Abstract
Transgenic zebrafish that express GFP under control of the T cell-specific tyrosine kinase (lck) promoter were used to analyze critical aspects of the immune system, including patterns of T cell development and T cell homing after transplant. GFP-labeled T cells could be ablated in larvae by either irradiation or dexamethasone added to the water, illustrating that T cells have evolutionarily conserved responses to chemical and radiation ablation. In transplant experiments, thymocytes from lck-GFP fish repopulated the thymus of irradiated wild-type fish only transiently, suggesting that the thymus contains only short-term thymic repopulating cells. By contrast, whole kidney marrow permanently reconstituted the T lymphoid compartment of irradiated wild-type fish, suggesting that long-term thymic repopulating cells reside in the kidney.
The zebrafish has emerged as an important vertebrate genetic model of development. Zebrafish embryos are transparent and develop rapidly ex utero, and most organ systems are fully developed by 5 days postfertilization (dpf). Forward genetic screens in the zebrafish have been instrumental in identifying gene mutations that affect development (1-3). Such genetic approaches have been used successfully to identify genes involved in patterning, regeneration, and the development of organs, including heart, eye, and blood (4, 5). Additionally, small-molecule-based screens in the zebrafish have identified chemicals that perturb normal development (6, 7), and drug screens have been proposed to identify compounds that suppress cancer-associated phenotypes (8-10).
Comparisons of fish and mammalian hematopoiesis indicate that the genetic programs underlying vertebrate blood development have been highly conserved throughout evolution (5). Like their mammalian counterparts, fish B cells express Ig proteins, and T cells express T cell receptor components (11); both of which require gene rearrangements mediated by the rag1 and rag2 proteins (12). Moreover, many homologs of the mammalian lymphocyte receptors have been identified in fish, including CD3 (13) and the CD8 coreceptor (14). Taken together, these findings suggest that T and B cell development in the fish relies on many of the same molecules and pathways used in mammalian lymphopoiesis (15, 16).
The development and functional anatomy of the thymus are also remarkably similar between fish and humans. The zebrafish thymic rudiment is completely developed by 60 h postfertilization (hpf) and by 68 hpf is colonized by T lymphocyte progenitors, which begin to transcribe rag1 and rag2 by 72 hpf (15, 17-19). As in mice and humans, mature T cells localize to the medulla of the adult zebrafish thymus, whereas immature T cells localize to the cortex (20).
The genetics underlying the development of the zebrafish lymphoid system is under active investigation, but functional studies of the zebrafish immune system have been limited by the lack of tools to facilitate the isolation and characterization of different lymphoid cell populations. For example, antibodies against cell lineage markers, such as CD2, CD19, T cell receptors, and Ig proteins, are not available, making the identification of zebrafish T and B cell populations difficult. Also, markers of discrete T cell subpopulations, such as CD3, CD4, and CD8, have yet to be identified in the zebrafish. Because of these limitations, studies in the zebrafish have relied on transgenic technology and on lineage-restricted gene promoters to drive the expression of GFP in specific blood cell populations (21, 22). Transgenic zebrafish lines that express GFP in lymphoid cells have been established with both the rag1 and rag2 promoters (23, 24); however, because rag1 and rag2 are expressed only in immature T and B cells, such lines cannot be used to track the development and migratory patterns of mature T cells.
Here we describe the generation of a transgenic zebrafish model in which the zebrafish T cell-specific tyrosine kinase (lck) gene promoter drives GFP expression in lymphocytes. Using lck-GFP and rag2-GFP transgenic fish, we identify T and B cell populations in the zebrafish. Using lck-GFP transgenic fish, we tracked the development of T cells in the zebrafish from embryogenesis to adulthood, evaluated the sensitivity of T cells in living larvae to chemical and radiation ablation, analyzed T cell homing in transplanted embryos, and assessed the hematopoietic engraftment potential of kidney marrow and thymus cell populations. The results of these experiments provide a foundation for studies of immune responses in the zebrafish.
Materials and Methods
Animals. Zebrafish maintenance, developmental staging, and in situ analysis were conducted as described (25, 26). The cloche (27) and bloodless (28) zebrafish mutant lines, which are deficient in hematopoietic stem cells, and van gogh, which lacks the thymus (29, 30), were used to assess T cell-specific expression of the lck gene at 4 dpf.
Isolation of cDNA Clones. A degenerate PCR strategy was used to isolate a fragment of the zebrafish lck cDNA. A full-length clone was obtained by screening a kidney cDNA library (Fig. 7, which is published as supporting information on the PNAS web site). Protein sequence comparisons were completed by using the Jotun Hein algorithm in the megalign program of the dnastar sequence analysis software package (DNASTAR, Madison, WI).
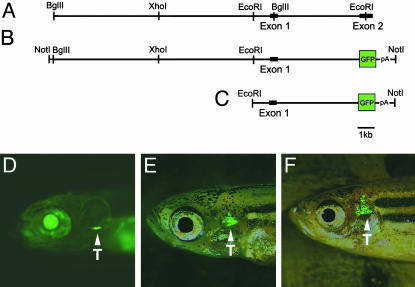
Isolation and Identification of the Zebrafish lck promoter. A genomic zebrafish lck clone (84-I6) was identified by screening a PAC library (Incyte, Palo Alto, CA) with the lck cDNA probe (Supporting Text, which is published as supporting information on the PNAS web site). The 5.5-kb sequence upstream of the lck start codon was obtained by sequencing the lck PAC clone, and PCR was used to amplify a 3.8-kb genomic fragment extending from the first noncoding exon to the translation initiation site contained within exon 2 (Fig. 1A). This fragment was cloned into a pBluescript vector containing the enhanced GFP transgene (p1x EGFP). The EGFP coding sequence is followed by a SV40 polyadenylation sequence that terminates with a NotI restriction digest site.
