Abstract
Initially discovered as an initiator protease in apoptosis mediated by death receptors, caspase-8 is now known to have an apparently confounding opposing effect in securing cell survival. It is required to allow mouse embryo survival, and the survival of hematopoietic cells during their development and activation. Classic models in which caspase-8 is depleted or inhibited frequently result in inhibition of apoptosis, and conversion to death through a necrotic pathway. This bewildering switch is now known to be driven by activation of a pathway dependent on protein kinases of the RIP family, which engage a pathway known as necroptosis. If caspase-8 does not control this pathway, necrotic death results. The pro-apoptotic and pro-survival functions of caspase-8 are regulated by a specific interaction with the pseudo-caspase cFLIP, and it is thought that the heterocomplex between these two partners alters the substrate specificity of caspase-8 in favor of inactivating components of the RIP kinase pathway. The description of how caspase-8 and cFLIP coordinate the switch between apoptosis and survival is just beginning. The mechanism is not known, the differential targets are not known, and the reason of why an apoptotic initiator has been co-opted as a critical survival factor is only guessed at. Elucidating these unknowns will be important in understanding mechanisms and possible therapeutic targets in autoimmune, inflammatory, and metastatic diseases.
Keywords: apoptosis, necrosis, necroptosis, autophagy
1. Introduction: a little history
1.1 Traditional role of caspase 8 in extrinsic apoptosis
The great genomics rush of the mid 1990s was a watershed event in the apoptosis field, where new members of the caspases and the Bcl-2 family were discovered on what seemed like a monthly schedule. In 1992, apoptosis was gaining interest following the revelations of the existence of a genetically-programmed pathway in C. elegans [1], but nobody had much of an idea of how the mammalian pathway was regulated. By 1998 pretty much all of the currently known protein components that participate in apoptosis had been defined in humans and laboratory mice [2, 3]. Spurring these advances was the discovery that caspases were comparatively easy to express in E. coli in active forms [4, 5], allowing for relatively straightforward characterization of the properties and fundamental distinguishing characteristics of these proteases, at least in vitro [6–8]. Exemplifying this trend was caspase 8 (casp8).
It was known that death ligands such as FasL and TNF (Tumor Necrosis Factor) transmit information from outside a cell to the cytosol by engaging their cognate receptors, via cytosolic adaptor molecules [9], and it was the discovery of casp8 through EST homology analysis [10] and interactive cloning [11] that paved the way to reveal the first proteolytic signal in the initiation of the extrinsic pathway of apoptosis.
1.2 New role in protection against RIPK-dependent death (necroptosis)
Given that casp8 was thought to be the primary mediator of extrinsic apoptosis (but see below for a discussion of caspase 10), it came as a surprise that deletion of the gene in mice [14] resulted in embryonic lethality with a phenotype reminiscent of degeneration rather than proliferation, an observation brought home by the discovery of a casp8 mutation in humans that decreased immune activation of naive lymphocytes [15]. The most parsimonious explanation for these apparently counterintuitive findings was that casp8 had dual roles: one pro-death and one pro-survival.
It had long been known that engagement of the (DR) receptor TNFRI in many cell types provided a proliferative stimulus that could be converted to apoptosis by treatment with protein translation inhibitors. This was classic casp8 mediated apoptosis. But treatment with the broad-spectrum caspase inhibitor Z-VAD-FMK (benzoxycarbonyl-Val-Ala-Asp-fluoromethyl ketone) paradoxically also resulted in cell death, with kinetics sometimes faster that the apoptotic outcome [16]. TNFRI engagement triggered another death pathway, and this pathway was countered by casp8, putting flesh onto the idea of a pro-survival role. Breakthroughs in understanding the putative pro-survival role were provided by a chemical biology approach that identified RIPK1 (Receptor Interacting Protein Kinase 1) as a mediator of the second death pathway [17] – frequently called necroptosis –with final validation by intercrossing mice defective in casp8 and/or RIPK1 and RIPK3 – reviewed in [18].
2.1 Activation Mechanism
To understand the pro-apoptotic and pro-survival roles of casp8 it is important to comprehend the mechanism of activation of this protease. All caspases are obligate homodimers in their active forms. The two monomers of the active molecule are required to provide mutual interactions that stabilize the catalytic site in a productive conformation [22, 23]. This means that caspases typically have two active sites – one per monomer. Effector caspase zymogens (Fig 1) are pre-formed dimers and require proteolysis in an intra-domain linker, which effectively releases a lock on the zymogen form, to allow transition to the catalytically competent conformation. In contrast, apical caspase zymogens are monomers, and simply require dimerization to gain catalytic competency. Thus the general mechanisms of caspase zymogen activation represent a perfectly harmonized pathway – when an apical protease is activated its catalytic power is used to activate a downstream member by proteolysis. The concept is somewhat comparable with receptor tyrosine kinase signaling, where receptor clustering activates an apical kinase and its catalytic power is used to activate a downstream member by phosphorylation [24].
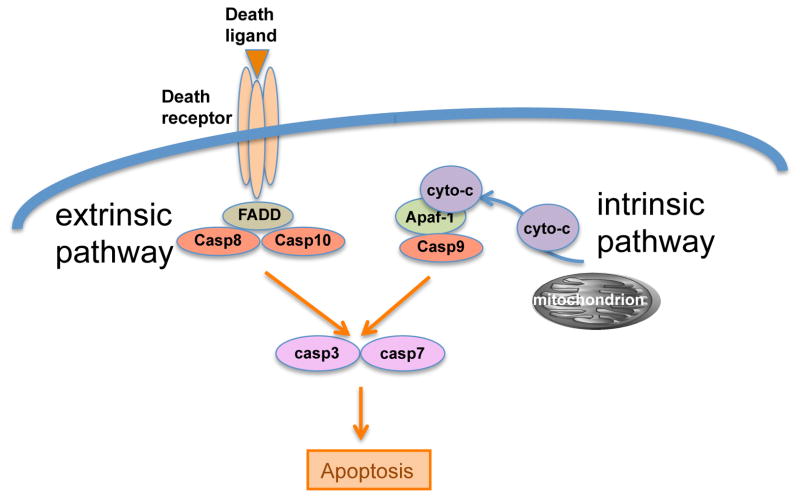
Figure 1. Extrinsic and Intrinsic Apoptosis Pathways.
