Abstract
This report describes the long term safety and efficacy of intrathecal therapy using Sufentanil for the management of chronic intractable neuropathic pain in 12 chronic pain patients. Standardized psychological screening was used to determine treatment suitability. Evaluation data included the Visual Analog Scale (VAS), Wong-Baker Faces Scale, Brief Pain Inventory (BPI), Disability of Arm, Shoulder, and Hand (DASH), McGill Quality of Life Questionnaire, and complications (granulomas, toxicity, withdrawal, or deaths). SPSS version 18 was used for data analysis. Pre- and post- treatment BPI measures and pain scale scores showed a statistically significant difference. There were no complications directly related to drug toxicity, nor drug withdrawals, granulomas, or deaths. Intrathecal therapy with Sufentanil therapy offers a good treatment alternative for those cases that have failed both surgery and standard pain treatment. Strict patient selection based on psychological screening, control of co-morbidities, a proper pain management may contribute to successful outcome.
Keywords: implantable infusion pump, intractable pain, neuropathic pain, sufentanil
Chronic intractable neuropathic pain can be profoundly disruptive to life, and often presents formidable treatment challenges which are non-responsive to conventional therapies. In a group of chronic limb pain patients (n = 87) with a pain duration average of 5.7 years, Global Assessment of Functioning scores for social, occupation, or school functioning showed 27.6% had serious difficulty functioning, 57.5% had moderate difficulty functioning, 13.79% had some difficulty functioning, and 1.15% had good functioning in all areas. When surveyed regarding suicidal thoughts (n = 90), 67.8% had none, 6.7% had thoughts of self-harm, 15.6% had suicide ideation, and 10% had made a suicide attempt [1].
Spine surgeries are among the most difficult chronic pain problems to treat. In 2003, spinal fusion surgeries represented 47% of total costs expended for back surgery in the United States, dramatically increased from the 14% reported in 1992 [2]. Emerging evidence suggests that for worker's compensation patients who have chronic low back pain, those who have spinal fusion surgery have lower return to work rates, higher complication rates, and higher rates of permanent disability. They also continued taking opioid drugs after surgery, at higher doses than those who did not have surgery [3].
The need for alternative therapies for patients with chronic, intractable pain who are unresponsive to conventional therapies is clear. Moderate level quality of evidence exists to support the effectiveness of continuous intrathecal infusions in controlling chronic non-malignant pain [4]. However, morphine, which is commonly used as the medication in the intrathecal device, has significant side effects which limit its usefulness. A promising alternative to morphine is Sufentanil, but there are few clinical reports on the safety, toxicology, and stability of its pH available to support its use in chronic pain [5]. This is an off-label use of Sufentanil.
The purpose of the study is to describe the long-term safety and efficacy of intrathecal therapy using Sufentanil for the management of chronic intractable neuropathic pain, including failed back surgery syndrome.
METHODS
This study was a pre-designed cohort review on patients undergoing treatment using Sufentanil intrathecal therapy. Institutional Review Board approval was obtained prior to the study from The University of Texas at El Paso, El Paso, Texas.
Patients were considered for treatment if they had failed standard treatment and used high doses of opioids for greater than two years. Standards of appropriate patient selection criteria for intrathecal therapy included assessment of pre-existing medical comorbidities, psychological status, associated social, technical, and economic issues, and response to a trial assessment. Screening included both a clinical interview and psychological tests. At each clinical visit, pain was assessed by self-report using an equidistant 0-10 numerical rating scale and the psychologist used the same scale to document usual, worst, and least pain during the last week. Psychological screening included the Oswestry Pain Questionnaire, Psychosocial Stressors Severity measure, and Global Assessment of Function. Approximately 80% (of the 5% chronic pain patient population of the first author) of the patients screened were not suitable for this therapy.