Fig. 1.
lck-GFP transgenic zebrafish. Diagrams of the genomic DNA sequence comprising the lck promoter (A) and the GFP construct (B) are shown. Enzyme digest sites used for cloning and restriction mapping of the minimal promoter are shown. lck-GFP transgenic fish expressing the 5.5-kb EcoRI-NotI fragment (C) are shown at 8 dpf (D), 45 dpf (E), and 80 dpf (F). Arrowheads denote GFP-labeled cells in the thymus (T). The views are lateral with anterior to the left.
Restriction enzyme mapping and Southern blot analysis of the lck PAC clone identified a 13-kb BglII genomic fragment that lies immediately upstream of the 3.8-kb fragment. This restriction fragment was cloned upstream of the 3.8-kb genomic p1xEGFP construct.
Generation of Stable Transgenic lck-GFP and rag2-GFP Transgenic Zebrafish. The lck promoter-containing vectors were digested with NotI alone or in combination with XhoI, EcoRI, or BglII (Fig. 1B and Supporting Text). Digestion products were resolved on an ethidium bromide-containing agarose gel, and DNA was purified (QIAquick gel extraction kit, Qiagen, Valencia, CA), resuspended in 0.5× Tris-EDTA buffer (pH 8.0) + 100 mM KCl, and injected as described (25). Transgenic rag2-GFP fish were generated as reported in refs. 24 and 25.
Immunocytochemistry and RNA in Situ Hybridization on Paraffin-Embedded Sections. Paraffin embedding and sectioning, in situ hybridization, and immunohistochemical analysis were performed essentially as described in ref. 25. cDNA probes were made by PCR amplification of plasmid DNA containing coding sequences for rag1, rag2, IgLC3, and lck, using primers containing T7 and Sp6 promoter sequences (Table 1, which is published as supporting information on the PNAS web site).
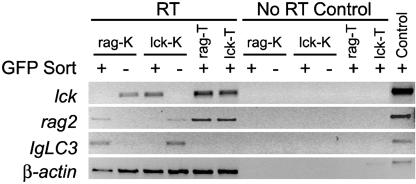
Fluorescence-Activated Cell Sorting (FACS) and RT-PCR Analysis. Kidney, thymus, spleen, and whole blood cell suspensions from wild-type AB or transgenic rag2-GFP or lck-GFP fish were analyzed by FACS based on forward and side scatter and GFP fluorescence as described in refs. 25 and 31. RNA was isolated from GFP-positive and -negative FACS sorted blood cell populations and used in reverse transcription reactions. Complementary DNA samples were analyzed by semiquantitative PCR with primers specific for lck, Ig Light Chain 3 (IgLC3), rag2, and β-actin (Table 2, which is published as supporting information on the PNAS web site).
T Cell Homing in Transplanted Zebrafish Embryos. Whole kidney marrow or whole thymus cells were isolated from 8-week-old transgenic fish, filtered, resuspended in 0.9× PBS + 5% FBS, and injected into the sinus venosus of 2-day-old AB-strain embryos. Approximately 0.5-1.0 × 103 kidney marrow cells or thymus cells were injected per embryo (31). T cell homing was assessed by GFP fluorescence at 24 and 48 h posttransplantation.
T Cell Ablation by γ-Irradiation. Six-day-old rag2-GFP and lck-GFP transgenic embryos were given a whole-body dose of γ-irradiation (15 Gy from a 137Cs source) and analyzed for GFP expression within the thymus at 1, 2, and 3 days posttreatment.
T Cell Ablation by Dexamethasone Treatment. Five-day-old rag2-GFP and lck-GFP transgenic embryos were treated with dexamethasone [250 μg·ml-1 (1% ethanol), 100 μg·ml-1 (0.4% ethanol), or 25 μg·ml-1 (0.1% ethanol)] or with ethanol vehicle alone (1%, 0.4%, and 0.1%) in egg water (60 mg·liter-1 instant ocean in distilled water containing methylene blue). Fish were analyzed at 1, 2, and 3 days posttreatment for T cell ablation as detected by loss of GFP fluorescence in the thymus.
Hematopoietic Cell Transplantation into Adult Zebrafish. Nonirradiated and irradiated (23 Gy from a 137Cs source 2 days before transplantation) 2-month-old AB wild-type fish were injected i.p. with either whole kidney marrow cells (3 × 105) or cells isolated from the thymi (1.0-1.5 × 106) of transgenic rag2-GFP and lck-GFP fish. Cells were resuspended in 0.9× PBS + 5% FBS, and 5 μl was injected i.p. with a 10-μl Hamilton syringe. Radiation doses of 20-23 Gy were chosen because these are sublethal, have been shown to be immunoablative in adult zebrafish (D.T., A. Winzeler, H. M. Stern, E. A. Mayhall, D.M.L., J. L. Kutok, A.T.L., and L.I.Z., unpublished data), and were used successfully for engraftment of T cell leukemias into irradiated fish (25).
