Abstract
Bronchoalveolar lavage (BAL) has been a useful initial diagnostic tool in the evaluation of pulmonary complications after hematopoietic stem cell transplantation (HSCT); however, the diagnostic sensitivity, prevalence, and outcome after BAL versus lung biopsy (LB) in pediatric HSCT patients remains to be determined. We reviewed 193 pediatric HSCT recipients who underwent a total of 235 HSCTs. Sixty-five patients (34%) underwent a total of 101 BALs for fever, respiratory distress, and/or pulmonary infiltrates on chest radiograph and/or computed tomography scan. The 1-year probability of undergoing BAL was 43.0% after allogeneic stem cell transplantation (alloSCT) and 8.5% after autologous stem cell transplantation (autoSCT) (P =.001). Sixteen of the 193 patients (8%) patients underwent 19 LBs. The probability of undergoing LB at 1 year after HSCT was 9.3%. No grade III or IV adverse events related to either procedure were observed. Of the 101 BALs performed, 40% (n = 40) were diagnostic, with a majority revealing a bacterial pathogen. Among the 19 LBs performed, 94% identified an etiology. In multivariate analysis, myeloablative conditioning alloSCT conferred the highest risk of requiring a BAL (hazard ratio [HR],8.5; P = .0002). The probability of 2-year overall survival was 20.2% in patients who underwent BAL, 17.5% for patients who underwent biopsy, and 67.4% for patients who had neither procedure. In multivariate analysis, only the requirement of a BAL was independently associated with an increased risk of mortality (HR, 2.96; P < .0001). In summary, in this cohort of pediatric HSCT recipients, BAL and LB were used in approximately 35% and 8% of pediatric HSCTs with diagnostic yields of approximately 40% and 94%, respectively, and were both associated with poor long-term outcomes.
Keywords: Bronchalveolar lavage, Lung biopsy, Pediatrics, Stem cell transplantation
INTRODUCTION
Pulmonary complications have been reported in approximately 25% of pediatric recipients after hematopoietic stem cell transplantation (HSCT) [1]. Infectious etiologies are the most prevalent causes of pulmonary dysfunction after HSCT [2]. Early pulmonary complications have been associated with a significantly decreased overall survival in children after allogeneic HSCT (alloSCT) [3]. HSCT recipients who present with clinical and radiological findings of pulmonary infiltrates often receive empiric broad-spectrum antimicrobial therapy [4]. However, a delay in establishing a definitive diagnosis has been shown to be a negative prognostic factor in immunocompromised patients [4,5]. To establish an earlier, more definitive diagnosis, bronchoalveolar lavage (BAL) is regarded as an initial diagnostic tool in pediatric immunocompromised patients with pulmonary dysfunction [6]. However, the diagnostic yield of BAL in pediatric HSCT recipients has been reported as varying from 29% to 68%, with a reduced yield in those patients with grade II to IV graft-versus-host disease (GVHD) and in those receiving immunosuppressive therapy [7,8]. It has been suggested that some of this variation could result from the procedure’s timing, with higher diagnostic yield and favorable impact on survival resulting from early use of BAL, but this requires further investigation [9].
In the absence of definitive findings from BAL, many patients continue to receive empiric antimicrobial treatments that can have deleterious toxicities, including but not limited to ototoxicity, renal insufficiency, and hepatotoxicity [10,11]. Furthermore, radiographic findings of pulmonary infiltrates in HSCT recipients may indicate noninfectious processes, including GVHD, disease recurrence, and treatment/ transplantation-associated toxicity [12].
After HSCT, children are also at risk for a variety of noninfectious pulmonary complications, which are unlikely to be diagnosed after a BAL, including GVHD, idiopathic pneumonia syndrome, and interstitial fibrosis [13–15]. Lung biopsy (LB) can have an increased diagnostic yield, given its ability to detect infectious and noninfectious etiologies. The diagnostic sensitivity of LB has been reported to be 60% to 100% in patients with pulmonary complications after HSCT [13–15]. However, diagnosis based on LB specimens has been reported to be less sensitive in immunocompromised children [14,16–18]. Additionally, the reported complication rates in children after open LB (OLB) vary widely, from 2% to 52%, depending on the patients’ underlying disease and degree of immunocompromise [17].
The impact of LB on overall survival has not yet been prospectively evaluated. In retrospective studies of pediatric populations, LB led to a specific diagnosis in most cases, with the most common organisms recovered being cytomegalovirus (CMV) and Aspergillus fumigatus; the overall mortality after LB ranged from 24% to 45% [13–15]. Single-institution experience demonstrated that OLB was very effective at identifying pulmonary pathology in pediatric HSCT recipients but had little impact on mortality. OLB identified noninfectious causes in 58% of the cases and an infectious organism in 30% of cases; postoperative complications were reported in 47% of patients [9].
Reduced-toxicity conditioning (RTC) has recently been employed in children before allogeneic HSCT, as we have previously reported [19–23]. Although this approach may reduce early mortality, there seems to be similar risk of viral and fungal infections compared with those receiving myeloablative conditioning (MAC) [21]. However, there have been no reported studies that evaluated the impact of pulmonary complications comparing MAC or RTC in pediatric HSCT recipients. Furthermore, there is a paucity of information reporting the safety and effectiveness of video-assisted thorascopy (VAT) or computed tomography (CT)–guided biopsy in pediatric HSCT recipients. Therefore, in the current study, we examined the diagnostic yield of BAL and LB, including OLB, VATs, and CT-guided biopsies, and also compared the survival of children with pulmonary complications after autologous and allogeneic HSCT with either prior RTC or MAC.
METHODS
Patients
We evaluated the safety and efficacy of BAL and LB in 193 consecutive children and adolescents who underwent HSCT at New York-Presbyterian Morgan Stanley Children’s Hospital. Patients received MAC or RTC before allogeneic transplantation (MAC alloSCT, RTC alloSCT) or MAC before autologous transplantation (MAC autoSCT). All patients were enrolled on institutional review board–approved research protocols and parents and/ or patients signed informed consent, as applicable, before to the initiation of therapy. All research was conducted in compliance with the Declaration of Helsinki. The following studies were registered with clinicaltrials.gov: NCT00669890, NCT01050439, NCT00802113, NCT00408447. This retrospective study was approved by the institutional review board of Columbia University Medical Center.