Extrinsic and intrinsic signals regulating apoptosis had been proposed in descriptive terms [12], and later revised to cover mechanistic events, such that death mediated by death receptors (DRs) became known as the extrinsic apoptosis pathway, and death mediated through mitochondria became the intrinsic apoptosis pathway [13]. The pathways activate distinct apical caspases (casp8 and 10 in the extrinsic pathway and casp9 in the intrinsic pathway), which converge on the activation of executioner caspases 3 and 7, leading to cleavage of an unknown number of proteins to express the apoptotic phenotype.
The fundamental concept for apical caspase activation – generation of the first protease active site - is described by the “induced proximity” model, introduced in its initial [25] and refined [26] forms. In essence, of the induced proximity model stipulates that apical caspases exist in their quiescent state as monomers that must be dimerized to gain activity. This is achieved on oligomeric signaling platforms, either the soluble “apoptosome” that activates caspase 9, or the membrane-associated DISC that activated casp8 and 10 [27]. These platforms serve to recruit monomers via homotypic recruitment domains, clustering the monomers in space and thereby enabling them to attain the dimeric, active, conformation. The concept is straightforward: clustering converts a three-dimensional diffusion process, where the Kd for dimerization is unfavorable, to a two-dimensional process that overcomes this unfavorable Kd [26, 28, 29]. Importantly, and unfortunately, this concept is sometimes mis-represented in the literature because the oligomerization of the platform (the driving force) is confused with the dimerization of the caspase (the activation process) and it is misleading to say that caspases are activated by oligomerization – it is correct to say that they are activated by dimerization within oligomeric platforms [30]. This mechanism has predictions that have been tested and validated [31, 32], with consequences for the specificity of death versus survival activity of casp8.
2.2 Substrate Specificity and the FLIP Switch
The homologue of caspase-8 referred to as cellular Flice Inhibitory Protein (cFLIP), which contains DEDs and therefore is also recruited to the DISC, contains inactivating mutations in the catalytic and substrate binding sites of the caspase homology domain (Fig 2). The short isoform of cFLIP (FLIPS) lacks the catalytic domain and therefore can, in principle, act as a negative regulator of DISC formation. However, the long form (FLIPL) contains the entire casp8 homology domains and confers polymorphic phenotypes upon cell transfection. FLIPL was concurrently identified by several groups, and data presented in these publications were controversial regarding whether FLIPL was pro- or anti-apoptotic. Some thought FLIPL to be an inhibitor of DISC formation and casp8 activation, hence the name, and others thought it to be an independent casp8-like cell death inducer [40–44]. This controversy was settled when it was recognized that FLIPL prevented cell death upon over-expression by occupying the majority of binding sites for caspase-8 at the DISC [45], but the field has now moved ahead and it seems that FLIP has an entirely different mechanism in securing cell survival.
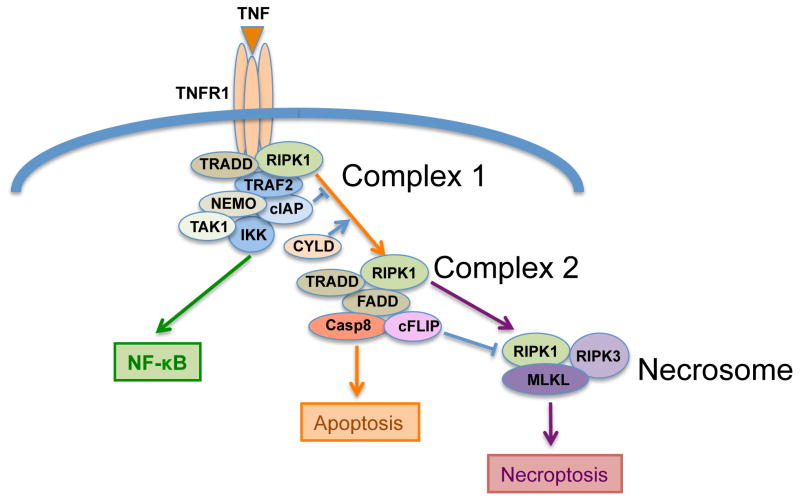
Figure 2. Pathways Emerging from TNFR1.
Upon ligation of TNFR1 three pathways emerge to program different cell outcomes. NK-κB activation is triggered through the IKK complex, and depending on the concentration of available adaptors, a separate complex that recruits components of the DISC is engaged (Complex 1). Complex 2 is considered to be the main functional apoptotic protein cluster, but it is likely that a continuous range of adaptor and effector recruitments evolves, with complex 1 and complex 2 as the extremes. The Necrosome [19], synonymous with the Ripoptosome [20, 21], is engaged. The outcome of apoptosis versus necroptosis is controlled by casp8 and cFLIP.
