Abstract
Background
Neuropathic pain induced by spinal or peripheral nerve injury is very resistant to common pain killers, nerve block, and other pain management approaches. Recently, several studies using stem cells suggested a new way to control the neuropatic pain. In this study, we used the spinal nerve L5 ligation (SNL) model to investigate whether intrathecal rat mesenchymal stem cells (rMSCs) were able to decrease pain behavior, as well as the relationship between rMSCs and reactive oxygen species (ROS).
Methods
Neuropathic pain of the left hind paw was induced by unilateral SNL in Sprague-Dawley rats (n = 10 in each group). Mechanical sensitivity was assessed using Von Frey filaments at 3, 7, 10, 12, 14, 17, and 24 days post-ligation. rMSCs (10 µl, 1 × 105) or phosphate buffer saline (PBS, 10 µl) was injected intrathecally at 7 days post-ligation. Dihydroethidium (DHE), an oxidative fluorescent dye, was used to detect ROS at 24 days post-ligation.
Results
Tight ligation of the L5 spinal nerve induced allodynia in the left hind paw after 3 days post-ligation. ROS expression was increased significantly (P < 0.05) in spinal dorsal horn of L5. Intrathecal rMSCs significantly (P < 0.01) alleviated the allodynia at 10 days after intrathecal injection (17 days post-ligation). Intrathecal rMSCs administration significantly (P < 0.05) reduced ROS expression in the spinal dorsal horn.
Conclusions
These results suggest that rMSCs may modulate neuropathic pain generation through ROS expression after spinal nerve ligation.
Keywords: mesenchymal stem cells, neuropathic pain, reactive oxygen species
INTRODUCTION
Neuropathic pain induced by nervous tissue damage is an incurable condition that affects about 3% of the population [1]. Neuropathic pain manifests specific symptoms such as spontaneous burning pain and allodynia (pain that arises due to non-noxious stimuli) due to sensory system damage or disease [1]. These symptoms have a significant impact on a patient's quality of life and social productivity. Pain control for neuropathic pain is still not at a satisfactory level despite the availability of various treatment methods. The failure of pain control may lead to suicide.
Recently, many studies have investigated mesenchymal stem cells (MSCs) for the treatment of incurable conditions. MSCs have the ability to renew themselves in an undifferentiated state, and the selective proliferation of MSCs can be achieved using a special culture media, causing differentiation into various limited tissue cells [2]. Bone marrow includes hematopoietic stem cells and MSCs [3]. MSCs can be differentiated into muscle cells, osteocytes, and nerve cells [4]. In addition, MSCs migrate to the damaged lesion and then settle and survive at the site for the functional recovery of the damaged lesion [5]. These cells have the potential to treat neuropathic pain due to nerve damage because they have genetically safe and strong immunosuppressive characteristics [6]. Therefore, this study was conducted to determine whether allogenic MSCs can be used clinically for the control of neuropathic pain.
Reactive oxygen species (ROS) are known to be involved in continuous chronic pain, including neuropathic pain and inflammatory pain [7,8]. A certain amount of ROS in the cell is useful in one sense, as these molecules block pathogens and activate various enzymes, but an increase in ROS destroys the cell composition factors and may lead to structural and functional defects in the cell [7]. ROS in nerve tissues are formed from various causes and are known to have an important role in the nerve ligation-induced neuropathic pain model [7]. A recent in vitro study reported that human MSCs can exist under oxidative stress and can also remove ROS [9]. However there is not any research of MSCs reduce the ROS in the neuropathic pain.
Therefore, we performed spinal nerve ligation (SNL) in rats to induce neuropathic pain and then carried out intrathecal administration of MSCs to investigate whether these cells decreased the withdrawal response. We also investigated the relationship between withdrawal response and ROS expression in the spinal dorsal horn to find a new strategy for the elimination of neuropathic pain.