Conditioning Regimens
Conditioning regimens were largely protocol driven and disease specific, and they consisted of both MAC (n = 172, 63%) and RTC (n = 72, 37%). Most MAC regimens consisted of total body irradiation (TBI, 1200 cGy) or busulfan (12.8 mg/kg in patients > 4 years of age, 16 mg/kg in patients ≤ 4 years of age) in combination with melphalan (135 mg/m2) or cyclophosphamide as follows: TBI/melphalan, TBI/cyclophosphamide, busulfan/cyclophosphamide, or busulfan/melphalan. Lung shielding was not used for TBI-containing regimens. Busulfan-containing conditioning regimens utilized busulfan pharmacokinetic dose adjustment and were targeted to achieve 600 to 900 ng/mL steady-state concentration, as we have previously reported [20,23]. RTC regimens were fludarabine-based (150 to 180 mg/m2) as follows: fludarabine/busulfan (12.8 mg/kg in patients > 4 years of age, 16 mg/kg in patients ≤ 4 years of age) and fludarabine/cyclophosphamide, as we have previously reported [23]. Many patients also received rabbit antithymocyte globulin or alemtuzumab as part of RTC prior to AlloSCT [19].
Cell Sources, HLA Typing, and Donor Chimerism Studies
Grafts were from unrelated and related donors, with cell sources of bone marrow, peripheral blood stem cells, or cord blood. HLA typing was performed, and transplantations were classified as fully matched or HLA-mismatched with 1 or 2 differences, as we previously described [19,24,25].
GVHD Prophylaxis
Acute GVHD (aGVHD) prophylaxis consisted of tacrolimus and mycophenolate mofetil (MMF). Tacrolimus was administered starting at .03 mg/ kg per day as continuous i.v. infusion or .12 mg/kg orally (PO) twice a day, with dosage adjustment to maintain blood levels between 5 and 20 ng/mL, starting on the first day of conditioning regimen or 1 day before transplantation (day −1), as we have previously reported [26,27]. MMF was administered at 15 to 30 mg/kg every 6 to 12 hours, either PO or i.v., starting the day after transplantation (day +1), as we have previously described [26,27]. For sibling donor transplant recipients, at day +30, MMF was stopped and tacrolimus was weaned over a 4 to 8 week period if patients had ≤ grade II aGVHD. For unrelated donor transplant recipients, MMF was stopped at day +30 and tacrolimus was continued until day +60, when it was weaned over a 4 to 8 week period, if patients had ≤ grade II aGVHD [26,27]. Acute GVHD and chronic GVHD (cGVHD) were graded according to Seattle consensus criteria [28]. All patients who achieved any level of donor chimerism were considered at risk for developing aGVHD. Only patients with sustained engraftment of donor hematopoiesis and surviving for more than 100 days after transplantation were evaluated for the development of cGVHD.
Supportive Care
Infectious disease prophylaxis consisted of the following: herpes simplex virus prophylaxis (from day −5 until neutrophil engraftment with acyclovir 250 mg/m2/dose i.v., every 8 hours), antifungal prophylaxis from day 0 to day 100 with liposomal amphotericin B 3 mg/kg i.v. daily as we have previously reported [29], Pneumocystis jirovecii prophylaxis (beginning when absolute neutrophil count (ANC) ≥ 500/mm3 × 2 days after transplantation) with trimethoprim sulfamethoxazole (TMP/SMX) 5 mg/kg/day PO divided twice daily thrice weekly or pentamidine 4 mg/kg i.v. every 2 weeks for patients unable to tolerate TMP/SMX, and cytomegalovirus (CMV) prophylaxis (when ANC ≥ 750/mm3 ×2 days after transplantation and donor and/or recipient were CMV+) with foscarnet 90 mg/kg i.v. every other day, alternating with ganciclovir 5 mg/kg i.v. every other day until day 100, as we have previously reported [30]. All patients received sargramostim (250 μg/m2 per day) i.v. daily from day 0 until the white blood cell count reached ≥ .3 × 109/L for 2 days and then were switched to filgrastim (10 μg/ kg per day) either i.v. or subcutaneously until an ANC ≥ 2.5 × 109/L was achieved for 3 days, as we previously described [31]. Intravenous immune globulin (IVIG) 200 mg/kg was administered starting on day −1 and continued every 3 weeks until day +100. IVIG was discontinued on day +100 for patients with < grade II aGVHD. For patients with < grade II aGVHD on day +100, treatment was continued until the severity of aGVHD was < grade II. Patients with IgA deficiency were given IVIG products low in IgA. Patients who developed cGVHD or relapse of greater than or equal to grade II aGVHD resumed IVIG prophylaxis until severity of aGVHD was less than grade II.
BAL and Biopsy Procedures
BAL was performed by a pediatric pulmonologist, using an age-adjusted flexible bronchoscope. Warmed sterile normal saline was instilled in 4 to 6 aliquots of 10 to 20 cc, which was suctioned and sent for pathology and microbiology evaluation.
LBs were by VATS, OLB, or CT-guided biopsies, at the discretion of the pediatric HSCT physician and pediatric surgeon. OLBs and VATS were performed by a pediatric surgeon. Further intervention or resection was at the discretion of the surgeon. CT-guided biopsies were performed by an interventional radiologist, obtaining fine needle aspiration and core biopsy samples.
Biopsy samples were analyzed by a pathologist. All BAL and biopsy samples were screened for infectious diseases with Gram, acid fast, Gomori methenamine silver stains, and viral immunostains. Samples were also cultured for bacteria, viruses, and fungi. Respiratory syncytial virus, adenovirus, influenza A, and parainfluenza were specifically screened with an ELISA and shell vial culture coupled with immunofluorescence stains. Flow cytometry was also performed in all patients with leukemia or lymphoma. A false-negative BAL was defined as a nondiagnostic BAL that was followed by an LB or autopsy within 2 weeks of BAL that identified at least 1 etiology for the patient’s pulmonary dysfunction.