Heterodimerization with FLIPL activates caspases 8 and 10 [46–48] because FLIPL utilizes the same dimer interface between the catalytic domains [49]. Thus FLIPL could be considered an activator of caspases 8 and 10. Perhaps more importantly, FLIPL is actually a better activator of casp8 and 10 than are these caspases themselves, because the entropic barrier for heterodimerization is lower than for homodimerization [46, 50]. Thus one could propose, although it is currently very difficult to test, that in the presence of FLIPL the first active enzyme at the DISC may actually be the casp8 (or 10) heterodimer with FLIPL. In purified systems, the heterodimer has a restricted substrate repertoire and reduced activity on the main pro-apoptotic targets downstream of caspase-8/10 activation, such as caspase-3 and Bid, as demonstrated in two independent studies utilizing in vitro generated caspase-8/FLIPL heterodimers [30, 50].
Extensive literature on post-translation regulation of casp8, primarily involving Tyr phosphorylation – reviewed in [33], and K63 [34] and K48 [35, 36] ubiquitylation, is available and readers are referred to these publications and additional reviews for thorough explorations of caspase post-translation modifications [37, 38]. We have not included a review of these here, since there is no evidence yet that the survival/death role of caspase-8 is impacted by such modifications, although this would be a fertile area to explore. However, we note that cFLIP isoforms are subjected to active isoform-specific ubiquitylation and degradation. Thus, FLIPS is degraded more rapidly that FLIPL [39], with the implication that the DISC-inhibitory function may persist more transiently than the casp8 and 10 activation function.
2.3 Evolution of caspases 8, 10 and cFLIP
By comparing the genome databases of caspases that have been identified in a wide variety of animals it seems that the sub-group of caspases of the extrinsic pathway, including the ancestors of caspases 8, 10 and cFLIP, was newly established in the fish lineage and later underwent duplication, variation, and deletion events in the mammalian lineage during evolution [51] – see Fig 2. If a function of cFLIPL is indeed to activate casp8, we predict this would also be true for the earlier casp8 paralogs, with the understanding that the survival function of casp8 goes back at least to fish. In this context, we note that the RIPK and MLKL components of the necroptosis pathway were already in place before the origin of bony fish, and thus predated the cFLIP and caspase-8 progenitors (Kay Hoffman, personal communication).
2.4 T/B cell caspase 8 – activation pathway
Perhaps one of the earliest clues that casp8 activity was not exclusively engaged in promoting apoptosis was uncovered through examination of lymphocytes following their antigen receptor stimulation. Numerous studies implicated death receptors (DR) of the TNF receptor family in the regulation of lymphocyte homeostasis and tolerance [53, 54]. Lpr mice, bearing a splicing defect in the Fas/CD95 gene, were found to present with lymphoproliferative disease [55]. Other studies revealed that mice bearing the Gld mutation [56], a mutation in Fas/CD95 ligand, also developed lymphadenopathies and lymphoproliferative diseases that were highly similar to those observed in Lpr mice [57, 58]. These studies supported the hypothesis that DR signaling via Fas/FasL is crucial to the maintenance of lymphocyte homeostasis. It was thus surprising that mice lacking the function of FADD [59, 60], an essential signaling adaptor required for DR-induced apoptosis [61], failed to develop lymphoproliferative disease [62–64]. Subsequent investigation also revealed that loss of casp8, normally recruited to the death inducing signaling complex (DISC) via FADD following DR stimulation [10, 11, 60, 65], similarly failed to cause lymphoproliferative disease in humans and mice [15, 66]. Supporting the notion that FADD and casp8 are required for processes distinct from caspase-induced apoptosis, germline deficiencies in FADD and casp8 result in embryonic lethality at roughly E10 [14, 64, 67]. Curiously, germline deletion of the non-catalytic casp8 homologue cFLIP also results in embryonic lethality and analogous lymphocyte proliferation defects [66, 68–70].
FADD, casp8 and cFLIP all are required for T and B cell clonal expansion, and also for lymphocyte homeostasis. Loss of FADD signaling via engineered null mutation or through ectopic expression of a dominantly interfering isoform, leads to significant T cell proliferative defects [62–64]. Such mice were found to have diminished peripheral T cell populations [71], especially CD8+ cells, demonstrating an important role for this DR adaptor in CD8+ T cell homeostasis and antiviral immunity [72]. Subsequent studies have revealed a similar requirement for FADD in proliferating B cells, although in B cells the proliferative defect appears to be dependent on the stimulus [73]. While FADD-deficient B cells respond normally to B cell receptor stimulation, Toll-like receptor (TLR) stimulation results in defective clonal expansion. casp8 and cFLIP function are similarly vital to lymphocyte cell clonal expansion and homeostasis. Loss of casp8 by virtue of conditional deletion in mice results in defective T cell proliferation and homeostasis [66], primarily due to enhanced cell death [74]. casp8 activity is required in B cells for stimulus dependent mitogenesis in a manner essentially identical to that of FADD [75]. cFLIP is also required for normal T cell mitogenesis and homeostasis, although its role in these processes is more complex due to its anti-apoptotic nature [69, 70, 76, 77]. Unsurprisingly, cFLIP also required for B cell proliferation and in vivo responsiveness [78, 79]. Taken together, FADD, casp8 and cFLIP all promote non-apoptotic signaling required for lymphocyte clonal expansion, homeostasis and immune function [80].