Results
lck mRNA Expression Is Confined to T Cells Throughout Development.Developmental expression of lck, rag1, and rag2 RNA was analyzed by using whole-mount in situ hybridization. lck mRNA expression was first detected in T cells located in the bilateral thymic lobes by 3 dpf (Fig. 8 C and G, which is published as supporting information on the PNAS web site), and by 7dpf, the number of lck-positive T cells in the thymus had increased (Fig. 8 D and H). The pattern of lck expression was similar to that of rag2 (Fig. 8 A, B, E, and F), but lck was not detected in the epithelial cells of the olfactory placode, as has been described for rag1 and rag2 (17, 23, 24), nor was lck detected in cloche, bloodless, or van gogh zebrafish mutants (Fig. 9, which is published as supporting information on the PNAS web site, and data not shown), all of which have defects resulting in the absence of T cells or the thymus (29, 30, 32).
To further analyze the anatomic subcompartments of the adult zebrafish thymus, we analyzed thymic sections from 3-month-old fish by RNA in situ hybridization for lck, rag1, rag2, and T cell receptor alpha (TCR-α) expression. lck transcripts were detected in cells throughout the thymus (Fig. 10, which is published as supporting information on the PNAS web site), whereas rag1 and rag2 transcripts were selectively expressed in the cortex, and TCR-α was predominantly localized to cells found in the medulla and the corticomedullary junction (20) (Fig. 10).
Generation of Stable lck-GFP Transgenic Zebrafish. A 17-kb genomic fragment 5′ to the lck translation start codon (Fig. 1 A and B) was found to drive expression of GFP in the T cells of transiently injected embryos (data not shown). To identify the minimal promoter fragment that is required for T cell-specific expression, we truncated the GFP-containing DNA construct, using NotI with XhoI, EcoRI, or BglII (Fig. 1B). The 11-kb XhoI/NotI and the 5.5-kb EcoRI/NotI fragments drove GFP expression in the thymic compartment, whereas the 3.8-kb BglII/NotI fragment was inactive (data not shown). Four stable lck-GFP transgenic zebrafish lines expressing the 5.5-kb EcoRI/NotI restriction fragment were generated (Fig. 1C and Supporting Text), two of which showed strong T cell-specific expression and were used for subsequent analyses (Fig. 1 D-F).
Localization of GFP-Labeled Lymphocytes in lck-GFP and rag2-GFP Transgenic Zebrafish. Because the location of T and B cell populations in adult zebrafish is largely unknown, we used anti-GFP immunostaining to identify sites of T and B cell accumulation in paraffin-embedded sections of adult transgenic fish. GFP-positive lymphocytes were found in the thymus (Fig. 11 A and G, which is published as supporting information on the PNAS web site), kidney (Fig. 11 B and H), and spleen (Fig. 11F and data not shown) of both lck-GFP and rag2-GFP adult fish. In adult lck-GFP transgenic fish, such cells were also detected in the gastrointestinal lining (Fig. 11C), the esophagus, gills, at the base of the olfactory epithelium (Fig. 11D), and surrounding regressing oocytes in the ovary (Fig. 11E). Except for the nose, none of these sites in rag2-GFP fish contained GFP-labeled cells (Fig. 11 I-K), indicating that immature T and B lymphocytes do not develop in or migrate to these tissues. In the nasal epithelium rag2-GFP-positive cells were histologically of nonlymphoid origin, and their location near the surface of the nasal epithelium did not overlap with that of lck-GFP-positive cells. We therefore conclude that the GFP signal in the nasal epithelium of rag2-GFP adult fish emanates from nonlymphoid tissues (Fig. 11J).
lck-GFP Expression Identifies Mature T Cells in the Kidney. Although the dogma in mammals is that T cells develop in the thymus, it is unknown whether T cell development occurs in tissues other than the thymus of the adult zebrafish. Given that the fish lack bone marrow and that the kidney is the primary site of definitive hematopoiesis in the fish, we wanted to determine whether immature T cells reside in the kidney marrow.
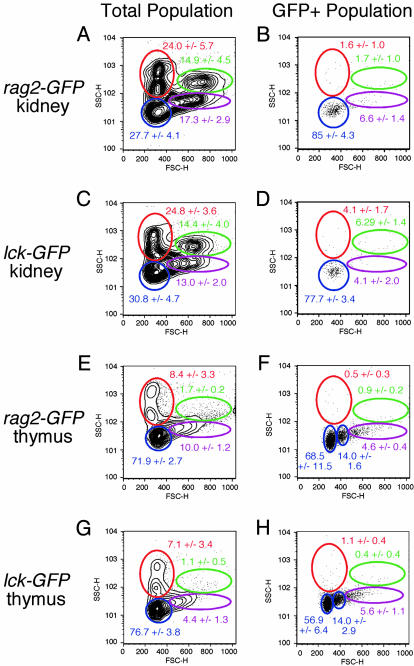
Suspensions of kidney cells consisted of ≈29% lymphocytes (Fig. 2 A and C). GFP-positive cells were largely confined to the lymphoid cell compartment as defined by Traver et al. (31) for both the rag2-GFP and lck-GFP transgenic kidney (Fig. 2 B and D). GFP-positive lymphocytes represented a much higher percentage of the total kidney lymphoid cells in rag2-GFP compared with lck-GFP transgenic fish (mean 38.8 ± 8.8% vs. mean 18.4 ± 5.9%, respectively), suggesting that rag2-expression identifies a distinct subset of lymphocytes in the kidney. RT-PCR analysis of FACS-sorted GFP-positive and GFP-negative kidney cells showed that GFP-labeled cells from rag2-GFP fish express both rag2 and IgLC3, but not lck, whereas GFP-labeled cells from lck-GFP fish do not express either IgLC3 or rag2 (Fig. 3). By contrast, GFP-negative blood cells in the rag2-GFP kidney express lck (Fig. 3) and low levels of IgLC3 when RT-PCR cycle number is increased (data not shown). Taken together, these results indicate that GFP-labeled kidney cells in rag2-GFP fish are B cells, whereas those in lck-GFP fish are mature T cells.
