Abstract
Several studies using rodent subjects have now shown that extra dietary choline may prevent or even reverse the deleterious effects of pre- and early post-natal ethanol administration. Choline supplementation has been shown to attenuate many, although not all, of ethanol’s effects on brain development and behavior. Our laboratory has consistently reported impaired habituation of the heart rate orienting response to a novel olfactory stimulus in animals exposed to ethanol on postnatal days (PD) 4–9. Here we examine whether supplemental choline given both during and after ethanol administration could alleviate these ethanol-induced deficits. Subjects were given 5 g/kg/day ethanol or sham intubations on PD 4–9. Half of the subjects in each group were given a single daily s.c. injection of choline chloride on PD 4–20, while the other half were injected daily with saline. Pups were tested for heart rate orienting and response habituation in a single test session on PD 23. Results replicated the ethanol-induced impairment in response habituation. However, choline supplementation had no effect on orienting or habituation in either neonatal treatment group. These findings indicate that habituation deficits induced by ethanol are not alleviated by extra dietary choline using these parameters. Choline holds great promise as a treatment for some fetal alcohol effects, but is not an effective treatment for all ethanol-related deficits.
Keywords: dietary supplement, choline, fetal alcohol, olfactory, orienting
1. Introduction
Prenatal exposure to ethanol in humans can result in notable impairments in attention, memory, and other cognitive processes [28]. Some of these deficits are measurable shortly after birth [33] and many are known to persist well into adulthood [3, 32]. Efforts at preventing maternal drinking during pregnancy have largely been unsuccessful; the incidence of Fetal Alcohol Syndrome (FAS) has not decreased since the identification of the syndrome more than 35 years ago [16]. It is estimated that nearly 1% of the population exhibits neurobehavioral problems associated with prenatal exposure to ethanol [30]. Because incidence rates seem to be relatively unchanged [6], more effective measures for prevention of maternal ethanol use are sorely needed. However, until that time, efforts to treat the afflicted individuals continue to be a major focus of research and practice.
Work with animal models has investigated approaches to treating neural and behavioral alterations caused by ethanol exposure. Neonatal handling [21], rearing in an enriched environment [10], and therapeutic motor training [19] all appear to mitigate some of the negative consequences of pre- or early post-natal ethanol exposure. Antioxidants, such as vitamin E and β-carotene, also offer promising avenues for treatment and perhaps prevention of alcohol-induced neural and behavioral deficits [2, 23]. Choline supplementation has also been found to attenuate many of the negative effects of neonatal alcohol exposure. In an extensive series of experiments, Thomas and colleagues have reported that supplemental choline given during and/or after ethanol exposure can improve measures of physical and behavioral development [34] and reverse hyperactivity [26, 36]. Choline has also been shown to rescue impairments in spatial memory [29, 35], visual discrimination learning [37], reversal learning [36], and trace eyeblink conditioning [39]. Wagner and Hunt [40] also demonstrated a beneficial effect of supplemental choline on ethanol-induced deficits in trace fear conditioning. Collectively, these findings lend support for the treatment potential of choline.
Another robust behavioral consequence of prenatal ethanol exposure is a lack of response habituation [11, 33], and we have previously observed that habituation to discrete cues is impaired in young rats exposed to ethanol during the neonatal period [13, 14, 27]. The assessment of habituation may be particularly useful as an early indicator of cognitive dysfunction [e.g., 9, 31]. A stimulus-elicited decrease in heart rate is one component of the orienting response [20], which undergoes rapid habituation with repeated stimulus presentations. Animals exposed to ethanol on PD 4–9 show virtually no response decrement to a novel olfactory cue throughout a 10-trial test session, whereas control subjects exhibit complete habituation within 4 or 5 trials [13, 27]. The present experiment was designed to assess whether choline supplementation would rescue these habituation deficits. The choline administration procedure was identical to that used by Wagner and Hunt [40], which was based on that described by Thomas, Garrison and O’Neill [36].
2. Methods
2.1 Animals
The subjects were offspring of 9 litters of Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), born and reared at the College of William and Mary. Pups from an additional 10 litters served as unhandled controls and underwent no treatment until the day of heart rate testing. Animal facilities were temperature- and humidity-controlled and maintained on a 14:10 h light:dark cycle (light onset 0600 h). Male and female breeders were maintained together in 50.8 × 40.6 × 21.6 cm clear polycarbonate cages with pine shavings and stainless steel lids. Animals had free access to high-protein food (LabDiet Formula 5008) and water. Cages were checked daily for pups, and the day of birth was designated as postnatal day (PD) 0. Litters were culled to 8–10 pups on PD 2. Pups were weaned from the home cage on PD 21, at which time they were group housed with all siblings in identical polycarbonate cages. Procedures were approved by the Institutional Animal Care and Use Committee of the College of William & Mary.
2.2 Apparatus
Heart rate was recorded as previously described [27] using two transcutaneous electrodes made from 27 ga stainless steel wire and shaped to resemble safety pins. Cardiac potentials were amplified with a Grass Instruments (Quincy, MA) Model P15 preamplifier. The R-spike activated a Schmitt trigger (Coulbourn Instruments, Allentown, PA). A computer stored each inter-beat interval (IBI) measured to the nearest millisecond, and controlled all timing sequences and data collection.