The basis for the proliferative defects observed in lymphocytes bearing defects in DISC factors (e.g. FADD, casp8, cFLIP) was the source of much consternation; how could it be that loss of a signaling cascade required to promote apoptosis not recapitulate the phenotype observed with the absence of the ligand or receptor at the apex of the cascade? It was clear that there must be divergence of function downstream of the DR, and the most obvious place for this would be at the level of casp8 activity. Indeed, caspase activity was observed in antigenically stimulated T cells, and the pan-caspase inhibitor Z-VAD-FMK was found to block clonal T cell expansion [81, 82]. However, the requirement for casp8 catalytic activity in T cell clonal expansion was not established until recently when it was observed that reconstitution of casp8 with wildtype, but not a catalytic mutant, restored the proliferation of casp8-deficient T cells [83]. This antigen receptor induced casp8 activity appears to be distinct from that induced by the DISC assembled around a ligated DR. DR ligation results in the catalytic activation (via induced proximity) and proteolytic cleavage of the casp8 p12 light chain [25, 50, 84], an event required for DR-induced apoptosis [85]. In contrast, catalytically active casp8 is detected within twenty-four hours of T cell receptor (TCR) ligation, but this is not associated with processed casp8. Moreover, casp8 processing appears to be dispensable for T cell clonal expansion [83]. Taken together, these studies lead to the conclusion that casp8 is activated in a specific fashion in response to DR vs. TCR ligation, a concept supported by the observation of unique post-translational modification of FADD and casp8 [83, 86] depending on the stimulus. However, the basis for this stimulus-dependent processing of casp8 remains poorly understood. Additionally, it is likely that the substrates targeted by casp8 following DR vs. TCR stimulation are distinct, an issue that requires additional clarification.
2.5 Autophagy
Cellular macroautophagy (herein referred to as “autophagy”) is a cellular process of self-eating, in which cytosolic macromolecules and organelles are engulfed by de novo generated autophagosomal membranes [87]. Following engulfment and generation of double membrane autophagosomes, these vesicles fuse with lysosomes, leading to the breakdown and recycling of macromolecules and damaged organelles. Lymphocytes are acutely dependent on autophagy, as loss of autophagy in B and T cells results in numerous defects in homeostasis and activation [88]. As autophagy is a key process to respond to intracellular stress and nutrient deprivation [87, 89], it is not surprising that it has been associated with different forms of cell death [90]. It is believed that autophagy may induce a unique form of cell death termed autophagic- or type-II cell death [91], and that this cell death occurs in a manner distinct from apoptotic death [92]. Supporting the concept that cell death and autophagy pathways are tightly interwoven, anti-apoptotic Bcl2 family members bind to Beclin-1, an important factor in the induction of autophagosome formation, and diminish autophagic flux [93].
Casp8 has also been found to modulate autophagy, as loss of casp8 in L929 cells results in heightened autophagy and an increase in type-II cell death [94]. In this study, loss of casp8 expression by virtue of siRNA mediated casp8 knockdown resulted in RIP1-dependent cell death (see below), implicating this caspase as an inhibitor of autophagic flux and associated type-II cell death [92]. FADD was also found to play a role in modulating autophagic signaling via direct binding to the autophagy regulator Atg5 [95]. Further tying DISC proteins to autophagy, cFLIP has been found to bind and inhibit Atg3 [96], an intermediate in the lipidation of the autophagosomal membrane protein LC3 [97]. Of note, T cells bearing defective FADD or casp8 function become hyperautophagic following TCR stimulation, and are susceptible to RIP1-dependent cell death following TCR stimulation [98, 99]. While it is unclear whether autophagy participates in the demise of T cells lacking FADD or casp8 signaling, necroptotic signaling has been definitively implicated. Jurkat T cells lacking FADD have also been found to be highly susceptible to TNF-induced necroptosis [100], and blockade of RIP1 using necrostatin-1 (Nec-1) rescues these cells [101]. Moreover, Nec-1 eliminates the embellished autophagic flux observed in FADD-deficient Jurkats treated with TNF, suggesting that this hyperautophagy may be due to the cellular response to necroptosis.
2.6 Necroptosis
Programmed necrosis is a form of cell death that ensues in a distinct fashion from caspase-dependent apoptosis. Termed “necroptosis” [101], this process of cell death was initially described in the context of the casp8 deficient Jurkat cell line JB-6 treated with anti-Fas antibodies [102]. Vandenabeele and coworkers had observed similar non-apoptotic cell death in the context of L929 cells treated with the pan-caspase inhibitor Z-VAD-FMK, particularly after stimulation with TNF-alpha [16]. Subsequent studies by Tschöpp and colleagues revealed similar non-caspase dependent cell death in a FADD-deficient Jurkat line [100]. This group also demonstrated that the catalytic activity of the serine/threonine kinase RIP1 was required for this non-apoptotic cell death. Using knowledge of this requirement, Yuan and colleagues screened a series of chemical compounds to identify inhibitors of necroptosis [101], a screen resulting in the identification of several “necrostatins.” These include Nec-1, an allosteric inhibitor of RIP1 that potently inhibits TNF-alpha induced death of FADD-deficient Jurkats [17]. Hitomi et al. employed an siRNA screen to identify a large signaling network that distinguishes RIP1-dependent necroptotic vs. caspase-dependent apoptotic cell death [103], demonstrating that these death processes occur in very different ways.
While there are several distinct forms of non-apoptotic cell death, necroptosis as described here refers specifically to that cell death that requires RIP1 activity [104]. This form of death was also likely observed in the context of L929 cells treated with casp8 siRNA, since it was found that RIP1 was involved in this caspase-independent cell death associated with autophagy [94]. Blockade of RIP1 via Nec-1 or shRNA-mediated knock down led to the restoration of T cell proliferation in mice lacking FADD or casp8 activity, demonstrating that the proliferative defects observed in DISC-protein deficient T cells were likely due to necroptosis [98, 99, 105]. In addition to RIP1, a homologous serine/threonine kinase called RIP3 is also required for necroptosis [106–110]. Upon RIP1 activation, RIP3 is subsequently phosphorylated and binds to RIP1, leading to the formation of stable RIP1/RIP3 containing “necrosomes” [111] – see Fig 3. Substantiating the hypothesis that RIP1/RIP3-mediated necroptosis promotes the demise of DISC-protein deficient lymphocytes upon mitogenic stimulation, T cells lacking FADD or casp8 function were observed to proliferate normally when they bore a compound deficiency in RIP3 [112–116]. These observations demonstrate that, in the absence of normal DISC function, lymphocyte mitogenic signaling results in RIP1/RIP3 dependent necroptosis, although the basis for this remains to be established.