Fig. 2.
FACS analysis of cells from the kidney (A-D) and thymus (E-H) of rag2-GFP and lck-GFP transgenic zebrafish. Gated populations of erythrocytes (red), lymphocytes (blue), granulocytes and monocytes (green), and blood cell precursors (purple) are outlined. Populations of cells within each gate are described as percentages of total live cells (±1 SD; n = 24 for A; n = 18 for C; and n = 8 for B, D, and E-H). Cell size is represented by forward scatter (FSC), and granularity is represented by side scatter (SSC). GFP-positive cells in the progenitor gate in thymus samples became confined to the lymphoid gate upon reanalysis, confirming that GFP-labeled populations in the progenitor gate are predominately lymphoid in origin.
Fig. 3.
Semiquantitative RT-PCR analysis of FACS-sorted blood cell populations in the kidney (K) and thymus (T) of lck-GFP and rag2-GFP transgenic fish. GFP-positive (+) and -negative (-) blood cell populations are shown. Results for “No RT Control” show absence of genomic DNA contamination in samples. The β-actin PCR control was completed on genomic DNA. Because the β-actin primers span an intron, PCR amplifies a 100-bp larger fragment than seen in RT samples.
FACS analysis of rag2-GFP and lck-GFP thymocytes revealed that the thymus harbors ≈73% lymphocytes (Fig. 2 E and G), with two distinct subpopulations of T cells (Fig. 2 F and H). Both subpopulations showed essentially the same granularity based on side scatter but differed markedly in cell size based on forward scatter. This finding suggests that zebrafish lymphocytes may change in size as they mature, in agreement with the positive correlation between lymphocyte size and maturational stage of development in mammals.
Whole blood and spleen samples from 10-week-old lck-GFP fish were analyzed for GFP expression by FACS. The spleen contained a mean of 3.2 ± 1.0% GFP-positive T cells, compared with 0.07% in whole blood (data not shown), indicating that only a minority of T cells are in circulation at any given time, as in humans and mice.
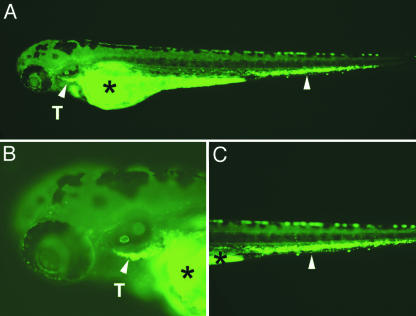
GFP-Labeled T Cells Home to the Thymus After Transplantation.Knowledge of the homing properties of transplanted zebrafish lymphocytes will be important for successful use of this model in studies of the immune system. Thymocytes isolated from adult lck-GFP fish homed to the thymus when injected into 2-day-old embryos (Fig. 4 A and B). Twenty-four hours after injection into the sinus venosus, the GFP-labeled cells were found in the thymus, in the circulation (Movies 1 and 2, which are published as supporting information on the PNAS web site), and in a region of the tail (Fig. 4C) where macrophages reside and hematopoietic activity takes place (32). These results indicate that a subset of thymocytes have the capacity to enter circulation and home to the thymus of 3-day-old zebrafish. Transplantation experiments in adult fish confirm that GFP-labeled T cells can home to the thymus, kidney, and peripheral sites of T cell accumulation (Supporting Text).
Fig. 4.
GFP-labeled thymic T cells obtained from adult lck-GFP transgenic fish home to the thymus of transplanted embryos. (A) GFP fluorescent microscopic images of a 4-day-old transplanted embryo (anterior to the left and dorsal to the top) are shown. (B and C) Enlarged views of the head and tail region, respectively. Arrowheads denote GFP-labeled cells in the thymus (T) and in the tail region. Asterisks denote autofluorescence of the yolk sac.
Thymic Engraftment Requires Ablation of the Immune System. To visualize T cell ablation, lck-GFP larvae were treated with a single dose of γ-irradiation (15 Gy) administered at 6 dpf. By 8 dpf, the nonirradiated control fish retained GFP-labeled T cells (Fig. 5A), whereas fish treated with γ-irradiation had lost them (Fig. 5B). T cells from adult fish were also radiation-sensitive and could be ablated with γ-irradiation (Supporting Text).
Fig. 5.
