Abstract
With age, many aspects of the brain structure undergo a pronounced decline, yet individuals generally function well until advanced old age. There appear to be several compensatory mechanisms in brain aging, but their precise nature is not well characterized. Here we provide evidence that the brain of older adults expends more energy when compared to younger adults, as manifested by an age-related increase (P=0.03) in cerebral metabolic rate of oxygen (CMRO2) (N=118, men=56, ages 18 to 74). We further showed that, before the mean menopausal age of 51 years old, female and male groups have similar rates of CMRO2 increase (P=0.015) and there was no interaction between age and sex effects (P=0.85). However, when using data from the entire age range, women have a slower rate of CMRO2 change when compared to men (P<0.001 for age × sex interaction term). Thus, menopause and estrogen level may have played a role in this sex difference. Our data also revealed a possible circadian rhythm of CMRO2 in that brain metabolic rate is greater at noon than in the morning (P=0.02). This study reveals a potential neurobiological mechanism for age-related compensation in brain function and also suggests a sex-difference in its temporal pattern.
Keywords: blood oxygenation, cerebral blood flow, cerebral metabolism, MRI, TRUST
Introduction
The human brain consumes about 20% of the total energy, although it only accounts for 2% of the total body weight (Attwell and Laughlin, 2001). In addition, most of the oxygen that brain consumes is used for neural activity (Buxton, 2002). Thus, the rate of oxygen consumption by the brain, referred to as cerebral metabolic rate of oxygen (CMRO2), is an important index for neural activity. Regulation of brain metabolism is critical for the maintenance of normal cognitive function. In the context of brain aging, earlier studies showed that resting CMRO2 were lower in older subjects (Aanerud et al., 2012; Eustache et al., 1995; Ibaraki et al., 2010; Yamaguchi et al., 1986), whereas activation data usually show that task-evoked fMRI signal (presumably reflecting task-evoked CMRO2 changes) increases with age (Cabeza et al., 2004; Cappell et al., 2010; Daselaar et al., 2003; Park et al., 2003). Therefore, the exact relationship between CMRO2 and age requires further examination.
Most of the prior studies on resting CMRO2 were conducted using Positron Emission Tomography (PET) (Aanerud et al., 2012; Eustache et al., 1995; Ibaraki et al., 2010; Yamaguchi et al., 1986), which until recently was the only method to measure CMRO2 in humans. Only a few of these had accompanying high-resolution (e.g. 1 mm3) MRI image to allow careful delineation of regions of interest (Aanerud et al., 2012), and none had corrected partial volume effect at high-resolution. A potential limitation of low-resolution images in the study of aging is that, as brain atrophy occurs, cerebral spinal fluid (CSF) volume fraction in the voxel increases and tissue fraction decreases, which could result in a CMRO2 reduction in the absence of any real tissue metabolic change. Recently, using a novel, MRI-based CMRO2 technique (Xu et al., 2009), we presented preliminary evidence that CMRO2 may, in fact, increase in older individuals (Lu et al., 2011). A limitation of that study is that it did not account for a possible age-related decline in hemoglobin concentration (Aanerud et al., 2012), which is important for accurate estimation of CMRO2. Indeed, when re-analyzing the data by including hematocrit changes, the age effect on CMRO2 now becomes a trend only. Additionally, CMRO2 measured in that study was based on blood flow determined at the level of cervical spine (Lu et al., 2011; Xu et al., 2009), which is not as accurate as that determined at a more proximal (relative to the brain) location of foramen magnum (Liu et al., 2013).
Another unexplored aspect in the prior study is that sex differences in the age-pattern. Sex differences in brain metabolism have yet to be characterized. Previous work by Baxter et al.(1987) showed that young women (age 28-39 years) have a higher cerebral metabolic rate of glucose (CMRglu) compared to young men (Baxter et al., 1987), which is consistent with animal research findings that estrogen injection enhances brain glucose metabolism (Namba and Sokoloff, 1984). On the other hand, estrogen levels in females are known to change with age, thus this enhancing effect, if present, may dissipate with age. Therefore, it is reasonable to expect that the age-pattern of oxygen metabolic rate may also be sex dependent.
In the present study, we determined global CMRO2 in a healthy cohort of 118 subjects across the adult life span. Our CMRO2 measure accounted for brain atrophy effect using a high- resolution (1×1×1 mm3) anatomic image. The dependence of CMRO2 on age and sex as well as the sex dependence of the age effect, i.e. the interaction between the variables, were examined. These findings were interpreted in the context of age-dependence of two constituent parameters, cerebral blood flow (CBF) and venous oxygenation (Yv). Finally, potential dependence of CMRO2 on circadian phase and ethnicity was examined.
Materials and Methods
Participants
The study population consisted of 118 healthy subjects (62 female and 56 male). The age range in our inclusion criteria was 18-74 years. The Health Insurance Portability and Accountability Act (HIPAA) compliant protocol was approved by the UT Southwestern Institutional Review Board and written informed consent was obtained from all participants. The participants were carefully screened and did not report neurological or psychiatric disorders according to self-completed questionnaires. The participants did not have MR contraindications such as metal implants, pacemaker, neurostimulator, body piercings, or claustrophobia. Since our hypothesis involved the effect of estrogen on brain metabolism, the history of postmenopausal hormone replacement therapy was also used as an exclusion criterion to avoid confounding factors. Demographic information of the participants is listed in Table 1. There was no significant difference in age distribution between women and men (mean age ± SD, 38 ± 18 y for women; 36 ± 16 y for men; Chi-square test, P=0.12). The ethnic makeup of the participants included Caucasian (53%), Asian (32%), and African American (15%).
Table 1.