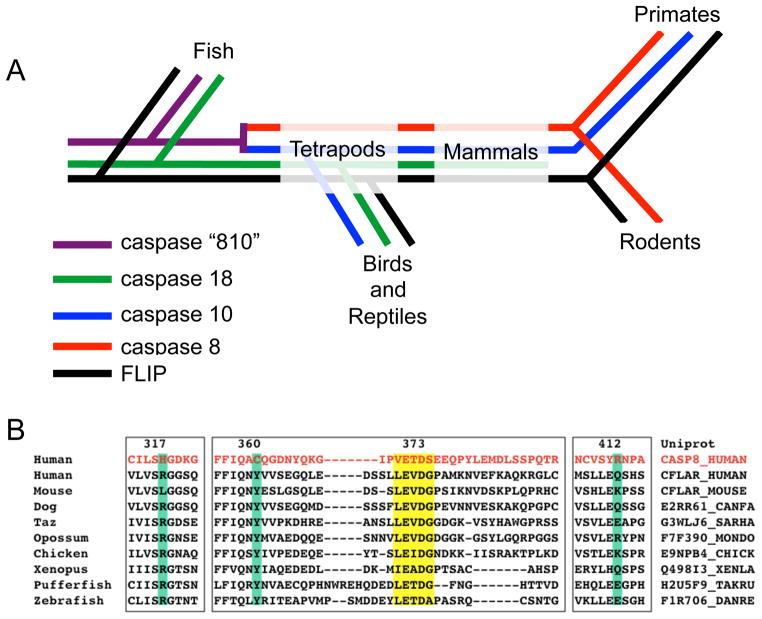
Figure 3. Caspase 8 and cFLIP phylogeny.
Distinct family members related to modern casp8 arose in the chordate branch of the eukaryotes. A) Caspase 18 and the ancestor of caspases 8 and 10 (which we call “caspase 810” in this schematic, are still found in modern fishes. Later on in evolution, caspase 8 and 10 likely branched from caspase 810. Birds and lizards express three apical caspases in the DR pathway: caspase 8, 10 and 18. Mammals subsequently lost caspase 18, while rodents lost both caspase 10 and 18, leaving them with a single apical caspase of the extrinsic pathway. cFLIP is maintained throughout the lineage. See also Eckhart et al. [52]. B) Human casp8 (red) aligned with cFLIP paralogs. In the case of cFLIP, the conservation of mutated active site residues is sustained from fish to mammals (green highlight), as is the conservation of the cleavage site between the large and small subunits (yellow highlight). Each representative species seems to have only one cFLIP paralog, with the exception of the Japanese pufferfish that has two (but not surprising given the duplication of the pufferfish genome).
The means by which casp8 prevents the induction of necroptosis remains to be firmly established. RIP1 is subject to cleavage by active casp8, and this event was originally thought to block its ability to promote anti-apoptotic signaling via NF kappa B [117, 118]. RIP3 is similarly cleaved by casp8, resulting in a loss of kinase activity and necroptotic signaling [106]. Thus, processing of RIP1 and RIP3 by casp8 may prevent the elaboration of necroptotic signaling in wildtype T and B cells following their mitogenic stimulation. Supporting this hypothesis is the observation that antigenic stimulation of T cells leads to cleavage of both RIP1 and RIP3, conditions that failed to induce PARP1 cleavage and apoptotic cell death [115]. These findings support the hypothesis that casp8 inhibits the assembly or function of RIP1/RIP3 containing necrosomes through direct cleavage of these proteins. Lending further support to this hypothesis, RIP1-deficient Jurkat cells [119] bearing a casp8-resistant isoform of RIP1 were hypersensitive to TNF-alpha induced necroptosis. Confounding the identification of key proteolytic target(s) of casp8 acting in its survival role is the finding that RIPK1 is cleaved very efficiently during apoptosis, calling into question whether this is an important substrate of casp-8 [120]. An alternative explanation for the embellished necroptotic sensitivity observed in casp8-deficient cells has been raised by Ting and colleagues, observing that casp8-mediated cleavage of the E3 ubiquitin ligase CYLD enhances RIP1/RIP3 dependent necroptosis [121].
It is still far from clear whethercasp8 has numerous targets in the different necroptotic pathways that are subject to cleavage by this protease, thus determining the cell death mechanism under distinct conditions, or whether there is a key substrate yet to be identified, maybe different substrates for necroptotic events driven by divergent stimuli.
3. Conclusions - Missing links
Stimulus dependent necrosome formation
As described above, RIP1/RIP3 containing necrosomes are formed in mitogenically stimulated T cells lacking FADD or casp8 activity [115], although the basis for this is poorly understood. Moreover, the rescue of embryonic lethality in FADD- and casp8-deficient mice by a compound deletion of RIP3 suggests that casp8 activity is required in specific cell types during embryogenesis to prevent necroptosis [112–114]. While it is possible that necrosome formation in such cells occurs via DR signaling, this may not necessarily be the case. Indeed, blockade of DR signaling failed to rescue T cells lacking casp8 [83]. In addition, casp8 processing appears to occur distinctly upon antigenic- vs. DR-stimulation, supporting the hypothesis that casp8 itself is recruited to and activated by different intracellular scaffolds. This is supported by studies demonstrating that cFLIP binding promotes casp8 activation without interdomain cleavage [50], and that FLIPL prevents necroptosis [114, 122]. The potential that differentially processed casp8 may target distinct substrates is also an important issue to clarify.