Twelve patients were reviewed (seven men and five women). The age range was 38-79 years, with an average age of 53.6 years. Seven patients had diagnoses of failed back surgery syndrome with an average of 6.5 procedures each, four had failed spinal cord stimulators, and five had neuropathic pain. The follow-up ranged from 18 months to 14 years, with an average of 5 years (median = 3.5 years). Ten patients had pumps with 40 ml capacity, and two patients had pumps with 20 ml capacity.
Following screening and baseline data collection, patients underwent a procedure for catheter placement. Patients were prepared under sterile conditions in the operating room suite. The catheter was placed at the lower thoracic spine level between T8-T11. Pumps were then refilled at 30-80 day intervals (dose ranges between 25-55 mcg/of Sufentanil/day), and the pH was measured of all fluids when pump was refilled. Patients were also monitored with routine neuropathy blood work every three months.
1. Pre/post measures
Baseline data included pain self-report ratings using a Visual Analog Scale (VAS), the Wong-Baker Faces Scale, the Brief Pain Inventory (BPI), and the Disability of Arm, Shoulder, and Hand (DASH). These same ratings were also collected at follow-up.
The VAS uses an equidistant 0-10 rating scale (no pain to worst pain possible) on a 100-mm line, and is commonly used in clinical practice. However, since the VAS is a one dimensional measurement, other measures must also be used to capture the multi-dimensional nature of pain. Research suggests that faces pain scales may be reliable and valid for use in adults, and can provide an added measurement component when literacy or understanding of English may compromise results of other measures [6]. The Wong-Baker Faces Scale provides an excellent supplemental measure for the multicultural population in this sample.
The BPI is a measure used to assess the severity and location of pain, the impact of pain on daily functions, pain medications being used, and pain relief obtained during the past 24 hours or past week. The BPI uses numeric rating scales that range from 0 to 10. Mild pain is defined as a pain score of 1-4, moderate pain as a score of 5-6, and severe pain as a score of 7-10 (http://www3.mdanderson.org/epts./prg/bpi.htm#detail_descript).