Subject demographic information
| Female subjects | Male subjects | All subjects | |
|---|---|---|---|
| Number of Caucasian | 36 | 27 | 63 |
| Number of Asian | 19 | 18 | 37 |
| Number of African American | 7 | 11 | 18 |
|
| |||
| Subtotal | 62 | 56 | 118 |
Experimental procedures
All experiments were conducted on a 3T MR system (Philips Medical System, Best, The Netherlands). The body coil was used for radiofrequency transmission and an eight-channel sensitivity encoding (SENSE) head coil was used for receiving. Foam padding was used to stabilize the head to minimize motion. A localizer scan was performed for slice positioning and a coil sensitivity scan was conducted for SENSE reconstruction. The CMRO2 data acquisition took approximately 5 minutes and is detailed below. Additionally, a 3D T1-weighted Magnetization- prepared-rapid-acquisition-of-gradient-echo (MPRAGE) scan was performed for anatomical reference and the estimation of brain volume. The MPRAGE sequence used the following imaging parameters: repetition time (TR)/ echo time (TE)/ flip angle=8.1 ms/3.7 ms/12°, shot interval 2100ms, inversion time (TI)=1100 ms, voxel size = 1 × 1 × 1 mm3, number of slices 160, sagittal slice orientation, and scan duration = 3 min 57s. These procedures did not use any exogenous tracers.
Measurement of CMRO2
The method used to quantify global CMRO2 followed techniques originally developed by Xu et al. (Xu et al., 2009) and was recently improved by Liu et al. (Liu et al., 2013). It is based on the Fick principle of the arteriovenous differences in oxygen content (Kety and Schmidt, 1948):
| [1] |
where tCMRO2 and tCBF are total CMRO2 and cerebral blood flow, respectively; Ya and Yv are oxygen saturation percentage in arterial and venous blood, respectively; and Ch is a constant representing the capacity of blood to carry O2 and is well established in physiology literature (Guyton and Hall, 2005). Here we used Ch values of 8.15 μmol O2/ml blood for young female and 8.56 μmol O2/ml blood for young male, based on assumed hematocrit of 0.40 and 0.42, respectively (Guyton and Hall, 2005). A recent study suggested that hematocrit may decrease with age (Aanerud et al., 2012). Thus, Ch of each individual was adjusted for this decline rate of 0.0079 μmol/ml per year in our calculation (Aanerud et al., 2012). Ya is close to unity and our earlier study has shown that both age and sex have a small but significant effect on this parameter: Ya= 99.77-0.036×age-1.235×sex+0.021×age×sex (Lu et al., 2011), where age is written in years and sex uses 0 and 1 for female and male, respectively. We therefore used this equation to estimate Ya of each individual according to their age and sex. The two parameters that are most variable are tCBF and Yv, which are experimentally determined as described below, from which tCMRO2 in units of μmol O2/min was calculated.
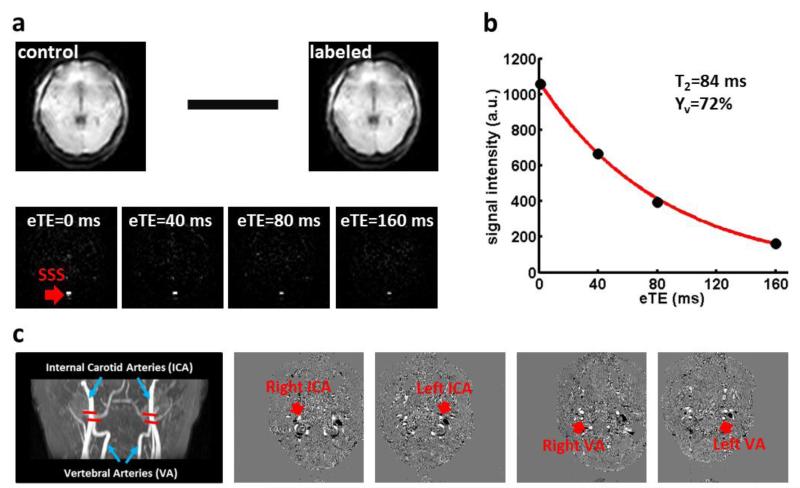
Global venous oxygenation, Yv, was noninvasively assessed from the superior sagittal sinus (SSS) using a validated approach T2-relaxation-under-spin-tagging (TRUST) MRI (Lu and Ge, 2008; Lu et al., 2012; Xu et al., 2012). The imaging parameters were: voxel size 3.44 × 3.44 × 5 mm3, TR=3000 ms, TI=1022 ms, four effective TEs: 0, 40, 80, 160 ms, labeling thickness 100 mm, gap 22.5 mm, and scan duration 1.2 min. For processing TRUST MRI data, pairwise subtraction between control and tag images was performed, the difference of which yields pure venous blood signal (Figure 1a). The venous blood signals were fitted to a monoexponential function to obtain T2 (Figure 1b), which was in turn converted to Yv via a calibration plot (Lu et al., 2012).
Figure 1.
Representative results of MRI-based measurement of CMRO2. (a) Images of TRUST MRI for the quantification of venous oxygenation (Yv) in the superior sagittal sinus (SSS). Top panel: raw images of control and labeled scans. Bottom panel: difference images, i.e. control- labeled, as a function of T2 weighting. eTE = effective echo time. Red arrow indicates the location of the SSS. (b) Blood signals in the SSS in TRUST MRI are fitted to a monoexponential function of eTE to yield blood T2, which can in turn be converted to Yv via a calibration plot. (c) Slice positions and typical results of phase-contrast (PC) MRI for the quantification of global CBF. Left panel: Slice positions of PC MRI scans overlaid on an angiogram image. Each red bar indicates the imaging slice of one PC MRI scan. Four PC MRI scans were performed in each subject to cover the four feeding arteries, respectively. The corresponding PC MRI images are displayed on the right panels. The target artery (red arrows) always appears in the center of the image and is easily identified.