GFP-labeled T cells in 8-day-old lck-GFP fish are ablated in response to γ-irradiation or dexamethasone treatment. (A) Nonirradiated control fish. (B) Fish 2 days postirradiation. (C) Control fish with 0.4% ethanol. (D) Fish 3 days after treatment with 100 μg·ml-1 dexamethasone (DEX). Asterisks denote autofluorescence of the yolk sac. Arrowheads denote GFP-labeled cells in the thymus (T). The views are lateral with anterior to the left.
Irradiation not only ablates the thymic cell compartment but also causes ablation of hemato-lymphoid cells in the kidney (D.T., A. Winzeler, H. M. Stern, G. A. Mayhall, D.M.L., J. L. Kutok, A.T.L., and L.I.Z., unpublished data). Thus, we wanted to test whether hematopoietic cell engraftment requires that recipients be irradiated before transplantation. rag2-GFP kidney marrow (3 × 105 cells) was injected into sublethally irradiated and nonirradiated recipient fish. Fluorescent microscopy enabled us to visualize reconstitution of the lymphoid compartment through GFP expression by thymic T cells. Kidney marrow cells from the rag2-GFP fish failed to engraft into nonirradiated recipient fish analyzed at 14 and 25 days postinjection (0 of 12), but engrafted well into γ-irradiated recipient fish (10 of 10) (Fig. 12, which is published as supporting information on the PNAS web site).
T Cells Can Be Ablated by Dexamethasone. To test whether zebrafish thymocytes are susceptible to chemical ablative agents, we treated lck-GFP transgenic fish with 250 μg·ml-1 or 100 μg·ml-1 of dexamethasone at 5 dpf. Control larvae treated with the ethanol vehicle retained GFP-labeled T cells at 8 dpf (Fig. 5C), whereas all surviving dexamethasone-treated larvae lacked these cells in the thymus when analyzed at 8 dpf (Fig. 5D). Lower doses of dexamethasone were able to ablate GFP-labeled T cells in transgenic larvae (Table 3 and Supporting Text, which are published as supporting information on the PNAS web site). These results indicate that 5- to 8-day-old zebrafish embryos are able to absorb chemicals from the water and that immature GFP-labeled T cells are responsive to chemical ablation by dexamethasone.
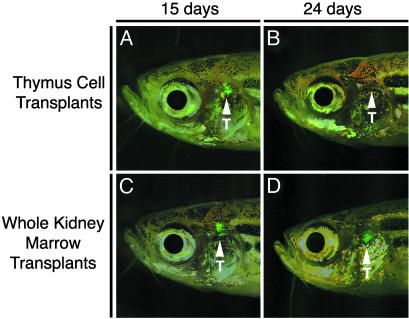
The Kidney Marrow Contains Long-Term Thymic Repopulating Cells.Next we asked whether long-term thymic repopulating cells reside in the kidney marrow or the thymus. Kidney marrow (3 × 105 cells) and thymic cell (1.0 to 1.5 × 106 cells) populations from lck-GFP fish were transplanted into 2-month-old irradiated recipient fish. GFP-positive cells were detected in the thymus of both transplant groups by 14 days posttransplantation (Fig. 6 A and C). Seven of ten fish transplanted with lck-GFP kidney marrow showed strong expression of GFP-positive T cells in the thymus by 14 days posttransplantation, compared with only 4 of 11 fish transplanted with cells from the thymus, indicating weak T cell reconstitution. By 24 days posttransplantation, the GFP-positive T cells could no longer be detected in fish transplanted with thymic cells (Fig. 6B), whereas fish given whole kidney marrow maintained GFP expression for greater than 6 months (Fig. 6D), the last time point at which the fish were analyzed.
Fig. 6.
Lymphopoiesis is fully reconstituted in irradiated adult recipients transplanted with lck-GFP kidney marrow. Transplants consisted of thymus cells (1.5 × 106 cells, A and B) or whole kidney marrow (3 × 105 cells, C and D) at 15 or 24 days posttransplantation. The views are lateral with anterior to the left. Arrowheads denote the location of the thymus (T).
Discussion
We have taken advantage of the fact that lck is expressed in both immature and mature T cells, and that the rag1 and rag2 genes are expressed in immature B and T cells, to characterize the zebrafish lymphoid system by using lck-GFP and rag2-GFP transgenic lines. GFP expression in the lck-GFP transgenic line faithfully recapitulates endogenous lck mRNA expression during embryogenesis. We show that lck is expressed in thymic T cells as early as 3 dpf and in both immature cortical and mature medullary thymocytes of adult fish. Similarly, lck-GFP transgenic fish have GFP-expressing T cells found in the thymus by 4 dpf and on into adulthood. Immunohistochemical studies of rag2-GFP transgenic fish reveal that GFP staining is detected in both the cortical and medullary regions of the thymus. This finding contrasts with the restricted expression of rag1 and rag2 mRNA expression in immature cortical thymocytes (20). These results can be explained by the stability of the GFP fluorophore and indicate that GFP protein expression may persist longer than the highly labile rag1 and rag2 proteins. Similar results have been reported in rag2-GFP transgenic mice (33).