Statistical Analysis
BAL and LB were considered to be diagnostic if any etiology for respiratory dysfunction was identified. We further examined the following variables: age at time of HSCT, gender, disease type (malignant or nonmalignant), disease risk status, type of transplantation (MAC autoSCT, RTC alloSCT, or MAC alloSCT), graft source (related or unrelated), HLA matching, recipient and donor CMV serologic status, graft manipulation (CD34+ selection), the presence of a primary immune deficiency, the use of T cell antibodies (antithymocyte globulin or alemtuzumab), and presence of aGVHD and cGVHD before or at the time of BAL or LB. Poor-risk disease was defined by patients with refractory malignant disease, patients not with complete response (CR) at the time of HSCT, patients in CR3 or beyond, patients with history of induction failure, and patients receiving a second alloSCT. The remainder of patients were classified as average risk. Adverse events associated with BAL or LB were also analyzed.
Continuous variables were summarized as mean ± standard deviation, and 2-sided t-tests were used for 2-group comparison. Categorical variables were summarized as percentages and chi-square tests were used for 2-group comparison. Kaplan-Meier curves and log-rank tests were used for comparing time to first BAL and time to death between 2 groups. Cox proportional hazards models were used to examine the effect of each covariate on time to the first BAL and time to death. Acute and chronic GVHD status were considered as time-dependent covariates in the Cox proportional hazards models. Covariates with P < .2 were included in the final multivariable Cox proportional hazards model. All statistical analyses were performed with the use of SAS version 9.2 software (SAS Institute, Cary, NC). The significance level was set to be .05.
RESULTS
Patient Demographic and Transplantation Characteristics
During the study period, 193 patients underwent 235 HSCTs. The majority of sequential transplantation were either planned tandem triple autologous transplantations in patients with central nervous system tumors (n = 4), planned autologous transplantations followed by reduced-intensity allogeneic transplantations in patients with lymphoma or neuroblastoma (n = 20), graft failure requiring a second alloSCT (n = 12), or relapse (n = 6). The demographics of the patients and transplantation characteristics are depicted in Table 1. Briefly, the mean age of the cohort was 8.6 years (range, 2.3 to 14.9), the cohort included 116 male and 77 female patients, there were 140 malignant and 53 nonmalignant diagnoses, and there were 38 MAC autoSCT, 83 MAC alloSCT, and 72 RTC alloSCT performed.
Table 1.
Patient Demographics and Characteristics
| All Patients (N = 193) | BAL Cohort (n = 65) | Biopsy Cohort* (n = 16) | No BAL/Biopsy Cohort (n = 126) | |
|---|---|---|---|---|
| Age, mean, SD | 8.59 (2.26–14.92 ± 6.33) | 9.98 (3.42–16.54 ± 6.56) | 10.63 (3.77–17.59 ± 6.86) | 7.84 (1.75–13.93 ± 6.09) |
| Sex | ||||
| Male | 116 | 37 | 10 | 77 |
| Female | 77 | 28 | 6 | 49 |
| Diagnosis | ||||
| Malignant | 140 | 44 | 13 | 94 |
| Nonmalignant | 53 | 21 | 3 | 32 |
| Risk status | ||||
| Average risk | 152 | 49 | 13 | 102 |
| Poor risk | 41 | 16 | 3 | 24 |
| Transplantation type | ||||
| MAC autoSCT | 38 | 3 | 4 | 34 |
| MAC alloSCT | 83 | 45 | 6 | 32 |
| RTC alloSCT | 72 | 17 | 6 | 54 |
| Graft | ||||
| Related | 58 | 24 | 4 | 34 |
| Unrelated | 97 | 38 | 8 | 58 |
| HLA match | ||||
| 6/6 or 10/10 | 54 | 27 | 3 | 37 |
| Other mismatch | 91 | 35 | 9 | 55 |
| T cell antibody† | ||||
| R-ATG | 88 | 32 | 4 | 55 |
| Alemtuzumab | 31 | 12 | 3 | 19 |
| None | 73 | 20 | 9 | 52 |
| CMV status | ||||
| Neg/neg | 99 | 19 | 5 | 78 |
| Other | 94 | 46 | 11 | 48 |
BAL indicates bronchoalveolar lavage; MAC, myeloablative conditioning; autoSCT, autologous stem cell transplantation; alloSCT, allogeneic stem cell transplantation; RTC, reduced-toxicity conditioning; R-ATG, rabbit antithymocyte globulin; CMV, cytomegalovirus; neg, negative.
Data presented are n, unless otherwise indicated.
All but 2 patients in the biopsy cohort are also reflected in the BAL group.
One patient received ATG and alemtuzumab.
Sixty-five patients (34%) underwent a total of 101 BALs for fever, respiratory distress, and/or pulmonary in-filtrate(s) on chest X-ray and/or CT scan. Ninety-five BALs (94%) were performed after alloSCT and 6 BAL (6%) were performed after autoSCT. The median time of BAL after alloSCT and autoSCT was day +95 and day +31, respectively. The 1-year probability of undergoing BAL after HSCT was 43.0% for alloSCT and 8.5% for autoSCT (P = .001).
Sixteen of the 193 patients (8%) patients underwent 19 LB for the following indications: nondiagnostic BAL within 30 days, suspected recurrent disease, solitary lung mass/abscess, and obstructive and/or restrictive lung disease. Eleven (58%) of the LB were surgical LB, consisting of 8 VATs and 3 OLBs, and 8 (42%) were CT-guided biopsies. Two of the 16 patients had not previously undergone a BAL (1 patient required surgical resection, 1 patient had suspected recurrent disease); they are, therefore, not included within the BAL patient cohort. The probability of undergoing LB at 1 year after HSCT was 9.3%.
The probabilities of developing grades II to IV aGVHD and cGVHD were 29.5% (n = 57) and 5.7 (n = 11), respectively. Among patients undergoing BAL or LB, the incidence was 31.3% (n = 21) and 7.5% (n = 5), respectively (P = not significant).