Phase-contrast (PC) flow velocity MRI was used to measure the total CBF to the entire brain. Before the flow measurements, time-of-flight angiogram was performed to obtain the anatomical information of the feeding arteries of the brain. Imaging parameters of the angiogram were: TR/TE/flip angle=23ms/3.45 ms/18°, field of view (FOV) = 160 × 160 × 70.5 mm3, voxel size = 0.3 × 0.3 × 1.5 mm3, number of slices = 47, one 60-mm saturation slab positioned above the imaging slab, and scan duration = 1.4 min. Based on the maximum intensity projection reconstruction of the angiogram, four PC MRI scans were then placed on the four feeding arteries of the brain: right internal carotid artery (right ICA), left internal carotid artery (left ICA), right vertebral artery (right VA), and left vertebral artery (left VA) (Figure 1c) (Liu et al., 2013). A region-of-interest (ROI) was then drawn on each of the 4 arteries based on the magnitude image (Aslan et al., 2010). The ROI mask was applied to the velocity map and the integration of the velocity within the ROI (i.e., velocity × area) yielded CBF in units of milliliters per minute. Scan parameters were as follows: one slice, FOV = 200 × 200 × 5 mm3, voxel size = 0.5 × 0.5 × 5 mm3, 4 averages, maximum velocity encoding = 80 cm/s, and scan duration = 0.5 min for one PC scan.
To obtain unit volume CMRO2 value and account for brain atrophy in aging, we first determined gray and white matter volumes using tissue segmentation (FSL software, FMRIB Software Library, Oxford University, UK) of the high-resolution T1 image. Then the total CMRO2 of the brain was normalized to these volume values. To account for the factors that gray and white matter volume loss with age may have different rates and that these two tissue types have different metabolic rate, the gray and white matter volume terms were used separately in the normalization equation, which yields unit-mass gray matter CMRO2 in μmol O2/100g gray matter (gm)/min:
| [2] |
where tCMRO2 is the total CMRO2 of the brain; Vgm and Vwm are the volumes of gray and white matter, respectively; ρ is the mass density of tissue and is assumed to be 1.06 g/ml (Herscovitch and Raichle, 1985). The coefficient, r, reflects the CMRO2 ratio between the white and gray matter. In our calculation, we tested a range of values, from 1 to 0.3 in steps of 0.1, to examine whether the relationship between and age is dependent on the r value used.
Statistical analysis
To examine the dependence of on age and sex, a linear regression analysis was performed, in which was assigned as the dependent variable while age and sex were used as the independent variables. To evaluate the sex differences in the age pattern, we conducted further analysis by including an age × sex interaction term in the regression model. If a significant interaction effect was observed, we then divided the data into female and male subgroups and conducted separate linear regression analysis as a function of age in each sex. Similar analyses were conducted for and oxygenation extraction fraction (OEF) (i.e. [Ya-Yv]/Ya).
We also tested the possibility of dependence on circadian phase by classifying the data into three categories according to the time of data collection: 7:00~10:00 (morning), 10:00~14:00 (noon), and 14:00~18:00 (afternoon). The data were then analyzed by one-way ANOVA for a categorical effect. Given the dependence of on age and sex, we first corrected for these two factors by calculating the residual value before performing the ANOVA. Specifically, we computed a corrected :
| [3] |
where the coefficients a, b and c were based on the results of the age and sex regression model. Thus, we essentially examined the sources of residual variance of across subjects. If a categorical effect was observed from the ANOVA analysis, pairwise comparisons were further conducted with a post-hoc Scheffe test. We also examined whether is different across Caucasian, Asian, and African American subjects using the values. The correlation between and OEF across individuals was tested as well. Since these two parameters are also dependent on age and sex, we corrected for their effects before conducting the correlation analysis.
A P value of 0.05 or less was considered statistically significant. For analyses conducted separately for each sex, a multiple comparison correction was performed by multiplying the original P value by 2.
Results
Dependence of CMRO2 on Age and Sex
Analysis of total CMRO2 (tCMRO2) values (not normalized by brain volume) showed that there was a significant age × sex interaction term (P=0.01). When separating the participants by sex, female showed a significant age-decrease in tCMRO2 (P=0.01) whereas male showed no age effect (P=0.32). It is important to note, however, that tCMRO2 reflects the total amount of oxygen that the brain consumes and does not account for brain atrophy associated with aging. The brain-volume corrected results are described below.
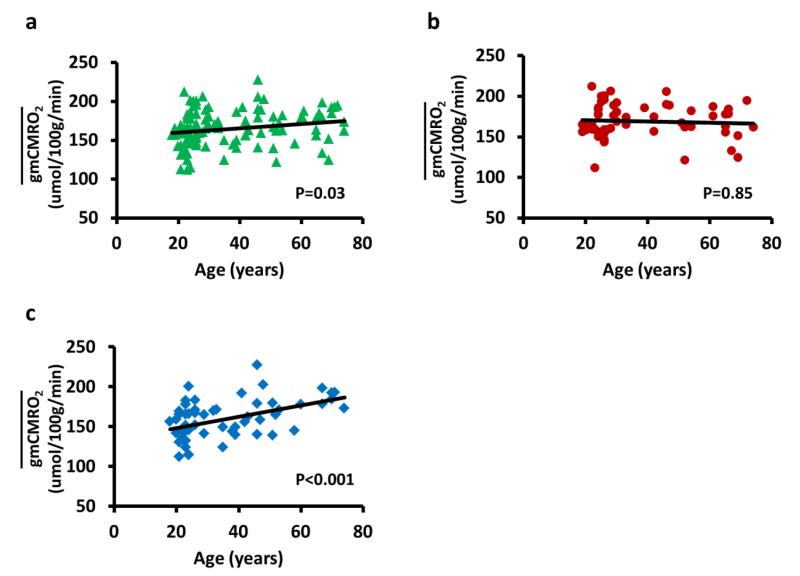
Figure 2a shows the scatter plot between (assuming r=1 in Eq. [2]) and age for all subjects. Regression analysis using age and sex as independent variables revealed that, as whole group, increased with age (P=0.03). Females were found to have a greater than male (P=0.03). When including the age × sex interaction term in the regression model and reanalyzing the data, it was found that all three variables, age (P<0.001), sex (P<0.001), and their interaction (P=0.001), had a significant effect on . Therefore, the data were divided into female (Figure 2b) and male (Figure 2c) subgroups and regression analyses were performed separately for each subgroup. It was found that the age-increase pattern in the whole group was primarily attributed to an age-related increase in in males (R2=0.22, P<0.001), while age had no effect on in females (R2=0.004, P=0.85). For male subjects, the rate of increase was 0.71 μmol/100g/min per year.