T cells have been identified in the thymus; however, until our study, additional sites of T cell accumulation had not, to our knowledge, been identified in the adult zebrafish. We used GFP-immunostaining of paraffin-embedded sections to identify lck-GFP-positive T cell populations in peripheral sites including the intestine, esophagus, ovary, and the base of the olfactory epithelium. In support of the olfactory placode being a preferred site of T cell accumulation in fish, Myc-transformed T cells have been shown to home to this region when transplanted into irradiated recipients (25). Given that the olfactory epithelium, esophagus, and intestine are constantly exposed to foreign microbes, and that mice and humans have T cell accumulation in these sites as well (34-36), it is not surprising that mature T cells would be present at these sites in the zebrafish. By contrast, GFP-positive cells from the rag2-GFP fish were not found in peripheral organs, with the exception of GFP-labeled epithelial cells in the olfactory bulb. Taken together, these results indicate that GFP-labeled lymphoid cells from the rag2-GFP line are likely restricted to thymocytes, and B cells in the kidney. In contrast to previous reports identifying the pancreas as a lymphoid organ during embryogenesis (37), we failed to detect GFP-labeled lymphocytes in the pancreas of developing fry or adult rag2-GFP and lck-GFP transgenic zebrafish (data not shown). This discrepancy may be due to a short transit time of developing B cells in the pancreas. If the time from onset of rag transcription to exit of the developing B cells from the pancreas is shorter than the time required for the GFP protein to mature, fluorescence would not be expected to be detectable in the pancreas. A second possibility is that the rag2 promoter used in our transgenic zebrafish lines is missing a critical regulatory element required for rag2 expression in immature pancreatic B cells. Finally, it is possible that B cells do not reside in the pancreas. Further experiments are needed to resolve this discrepancy.
In mammals, T cell development occurs predominantly in the thymus. However, the presence of rag2-GFP-positive cells in the kidney prompted us to question whether a portion of these cells are immature T cells. RT-PCR analysis of FACS sorted GFP-positive and -negative kidney cells revealed that rag2-positive lymphocytes express IgLC3 but not lck, indicating that these cells are B cells. By contrast, we found that lck-positive cells in the kidney fail to express B cell markers or the rag genes, indicating that these cells are likely mature T cells. Based on RT-PCR analysis of fluorescence-activated cell-sorted GFP-labeled cell populations from the kidney and GFP immunostaining of paraffin-embedded sections, we conclude that the thymus is the only site of T cell development in the zebrafish, and that B cell development occurs in the kidney marrow.
The thymus contains T cells at various stages of development. To assess whether a subset or all of the thymocytes are able to home to the thymus, we transplanted thymus cells from lck-GFP transgenic fish into 2-day-old embryos. Only a subset of GFP-labeled thymocytes is able to enter circulation and home to the thymus of transplanted 3-day-old embryos by 24 h posttransplantation. The remaining GFP-labeled cells entered circulation or became confined to a region of the tail. These results indicate that a subset of thymocytes are able to home back to the thymus, a process that can be easily visualized by fluorescence microscopy. Using lck-GFP transgenic fish, we will be able to assess whether immature thymocytes and mature T cells have different homing potential. Finally, these experiments suggest that the thymus of 3-day-old fish expresses receptors and/or chemoattractants that facilitate the migration of immature T cell progenitors back into the thymus.
Using lck-GFP transgenic fish, we show that GFP-labeled T cells are responsive to irradiation and chemical ablation in a similar manner as has been described in mouse and man. In mice, dexamethasone is administered by i.p. injection, with ≈90% of thymocytes dying by 2 days posttreatment (38, 39). Our experiments show that the zebrafish absorb the steroid from the water and respond to treatment within 3 days. Because fish must pass water over the gills to obtain oxygen and because the zebrafish thymus remains contiguous with the gill arches throughout development until 15 weeks postfertilization (20), the thymus is likely constantly exposed to environmental agents, and thus, thymocytes may directly absorb dexamethasone from the water.
In mice, long-term engraftment of adult bone marrow cells requires ablation of the host hematopoietic system. However, it was unknown whether transplant engraftment in fish would be similar. Nonirradiated adult fish transplanted with lck-GFP marrow failed to develop GFP-labeled T cells in the thymus by 25 days postinjection. By contrast, whole kidney marrow cells isolated from lck-GFP fish were able to reconstitute lymphopoiesis in recipient fish after a sublethal dose of irradiation for >6 months posttransplantation. Previously, long-term reconstitution of the erythroid lineage was reported in zebrafish embryos transplanted with kidney marrow (31). To our knowledge, our results are the first demonstration of long-term reconstitution of a hematopoietic cell lineage in irradiated adult zebrafish transplanted with whole kidney marrow. Taken together, these results suggest that either donor cells were rejected in nonirradiated recipients or that engraftment of donor hematopoietic cells must overcome competition with endogenous cells. In the latter case, hematopoietic engraftment likely occurs in irradiated fish because of the opening of niche space in the kidney.
To determine whether the thymus contains T cell progenitors that have the capacity to reconstitute T cell lymphopoiesis in the thymus, we injected irradiated adult wild-type fish with thymocytes from lck-GFP fish. T cell reconstitution of the thymic compartment was only transient, indicating that progenitor cells home to the thymus and differentiate into mature T cells, but these progenitor cells lack the capacity for self-renewal. Similar results have been reported in mice, in that bone marrow transplantation into irradiated recipient mice led to full reconstitution of the immune system (40). However, transplantation of thymus cells could only transiently reconstitute the thymic compartment (41). Our experiments in the zebrafish indicate that hematopoietic cells in the kidney marrow give rise to T cell progenitors that migrate to the thymus throughout life. It is these committed thymic progenitor cell populations that give rise to mature T cells in the thymus.