Diagnostic Yield
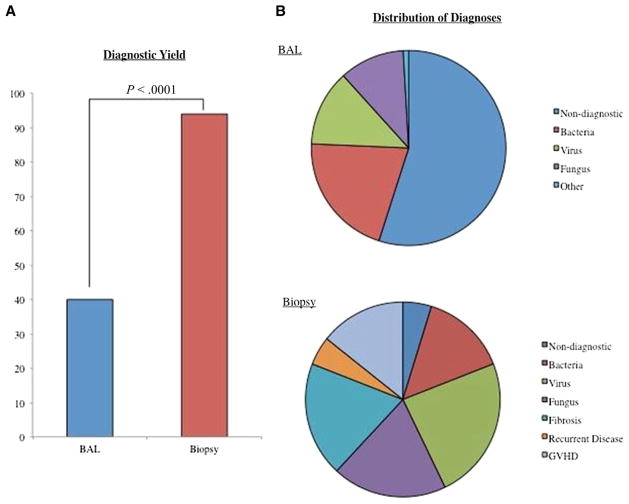
Of the 101 BALs performed in 65 patients, 40% (n = 40) were diagnostic. All diagnostic BAL revealed an infectious etiology, with bacteria being the most prevalent isolated pathogens (Figure 1A,B). Four BALs identified 2 microorganisms (Mycobacterium avium complex and vancomycin-resistant Enterococci [n = 2], coagulase-negative staphylococcus and Pseudomonas aeruginosa [n = 1], and Pseudomonas aeruginosa and Candida glabrata [n = 1]) and 3 BAL identified 3 pathogens (Enterococcus faecium, Candida albicans, Candida krusei [n = 1]; Staphylococcus epidermidis, Streptococcus group D, and Candida not otherwise specified [n = 1]; and CMV, adenovirus, and parainfluenza type 3 [n = 1]). All pathogens isolated from BAL specimens are described in Table 2.
Figure 1.
(A) Diagnostic yield of bronchoaleolar lavage (BAL) versus lung biopsy (P < .0001). (B) Distribution of diagnosis after bronchoalveolar lavage (BAL) versus lung biopsy.
Table 2.
Pathogen Identified with BAL
| Pathogen | n |
|---|---|
| Bacteria | 23 |
| Mycobacterium avium complex | 6 |
| Staphylococcus epidermdis | 4 |
| Vancomycin-resistant Enterococcus | 3 |
| Pseudomonas aeruginosa | 3 |
| Coagulase-negative Staphylococcus NOS | 2 |
| Legionella sp | 1 |
| Serratia marcescens | 1 |
| Streptococcus group D | 1 |
| Enterococcus faecium | 1 |
| Lactobacillus sp | 1 |
| Virus | 14 |
| CMV | 6 |
| Adenovirus | 5 |
| Parainfluenza 3 | 2 |
| Influenza B | 1 |
| RSV | 1 |
| Fungus | 12 |
| Candida NOS | 4 |
| Aspergillus fumigatus | 2 |
| Candida krusei | 2 |
| Candida albicans | 1 |
| Candida glabrata | 1 |
| Trichosporon ashaii | 1 |
| Fungus NOS | 1 |
| Other | 1 |
| Pneumocystis jirovecii | 1 |
BAL indicates bronchoalveolar lavage; NOS, not otherwise specified; CMV, cytomegalovirus; RSV, respiratory syncytial virus.
Data presented are n.
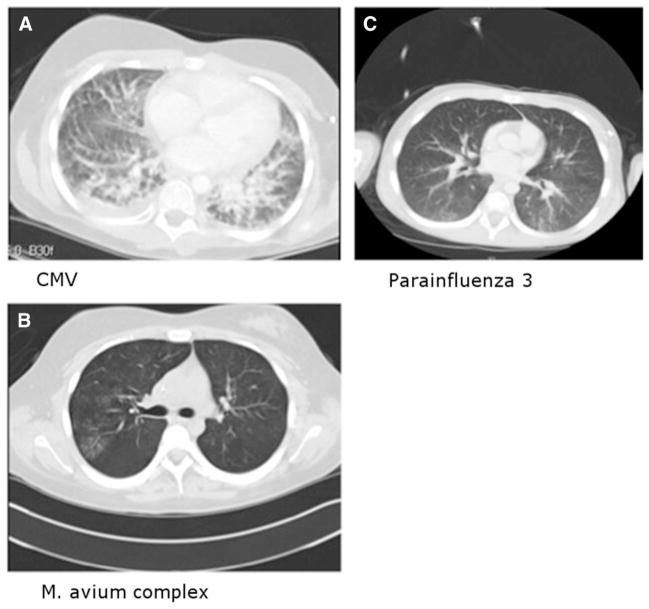
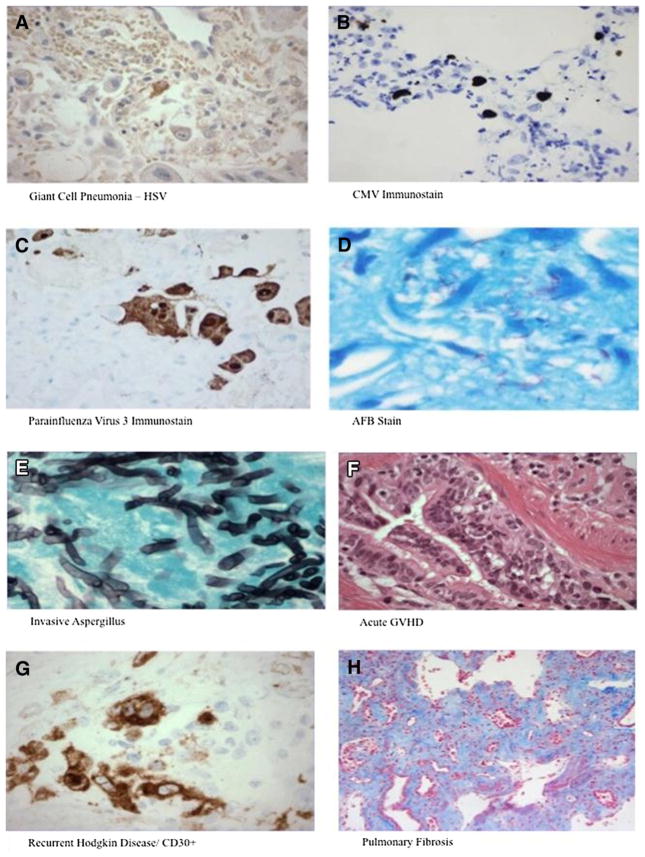
Overall, no pathogens were detected in 61% (n = 61) of BALs tested. Among BALs performed in the first 30 days after HSCT, 67% were diagnostically unrevealing (“negative”). There was no statistically significant difference in the diagnostic yield of BAL performed within the first 30 days after HSCT compared with after 30 days. Two negative BALs were followed within 2 weeks by diagnostic BAL (both positive for CMV). Thirteen negative BALs were followed within 30 days (median, 13 days) by biopsy (n = 7) or autopsy (n = 6). Of the 7 biopsies, all were diagnostic and 6 (86%) identified at least 1 infectious pathogen; 2 biopsies yielded 2 etiologies. Five of the autopsies were diagnostic and 4 identified at least 1 infectious pathogen. Representative chest CT scans from patients with falsely negative BAL are shown in Figure 2A-C. A more detailed representation of the etiologies identified by biopsy and autopsy after initial negative BAL is provided in Table 3. A representative pathological sample of a post-HSCT pediatric recipient with a negative BAL (false-negative) and a positive LB is depicted in representative pathology specimens in Figure 3A–H. There was a significant increase in diagnostic yield between BAL versus LB (40% versus 94%, P < .001) (Figure 1A,B).