Crosstalk between necroptosis and autophagy
Autophagy and RIP1/RIP3 dependent necroptosis are often observed to occur in tandem, suggesting that they are linked mechanistically [123]. The RIP1 inhibitor Nec-1 blocks the induction of hyper-autophagy in antigenically stimulated primary T cells [98] and TNF-alpha treated, FADD-deficient Jurkat cells [101]. Thus, the induction of necroptosis may lead to autophagy, perhaps in response to depletion of cellular energy stores during necroptosis [80]. Alternatively, since Nec-1 interferes with RIP1 activity allosterically, RIP1 may induce autophagy independent of its role in promoting necroptosis. Thus, it will be important to discern how autophagic and necroptotic signaling cascades are interwoven.
Does FLIPL activate or inhibit caspase-8?
Models proposing cFLIP as an inhibitor of DISC formation have been proposed several times, on the assumption that recruitment to the DISC of this inactive caspase paralog prevents casp8 or 10 recruitment. Yet as we have described, FLIPL but not FLIPS can activate casp8 and 10. Thus the relative concentrations of FLIPL and FLIPS are likely to define whether casp8 and 10 are activated or inhibited, and clear data on this are missing because most experimental data are derived from ectopic expression. Defining the conditions and situations, likely to be heavily cell-type dependent, that the different cFLIP forms use to influence caspase activity will probably be decisive in understanding death versus survival outcomes.
Caspase-8 survival substrates
A few proteins engaged in necroptotic signaling have been proposed as targets of casp8 (or casp8/FLIP) but there is as yet no consensus. Different reports implicate different proteins. Again, the key substrates may be cell-type dependent, there may be one key substrate, or there may be several. Emergent focused proteomic and genome editing tools are likely to be required to tease out this issue.
Highlights.
Caspase-8 is required for death receptor apoptosis and, paradoxically, survival of some cell types
This switch is requires protein kinases of the RIP family, which engage a pathway known as necroptosis
The pro-apoptotic and pro-survival functions of caspase-8 are regulated by the pseudo-caspase cFLIP
The mechanism of how caspase-8 and cFLIP coordinate the switch between apoptosis and survival is unknown
The reason why an apoptotic initiator has been co-opted as a critical survival factor is unknown
Acknowledgments
This work was supported by NIH grants 1R01 GM099040 and 5P30 CA030199
Abbreviations
- DED
Death Effector Domain
- DISC
Death Inducing Signaling Complex
- TNF
Tumor necrosis factor
- TNFRI
Tumor necrosis factor receptor I
- Z-VAD-FMK
benzoxycarbonyl-Val-Ala-Asp-fluoromethyl ketone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Guy S. Salvesen, Email: gsalvesen@sanfordburnham.org, Program in Cell Death and Survival Networks, Sanford-Burnham Medical Research Institute, La Jolla, CA 92037, USA
Craig M. Walsh, Email: cwalsh@uci.edu, Department of Molecular Biology and Biochemistry, Institute for Immunology, University of California, Irvine, CA 92697, USA
References
- 1.Horvitz HR. Nobel lecture. Worms, life and death. Biosci Rep. 2003;23(5–6):239–303. doi: 10.1023/b:bire.0000019187.19019.e6. [DOI] [PubMed] [Google Scholar]
- 2.Alnemri E, et al. Human ICE/CED-3 protease nomenclature [letter] Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 3.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 4.Thornberry N, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272(29):17907–11. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 5.Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. J Biol Chem. 1997;272(41):25719–23. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 6.Thornberry NA, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272(29):17907–11. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 7.Talanian RV, et al. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272(15):9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 8.Stennicke HR, et al. Internally quenched fluorescent peptide substrates disclose the subsite preferences of human caspases 1, 3, 6, 7 and 8. Biochem J. 2000;350(Pt 2):563–568. [PMC free article] [PubMed] [Google Scholar]
- 9.Vandenabeele P, et al. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5(10):392–9. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 10.Muzio M, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85(6):817–27. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 11.Boldin M, et al. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85(6):803–15. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 12.Anilkumar TV, Sarraf CE, Alison MR. The biology and pathology of programmed cell death (apoptosis) Vet Hum Toxicol. 1992;34(3):251–4. [PubMed] [Google Scholar]
- 13.Stennicke HR, Salvesen GS. Caspases - controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta. 2000;1477(1–2):299–306. doi: 10.1016/s0167-4838(99)00281-2. [DOI] [PubMed] [Google Scholar]
- 14.Varfolomeev EE, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9(2):267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 15.Chun HJ, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419(6905):395–9. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 16.Vercammen D, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187(9):1477–85. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degterev A, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green DR, et al. RIPK-dependent necrosis and its regulation by caspases: a mystery in five acts. Mol Cell. 2011;44(1):9–16. doi: 10.1016/j.molcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallach D, Kovalenko A, Kang TB. ‘Necrosome’-induced inflammation: must cells die for it? Trends Immunol. 2011;32(11):505–9. doi: 10.1016/j.it.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Feoktistova M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43(3):449–63. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenev T, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43(3):432–48. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284(33):21777–81. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKenzie SH, Clark AC. Death by caspase dimerization. Adv Exp Med Biol. 2012;747:55–73. doi: 10.1007/978-1-4614-3229-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muzio M, et al. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273(5):2926–30. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 26.Boatright KM, et al. A unified model for apical caspase activation. Mol Cell. 2003;11(2):529–41. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 27.Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8(5):405–13. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 28.Donepudi M, et al. Insights into the regulatory mechanism for caspase-8 activation. Mol Cell. 2003;11:543–549. doi: 10.1016/s1097-2765(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 29.Renatus M, et al. Dimer formation drives the activation of the cell death protease caspase 9. Proc Natl Acad Sci USA. 2001;98:14250–14255. doi: 10.1073/pnas.231465798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes MA, et al. Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell. 2009;35(3):265–79. doi: 10.1016/j.molcel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Pop C, et al. Role of proteolysis in caspase-8 activation and stabilization. Biochemistry. 2007;46(14):4398–407. doi: 10.1021/bi602623b. [DOI] [PubMed] [Google Scholar]