MATERIALS AND METHODS
1. Animal care
Six-week-old male rats (Sprague-Dawley) with weights from 180 g to 200 g were used in this study. Sawdust was laid and water and feed were freely available in the cage. Day and night were controlled every 12 hours, and the indoor temperature of 22 ± 2℃ and humidity were maintained. All experiments were conducted according to regulations approved (CNUH-P0005-R1) by the animal experimentation committee at the Chungnam National University Hospital and guidelines of the International Association for the Study of Pain.
2. Preparation of the pain model
The selected rats went under anesthesia with isoflurane 1.5%-2% (Baxter, USA). Then, in the prone position, the fur on the back was shaved, followed by disinfection with potadine. The skin was incised, left transverse process was removed, and then the L5 nerve was isolated and tightly ligated with black silk. The muscle and skin were then sutured for the completion of the procedure. The same personnel carried out all of the procedures with the same methods. The pain threshold was measured by using Von Frey filament. Rat mesenchymal stem cells (rMSCs) were injected intrathecally at 7 days post-ligation.
3. Pain threshold assessment
Mechanical paw withdrawal thresholds were confirmed by measuring the withdrawal response to stimulating the rats' left soles with Von Frey filaments. The rats were stabilized for 30 minutes as they were placed on the wire mesh, which was enclosed with a plastic box without the bottom surface. The stimulation was gradually increased, and a positive response was classified as dodging, shaking, or flinching from the stimulation. The level of stimulation was also recorded. For accurate measurement, pressure was exerted until the Von Frey filament was slightly bended. The measurement was repeated 5 times and performed between 11 a.m. and 3 p.m. [10]. The pain threshold was recorded in grams (g), which were indicated on the Von Frey filaments.
Pain threshold test was performed on prior to and on days 3 and 7 following SNL procedure. rMSCs were administered intrathecally in the rats presenting with pain behavior on day 7 following SNL procedure, and then the withdrawal response was also measured with Von Frey filaments on days 3, 5, 10, and 17 after intrathecal injection.
4. Rat mesenchymal stem cell isolation
Adult male Sprague-Dawley rats (weight 180g to 200g) were used, with the cell culture method described by Soleimani and Nadri [11]. The animals were anesthetized with isoflurane 1.5-2% and killed by the cervical dislocation method. Under sterile conditions, both femurs and tibia from each rat were excised. The muscles and entire connective tissue were detached. The ends of the bones were removed, and a 27-gauge needle, inserted into the shaft of the bone marrow, was extruded by flushing with DMEM (Dulbecco's modified Eagle medium-low glucose) supplemented with 10% FBS, 100 µ/ml penicillin, and 100 µg/ml streptomycin, which was also used as the growth medium. Marrow plug suspension was dispersed by pipetting, successively filtered through a 70 mm mesh nylon filter (BD Biosciences, Bedford, MA, USA), and centrifuged at 1,500 rpm for 5 min. The cells from one rat were seeded onto one 100-mm plastic tissue culture flask (BD Biosciences) and incubated at 37℃ in an incubator containing 5% CO2 for 1 day. The rMSCs were isolated on the basis of their ability to adhere to the culture plates. The rMSCs were washed with phosphate-buffered saline (PBS) and detached by incubating with a 0.25% trypsin-EDTA solution (Invitrogen/GIBCO) for 5-10 min at 37℃. The cells were centrifuged at 200 g for 10 min, followed by cell dilution of 1 × 105/ml in PBS for administration. The amount of cells and their concentration were 1 × 105 cells/ml, chosen according to Yang et al. [12].
5. Intrathecal rMSC administration
The pain response was observed for up to 7 days after SNL, and the model rats were selected based on withdrawal response threshold and randomized into three groups. Animals were divided into a pain group (N = 10), a PBS group (N = 10) and an rMSC group (N = 10). rMSCs or PBS was injected intrathecally through the L5-L6 interspace at 7 days post-ligation. The pain group had neuropathic pain after SNL without intrathecal injection; the PBS group underwent intrathecal injection of PBS to the neuropathic pain rats at 7 days post-ligation; and the rMSCs group underwent intrathecal administration of rMSCs to the neuropathic pain rats at 7 days post-ligation. Under isoflurane anesthesia, intrathecal injections were performed by lumbar puncture (L5-L6) with a 25 G needle [13]. Total volume of 10 µl 1 × 105 of rMSCs or 10 µl PBS was injected using microsyringe pump for 10 seconds [12].