Figure 2.
(A) Patient with cytomegalovirus: chest CT with contrast shown in lung windows reveals interlobular septal thickening, ground glass opacification and a small right pleural effusion. Findings suggest diffuse lung disease but are not specific for 1 entity. (B) Patient with Mycobacterium Avium Intracellulare: noncontrast chest CT shown in lung windows reveals tree-in-bud opacities posteriorly in the right upper lobe. Similar opacities were seen throughout the lungs (not shown), particularly in the right middle lobe and lingula. The appearance and distribution suggests the presence of M. avium intracellulare. (C) Patient with parainfluenza 3: chest CT scan with contrast shown in lung windows reveals ground glass and mild confluent nodular opacities present posteriorly in the lungs bilaterally.
Table 3.
Diagnostic Biopsy and Autopsy after Negative BAL
| Biopsy | n | Autopsy | n | |
|---|---|---|---|---|
| Infectious | Mycobacterium avium complex* | 1 | Adenovirus | 2 |
| CMV | 1 | CMV+HSV | 1 | |
| Parainfluenza+influenza | 1 | CMV | 1 | |
| Parainfluenza 3 | 1 | |||
| HSV-1 | 1 | |||
| Aspergillus fumigatus | 1 | |||
| Noninfectious | GVHD* | 2 | Fibrosis | 1 |
BAL indicates bronchoalveolar lavage; CMV, cytomegalovirus; HSV, herpes simplex virus; GVHD, graft-versus-host disease.
One biopsy demonstrated GVHD and Mycobactrium avium complex.
Figure 3.
(A) Lung biopsy section immunostained for herpes simplex virus (HSV)-1. Rare cells are positive, consistent with HSV infection. (B) Lung tissue section immunostained for cytomegalovirus (CMV). Positive cells are seen, consistent with CMV infection. (C). Lung tissue section immunostained for human parainfluenza virus (HPIV). Positive cells are seen, consistent with parainfluenza virus infection. (D) Lung tissue section. Numerous acid fast bacilli (AFB) are seen on AFB special stain, consistent with a mycobacterial infection. (E) Lung tissue section. Fungi (branched, septate hyphae), morphologically compatible with Aspergillus are identified with Gomori methenamine stain (GMS), consistent with Aspergillus infection. (F) Lung tissue section. A small airway shows scarring of the wall, associated lymphocytic inflammation involving the epithelium and wall of the airway, and rare apoptotic debris in the epithelium, consistent with pulmonary graft-versus-host disease (GVHD), causing obliterative bronchiolitis (constrictive bronchiolitis). (G) Lung tissue section immunostained for CD30. Multiple large Hodgkin cells are seen, consistent with recurrent classical Hodgkin lymphoma. (H) Lung tissue section. On trichrome special stain, diffuse, panlobular interstitial fibrosis is seen.
Among the 19 LB performed, 94% of the biopsies identified at least 1 etiology: infection (n = 11), fibrosis (n = 4), GVHD (n = 3), and recurrent malignant disease (n = 1) (Figure 1A,B). Overall, lung autopsies were performed in a total of 14 patients (43%). Seven of 14 autopsies (50%) provided at least 1 pathogen previously unidentified after a falsely negative BAL.
Respiratory Status before and after BAL and/or LB
Records regarding ventilatory status surrounding the time of BAL were available for 88 of 101 cases. Of these cases, 39% (n = 34) of patients were intubated for > 48 hours after BAL, and of these cases, 73% (n = 25) were intubated for respiratory distress before the BAL. The median duration of intubation was 8.5 days. Of the 19 LBs, 5 patients remained intubated for > 48 hours after the procedure, 2 of whom had been intubated before the procedure. The median duration of intubation was 4 days.
Complications and Adverse Events after BAL and/or LB
Eight patients required chest tube placement after OLB, which was transient and did not progress to grade III toxicity. One patient developed postoperative seizures, felt to be unrelated to the OLB. One patient subsequently developed a grade III hemorrhage after CT-guided LB. This patient was intubated and died within 48 hours after the procedure; the biopsy was positive for Aspergillus fumigatas and the death was considered to be secondary to pulmonary aspergillosis and unrelated to CT-guided biopsy.
