||
| Tumor size, mm (mean ± SD) | 20.8 (1.164) | 29.1 (1.659) | <0.0001* | 24.44 (1.461) |
| Tumor size (mean ± SD) | ||||
| <20 mm | 458 (61.73) | 211 (36.19) | 669 (50.49) | |
| 20–40 mm | 246 (33.15) | 286 (49.06) | 532 (40.15) | |
| >40 mm | 38 (5.12) | 86 (14.75) | <0.0001* | 124 (9.36) |
| Grade | ||||
| I | 143 (19.27) | 72 (12.35) | 215 (16.23) | |
| II | 415 (55.93) | 320 (54.89) | 735 (55.47) | |
| III | 184 (24.80) | 191 (32.76) | 0.0002* | 375 (28.30) |
| Pathological factors | ||||
| Estrogen receptor | ||||
| Negative | 256 (34.50) | 177 (30.36) | 433 (32.68) | |
| Positive | 486 (65.50) | 406 (69.64) | 0.1106 | 892 (67.32) |
| Progesterone receptor | ||||
| Negative | 291 (39.22) | 183 (31.39) | 474 (35.77) | |
| Positive | 451 (60.78) | 400 (68.61) | 0.0032* | 851 (64.23) |
| HER2 | ||||
| Negative | 616 (83.02) | 444 (76.16) | 1060 (80.00) | |
| Positive | 126 (16.98) | 139 (23.84) | 0.0019* | 265 (20.00) |
| Lymphovascular invasion | ||||
| No | 585 (78.84) | 167 (28.64) | 752 (56.75) | |
| Yes | 157 (21.16) | 416 (71.36) | <0.0001* | 573 (43.25) |
p<0.01.
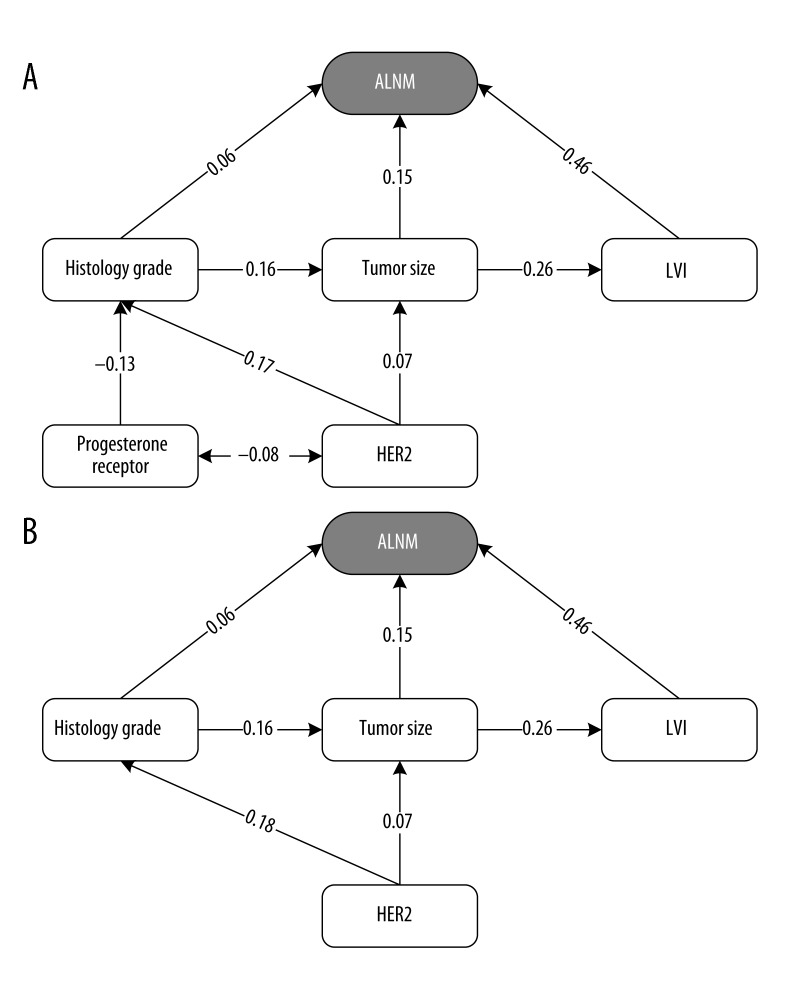
Univariate Cox regression analysis of factors associated with axillary lymph node metastases was performed (Table 2). Five variables were found to be significant in the univariate analysis. They included tumor size, progesterone receptor, HER2 status, LVI, and histology grade. Tumor size was highly associated with axillary nodal involvement and increasing size with an increasing risk of ALNM (odds ratio =1.56, 95% confidence interval: 1.423 to 1.708, p<0.0001). A poor histology grade (odds ratio =1.69, p=0.0008) and presence of LVI (odds ratio =9.282, p<0.0001) were also a significant factor for axillary nodal disease. Progesterone receptor positivity (p=0.0032) and HER2 over-expression (p=0.002) were significantly associated with positive axillary status. A multivariate logistic regression model was applied and results are presented in Table 2. In this model, the data showed that tumor size, LVI, and histology grade were associated with statistically significant differences in axillary nodal metastases (p<0.05). The relation between clinical and pathologic factors with ALNM is shown in Table 3. The clinical and pathology factors, as well as tumor size, progesterone receptor, HER2, LVI, and histology grade, were highly correlated with ALNM. To determine the causal relationship among those factors, we conducted a structured equation model to build a pathway analyses. We used SEM to test the proposed model because it showed the correlation among all the variables, as