6. Fluorescent dye of ROS expression
Dihydroethidium (DHE) or hydroethidine is a cell-permeable compound that, upon entering the cells, interacts with O2·-to form oxyethidium. This, in turn, interacts with nucleic acids to emit a bright red color detectable qualitatively by fluorescent microscope (Carl Zeiss, Oberkochen, German).
Dihydroethidium (DHE), an oxidative fluorescent dye, was used to detect superoxide according to the method described by Harraz et al. [14]. The production of ROS was detected at 17 days (24 days post-ligation) after intrathecal injection in the pain (N = 7), rMSC (N = 3), PBS (N = 3) groups, and naive (N = 3) rats. Rats were euthanized with an overdose of isoflurane. Lumbar enlargement (L4-L6) regions of the spinal cords were removed immediately, immersed in 4% paraformaldehyde fixative overnight, and then embedded in paraffin. Four-micrometer sections of the paraffin-embedded tissue arrays were deparaffinized and rehydrated in a graded alcohol series. Sections were rinsed in PBS containing 100 µM rotenone to inhibit mitochondrial respiration prior to incubation with 1 µM DHE (Invitrogen) for 5 minutes in the dark in the continued presence of 100 µM rotenone. Sections were then rinsed in PBS and cover slipped with Vectashield mounting media containing DAPI. Images were obtained with a fluorescent microscope. The excitation wavelength was 488 nm, and emission fluorescence was detected with the use of a 585 nm filter. The reactive oxygen expression was quantitatively counted at the dorsal horn.
For quantitative analysis of DHE reactive cells, stained sections from the spinal dorsal horn compared the number of DHE to DAPI cells using Adobe Photoshop (version 7.0.1, Adobe). All quantitative procedures were performed uninformed of each animal's experimental condition.
7. Statistical analysis
SPSS (version 12.0, SPSS Inc.) was used for the statistical analysis and compared the pain threshold using one-way ANOVA followed by individual post hoc comparisons (Tukey post hoc tests). All of the values were recorded as the mean ± the standard error of the mean (SEM), and statistical significance was defined as a P value of less than 0.05.
RESULTS
1. Result of the measurement of pain threshold
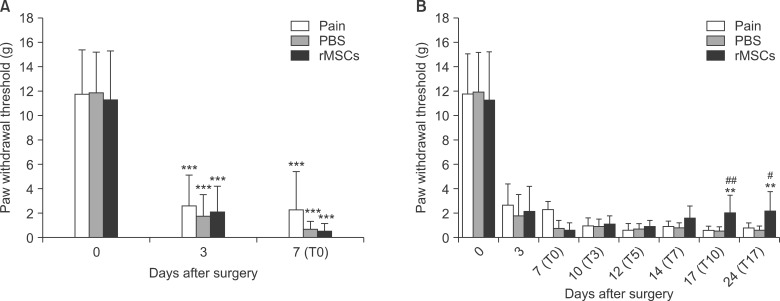
After the procedure, the rats did not completely touch their left hind paw (the side affected by the procedure) onto the ground, and their toes were curled toward the hind paw. The rats presented signs of spontaneous pain, such as licking or lifting the left hind paw. The withdrawal response thresholds due to stimulation of the left hind paw by Von Frey filaments prior to and on days 3 and 7 post-ligation procedure were 11.8 ± 3.6 g, 2.6 ± 2.5 g, and 2.3 ± 3.1 g, respectively, in the pain group; 11.9 ± 3.3 g, 1.8 ± 1.8 g, and 0.7 ± 0.6 g, respectively, in the PBS group; and 11.3 ± 4.0 g, 2.2 ± 2.0 g, and 0.6 ± 0.5 g, respectively, in the rMSCs group. In all 3 groups, the pain thresholds showed decreases that were statistically significantly (P < 0.001) on days 3 and 7 following the spinal nerve ligation compared with the values prior to the procedure (Fig. 1A). The sham surgery didn't induced pain behavior and no different with naive (data not show).