Univariate and Multivariate Analysis on Factors Associated with BAL
The probability of undergoing BAL was 7.9% for MAC autoSCT patients, 23.6% for RTC alloSCT patients, and 54.2% for MAC alloSCT patients, representing a significantly higher probability in the MAC alloSCT group (hazard ratio [HR], 7.4; 95% confidence interval [CI], 2.7% to 20.8%; P <.0001). Other statistically significant factors in the univariate analysis included age at HSCT, which had a hazard ratio of 1.05 (95% CI, 1.0 to 1.1; P =.01) and grade II to IV aGVHD (HR, .105; 95% CI, .01 to .03; P = .027). When controlling for other factors in multivariate analysis, MAC alloSCT conferred the highest risk of requiring a BAL (HR, 8.5; 95% CI, 2.7 to 26.3; P = .0002) (Table 4). In univariate analysis, there were no statistically significant variables in predicting positive, compared with nondiagnostic, BAL. In the univariate analysis, there were no statistically significant predictors for risk of requiring an LB; however, the number of patients was small.
Table 4.
Multivariate Analysis of Predictors of BAL
| Variable | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age | 1.056 | 1.012–1.101 | .0122 |
| Risk | |||
| Average | Reference (1.0) | ||
| Poor | 1.427 | .802–2.540 | .2262 |
| Chronic GVHD | .217 | .030–1.585 | .1321 |
| Acute GVHD | .066 | .009–.485 | .0076 |
| Transplantation type | |||
| MAC autoSCT | Reference (1.0) | ||
| MAC alloSCT | 8.464 | 2.725–26.293 | .0002 |
| RTC alloSCT | 2.613 | .751–9.083 | .1309 |
| T cell antibody | |||
| None | Reference (1.0) | ||
| R-ATG | .785 | .417–1.478 | .4531 |
| Alemtuzumab | 1.223 | .543–2.754 | .6270 |
BAL indicates bronchoalveolar lavage; GVHD, graft-versus-host disease; MAC, myeloablative conditioning; autoSCT, autologous stem cell transplantation; alloSCT, allogeneic stem cell transplantation; RTC, reduced-toxicity conditioning; R-ATG, rabbit antithymocyte globulin.
Survival Analysis
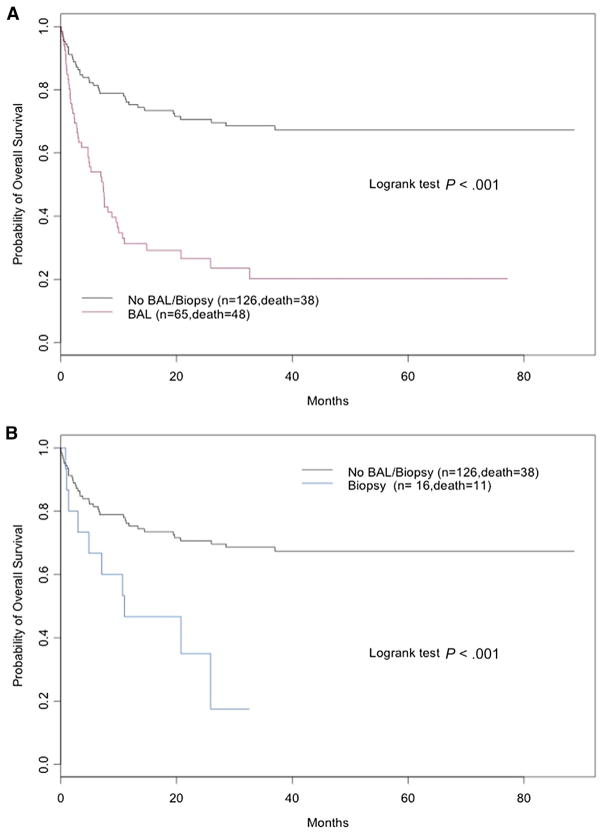
The probability of overall survival was 20.2% in pediatric HSCT recipients who required BAL, 17.5% in those who underwent a LB, and 67.4% in those who had neither procedure (P = .001) (Figure 4A,B). In the univariate analysis, variables having a statistically significant mortality risk included BAL, age, disease risk, HLA matching, and transplantation type. When controlling for other factors in multivariate analysis, only the requirement of a BAL was independently associated with an increased risk of mortality after HSCT in pediatric recipients (HR, 2.96; 95% CI, 1.8 to 4.9; P <.0001). Of patients with at least 1 positive BAL, 10% of patients survived, whereas in patients with a nondiagnostic BAL, 56% of patients survived (P < .0001).
Figure 4.
(A) Probability of overall survival in pediatric HSCT recipients stratified for patients requiring bronchoalveolar lavage (BAL) versus no BAL/LB. (B) Probability of overall survival in pediatric HSCT recipients stratified for patients requiring lung biopsy versus no lung biopsy/bronchoalveolar lavage.
DISCUSSION
To our knowledge, this study is the largest cohort of pediatric HSCT recipients analyzing invasive diagnostic procedures for pulmonary dysfunction to date. The cohort described is a heterogeneous group, including patients with differing underlying diseases and conditioning regimens, including MAC and RTC regimens, as well as both autologous and allogeneic donor sources. In pediatric patients with pulmonary complications after HSCT, BAL remains the first-line diagnostic procedure for patients with pulmonary dysfunction [7]. Our analysis demonstrates that, among our diverse patient population, the diagnostic yield of BAL was 40%. This is consistent with the diagnostic yield of BAL in pediatric HSCT recipients described by others in smaller series, which range from 29% to 68% [7,8].
As expected, the diagnostic yield we observed from LB was substantially higher (94%) than BAL at 40%, consistent with the post-HSCT LB diagnostic yield reported by others [12–14]. In particular, LB greatly increased the yield for certain disease-specific diagnoses, including GVHD, pulmonary toxicity, and rare infectious organisms. In a smaller retrospective study, OLB yielded new information that led to a change in therapy more often than BAL or CT-guided biopsy; furthermore, although CT-guided biopsy had a high diagnostic yield of 60%, the diagnostic value was diminished by the fact that they yielded only organisms that had already been isolated from peripheral culture (blood or sputum) [32].