Figure 2.
Scatter plots between and age. (a) Data from all subjects. Each symbol represents data from one subject. Black curve indicates the trend line. showed a significant increase with age (P=0.03). (b) Data from female subjects only. There was not a significant age effect on . (c) Data from male subjects only. Regression analysis showed that increases significantly with age (P<0.001).
We have also examined the results when assuming a different r (white matter to gray matter ratio) value in Eq. [2]. It was found that all of the significant effects reported above remained within the r range tested (P<0.005 for all effects). The only difference is that the rate of increase is greater when a lower r value is used, ranging from 0.71 μmol/100g/min per year using r=1 to 1.41 μmol/100g/min per year using r=0.3.
To test our hypothesis that the sex difference of the age effect is mainly attributed to the onset of menopause in females, we conducted additional regression analyses in which only subjects (including 45 females and 46 males) equal or less than 51 years old (the average menopause age according to reports from National Institute on Aging, http://www.nia.nih.gov/health/publication/menopause) were used. It was found that the age- dependence of in this age range was comparable for male and female groups, i.e., there was a significant age effect (R2=0.19, P=0.015) but not the interaction effect (P=0.85). Once again, the value of r used did not affect the significance levels of these findings.
Dependence of CBF and OEF on Age and Sex
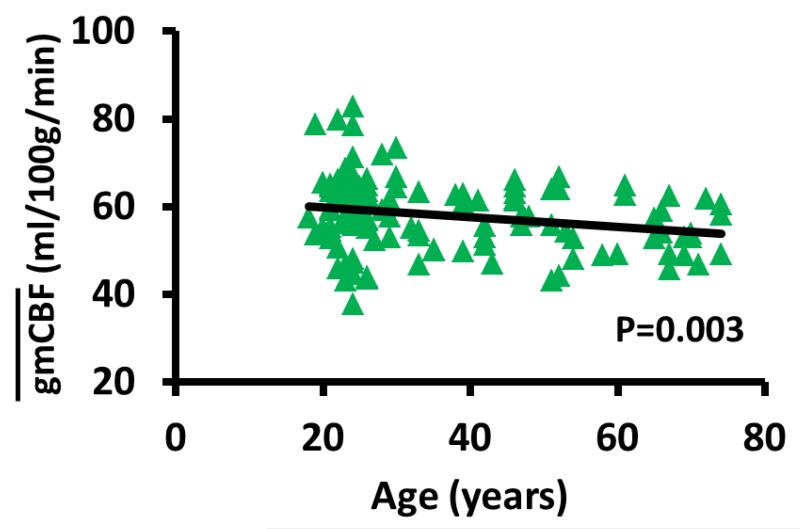
The scatter plot between and age for all subjects is displayed in Figure 3. Regression analysis revealed that reduces with age at a rate of 0.12 ml/100g/min per year (R2=0.21, P=0.003). of female subjects was significantly greater than that of male by 6.6 ml/100g/min (P<0.001). No age × sex interaction effect was observed (P=0.13). Thus, the female and male age data are not plotted separately.
Figure 3.
Scatter plots between and age for all subjects. Regression analysis showed that decreases significantly with age (P=0.003). There was not a significant age × sex interaction effect. Thus, no separate, sex-specific analyses were conducted.
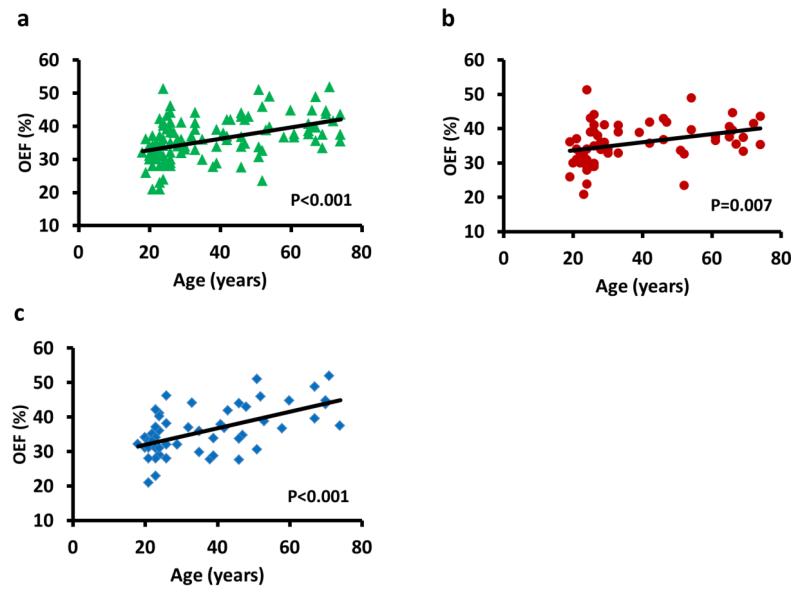
Figure 4a exhibits the scatter plot between OEF and age for all subjects. Regression analysis showed that there was a significant age × sex interaction effect (P=0.05), indicating a significant difference in the rates of increase between sex. Thus, female and male data were analyzed separately (Figures 4b and c). Both sex showed an age-related increase in OEF. In females, the rate of OEF increase was 0.12% per year (P=0.007) while in males the rate was 0.24% per year (P<0.001). Thus, it appears that the increased oxygen consumption with age is primarily fueled by extracting a larger fraction of oxygen per unit volume blood.
Figure 4.
Scatter plots between oxygen extraction fraction (OEF) and age. (a) Data from all subjects. Each symbol represents data from one subject. Black curve indicates the trend line. OEF showed a significant increase with age (P<0.001). (b) Data from female subjects only. (c) Data from male subjects only. OEF increases with age in both female (P=0.007) and male (P<0.001) participants. But the rate of decline was significantly steeper (P=0.05) in males.