well as showing the direction of the path among all the variables. Within the model, the clinical and pathology factors were defined as those variables highly associated with ALNM. Figure 1A presents the proposed model of this study. This model was used to test the hypothesis. All variables, including clinical factors and pathological factors, were calculated in the model, including HER2, progesterone receptor, histology grade, tumor size, and LVI. The model was further modified to improve the fit. Figure 1B was the final model of this study. In this model, 4 independent predictors of ALNM were identified. The optimal pathway among the 4 factors in the final model was that HER2 status might have influenced ALNM through histology grade (β=0.18), and then tumor size (β=0.16). Tumor size was highly relevant to LVI and influenced ALNM through LVI (β=0.26). The strongest predictor of ALNM in the final model was LVI (β=0.46), followed by histology grade, tumor size, and HER2 status. LVI was the highest risk factor for ALNM (odds ratio =9.282; 95% confidence interval: 7.218–11.936).
Table 2.
Logistic regression analysis of clinical and pathologic factors with axillary lymph node metastases.
| Variables | Axillary lymph node metastases | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| Clinical factors | ||||
| Tumor size, mm | 1.559 (1.423–1.708) | <0.0001* | 1.356 (1.228–1.498) | <0.0001* |
| Pathologic factors | ||||
| Estrogen receptor | ||||
| Positive vs. negative | 1.208 (0.958–1.525) | 0.1108 | ||
| Progesterone receptor | ||||
| Positive vs. negative | 1.410 (1.122–1.773) | 0.0032* | 1.329 (0.898–1.969) | 0.156 |
| HER2 | ||||
| Positive vs. negative | 1.531 (1.168–2.005) | 0.0020* | 0.977 (0.531–1.799) | 0.942 |
| Lymphovascular invasion | ||||
| Positive vs. negative | 9.282 (7.218–11.936) | <0.0001* | 8.219 (6.323–10.684) | <0.0001* |
| Grade | ||||
| II, III vs. I | 1.694 (1.247–2.303) | 0.0008* | 1.492 (1.034–2.152) | 0.032** |
p<0.01;
p<0.05;
CI – confidence interval.
Table 3.
Correlation coefficients among clinical and pathologic factors with axillary lymph node metastases.
| Correlations | |||||
|---|---|---|---|---|---|
| ALNM | Tumor size | PR | HER2 | LVI | |
| Tumor size | 0.281* | 1 | |||
| Progesterone receptor | 0.081* | −0.017 | 1 | ||
| HER2 | 0.085* | 0.102* | −0.080* | 1 | |
| LVI | 0.503* | 0.262* | 0.003 | 0.036 | 1 |
| Grade | 0.113* | 0.168* | −0.148* | 0.178* | 0.069** |
Data were analyzed with Pearson correlation analysis.
Correlation is significant at the 0.01 level (two-tailed);
Correlation is significant at the 0.05 level (two-tailed).
Figure 1.