Fig. 1.
Von Frey filament testing results. (A) Effect of spinal nerve ligation on the development of neuropathic pain. A significant decrease in the paw withdrawal threshold in the Von Frey filament test is induced 3 days after SNL. ***P < 0.001 vs. before surgery. (B) Effect of rMSCs on paw withdrawal reflex responses to Von Frey filament stimuli in rat pain models. rMSCs treatment on day 7 after SNL significantly increased the withdrawal threshold on days 10 and 17 after administration. **P < 0.01 vs. PBS group and #P < 0.05, ##P < 0.01 vs. Pain group. Values are expressed as the mean ± SEM (n = 10 in each group) Abbreviations: T, post-administration.
The withdrawal response thresholds on day 10 (17 days post-ligation) following the intrathecal injection in the pain group, PBS group, and rMSCs group were 0.6 ± 0.3 g, 0.5 ± 0.3 g, and 2.0 ± 1.4 g respectively. The pain threshold in the rMSCs group was significantly (P < 0.01) increased relative to the pain and PBS group. In addition, the withdrawal response thresholds on day 17, the values were 0.8± 0.3 g, 0.5 ± 0.4 g, and 2.2 ± 1.6 g respectively. Thus, the pain threshold in the rMSCs group was significantly increased relative to the pain and PBS groups (P < 0.05, P < 0.01) (Fig. 1B). The immune rejection response was not observed after the administration of MSCs, and no other adverse reactions were observed during the course of the study. No tumors were found in the cerebrospinal fluid analysis after the study.
2. ROS expression
ROS expression was observed at the L5 spinal dorsal horn on day 24 following SNL (on day 17 following the intrathecal administration) in each group. The DHE fluorescence expression in the naive and rMSC groups was less than in the Pain and PBS groups (Fig. 2A).
Fig. 2.
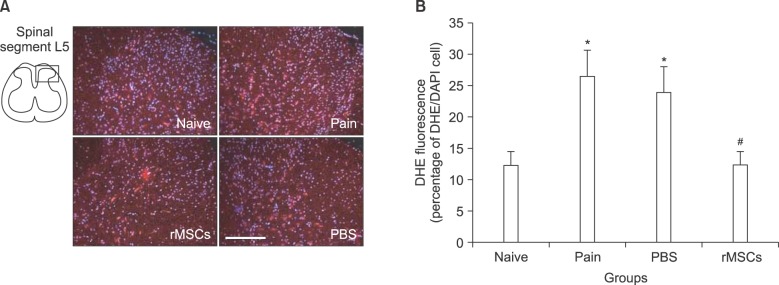
DHE staining of the L5 spinal dorsal horn on day 24 following the spinal nerve ligation. (A) The L5 spinal dorsal horn segments (naive, pain, PBS, rMSCs groups) were stained with ROS-sensitive dye DHE (dihydroethidium). ROS (red) expression was decreased in the spinal cord of the rMSCs group (scale bar, 200 µm). (B) Fluorescent intensities of DHE signals were measured and compared with other groups. The intensities of DHE in the pain and PBS groups show a significant difference at *P < 0.05 compared with the naïve rats. The rMSCs group shows a statistical difference at #P < 0.05 compared with the pain or PBS groups. Values are expressed as the mean ± SEM.
The reactive oxygen expression was quantitatively compared at the dorsal horn. The ROS fluorescence expression in the PBS and pain groups was significantly (P < 0.05) increased relative to the naive rats. The ROS fluorescence expression in the rMSCs group was significantly (P < 0.05) reduced relative to the pain and PBS groups. There was no significant difference between the naive and rMSCs groups, and between the pain and PBS groups (Fig. 2B).