Although the diagnostic yield was higher after LB compared with BAL, the former represents a more invasive procedure. However, although our sample size is small, we did not observe grade III or IV toxicities related to LB in our cohort. Although 34% of BAL patients and 26% of LB patients required intubation after the procedure, it is not possible to know from this analysis whether these intubations were a result of the patient’s significant underlying pulmonary disease, a result of the diagnostic procedure (whether BAL or LB), or a combination of these factors, especially given that most of these patients had already been intubated because of respiratory distress before the procedure. Thus, it is important to consider the value of the possible diagnostic yield for these procedures compared with the risks of intubation in these patients.
Patients undergoing MAC alloSCT were the most likely to require BAL, and although there are no studies directly comparing pulmonary complications in children undergoing MAC versus RTC, this result is not unexpected based on our previous work [21]. It is important to consider that the patients at lowest risk for undergoing BAL were the MAC autoSCT group. This suggests that both the conditioning regimen and graft source are potentially powerful influences associated with pulmonary dysfunction and the need for a BAL.
Patients undergoing BAL and LB were observed to have higher mortality than those who did not. This is most likely related to the morbidity and mortality involved in their underlying pulmonary disease and they may have received a MAC AlloSCT. These results are similar to other reports in the literature [7]. Additionally, it is important to consider that given the retrospective nature of these studies, it is not possible to know whether these patients would have increased or decreased survival without these diagnostic procedures.
As mentioned, 1 of the largest limitations of this study is its retrospective nature. In addition to the limitations of retrospective analysis, this includes the fact that the decision to perform BAL or LB was decided by attending HSCT physicians in consultation with the consulting pediatric pulmonologist, intensive care physician, surgeon, and radiologist based on the individual clinical features in each case. Another potential limitation of this study was the inability to determine the significance of timing of the BAL from clinical onset versus the percent of successful diagnostic yield. The standard of care in our HSCT center was to attempt to perform a diagnostic BAL within 72 hours of onset of clinical symptoms and CT findings of bilateral ground glass opacities. This occurred in over 95% of cases and, therefore, the numbers were too small to determine whether the timing of BAL was significantly associated with a successful diagnostic yield from the BAL. The cohort of patients in our study is quite diverse with respect to underlying disease, graft source, and conditioning regimen, and although this heterogeneity can seem to make for a diverse patient group, it may suggest applicability of the results to a more diverse population of pediatric HSCT recipients. Additionally, the number of patients who had autoSCT was quite small, consistent with the limited use of autoSCT in pediatric diseases.
In summary, we have demonstrated a high diagnostic yield of approximately 40% for BAL and 94% for LB for the diagnosis of pulmonary complications in pediatric HSCT recipients. Based on this study, we establish that approximately 34% and 8% of pediatric HSCT recipients will require a BAL and LB, respectively, sometime after their HSCT. In our cohort, both BAL and LB were safe and well tolerated with a significant diagnostic yield. Although BAL is traditionally considered the initial diagnostic procedure of choice in pediatric HSCT recipients with pulmonary dysfunction of unclear etiology, LB should be considered as an initial diagnostic procedure and performed early in certain circumstances, particularly when GVHD, pulmonary fibrosis, treatment toxicity, or atypical infectious organisms are being considered. Future studies should be performed to improve the long-term survival in pediatric HSCT recipients with pulmonary dysfunction who require these types of diagnostic procedures.
Acknowledgments
The authors thank the faculty, nurses, and staff and the patients and families of the Pediatric Blood and Marrow Transplantation Program at New York Presbyterian Hospital and Columbia University Medical Center.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: E.Q. and P.S. are coprimary authors.
Financial disclosure: Supported by grants from the Pediatric Cancer Research Foundation, Doris Duke Charitable Foundation, National Institute of Arthritis and Musculoskeletal Diseases (AR49330), Marisa Fund, and Paul Luisi Foundation.
References
- 1.Eikenberry M, Bartakova H, Defor T, et al. Natural history of pulmonary complications in children after bone marrow transplantation. Biol Blood Marrow Transplant. 2005;11:56–64. doi: 10.1016/j.bbmt.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Griese M, Rampf U, Hofmann D, et al. Pulmonary complications after bone marrow transplantation in children: twenty-four years of experience in a single pediatric center. Pediatr Pulmonol. 2000;30:393–401. doi: 10.1002/1099-0496(200011)30:5<393::aid-ppul5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Kaya Z, Weiner DJ, Yilmaz D, et al. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. 2009;15:817–826. doi: 10.1016/j.bbmt.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest. 1997;111:135–141. doi: 10.1378/chest.111.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Rano A, Agusti C, Benito N, et al. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2002;122:253–261. doi: 10.1378/chest.122.1.253. [DOI] [PubMed] [Google Scholar]
- 6.Efrati O, Gonik U, Bielorai B, et al. Fiberoptic bronchoscopy and bronchoalveolar lavage for the evaluation of pulmonary disease in children with primary immunodeficiency and cancer. Pediatr Blood Cancer. 2007;48:324–329. doi: 10.1002/pbc.20784. [DOI] [PubMed] [Google Scholar]
- 7.Collaco JM, Gower WA, Mogayzel PJ., Jr Pulmonary dysfunction in pediatric hematopoietic stem cell transplant patients: overview, diagnostic considerations, and infectious complications. Pediatr Blood Cancer. 2007;49:117–126. doi: 10.1002/pbc.21061. [DOI] [PubMed] [Google Scholar]