Dependence of on Other Factors
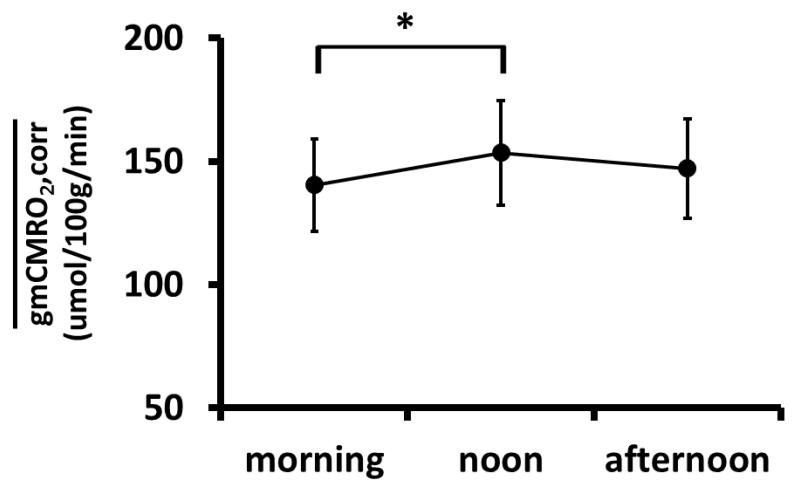
We examined whether at rest is dependent on other factors, such as circadian phase and ethnicity, in addition to age and sex. We first corrected for the age and sex effects by calculating the residual value using the regression equation. ANOVA analysis revealed that there was a significant difference (P=0.023) among measured during the three time blocks (Figure 5), 7:00~10:00 (morning, N=29), 10:00~14:00 (noon, N=52), and 14:00~18:00 (afternoon, N=37). Post-hoc Scheffe test showed that there was a significant difference in comparing morning to noon (P=0.022). ANOVA analysis on ethnicity did not find a significant difference among Caucasian (N=63), Asian (N=37), and African American (N=18) subjects.
Figure 5.
Comparison of by groups based on acquisition time of the day. ANOVA analysis using all three groups revealed a significant group difference (P=0.023). Post-hoc test showed that at noon was significantly higher than that in the morning (P=0.022). Note that the values used in this analysis have factored out the age and sex effects, by using the age/sex regression equation. Thus, this analysis examines the residual variance of across subjects.
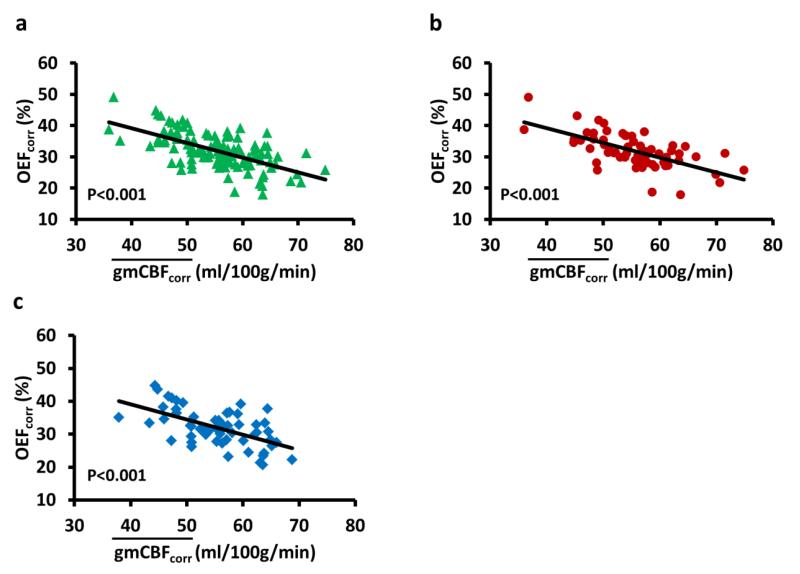
We also examined whether the two constituents of the CMRO2 estimation, CBF and OEF, were related. As shown in Figure 6a, there was a strong negative correlation (R2=0.38, P<0.001) between and OEF. That is, an individual who had a lower tended to have a greater OEF, and vice versa. Further analyses conducted separately for female and male subjects demonstrated similar results (Figures 6b and c), suggesting that this relationship was independent of sex.
Figure 6.
The relationship between and OEF for (a) all subjects, (b) female subjects, and (c) male subjects. A strong correlation is observed in all three plots, suggesting that an individual with a higher tends to have a lower OEF (i.e. higher Yv), thus simultaneous measurement of and OEF may allow a reduction in data variation. Again, the values used in these analyses have factored out the age and sex effects, by using the age/sex regression equation. Thus, they examine the residual variance across subjects.
Discussion
This present study provides strong evidence that age-related differences in cerebral hemodynamics are characterized by an increase in energy consumption and oxygen metabolic rate, particularly in males. Previous cognitive aging literature has suggested this concept in the setting of task-evoked neural activity using fMRI techniques (Cabeza et al., 2004; Hutchison et al., 2013; Park and Reuter-Lorenz, 2009). Our study demonstrates that a similar age change is present for resting-state brain metabolism. A consistent age effect on resting and evoked activity is congruent because the brain is highly active even at rest (Raichle et al., 2001) and there is no clear definition to distinguish rest from activated state.