DISCUSSION
In this study, neuropathic pain was induced by spinal nerve ligation in rats. The rats presented pain behavior, the typical withdrawal response, on day 3 of the procedure, and this continued for up to 4 weeks post-ligation. To obtain a firm pain model, the pain response was observed for up to 7 days after SNL, and the model rats were selected based on withdrawal response threshold and randomized into three groups. Then, rMSCs isolated from bone marrow were administered intathehecally in rMSCs group to observe the change in pain threshold for 3 weeks post-administration.
Neuropathic pain presents as nerve damage or functional defects [15]. The spinal dorsal horn is an important component that transmits and controls the nociception transmitted from the peripheral nociceptors [16,17]. In the spinal dorsal horn of the pain model, various neurotransmitters (excitatory amino acids, substance P, GABA, NO) are changed [13]. Neuropathic pain involves the expression of receptors in the nervous system or nociceptive pathway, as well as changes in enzymes or voltage-dependent ion channels [18,19]. ROS expression is increased at the dorsal horn when the cells such as glia becomes activated [20].
There are various neuropathic pain treatment methods based on the number of pain mechanisms and symptoms. Medications (anticonvulsants, antidepressants, opioids) or spinal cord stimulation are clinically used for treatment [1]. In addition, sodium and calcium channels, GABA receptors, neurotrophic factors [21], and genes are being targeted and experimented with in laboratories to develop treatments. However, control of neuropathic pain is very difficult.
Currently, the treatment of incurable conditions with stem cells is the area of particular interest. Stem cells are the fundamental cells for the formation of all cells and tissues in the body, and there are divided embryonic stem cells and adult stem cells. Embryonic stem cells are isolated from miscarried embryos between implantation and 8-12 weeks of pregnancy, and these cells are multi-differentiable. Adult stem cells can be isolated from tissues such as the skin, liver, heart, kidney, and bone marrow [22]. MSCs are among the adult stem cells and can be isolated from hematopoietic stem cells and bone marrow [3].
MSCs are multi-differentiable, self-proliferating cells that migrate along damaged tissues in the brain, spinal cord, and peripheral nerves to repair the functional damage [23]. MSCs can be differentiated into neurons and astrocytes both in-vivo and in-vitro [24]. In addition, for conditions such as Parkinson's disease [25], stroke [24], paraplegia [26], and infarction [27], stem cells have been transplanted in animal models, and their migration to the damaged site and functional recovery have been observed.
In this study, intrathecal administration of rMSCs in rats with neuropathic pain induced by L5 spinal nerve ligation improved pain behaviors (Fig. 1). The results suggest that intrathecal rMSCs may have certain roles in reducing the pain generated in the spinal cord of SNL neuropathic rats.
ROS have been implicated in many neurodegeneration diseases. Recent studies reported that ROS plays an important role in the induction and maintenace of neuropatic pain in the spinal nerve ligation [7,28]. Human MSCs have a high resistance to oxidative induced cell death [9]. This correlates with low levels of intracellular reactive species and constitutive expression of enzymes required to manage oxidative stress in vitro. It suggests that MSCs may reduce ROS and improve neuropathic pain.
In this study, we used in-vivo conditions to determine whether the intrathecal injection of rMSCs in the pain model due to spinal nerve ligation can reduce oxidative stress and result in pain control. rMSCs were administration on day 7 following the spinal nerve ligation. The withdrawal response threshold in the rats with rMSC administration was significantly increased starting on day 10 post-administration (17 days after the spinal nerve ligation) compared with the other groups, and the withdrawal response threshold was persistently high until day 17 post-administration. In addition, in the group with transplanted MSCs, the ROS was significantly reduced in the dorsal horn on day 17 post-administration. The degree of ROS expression of the dorsal horn in rMSC-administration rats was similar to that in the naive rats, which implies that the injected rMSCs contributed to the reduction of ROS expression. However, the ROS expression significantly increased in the pain and PBS groups compared with the naive rats. Therefore, our in-vivo study using rMSCs administration in the neuropathic pain model supports Valle-Prieto's in-vitro study that human MSCs have high resistance to oxidative induced cell death correlating with low levels of intracellular reactive species [9].