The results of these experiments provide a foundation for studies of immune responses in the zebrafish and illustrate both the remarkable evolutionary conservation of T cell immunobiology and the power of the zebrafish to visualize T cell responses in vivo through the use of GFP transgenic technology. Given that the response to chemical and radiation-induced T cell ablation can be easily monitored by loss of GFP-labeled T cells in the thymus, our lck-GFP transgenic zebrafish lines represent a new platform for the development of high-throughput in vivo-based drug screens designed to identify new T cell ablative agents. Finally, the lck-GFP transgenic zebrafish model will be useful for studying the genetics underlying T cell development and for developing the next generation of in vivo genetic screens designed to dissect genetic pathways that affect T cell homing and activation.
Supplementary Material
Acknowledgments
We thank B. Gregory, Y. Yang, and Matt Keefe for expert technical assistance; and J. Vinokur, G. Kourkoulis, and W. Saganic for fish care. This work was supported by National Institutes of Health Grants CA-68484 (to A.T.L.), CA-06516 (to J.L.K.), HL04233-04 (to N.S.T.), 1R21 DK063660-02 (to N.S.T.), and R01 HL48801-09 (to L.I.Z.). D.M.L. is a National Sciences Foundation predoctoral fellow, D.T. is an Irvington Institute for Immunological Research fellow, and A.A.F. is a fellow of the Leukemia and Lymphoma Society.
Abbreviations: dpf, days postfertilization; FACS, fluorescence-activated cell sorting; lck, T cell-specific tyrosine kinase.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY390224).
References
- 1.Driever, W., Solnica-Krezel, L., Schier, A. F., Neuhauss, S. C., Malicki, J., Stemple, D. L., Stainier, D. Y., Zwartkruis, F., Abdelilah, S., Rangini, Z., et. al. (1996) Development (Cambridge, U.K.) 123, 37-46. [DOI] [PubMed] [Google Scholar]
- 2.Haffter, P., Granato, M., Brand, M., Mullins, M. C., Hammerschmidt, M., Kane, D. A., Odenthal, J., van Eeden, F. J., Jiang, Y. J., Heisenberg, C. P., et. al. (1996) Development (Cambridge, U.K.) 123, 1-36. [DOI] [PubMed] [Google Scholar]
- 3.Golling, G., Amsterdam, A., Sun, Z., Antonelli, M., Maldonado, E., Chen, W., Burgess, S., Haldi, M., Artzt, K., Farrington, S., et. al. (2002) Nat. Genet. 31, 135-140. [DOI] [PubMed] [Google Scholar]
- 4.Poss, K. D., Nechiporuk, A., Hillam, A. M., Johnson, S. L. & Keating, M. T. (2002) Development (Cambridge, U.K.) 129, 5141-5149. [DOI] [PubMed] [Google Scholar]
- 5.Thisse, C. & Zon, L. I. (2002) Science 295, 457-462. [DOI] [PubMed] [Google Scholar]
- 6.Peterson, R. T., Link, B. A., Dowling, J. E. & Schreiber, S. L. (2000) Proc. Natl. Acad. Sci. USA 97, 12965-12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson, R. T., Mably, J. D., Chen, J. N. & Fishman, M. C. (2001) Curr. Biol. 11, 1481-1491. [DOI] [PubMed] [Google Scholar]
- 8.Stern, H. M. & Zon, L. I. (2003) Nat. Rev. Cancer 3, 533-539. [DOI] [PubMed] [Google Scholar]
- 9.Langheinrich, U., Hennen, E., Stott, G. & Vacun, G. (2002) Curr. Biol. 12, 2023-2028. [DOI] [PubMed] [Google Scholar]
- 10.Pichler, F. B., Laurenson, S., Williams, L. C., Dodd, A., Coop, B. R. & Love, D. R. (2003) Nat. Biotechnol. 21, 879-883. [DOI] [PubMed] [Google Scholar]
- 11.Haire, R. N., Rast, J. P., Litman, R. T. & Litman, G. W. (2000) Immunogenetics 51, 915-923. [DOI] [PubMed] [Google Scholar]
- 12.Wienholds, E., Schulte-Merker, S., Walderich, B. & Plasterk, R. H. (2002) Science 297, 99-102. [DOI] [PubMed] [Google Scholar]
- 13.Park, C. I., Hirono, I., Enomoto, J., Nam, B. H. & Aoki, T. (2001) Immunogenetics 53, 130-135. [DOI] [PubMed] [Google Scholar]
- 14.Hansen, J. D. & Strassburger, P. (2000) J. Immunol. 164, 3132-3139. [DOI] [PubMed] [Google Scholar]
- 15.Trede, N. S. & Zon, L. I. (1998) Dev. Comp. Immunol. 22, 253-263. [DOI] [PubMed] [Google Scholar]
- 16.Trede, N. S., Zapata, A. & Zon, L. I. (2001) Trends Immunol. 22, 302-307. [DOI] [PubMed] [Google Scholar]