- 32.Pop C, et al. The apoptosome activates caspase-9 by dimerization. Mol Cell. 2006;22(2):269–75. doi: 10.1016/j.molcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Graf RP, et al. Caspase-8 as a Regulator of Tumor Cell Motility. Curr Mol Med. 2014 doi: 10.2174/1566524014666140128111951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Z, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137(4):721–35. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalvez F, et al. TRAF2 Sets a threshold for extrinsic apoptosis by tagging caspase-8 with a ubiquitin shutoff timer. Mol Cell. 2012;48(6):888–99. doi: 10.1016/j.molcel.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 36.Zhang HG, et al. Regulation of apoptosis proteins in cancer cells by ubiquitin. Oncogene. 2004;23(11):2009–15. doi: 10.1038/sj.onc.1207373. [DOI] [PubMed] [Google Scholar]
- 37.Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138(5):838–54. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Raam BJ, Salvesen GS. Proliferative versus apoptotic functions of caspase-8 Hetero or homo: the caspase-8 dimer controls cell fate. Biochim Biophys Acta. 2012;1824(1):113–22. doi: 10.1016/j.bbapap.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toivonen HT, et al. Modeling reveals that dynamic regulation of c-FLIP levels determines cell-to-cell distribution of CD95-mediated apoptosis. J Biol Chem. 2011;286(21):18375–82. doi: 10.1074/jbc.M110.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han DKM, et al. MRIT, a novel death-effector domain-containing protein, interacts with caspases and Bcl-XL and initiates cell death. Proc Natl Acad Sci USA. 1997;94:11333–11338. doi: 10.1073/pnas.94.21.11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu S, et al. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J Biol Chem. 1997;272(28):17255–7. doi: 10.1074/jbc.272.28.17255. [DOI] [PubMed] [Google Scholar]
- 42.Rasper DM, et al. Cell death attenuation by ‘Usurpin’, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 1998;5(4):271–88. doi: 10.1038/sj.cdd.4400370. [DOI] [PubMed] [Google Scholar]
- 43.Shu HB, Halpin DR, Goeddel DV. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity. 1997;6(6):751–63. doi: 10.1016/s1074-7613(00)80450-1. [DOI] [PubMed] [Google Scholar]
- 44.Irmler M, et al. Inhibition of death receptors signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 45.Scaffidi C, et al. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274(3):1541–8. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 46.Boatright KM, et al. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382(Pt 2):651–7. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dohrman A, et al. Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-kappa B activation. J Immunol. 2005;174(9):5270–8. doi: 10.4049/jimmunol.174.9.5270. [DOI] [PubMed] [Google Scholar]
- 48.Micheau O, et al. The Long Form of FLIP Is an Activator of Caspase-8 at the Fas Death- inducing Signaling Complex. J Biol Chem. 2002;277(47):45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 49.Yu JW, Jeffrey PD, Shi Y. Mechanism of procaspase-8 activation by c-FLIPL. Proc Natl Acad Sci U S A. 2009;106(20):8169–74. doi: 10.1073/pnas.0812453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pop C, et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J. 2011;433(3):447–57. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakamaki K, Satou Y. Caspases: evolutionary aspects of their functions in vertebrates. J Fish Biol. 2009;74(4):727–53. doi: 10.1111/j.1095-8649.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckhart L, et al. Identification of novel mammalian caspases reveals an important role of gene loss in shaping the human caspase repertoire. Mol Biol Evol. 2008;25(5):831–41. doi: 10.1093/molbev/msn012. [DOI] [PubMed] [Google Scholar]
- 53.Ashkenazi A, Dixit V. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 54.Walsh CM, Luhrs KA, Arechiga AF. The “fuzzy logic” of the death-inducing signaling complex in lymphocytes. J Clin Immunol. 2003;23(5):333–53. doi: 10.1023/a:1025313415487. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe-Fukunaga R, et al. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356(6367):314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 56.Roths JB, Murphy ED, Eicher EM. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984;159(1):1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suda T, et al. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75(6):1169–78. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76(6):969–76. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 59.Chinnaiyan AM, et al. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81(4):505–12. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 60.Kischkel F, et al. Cytotoxicity-dependent APO-1 Fas/CD95 -associated proteins form a death-inducing signaling complex DISC with the receptor. EMBO J. 1995;14:5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chinnaiyan A, et al. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271(9):4961–5. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 62.Newton K, et al. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17(3):706–18. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh C, et al. A role for FADD in T cell activation and development. Immunity. 1998;8(4):439–49. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, et al. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392(6673):296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasula S, et al. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci U S A. 1996;93(25):14486–91. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salmena L, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17(7):883–95. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeh WC, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279(5358):1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 68.Yeh WC, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12(6):633–42. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 69.Zhang N, He YW. An essential role for c-FLIP in the efficient development of mature T lymphocytes. J Exp Med. 2005;202(3):395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chau H, et al. Cellular FLICE-inhibitory protein is required for T cell survival and cycling. J Exp Med. 2005;202(3):405–13. doi: 10.1084/jem.20050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, et al. Conditional Fas-associated death domain protein (FADD): GFP knockout mice reveal FADD is dispensable in thymic development but essential in peripheral T cell homeostasis. J Immunol. 2005;175(5):3033–44. doi: 10.4049/jimmunol.175.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beisner DR, et al. The requirements for Fas-associated death domain signaling in mature T cell activation and survival. J Immunol. 2003;171(1):247–56. doi: 10.4049/jimmunol.171.1.247. [DOI] [PubMed] [Google Scholar]