Our finding is opposite to several previous studies on age-related changes in brain metabolism using PET imaging methods (Aanerud et al., 2012; Eustache et al., 1995; Ibaraki et al., 2010; Yamaguchi et al., 1986). One possible reason may be associated with the degree to which partial volume effect is corrected in these studies, which is important in aging studies. In the present study, although the data has no spatial specificity, the partial volume effect between parenchyma and CSF is corrected to a resolution of 1 mm. Most of the previous studies do not have high-resolution MRI data available (Eustache et al., 1995; Ibaraki et al., 2010; Yamaguchi et al., 1986), thus the partial voluming correction could only be conducted at the intrinsic resolution of nuclear imaging methods, which are on the order of 4 mm. A more recent PET study has provided some interesting data in that both PET and high-resolution MRI (~1 mm3 voxel size) data were collected in the same subjects (Aanerud et al., 2012). In that report, the authors primarily focused on regional changes in CMRO2, thus high-resolution MRI images were mainly used for segmentation but not for partial volume correction (because voxel-by-voxel correction of partial volume requires perfect co-registration between PET and MRI images, which is not trivial). From a global CMRO2 point-of-view, on the other hand, the data in Aanerud et al. can be potentially used to estimate global CMRO2 with partial volume correction to a resolution of 1 mm, similar to what was estimated in the present study. This could be carried out by adding up all CMRO2 values in the brain (e.g. yielding a total CMRO2), followed by dividing by the brain volume measured from the high-resolution MRI image. Another possibility to explain the discrepancy is that some of the previous studies provided gray-matter-specific change in CMRO2 whereas our measure of brain oxygen consumption cannot distinguish gray and white matter contributions to the age-change. If gray and white matter shows differential rate of volume change with age and this results in an alteration in relative gray/white volume fraction between younger and older brain, CMRO2 could manifest a change without an actual alteration in either gray or white matter metabolic rate. This possibility was examined by separately including gray and white matter volume in the normalization equation (Eq. [2]) and by the inclusion of a gray/white metabolic ratio term (see below for more discussion).
An age-related increase in CMRO2 has been suggested on our previous study (Lu et al., 2011). However, the findings in that study were not conclusive for several reasons. For one, a potential age-related decrease in hemoglobin centration was not accounted for. Therefore, the prior finding could be attributed to an age-decrease in hematocrit, thereby a reduced capacity of the blood to carry oxygen. Age dependence of hematocrit has been suggested by a recent study, which showed that, with age, blood hemoglobin concentration could decrease at a rate of 0.0079mmol/L per year (Aanerud et al., 2012). By including the hematocrit effect in our calculation, we re-analyzed the data in (Lu et al., 2011) and found that the CMRO2 dependence on age is no longer significant, although still showing a trend (P=0.06). One of the reasons for insufficient power to detect the effect could be related to the accuracy of the CMRO2 measurement technique. Most notably, in the prior study (Lu et al., 2011), CBF was measured at the level of cervical spine 2 (Xu et al., 2009), at which location the four arteries are expected to be parallel in orientation, thus their flow rate can be measured with a single phase-contrast MRI scan. While time-efficient, this imaging location is distal to the brain and, more importantly, distant from the isocenter of the MRI scanners. A technical consequence of an offcenter location is that the actual flow-encoding gradient may be lower than the prescribed value due to limited gradient coil coverage, thereby causing an under-estimation of CBF (unpublished data). This may reduce the reliability of CMRO2 measurement. Therefore, in the present study, we used an improved method in which flow in the feeding arteries was determined at a more proximal location of foramen magnum (reducing gradient imperfection) and four separate phase-contrast scans were performed for the four arteries, respectively (ensuring perpendicular slice orientation). Technical investigations have shown that the coefficient of variation (CoV) of CMRO2 measured with this method was 3.8% (Liu et al., 2013). Therefore, these improved physiological and technological considerations entailed a further examination of the relationship between CMRO2 and age conducted in the present study, which showed not only an age effect but also an age-by-sex interaction effect, using a sample size half of that of the prior study.
Age-related increase in the context of task-evoked activation has been attributed to a compensatory or scaffolding mechanism in cognitive aging (see (Park and Reuter-Lorenz, 2009) for a review). For the increase in resting-state metabolism observed in the present study, we hypothesize that the following possible mechanisms may be responsible. First, age-related increase in metabolism per unit mass tissue may be compensatory to a loss of tissue volume with age. Brain volume decreases with age. Thus, the number of neural processing units (e.g. numbers of neurons or synapses) available for cognitive computation is expected to be reduced as well (Pakkenberg and Gundersen, 1997). Therefore, it is reasonable to expect that the remaining neural processing units would have to expend more energy and undertake more processing load in order to maintain the same functionality. Second, the computational efficiency and specificity of the neural activity in the elderly individuals may be reduced and the brain has to engage greater neural activity to achieve the same behavioral outcome. For example, there is compelling evidence that the brain activation patterns in the elderly are less specific compared to younger individuals. Park et al. (2004) showed that the brain has specific regions in visual and hippocampal areas differentially responding to faces, scenes and words in younger adults, but these specificities were markedly attenuated in older participants (Park et al., 2004). Similarly, using resting state fMRI, a number of researchers have shown that the brain’s functional connectivity may lose specificity or modularity with age in that nodes in the older brain may reveal connectivity with regions outside its own network (Geerligs et al., 2014a; Geerligs et al., 2014b; Rieckmann et al., 2011). These additional connectivities are likely energy-consuming, and may have contributed to a greater CMRO2 in elderly. Finally, the cellular machineries that are responsible for generating neural activity may themselves decline with age. Thus, more oxygen consumption is needed to produce the same amount of neural activity. For example, it is known that the oxidative phosphorylation process in mitochondria is not perfect and as much as 20% of the proton gradient that would otherwise generate high-energy ATP is wasted through proton leak (Attwell and Laughlin, 2001). It is possible that the fraction of proton leak may increase with age, resulting in less efficient energy use in older adults.
Another novel finding in the present study is that there appears to be difference in age changes in between sex. In the multiple regression analysis, a highly significant (P<0.001) age × sex interaction effect was observed. When separately analyzing the female and male results, it was found that males showed a more pronounced age-increase effect, whereas the age effect in females was not significant. We posit that estrogen may play a role in the age changes in females. That is, aside from the above-described compensatory mechanisms that may cause increased metabolism in the elderly, in females an additional factor may influence the metabolic changes with age. Estradiol is known to be protective for mitochondria against toxicity from beta-amyloid and reactive oxygen species (Vina and Lloret, 2010). There is also evidence that estradiol could increase glucose utilization throughout the brain (Namba and Sokoloff, 1984). Therefore, when elderly women lose up to 90% of their premenopausal estrogen level after menopause (Wharton et al., 2009), mitochondria function declines and results in a slower rate of metabolic change compared to elderly men. This reduced ability to increase metabolism and perform compensatory neural processing may be one of the reasons for a higher prevalence of Alzheimer’s disease in elderly females (Barnes et al., 2005; Vina and Lloret, 2010).