- 17.Willett, C. E., Zapata, A. G., Hopkins, N. & Steiner, L. A. (1997) Dev. Biol. 182, 331-341. [DOI] [PubMed] [Google Scholar]
- 18.Willett, C. E., Cortes, A., Zuasti, A. & Zapata, A. G. (1999) Dev. Dyn. 214, 323-336. [DOI] [PubMed] [Google Scholar]
- 19.Schorpp, M., Wiest, W., Egger, C., Hammerschmidt, M., Schlake, T. & Boehm, T. (2000) Curr. Top. Microbiol. Immunol. 251, 119-124. [DOI] [PubMed] [Google Scholar]
- 20.Lam, S. H., Chua, H. L., Gong, Z., Wen, Z., Lam, T. J. & Sin, Y. M. (2002) Dev. Dyn. 225, 87-94. [DOI] [PubMed] [Google Scholar]
- 21.Long, Q., Meng, A., Wang, H., Jessen, J. R., Farrell, M. J. & Lin, S. (1997) Development (Cambridge, U.K.) 124, 4105-4111. [DOI] [PubMed] [Google Scholar]
- 22.Ward, A. C., McPhee, D. O., Condron, M. M., Varma, S., Cody, S. H., Onnebo, S. M., Paw, B. H., Zon, L. I. & Lieschke, G. J. (2003) Blood 102, 3238-3240. [DOI] [PubMed] [Google Scholar]
- 23.Jessen, J. R., Willett, C. E. & Lin, S. (1999) Nat. Genet. 23, 15-16. [DOI] [PubMed] [Google Scholar]
- 24.Jessen, J. R., Jessen, T. N., Vogel, S. S. & Lin, S. (2001) Genesis 29, 156-162. [DOI] [PubMed] [Google Scholar]
- 25.Langenau, D. M., Traver, D., Ferrando, A. A., Kutok, J. L., Aster, J. C., Kanki, J. P., Lin, S., Prochownik, E., Trede, N. S., Zon, L. I., et. al. (2003) Science 299, 887-890. [DOI] [PubMed] [Google Scholar]
- 26.Bennett, C. M., Kanki, J. P., Rhodes, J., Liu, T. X., Paw, B. H., Kieran, M. W., Langenau, D. M., Delahaye-Brown, A., Zon, L. I., Fleming, M. D., et. al. (2001) Blood 98, 643-651. [DOI] [PubMed] [Google Scholar]
- 27.Stainier, D. Y., Weinstein, B. M., Detrich, H. W., III, Zon, L. I. & Fishman, M. C. (1995) Development (Cambridge, U.K.) 121, 3141-3150. [DOI] [PubMed] [Google Scholar]
- 28.Liao, E. C., Trede, N. S., Ransom, D., Zapata, A., Kieran, M. & Zon, L. I. (2002) Development (Cambridge, U.K.) 129, 649-659. [DOI] [PubMed] [Google Scholar]
- 29.Piotrowski, T., Schilling, T. F., Brand, M., Jiang, Y. J., Heisenberg, C. P., Beuchle, D., Grandel, H., van Eeden, F. J., Furutani-Seiki, M., Granato, M., et. al. (1996) Development (Cambridge, U.K.) 123, 345-356. [DOI] [PubMed] [Google Scholar]
- 30.Piotrowski, T., Ahn, D. G., Schilling, T. F., Nair, S., Ruvinsky, I., Geisler, R., Rauch, G. J., Haffter, P., Zon, L. I., Zhou, Y., et. al. (2003) Development (Cambridge, U.K.) 130, 5043-5052. [DOI] [PubMed] [Google Scholar]
- 31.Traver, D., Paw, B. H., Poss, K. D., Penberthy, W. T., Lin, S. & Zon, L. I. (2003) Nat. Immunol. 4, 1238-1246. [DOI] [PubMed] [Google Scholar]
- 32.Liao, E. C., Paw, B. H., Oates, A. C., Pratt, S. J., Postlethwait, J. H. & Zon, L. I. (1998) Genes Dev. 12, 621-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu, W., Misulovin, Z., Suh, H., Hardy, R. R., Jankovic, M., Yannoutsos, N. & Nussenzweig, M. C. (1999) Science 285, 1080-1084. [DOI] [PubMed] [Google Scholar]
- 34.Johansson-Lindbom, B., Svensson, M., Wurbel, M. A., Malissen, B., Marquez, G. & Agace, W. (2003) J. Exp. Med. 198, 963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahnsen, F. L., Farstad, I. N., Aanesen, J. P. & Brandtzaeg, P. (1998) Am. J. Respir. Cell Mol. Biol. 18, 392-401. [DOI] [PubMed] [Google Scholar]
- 36.Hirata, N., Takeuchi, K., Majima, Y. & Sakakura, Y. (2000) Scand. J. Immunol. 52, 380-384. [DOI] [PubMed] [Google Scholar]
- 37.Danilova, N. & Steiner, L. A. (2002) Proc. Natl. Acad. Sci. USA 99, 13711-13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundberg, K. (1991) Biochem. Biophys. Res. Commun. 178, 16-23. [DOI] [PubMed] [Google Scholar]
- 39.Sentman, C. L., Shutter, J. R., Hockenbery, D., Kanagawa, O. & Korsmeyer, S. J. (1991) Cell 67, 879-888. [DOI] [PubMed] [Google Scholar]
- 40.Brecher, G., Bookstein, N., Redfearn, W., Necas, E., Pallavicini, M. G. & Cronkite, E. P. (1993) Proc. Natl. Acad. Sci. USA 90, 6028-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry, S. S., Pierce, L. J., Slayton, W. B. & Spangrude, G. J. (2003) J. Immunol. 170, 1877-1886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.