The models of this study. (A) The proposed causal model. χ2=21.369 (p=0.002); df=6; χ2/df=3.562; RMSEA=0.044; GFI=0.995; AGFI=0.981; NFI=0.968; CFI=0.977. (B) The final model. χ2=4.564 (0.207); df=3; χ2/df=1.521; RMSEA=0.020; GFI=0.999; AGFI=0.993; NFI=0.993; CFI=0.997.
Discussion
Axillary nodal status is the crucial factor in cancer staging of female breast cancer. It is also an important prognostic factor for cancer survival [7–9]. Complete axillary lymph node dissection is the criterion standard for assessment of metastases. With advancements in surgical technique, sentinel lymph node (SLN) biopsy has become popular in clinical axillary node negative patients [10]. SLN biopsy reduces the morbidity and complications of axillary lymphatic drainage and major vessel and nerve injury [11]. Breast cancer screening program in Taiwan have evolved since 1995 [12]. An increase in breast cancer incidence was noted in Taiwan; fortunately, these tumors were being found at a smaller size than before and usually with less invasive axillary involvement; therefore, patients with small tumors might be benefit from SLN biopsy. Assessment of axillary status has become a key step in cancer counseling and pre-operative planning.
Clinical features and pathologic characteristics are important information in the pre-operative treatment of breast cancer. Physicians have been searching for favorable categories in axillary node evaluation [4,13–15]. There remains much debate about the correct clinical pathway of ALNM. In 1997 Barth et al. found LVI, tumor size, and histology grade can be used to estimate the risk of ALNM [15]. Among these characteristics, LVI is the strongest predictor of ALNM [15–23]. The presence of LVI is highly associated with ALNM. The odds ratio (LVI presence vs. negative) is high in extensive axillary nodal involvement. A low percentage of ALNM is found in small tumors with negative LVI [16]. Previous research showed that the predictive power was strong in non-SLN metastases [23]. LVI also increases the incidence of isolated tumor cells in SLN [24]. In our study, LVI was the strongest predictor of ALNM (odds ratio =8.2), which is concordant with the other published series referenced above. Patients with LVI are not good candidates for SLN biopsy and should be treated more aggressively.
Tumor size is a traditional predictor in axillary lymph node status. Our study demonstrates tumor size is the significant independent predictive factor in univariant and multivariant analysis. Patients with tumor size more than 40 mm are 3 times more likely to have axillary nodal involvement (Table 1). In agreement with previous publications, patients with larger tumor size have increased risk of ALNM [14,15,25–27]. Breast cancer with smaller tumors (<20 mm in our study) might benefit from sentinel lymph node biopsy [28]. In patients with moderate-sized tumors (20–40 mm), the risk of ALNM is relatively high and should be evaluated based on tumor characteristics and other examination tools because of the probability of need for a secondary axillary operation. In patients with large tumor size (>40 mm), complete axillary lymph node dissection is a preferred surgical procedure, based on the high probability of ALNM.
HER2 is associated with higher aggressiveness in invasive breast cancer, and is accepted as an important prognostic factor in breast cancer patients [29]. However, the association between HER2 and ALNM is not clearly identified. In our research, HER2 is a significant predictor in univariant analysis (odds ratio =1.53). We constructed an optimal predictive model to differentiate the clinical pathway of ALNM and tumor characteristics.
In the structure equation model (also called the causal model), pathway analysis is a statistical method for representing causal relationships among the variables in the model [30,31]. We believe this is the first series revealing the clinical pathway in tumor characteristics. Figure 1B shows the final model in this study. In this model, LVI, tumor size, and histology grade are important predictors of ALNM, which is compatible with findings reported in previous publications [15–28]. Presence of an HER2-positive tumor was identified as a predictor in the base of the model. HER2 status might influence ALNM through histology grade (β=0.18), and then tumor size (β=0.16). Tumor size was highly relevant to lymphovascular invasion and influenced ALNM through LVI (β=0.26). To apply this model in clinical practice, we suggest that LVI, tumor size, histology grade, and HER2 status are important predictors in assessment of axillary nodal status. If a patient is at high risk of ALNM in pre-operative cancer counseling, other advanced examinations (e.g., breast MR) and the possible need for a second axillary operation should be considered pre-operatively.