Since the present study measured CBF and OEF of each participant, our findings also have a few interesting implications on metabolism-vascular coupling in elderly. First, the age- increase in CMRO2 (in particularly in male) was not accompanied by a corresponding increase in CBF. In fact, with age, CBF decreased in both female and male, an observation noted by many other researchers (Bertsch et al., 2009; Chen et al., 2011). Thus, the notion used widely in brain activation studies that an increase in neural activity and metabolism should be accompanied by an increase in CBF is not applicable in age-related changes. This is because vascular property differs between young and older individuals, due to multiple factors such as atherosclerosis, arterial sclerosis, and cerebral amyloid angiopathy occurring with age. Therefore, the greater metabolic demand in the older brain is met by extracting more oxygen (i.e. larger OEF as shown in this study) from existing blood supply, rather than by attracting more blood supply. Second, as far as the sex difference is concerned, our data suggest that blood supply is not the rate-limiting factor that prevented older female from enhancing their brain metabolism. This is evidenced by the results that female has a greater CBF and lower OEF (Figure 4) compared to male, even at advanced age. Therefore, our interpretation is that female has sufficient oxygen extraction reserve (at least until the age limit of this study), should the neurons want to use more oxygen. Thus, we speculate that the reason behind a lack of metabolic increase in older women is due to a decline in cellular machinery responsible for oxidative metabolism (e.g. mitochondria), in that their function is compromised due to insults such as beta-amyloid and reactive oxygen species thus the cells cannot enhance their oxygen metabolic rate even though there is plenty of oxygen available.
When normalizing total CMRO2 by the tissue mass to obtain values, it is important to separately consider gray and white matter volumes. This is because gray matter is expected to have a greater metabolic rate than white matter. Therefore, when an individual is found to have a higher CMRO2, it could be due to a higher fraction of gray matter relative to white matter, rather than due to a true enhancement of metabolic rate. In the context of aging, the gray/white metabolic difference does not necessarily cause a bias in the age-pattern, when the rate of volume change is similar between them. However, a few literatures have suggested that the rate of atrophy is different between the gray and white matter, with some reporting a faster rate in the white matter (Guttmann et al., 1998; Resnick et al., 2000) and other suggesting a faster rate in the gray matter (Ge et al., 2002; Lim et al., 1992; Pfefferbaum et al., 1994; Taki et al., 2011). Therefore, to account for tissue specific volume changes, the present study listed the gray and white matter volume separately in the normalization equation (Eq. [2]), with a factor r indicating their relative CMRO2 ratio. The value of r is not well established in the literature, but is expected to be similar to that for CBF since OEF is thought to be homogeneous across tissue types. We therefore tested the range of 0.3 to 1 in the present study, and found that the significant results were not dependent on the r value used, although the actual value of the slope varies.
We also investigated whether brain metabolic rate showed physiologic fluctuations within a day. A time-dependence in was observed using ANOVA analysis and post- hoc tests suggested that was significantly greater at noon when compared to that in the morning. There is some evidence in the literature that cerebral metabolic level is reduced during sleep (Madsen and Vorstrup, 1991; Sawaya and Ingvar, 1989). Thus, our finding supports a circadian rhythm of brain metabolism and energy consumption. One potential confounding factor in this analysis of time dependence of is that participants may have a greater amount of caffeine intake in the morning, which may cause a variation in CMRO2 (Griffeth et al., 2011; Tal et al., 2013). We therefore also tested to analyze the data using only subjects who reported no caffeine intake within two hours of the MRI scan (N=84). The results showed that CMRO2 in the morning was still significantly (P=0.037) lower than that at noon.
This study also provides some interesting insights on normal physiology of the brain. Most CBF and OEF studies have shown a considerable variation in these parameters across individuals (Bertsch et al., 2009; Liu et al., 2013). Our data suggest that a large portion of these variations can be explained by physiological reasons, in that intersubject variations in CBF and OEF are highly correlated (Figure 6). An individual with a higher CBF tends to have a lower OEF.
It is important to point out the limitations of the current study. The values reported here are whole-brain measures without regional or tissue-type information. Thus, it is possible that age-related changes in metabolism are region dependent (Aanerud et al., 2012). It is also possible that gray and white matter may show different patterns in metabolic changes with age. That is, the coefficient r in Eq. [2] may be age-dependent, which is not accounted for in the present study. Another weakness of the present study is that estrogen level was not measured in our participants. Thus, the notion that estrogen may have played a significant role in CMRO2 changes in females is still an untested hypothesis. This hypothesis would be supported by evidence that participants with a higher estrogen level manifest a greater CMRO2 compared to age-matched females with lower estrogen level. Furthermore, the inclusion of a group of older female adults undergoing hormone replacement therapy could also shed more light on the influence of estrogen on CMRO2. These works shall be the focus of future studies.
Conclusion
The present study provides evidence that resting-state brain metabolic rate for oxygen is increased with age. Furthermore, we showed that female and male groups have different temporal patterns of metabolic change, as evidenced by a significant age × sex interaction term. Males on average have a higher rate of CMRO2 increase when compared to females. Our data also revealed a possible circadian rhythm of CMRO2 in that brain metabolic rate is greater at noon than in the morning.