Subjects were tested individually in a 25-cm long cylindrical Plexiglas chamber (14 cm diameter) mounted horizontally inside a sound attenuating shell. The olfactory stimulus (0.5 ml amyl acetate + 40 ml water) was introduced into the chamber by means of a custom-made olfactometer system. The temperature inside the chamber was maintained at 28°C by a heated airstream. The olfactory stimulus was evacuated from the chamber by negative pressure generated by an exhaust fan.
2.3 Ethanol administration
On PD 4, all pups from the litter were removed from the home cage and assigned to one of four groups, designated by ethanol treatment [ethanol (EtOH) or sham intubated (Sham)] and choline supplementation [choline or saline injections]. Data from like-treated pups within a litter were averaged so that each of the 9 litters contributed one data point per treatment group. Subjects in the Unhandled group (1 pup per litter) were not treated during this time.
Animals assigned to group EtOH were administered a total of 5.0 g/kg/day ethanol via intragastric intubation. The total daily dose was divided into two doses of 2.5 g/kg each that were administered 2 h apart (at 0900 and 1100 h). The ethanol solution was 11.9% v/v, dissolved in Similac™. A third feeding, given 2 h after the second ethanol dose (1300 h), consisted of the Similac™ vehicle alone [7]. Sham controls received the tube-insertion procedure three times per day, but were not given any fluid [7, 23]. Intubations were achieved using a 10 cm length of PE-10 tubing (Clay Adams, Parsippany, NJ). Animals were returned to the home cage after each feeding session. This procedure occurred daily from PD 4 through PD 9.
2.4 Choline administration
Supplemental choline injections began on the first day of ethanol administration, PD 4, and continued daily through PD 20. A single subcutaneous injection of choline chloride or saline was given to subjects immediately after the last feeding session on PD 4–9 (1300 h), and at the same time of day on PD 10–20. A constant volume of 0.10 ml of an 18.8 mg/ml solution of choline chloride (Sigma, St. Louis, MO) or saline vehicle was injected on each day of treatment [36, 40].
2.5 Behavioral testing
All animals were tested for heart rate orienting responses and response habituation on PD 23 (+/−1 day). Heart rate electrodes were acutely implanted on the dorsal surface, one at the nape of the neck and the other approximately 1 cm from the base of the tail. Animals were placed into the test chamber for a 10 min period of adaptation. Next, subjects were given 10 odor presentations. Each odor was 10 s in duration, and trials were separated by 100–200 s intervals. Heart rate was recorded for 1 sec prior to each odor trial to provide a measure of baseline heart rate, during the 10 s odor presentation, and for 5 s after stimulus offset.
2.6 Treatment of heart rate data
Inter-beat intervals were converted into a beats-per-minute (BPM) measure for analysis. Heart rate recorded during the 1 s baseline period was subtracted from that recorded during each second of the stimulus and post-stimulus periods to obtain difference scores. Negative difference scores reflected a decrease in heart rate (bradycardia) that defines the heart rate orienting response [20], whereas positive difference scores reflected an increase in heart rate (tachycardia).
2.7 Statistical analyses
Heart rate data were analyzed using mixed-factor Analysis of Variance (ANOVA), with ethanol treatment and choline supplementation as between-groups variables, and seconds (orienting response) or trial block (habituation) as the within-subjects variable. In all cases involving a repeated measure, the Greenhouse-Geisser correction procedure was used to control for possible inflation of probability values. Post hoc comparisons were made using Newman-Keuls tests (p < .05). The second-by-second changes from baseline heart rate obtained from the first trial were analyzed separately to assess the integrity of the orienting response. Habituation was assessed by analysis of the peak change in heart rate averaged across blocks of 2 trials. The peak change was defined as the largest change observed, either positive or negative [13, 14]. Data from the Unhandled control group were compared separately to ethanol- and sham-treated groups using ANOVAs.
3. Results
3.1 Body weights
Body weights of EtOH and Sham subjects recorded during the ethanol administration procedure (PD 4–9) and on the last day of choline administration (PD 20) were analyzed using a 2 (ethanol treatment) × 2 (choline supplementation) × 7 (day) mixed-factor ANOVA. The analysis yielded significant main effects of ethanol treatment [F (1, 32) = 12.02, p = .002] and day [F (6, 192) = 3795.17, p < .001] and an Ethanol Treatment × Day interaction [F (6, 192) = 10.70, p = .001]. Post hoc comparisons indicated that both EtOH-treated groups weighed less than the Sham groups on PD 6–9 and also on PD 20. Analysis of body weights on the test day was with a one-way ANOVA comparing all five treatment groups. A significant effect of Treatment was obtained, F (4, 41) = 3.90, p = .009. Ethanol-treated subjects weighed less on the test day than sham-treated and unhandled controls (Mean +/− SEM; EtOH = 60.11 g +/− 1.41, Sham = 69.52 g +/− 2.56, Unhandled = 69.7 +/− 1.41). Sham-treated and Unhandled groups did not differ. Choline had no effect on body weights at any time.