Recent data from the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial suggest that ALND may be omitted in selected patients with less than 2 positive SLNs [32]. In this study, 856 patients were enrolled and divided into 2 groups: ALND and SLND alone. Patient characteristics, including tumor size, ER, PR status, LVI, histology grade, tumor type, and lymph nodes metastases, were similar between the 2 groups. ALND may no longer be required in patients with the following characteristics: tumors smaller than 5 cm; fewer than 2 positive SLNs; without extracapsular extension; good patient acceptance; completion of whole breast radiation; and completion of adjuvant therapy, including hormonal, cytotoxic, or both. In this study, as in the ACOSOG study, tumor size was related with LVI, and LVI is the strongest predictor of ALNM. However, the aim of this study is different to that of the ACOSOG study. If a patient fulfills the ACOSOG study criteria, but with a larger tumor (T2) and LVI, according to our results the risk of ALNM is relatively higher. This study might suggest another option in surgical procedures (ALND or SLND) and post-operative treatment (e.g., chemotherapy and hormonal therapy).
Conclusions
Four independent predictors of ALNM were identified. The strongest predictor of ALNM was LVI, followed by histology grade, tumor size, and HER2 status. Our structure equation model presented the relation of these important predictors. This model might help physicians to assess axillary nodal condition and determine appropriate surgical procedures.
Acknowledgements
We thank Dr. Ping-Yi Lin for her statistical assistance.
Abbreviations
- ALNM
axillary lymph node metastasis
- HER2
human epidermal growth factor receptor 2
- LVI
lymphovascular invasion
- SD
standard deviation
- SEM
structure equation model
- SLN
sentinel lymph node
Footnotes
Conflict of interest statement
None declared.
Source of support: Self financing
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health Consensus Development Panel. Consensus statement: treatment of early-stage breast cancer. J Natl Cancer Inst Monogr. 1992:1–5. [PubMed] [Google Scholar]
- 3.NIH Consensus Conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–95. [PubMed] [Google Scholar]
- 4.Silverstein MJ, Gierson ED, Waisman JR, et al. Axillary lymph node dissection for T1a breast carcinoma. Is it indicated? Cancer. 1994;73:664–67. doi: 10.1002/1097-0142(19940201)73:3<664::aid-cncr2820730326>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551–57. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Andree C, Schmidt VJ, Munder BI, et al. Detecting of breast cancer metastasis by means of regional lymph node sampling during autologous breast reconstruction – a screening of 519 consecutive patients. Med Sci Monit. 2012;18(10):CR605–10. doi: 10.12659/MSM.883486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th edn. Springer; New York, NY: 2010. [Google Scholar]
- 8.Rosen PP, Groshen S. Factors influencing survival and prognosis in early breast carcinoma (T1N0M0-T1N1M0). Assessment of 644 patients with median follow-up of 18 years. Surg Clin North Am. 1990;70:937–62. doi: 10.1016/s0039-6109(16)45190-x. [DOI] [PubMed] [Google Scholar]
- 9.Mustafa IA, Cole B, Wanebo HJ, et al. Prognostic analysis of survival in small breast cancers. J Am Coll Surg. 1998;186:562–69. doi: 10.1016/s1072-7515(98)00076-3. [DOI] [PubMed] [Google Scholar]
- 10.Pazaiti A, Fentiman IS. Which patients need an axillary clearance after sentinel node biopsy? Int J Breast Cancer. 2011;2011:195892. doi: 10.4061/2011/195892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jatoi I. Management of the axilla in primary breast cancer. Surg Clin North Am. 1999;79:1061–73. doi: 10.1016/s0039-6109(05)70061-x. [DOI] [PubMed] [Google Scholar]
- 12.Wu GH, Chen LS, Chang KJ, et al. Evolution of breast cancer screening in countries with intermediate and increasing incidence of breast cancer. J Med Screen. 2006;13(Suppl 1):S23–27. [PubMed] [Google Scholar]
- 13.Lin PP, Allison DC, Wainstock J, et al. Impact of axillary lymph node dissection on the therapy of breast cancer patients. J Clin Oncol. 1993;11:1536–44. doi: 10.1200/JCO.1993.11.8.1536. [DOI] [PubMed] [Google Scholar]