- 8.Kasow KA, King E, Rochester R, et al. Diagnostic yield of bronchoalveolar lavage is low in allogeneic hematopoietic stem cell recipients receiving immunosuppressive therapy or with acute graft-versus-host disease: the St. Jude experience, 1990–2002. Biol Blood Marrow Transplant. 2007;13:831–837. doi: 10.1016/j.bbmt.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Shannon VR, Andersson BS, Lei X, et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:647–655. doi: 10.1038/bmt.2009.203. [DOI] [PubMed] [Google Scholar]
- 10.Blyth CC, Hale K, Palasanthiran P, et al. Antifungal therapy in infants and children with proven, probable or suspected invasive fungal infections. Cochrane Database Syst Rev. 2010:CD006343. doi: 10.1002/14651858.CD006343.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerner SA, Schmitt BA, Seligsohn R, Matz GJ. Comparative study of ototoxicity and nephrotoxicity in patients randomly assigned to treatment with amikacin or gentamicin. Am J Med. 1986;80:98–104. doi: 10.1016/0002-9343(86)90486-9. [DOI] [PubMed] [Google Scholar]
- 12.Forslow U, Remberger M, Nordlander A, Mattsson J. The clinical importance of bronchoalveolar lavage in allogeneic SCT patients with pneumonia. Bone Marrow Transplant. 2010;45:945–950. doi: 10.1038/bmt.2009.268. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham I. Pulmonary infections after bone marrow transplant. Semin Respir Infect. 1992;7:132–138. [PubMed] [Google Scholar]
- 14.Gentile G, Micozzi A, Girmenia C, et al. Pneumonia in allogeneic and autologous bone marrow recipients. A retrospective study. Chest. 1993;104:371–375. doi: 10.1378/chest.104.2.371. [DOI] [PubMed] [Google Scholar]
- 15.Snyder CL, Ramsay NK, McGlave PB, et al. Diagnostic open-lung biopsy after bone marrow transplantation. J Pediatr Surg. 1990;25:871–876. doi: 10.1016/0022-3468(90)90194-e. discussion 876–877. [DOI] [PubMed] [Google Scholar]
- 16.Coren ME, Nicholson AG, Goldstraw P, et al. Open lung biopsy for diffuse interstitial lung disease in children. Eur Respir J. 1999;14:817–821. doi: 10.1034/j.1399-3003.1999.14d16.x. [DOI] [PubMed] [Google Scholar]
- 17.Davies L, Dolgin S, Kattan M. Morbidity and mortality of open lung biopsy in children. Pediatrics. 1997;99:660–664. doi: 10.1542/peds.99.5.660. [DOI] [PubMed] [Google Scholar]
- 18.Kramer MR, Berkman N, Mintz B, et al. The role of open lung biopsy in the management and outcome of patients with diffuse lung disease. Ann Thorac Surg. 1998;65:198–202. doi: 10.1016/s0003-4975(97)01081-3. [DOI] [PubMed] [Google Scholar]
- 19.Geyer MB, Jacobson JS, Freedman J, et al. A comparison of immune reconstitution and graft-versus-host disease following myeloablative conditioning versus reduced toxicity conditioning and umbilical cord blood transplantation in paediatric recipients. Br J Haematol. 2011;155:218–234. doi: 10.1111/j.1365-2141.2011.08822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gall JB, Milone MC, Waxman IM, et al. The pharmacokinetics and safety of twice daily i.v. BU during conditioning in pediatric allo-SCT recipients. Bone Marrow Transplant. 2013;48:19–25. doi: 10.1038/bmt.2012.105. [DOI] [PubMed] [Google Scholar]
- 21.Satwani P, Baldinger L, Freedman J, et al. Incidence of viral and fungal infections following busulfan-based reduced-intensity versus myeloablative conditioning in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2009;15:1587–1595. doi: 10.1016/j.bbmt.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Satwani P, Morris E, Bradley MB, et al. Reduced intensity and non-myeloablative allogeneic stem cell transplantation in children and adolescents with malignant and non-malignant diseases. Pediatr Blood Cancer. 2008;50:1–8. doi: 10.1002/pbc.21303. [DOI] [PubMed] [Google Scholar]
- 23.Styczynski J, Tallamy B, Waxman I, et al. A pilot study of reduced toxicity conditioning with BU, fludarabine and alemtuzumab before the allogeneic hematopoietic SCT in children and adolescents. Bone Marrow Transplant. 2011;46:790–799. doi: 10.1038/bmt.2010.209. [DOI] [PubMed] [Google Scholar]
- 24.Bradley MB, Satwani P, Baldinger L, et al. Reduced intensity allogeneic umbilical cord blood transplantation in children and adolescent recipients with malignant and non-malignant diseases. Bone Marrow Transplant. 2007;40:621–631. doi: 10.1038/sj.bmt.1705785. [DOI] [PubMed] [Google Scholar]
- 25.Geyer MB, Ricci AM, Jacobson JS, et al. T cell depletion utilizing CD34(+) stem cell selection and CD3(+) addback from unrelated adult donors in paediatric allogeneic stem cell transplantation recipients. Br J Haematol. 2012;157:205–219. doi: 10.1111/j.1365-2141.2012.09048.x. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia M, Militano O, Jin Z, et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16:333–343. doi: 10.1016/j.bbmt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Osunkwo I, Bessmertny O, Harrison L, et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2004;10:246–258. doi: 10.1016/j.bbmt.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Roman E, Osunkwo I, Militano O, et al. Liposomal amphotericin B prophylaxis of invasive mold infections in children post allogeneic stem cell transplantation. Pediatr Blood Cancer. 2008;50:325–330. doi: 10.1002/pbc.21239. [DOI] [PubMed] [Google Scholar]
- 30.Shereck EB, Cooney E, van de Ven C, et al. A pilot phase II study of alternate day ganciclovir and foscarnet in preventing cytomegalovirus (CMV) infections in at-risk pediatric and adolescent allogeneic stem cell transplant recipients. Pediatr Blood Cancer. 2007;49:306–312. doi: 10.1002/pbc.21043. [DOI] [PubMed] [Google Scholar]
- 31.Waxman IM, Militano O, Baldinger L, et al. Sequential administration of sargramostim and filgrastim in pediatric allogeneic stem cell transplantation recipients undergoing myeloablative conditioning. Pediatr Transplant. 2009;13:464–474. doi: 10.1111/j.1399-3046.2008.01000.x. [DOI] [PubMed] [Google Scholar]
- 32.Armenian SH, Hoffman JA, Butturini AM, et al. Invasive diagnostic procedures for pulmonary infiltrates in pediatric hematopoietic stem cell transplant recipients. Pediatr Transplant. 2007;11:736–742. doi: 10.1111/j.1399-3046.2007.00733.x. [DOI] [PubMed] [Google Scholar]