Funding/Acknowledgements
This study was supported, in part, by the National Institutes of Health (R21 AG034318, R37 AG006265, R21 NS078656, R01 NS067015, R01 DA023203, R01 MH084021, R01 AG042753, and K23 MH093684) and by the Southern Methodist University Dedman College Dean’s Research Council Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aanerud J, Borghammer P, Chakravarty MM, Vang K, Rodell AB, Jonsdottir KY, Moller A, Ashkanian M, Vafaee MS, Iversen P, Johannsen P, Gjedde A. Brain energy metabolism and blood flow differences in healthy aging. Journal of Cerebral Blood Flow and Metabolism. 2012;32:1177–1187. doi: 10.1038/jcbfm.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, Lu H. Estimation of Labeling Efficiency in Pseudocontinuous Arterial Spin Labeling. Magnetic Resonance in Medicine. 2010;63:765–771. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62:685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. Cerebral Glucose Metabolic Rates in Normal Human Females Versus Normal Males. Psychiatry Research. 1987;21:237–245. doi: 10.1016/0165-1781(87)90028-x. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, Naumann E. Resting cerebral blood flow, attention, and aging. Brain Research. 2009;1267:77–88. doi: 10.1016/j.brainres.2009.02.053. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Introduction to functional magnetic resonance imaging: Principles and Techniques. Cambridge Univ. Press; Cambridge, UK: 2002. [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task- independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage. 2011;55:468–478. doi: 10.1016/j.neuroimage.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Eustache F, Rioux P, Desgranges B, Marchal G, Petit-Taboue MC, Dary M, Lechevalier B, Baron JC. Healthy aging, memory subsystems and regional cerebral oxygen consumption. Neuropsychologia. 1995;33:867–887. doi: 10.1016/0028-3932(95)00021-t. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Maurits NM, Renken RJ, Lorist MM. Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp. 2014a;35:319–330. doi: 10.1002/hbm.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cerebral Cortex. 2014b doi: 10.1093/cercor/bhu012. [DOI] [PubMed] [Google Scholar]
- Griffeth VE, Perthen JE, Buxton RB. Prospects for quantitative fMRI: investigating the effects of caffeine on baseline oxygen metabolism and the response to a visual stimulus in humans. Neuroimage. 2011;57:809–816. doi: 10.1016/j.neuroimage.2011.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Respiration. In: Guyton AC, Hall JE, editors. Textbook of medical physiology. Saunders, Elsevier; Philadelphia (PA): 2005. pp. 502–512. [Google Scholar]
- Herscovitch P, Raichle ME. What Is the Correct Value for the Brain Blood Partition- Coefficient for Water. Journal of Cerebral Blood Flow and Metabolism. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- Hutchison JL, Shokri-Kojori E, Lu H, Rypma B. A BOLD Perspective on Age- Related Neurometabolic-Flow Coupling and Neural Efficiency Changes in Human Visual Cortex. Front Psychol. 2013;4:244. doi: 10.3389/fpsyg.2013.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaraki M, Shinohara Y, Nakamura K, Miura S, Kinoshita F, Kinoshita T. Interindividual variations of cerebral blood flow, oxygen delivery, and metabolism in relation to hemoglobin concentration measured by positron emission tomography in humans. J Cereb Blood Flow Metab. 2010;30:1296–1305. doi: 10.1038/jcbfm.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The Effects of Altered Arterial Tensions of Carbon Dioxide and Oxygen on Cerebral Blood Flow and Cerebral Oxygen Consumption of Normal Young Men. Journal of Clinical Investigation. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Zipursky RB, Watts MC, Pfefferbaum A. Decreased gray matter in normal aging: an in vivo magnetic resonance study. J Gerontol. 1992;47:B26–30. doi: 10.1093/geronj/47.1.b26. [DOI] [PubMed] [Google Scholar]
- Liu P, Xu F, Lu H. Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magnetic Resonance in Medicine. 2013;69:675–681. doi: 10.1002/mrm.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2- Relaxation-Under-Spin-Tagging MRI. Magnetic Resonance in Medicine. 2008;60:357–363. doi: 10.1002/mrm.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med. 2012;67:42–49. doi: 10.1002/mrm.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng YM, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in Cerebral Metabolic Rate and Blood Supply across the Adult Lifespan. Cerebral Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen PL, Vorstrup S. Cerebral Blood-Flow and Metabolism during Sleep. Cerebrovascular and Brain Metabolism Reviews. 1991;3:281–296. [PubMed] [Google Scholar]
- Namba H, Sokoloff L. Acute Administration of High-Doses of Estrogen Increases Glucose-Utilization Throughout Brain. Brain Research. 1984;291:391–394. doi: 10.1016/0006-8993(84)91276-9. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, Noll DC, Taylor SF. Working memory for complex scenes: Age differences in frontal and hippocampal activations. Journal of Cognitive Neuroscience. 2003;15:1122–1134. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cerebral Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Karlsson S, Fischer H, Backman L. Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. J Neurosci. 2011;31:14284–14290. doi: 10.1523/JNEUROSCI.3114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya R, Ingvar DH. Cerebral Blood-Flow and Metabolism in Sleep. Acta Neurologica Scandinavica. 1989;80:481–491. doi: 10.1111/j.1600-0404.1989.tb03915.x. [DOI] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS One. 2011;6:e22734. doi: 10.1371/journal.pone.0022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal O, Diwakar M, Wong CW, Olafsson V, Lee R, Huang MX, Liu TT. Caffeine-Induced Global Reductions in Resting-State BOLD Connectivity Reflect Widespread Decreases in MEG Connectivity. Front Hum Neurosci. 2013;7:63. doi: 10.3389/fnhum.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J, Lloret A. Why Women Have More Alzheimer’s Disease Than Men: Gender and Mitochondrial Toxicity of Amyloid-beta Peptide. Journal of Alzheimers Disease. 2010;20:S527–S533. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- Wharton W, Gleason CE, Lorenze KR, Markgraf TS, Ries ML, Carlsson CM, Asthana S. Potential role of estrogen in the pathobiology and prevention of Alzheimer’s disease. American Journal of Translational Research. 2009;1:131–147. [PMC free article] [PubMed] [Google Scholar]
- Xu F, Ge Y, Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med. 2009;62:141–148. doi: 10.1002/mrm.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Uh J, Liu P, Lu H. On improving the speed and reliability of T2-relaxation- under-spin-tagging (TRUST) MRI. Magnetic Resonance in Medicine. 2012;68:198–204. doi: 10.1002/mrm.23207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Kanno I, Uemura K, Shishido F, Inugami A, Ogawa T, Murakami M, Suzuki K. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke. 1986;17:1220–1228. doi: 10.1161/01.str.17.6.1220. [DOI] [PubMed] [Google Scholar]