- 14.Aitken E, Osman M. Factors affecting nodal status in invasive breast cancer: a retrospective analysis of 623 patients. Breast J. 2010;16:271–78. doi: 10.1111/j.1524-4741.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- 15.Barth A, Craig PH, Silverstein MJ. Predictors of axillary lymph node metastases in patients with T1 breast carcinoma. Cancer. 1997;79:1918–22. [PubMed] [Google Scholar]
- 16.Wong JS, O‘Neill A, Recht A, et al. The relationship between lymphatic vessell invasion, tumor size, and pathologic nodal status: can we predict who can avoid a third field in the absence of axillary dissection? Int J Radiat Oncol Biol Phys. 2000;48:133–37. doi: 10.1016/s0360-3016(00)00605-2. [DOI] [PubMed] [Google Scholar]
- 17.Davis BW, Gelber R, Goldhirsch A, et al. Prognostic significance of peritumoral vessel invasion in clinical trials of adjuvant therapy for breast cancer with axillary lymph node metastasis. Hum Pathol. 1985;16:1212–18. doi: 10.1016/s0046-8177(85)80033-2. [DOI] [PubMed] [Google Scholar]
- 18.Ravdin PM, De Laurentiis M, Vendely T, Clark GM. Prediction of axillary lymph node status in breast cancer patients by use of prognostic indicators. J Natl Cancer Inst. 1994;86:1771–75. doi: 10.1093/jnci/86.23.1771. [DOI] [PubMed] [Google Scholar]
- 19.Tan YY, Wu CT, Fan YG, et al. Primary tumor characteristics predict sentinel lymph node macrometastasis in breast cancer. Breast J. 2005;11:338–43. doi: 10.1111/j.1075-122X.2005.00043.x. [DOI] [PubMed] [Google Scholar]
- 20.Weiser MR, Montgomery LL, Tan LK, et al. Lymphovascular invasion enhances the prediction of non-sentinel node metastases in breast cancer patients with positive sentinel nodes. Ann Surg Oncol. 2001;8:145–49. doi: 10.1007/s10434-001-0145-y. [DOI] [PubMed] [Google Scholar]
- 21.Nos C, Harding-MacKean C, Freneaux P, et al. Prediction of tumour involvement in remaining axillary lymph nodes when the sentinel node in a woman with breast cancer contains metastases. Br J Surg. 2003;90:1354–60. doi: 10.1002/bjs.4325. [DOI] [PubMed] [Google Scholar]
- 22.Turner RR, Chu KU, Qi K, et al. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer. 2000;89:574–81. doi: 10.1002/1097-0142(20000801)89:3<574::aid-cncr12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23.Hwang RF, Krishnamurthy S, Hunt KK, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10:248–54. doi: 10.1245/aso.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Mittendorf EA, Sahin AA, Tucker SL, et al. Lymphovascular invasion and lobular histology are associated with increased incidence of isolated tumor cells in sentinel lymph nodes from early-stage breast cancer patients. Ann Surg Oncol. 2008;15:3369–77. doi: 10.1245/s10434-008-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivadeneira DE, Simmons RM, Christos PJ, et al. Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: analysis in more than 900 patients. J Am Coll Surg. 2000;191:1–6. doi: 10.1016/s1072-7515(00)00310-0. discussion 6–8. [DOI] [PubMed] [Google Scholar]
- 26.Harden SP, Neal AJ, Al-Nasiri N, et al. Predicting axillary lymph node metastases in patients with T1 infiltrating ductal carcinoma of the breast. Breast. 2001;10:155–59. doi: 10.1054/brst.2000.0220. [DOI] [PubMed] [Google Scholar]
- 27.Nixon AJ, Schnitt SJ, Gelman R, et al. Relationship of tumor grade to other pathologic features and to treatment outcome of patients with early stage breast carcinoma treated with breast-conserving therapy. Cancer. 1996;78:1426–31. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1426::AID-CNCR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Coombs N, Chen W, Taylor R, Boyages J. A decision tool for predicting sentinel node accuracy from breast tumor size and grade. Breast J. 2007;13:593–98. doi: 10.1111/j.1524-4741.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–78. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 30.Wright S. Correlation and causation. J Agric Res. 1921;20:557–85. [Google Scholar]
- 31.Simon HA. Causal ordering and identifiability. In: Hood WC, Koopmans TC, editors. Studies in econometric method. John Wiley & Sons; New York: 1953. pp. 49–74. [Google Scholar]
- 32.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]