The DASH measure is a 30-item, self-report questionnaire designed to measure physical function and symptoms in people with musculoskeletal disorders of the upper limb. Scoring is divided into two sections: (a) disability and symptoms (30 items, scored 1-5) and an optional sport/music or work section (4 items, scored 1-5) (http://www.dash.iwh.on.ca/).
2. Outcome measures
In addition to the above measures, the McGill Quality of Life Questionnaire was used as an outcome measure. It is a patient-reported instrument that was designed to measure quality of life in populations with life-threatening illnesses. Subscale scores and overall index scores can range from 0 to 10, helping to identify areas that need attention if the score is low. This measure was chosen to supplement the outcome results.
Complications such as equipment problems, granulomas, toxicity, withdrawal, or deaths were reviewed. CT myelograms were used to detect any asymptomatic catheter granulomas. All data were analyzed using SPSS version 18.
RESULTS
1. Pre/post measures
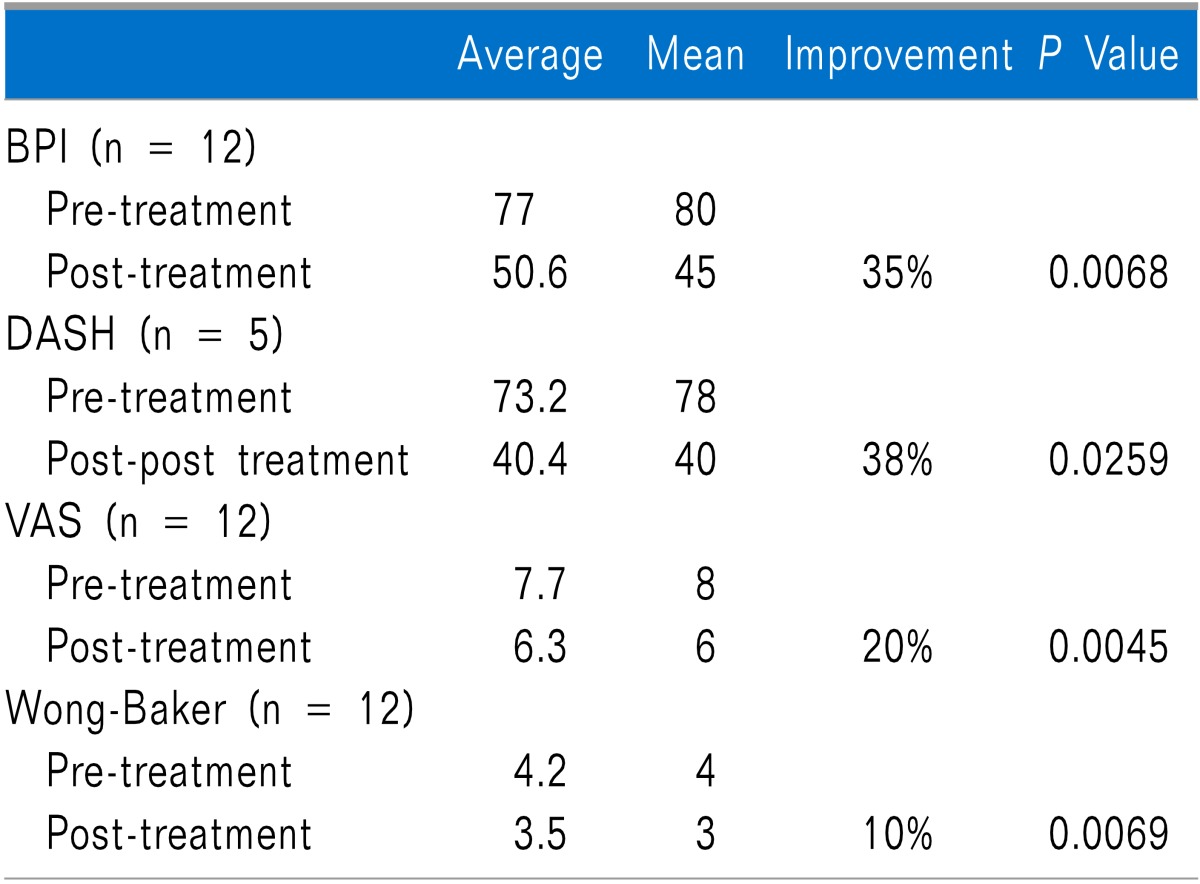
At pre-treatment baseline, the overall average BPI score was 77, and the mean was 80. Following treatment, overall scores were improved by 35% and paired T-test results showed statistical significance at a P value of 0.0068. At pre-treatment baseline, the overall average DASH score was 73.2 with a mean of 78 (Only five patients had upper limb pain, which is why only 5 are reported taking the DASH). Following treatment, overall scores were improved by 38% and paired T-test results showed statistical significance at a P value of 0.0259. At pre-treatment baseline, the overall average VAS score was 7.7, and the mean was 8. Following treatment, overall scores were improved by 20% and paired T-test results showed statistical significance at a P value of 0.0045. The 20% improvement in pain intensity demonstrates minimally important changes [7]. At pre-treatment baseline, the overall average Wong-Baker score was 4.2, and the mean was 4. Following treatment, overall scores were improved by 10% and paired T-test results showed statistical significance at a P value of 0.0069 (Table 1).
Table 1.