The pain response was reduced starting on day 10 post-administration, but the pain threshold observed for up to 17 days post-administration did not reach the same level as that observed pre-ligation. The reason for these results may be that the amount of transplanted MSC self-proliferation was too limited to result in complete pain control or that the time for these cells to exert their full effect is beyond the timeframe adopted in this study.
Overall, rMSCs administration in the rat neuropathic pain model through spinal nerve ligation resulted in a higher (P < 0.01) pain threshold compared with the pain and PBS groups, and the ROS expression was significantly (P < 0.05) reduced compared with the pain and PBS groups. It is still difficult to determine whether the rMSCs migrated directly to the damaged area and indirectly reduced ROS or whether these cells directly affected the reduction of oxidative stress due to ROS expression. Therefore, further in-vivo studies need to be warranted with regard to the role of MSCs in the improvement of neuropathic pain.
ACKNOWLEDGEMENTS
This study was financially supported by research fund of Chungnam National University in 2010.
References
- 1.Gilron I, Watson CP, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. CMAJ. 2006;175:265–275. doi: 10.1503/cmaj.060146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 3.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Zhao C, Liu Y, Sun X, Duan C, Ji M, et al. Therapeutic benefit of TH-engineered mesenchymal stem cells for Parkinson's disease. Brain Res Brain Res Protoc. 2005;15:46–51. doi: 10.1016/j.brainresprot.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Yang LY, Huang TH, Ma L. Bone marrow stromal cells express neural phenotypes in vitro and migrate in brain after transplantation in vivo. Biomed Environ Sci. 2006;19:329–335. [PubMed] [Google Scholar]
- 6.Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 7.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 9.Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19:1885–1893. doi: 10.1089/scd.2010.0093. [DOI] [PubMed] [Google Scholar]
- 10.Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 11.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 12.Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008;3:e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papir-Kricheli D, Frey J, Laufer R, Gilon C, Chorev M, Selinger Z, et al. Behavioural effects of receptor-specific substance P agonists. Pain. 1987;31:263–276. doi: 10.1016/0304-3959(87)90041-8. [DOI] [PubMed] [Google Scholar]
- 14.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Ruchi R, James SR, Chidiac EJ. Gene therapy for chronic neuropathic pain: how does it work and where do we stand today? Pain Med. 2011;12:808–822. doi: 10.1111/j.1526-4637.2011.01120.x. [DOI] [PubMed] [Google Scholar]
- 16.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 17.Hill RG. Molecular basis for the perception of pain. Neuroscientist. 2001;7:282–292. doi: 10.1177/107385840100700405. [DOI] [PubMed] [Google Scholar]
- 18.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 19.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A. 2010;107:14851–14856. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siniscalco D, Giordano C, Rossi F, Maione S, de Novellis V. Role of neurotrophins in neuropathic pain. Curr Neuropharmacol. 2011;9:523–529. doi: 10.2174/157015911798376208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 23.Siniscalco D, Giordano C, Galderisi U, Luongo L, Alessio N, Di Bernardo G, et al. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci. 2010;67:655–669. doi: 10.1007/s00018-009-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savitz SI, Dinsmore JH, Wechsler LR, Rosenbaum DM, Caplan LR. Cell therapy for stroke. NeuroRx. 2004;1:406–414. doi: 10.1602/neurorx.1.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy YS, Bahat-Stroomza M, Barzilay R, Burshtein A, Bulvik S, Barhum Y, et al. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson's disease. Cytotherapy. 2008;10:340–352. doi: 10.1080/14653240802021330. [DOI] [PubMed] [Google Scholar]
- 26.Zurita M, Vaquero J. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport. 2004;15:1105–1108. doi: 10.1097/00001756-200405190-00004. [DOI] [PubMed] [Google Scholar]
- 27.Kim CH, Kim YW, Jang SH, Chang CH, Jung JH, Kim SH. Motor function recovery after adipose tissue derived mesenchymal stem cell therapy in rats with cerebral infarction. J Korean Neurosurg Soc. 2006;40:267–272. [Google Scholar]
- 28.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]