- 73.Imtiyaz HZ, et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J Immunol. 2006;176(11):6852–61. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arechiga AF, et al. Cutting Edge: FADD Is Not Required for Antigen Receptor-Mediated NF-{kappa}B Activation. J Immunol. 2005;175(12):7800–4. doi: 10.4049/jimmunol.175.12.7800. [DOI] [PubMed] [Google Scholar]
- 75.Beisner DR, et al. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175(6):3469–73. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- 76.Zhang N, Hopkins K, He YW. c-FLIP protects mature T lymphocytes from TCR-mediated killing. J Immunol. 2008;181(8):5368–73. doi: 10.4049/jimmunol.181.8.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang N, Hopkins K, He YW. The long isoform of cellular FLIP is essential for T lymphocyte proliferation through an NF-kappaB-independent pathway. J Immunol. 2008;180(8):5506–11. doi: 10.4049/jimmunol.180.8.5506. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H, et al. A role for cFLIP in B cell proliferation and stress MAPK regulation. J Immunol. 2009;182(1):207–15. doi: 10.4049/jimmunol.182.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coffey F, Manser T. Expression of cellular FLIP by B cells is required for their participation in an immune response. J Immunol. 2010;184(9):4871–9. doi: 10.4049/jimmunol.0903506. [DOI] [PubMed] [Google Scholar]
- 80.Lu JV, Walsh CM. Programmed necrosis and autophagy in immune function. Immunol Rev. 2012;249(1):205–17. doi: 10.1111/j.1600-065X.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kennedy NJ, et al. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190(12):1891–6. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alam A, et al. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med. 1999;190(12):1879–90. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leverrier S, Salvesen GS, Walsh CM. Enzymatically active single chain caspase-8 maintains T-cell survival during clonal expansion. Cell Death Differ. 2011;18(1):90–8. doi: 10.1038/cdd.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salvesen GS, V, Dixit M. Caspase activation: The induced-proximity model. Proc Natl Acad Sci U S A. 1999;96(20):10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang TB, et al. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181(4):2522–32. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- 86.O’Reilly LA, et al. Modifications and intracellular trafficking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell Death Differ. 2004;11(7):724–36. doi: 10.1038/sj.cdd.4401408. [DOI] [PubMed] [Google Scholar]
- 87.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pua HH, He YW. Autophagy and lymphocyte homeostasis. Curr Top Microbiol Immunol. 2009;335:85–105. doi: 10.1007/978-3-642-00302-8_4. [DOI] [PubMed] [Google Scholar]
- 89.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6(6):439–48. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 90.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16(6):663–9. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 91.Galluzzi L, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–20. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004;3(9):1124–6. [PubMed] [Google Scholar]
- 93.Pattingre S, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Yu L, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304(5676):1500–2. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 95.Pyo JO, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280(21):20722–9. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 96.Lee JS, et al. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol. 2009;11(11):1355–62. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walsh CM, Edinger AL. The complex interplay between autophagy, apoptosis, and necrotic signals promotes T-cell homeostasis. Immunol Rev. 2010;236:95–109. doi: 10.1111/j.1600-065X.2010.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bell BD, et al. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105(43):16677–82. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ch’en IL, et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc Natl Acad Sci U S A. 2008;105(45):17463–8. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–95. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 101.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 102.Kawahara A, et al. Caspase-independent cell killing by Fas-associated protein with death domain. J Cell Biol. 1998;143(5):1353–60. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hitomi J, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311–23. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9(5):378–90. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 105.Osborn SL, et al. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci U S A. 2010;107(29):13034–9. doi: 10.1073/pnas.1005997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19(10):2056–67. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 107.Zhang DW, et al. RIP3, an Energy Metabolism Regulator that Switches TNF-Induced Cell Death from Apoptosis to Necrosis. Science. 2009 doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 108.He SW, Lai, Miao Lin, Wang Tao, Du Fenghe, Zhao Liping, Wang Xiaodong. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 109.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138(2):229–32. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 111.Vandenabeele P, et al. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3(115):re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 112.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang H, et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471(7338):373–6. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–7. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu JV, et al. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci U S A. 2011;108(37):15312–7. doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ch’en IL, et al. Mechanisms of necroptosis in T cells. J Exp Med. 2011;208(4):633–41. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin Y, et al. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13(19):2514–26. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kelliher M, et al. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 119.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. Embo J. 1996;15(22):6189–96. [PMC free article] [PubMed] [Google Scholar]
- 120.van Raam BJ, et al. Intrinsic cleavage of receptor-interacting protein kinase-1 by caspase-6. Cell Death Differ. 2013;20(1):86–96. doi: 10.1038/cdd.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O’Donnell MA, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13(12):1437–42. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dillon CP, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1(5):401–7. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bell BD, Walsh CM. Coordinate regulation of autophagy and apoptosis in T cells by death effectors: FADD or foundation. Autophagy. 2009;5(2):238–40. doi: 10.4161/auto.5.2.7512. [DOI] [PubMed] [Google Scholar]