Pre and Post Treatment BPI, DASH, VAS, and Wong-Baker Scores
2. Outcome measures
1) McGill quality of life questionnaire
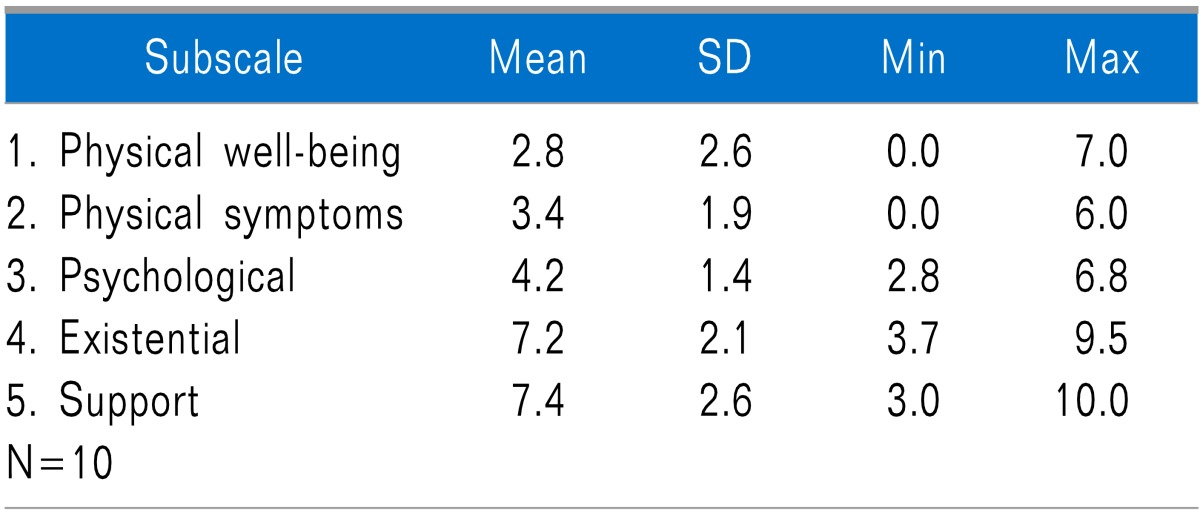
Because a low score can help identify areas that need attention, this measure was chosen to supplement the outcome results. Of interest is the high subscale score of 7.2 for the existential domain, which is considered important to wellbeing. The support subscale was also notable at 7.4.
Complications were limited to one catheter recall and one pump recall. One pump had to be replaced early for motor stall. There were no granulomas, toxicity, withdrawal, or deaths (Table 2).
Table 2.
McGill Quality of Life Questionnaire Scores
DISCUSSION AND CONCLUSION
In the larger cohort of 19 patients, a total of 47 pumps have been implanted. In the 12 patient group, 42 pumps with Sufentanil have been implanted without complications related to toxicity, drug withdrawals, granulomas, or deaths. The pain improvement of greater than 30% is clinically significant 7 and had a positive impact on quality of life.
Intrathecal therapy using Sufentanil offers an acceptable treatment alternative for those cases that have failed both surgery and standard pain treatment including dorsal spinal cord stimulators. Strict patient selection based on psychological screening, control of co-morbidities, and proper pain management may contribute to successful outcome.
References
- 1.Monsivais JJ, Robinson K. Psychological profile and work status of a predominantly Hispanic worker's compensation population with chronic limb pain. Hand (N Y) 2008;3:352–358. doi: 10.1007/s11552-008-9123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberman IH. Disc bulge bubble: spine economics 101. Spine J. 2004;4:609–613. doi: 10.1016/j.spinee.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TH, Randolph DC, Talmage J, Succop P, Travis R. Long-term outcomes of lumbar fusion among workers' compensation subjects: a historical cohort study. Spine (Phila Pa 1976) 2011;36:320–331. doi: 10.1097/BRS.0b013e3181ccc220. [DOI] [PubMed] [Google Scholar]
- 4.Hayek SM, Deer TR, Pope JE, Panchal SJ, Patel VB. Intrathecal therapy for cancer and non-cancer pain. Pain Physician. 2011;14:219–248. [PubMed] [Google Scholar]
- 5.Waara-Wolleat KL, Hildebrand KR, Stewart GR. A review of intrathecal fentanyl and sufentanil for the treatment of chronic pain. Pain Med. 2006;7:251–259. doi: 10.1111/j.1526-4637.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 6.Stuppy DJ. The faces pain scale: reliability and validity with mature adults. Appl Nurs Res. 1998;11:84–89. doi: 10.1016/s0897-1897(98)80229-2. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]