3.2 Baseline Heart Rate
Baseline heart rates recorded prior to each odor presentation were averaged across blocks of 2 trials and analyzed using a 2 (ethanol treatment) × 2 (choline supplementation) × 5 (trial block) mixed-factor ANOVA. The analysis revealed a main effect of ethanol treatment, F (1, 32) = 14.26, p = .001. There was no effect of choline supplementation or trial block. Overall, EtOH-treated subjects had higher baseline heart rates than sham controls. A separate ANOVA indicated that sham controls had higher baselines than unhandled subjects, F (2, 25) = 5.41, p = .011 (M +/− SEM; EtOH = 570.9 +/− 4.9, Sham = 540.2 +/− 6.2, Unhandled = 504.8 +/− 8.6). Baseline heart rates remained stable during the test session in all groups.
3.3 Heart Rate Orienting Response
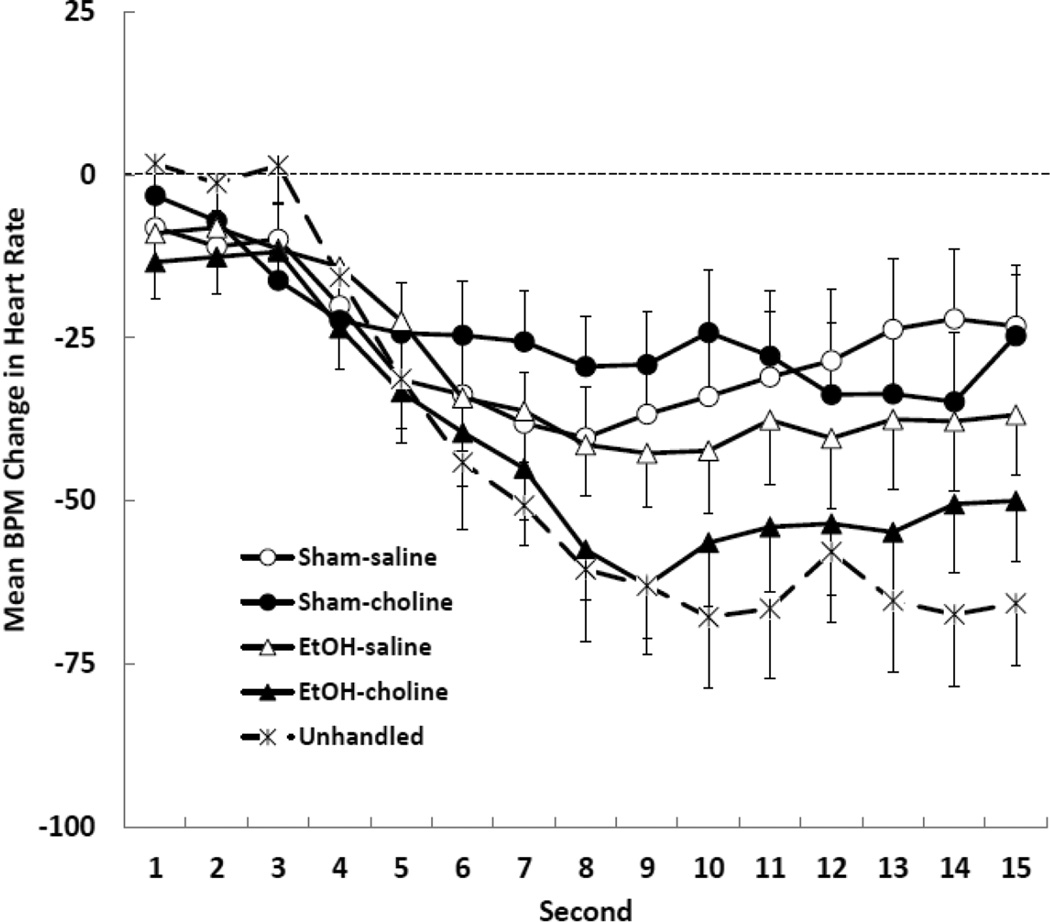
The second-by-second changes in heart rate recorded on the first stimulus presentation were analyzed using a 2 (ethanol treatment) × 2 (choline supplementation) × 15 (second) mixed-factor ANOVA. The analysis indicated a significant main effect of second, F (14, 448) = 15.69, p < .001. There was no effect of ethanol treatment or choline supplementation on the form or magnitude of the orienting response measured on the first trial. The response of the Unhandled controls was compared with the other groups, and was found to be significantly larger than the response of the Sham subjects [F (28,350) = 3.83, p = .001] but not different from the response of the ethanol subjects [F < 1]. The orienting response of all groups is shown in Figure 1.
Figure 1.
Mean (+/− SEM) heart rate orienting response recorded on the first trial for all groups. Heart rate was measured for 1 s prior to stimulus onset (baseline heart rate), during the 10 s olfactory stimulus, and for 5 s after stimulus offset. The data shown are changes in heart rate (beats-per-minute; BPM) from pre-stimulus baseline.
3.4 Habituation of the orienting response
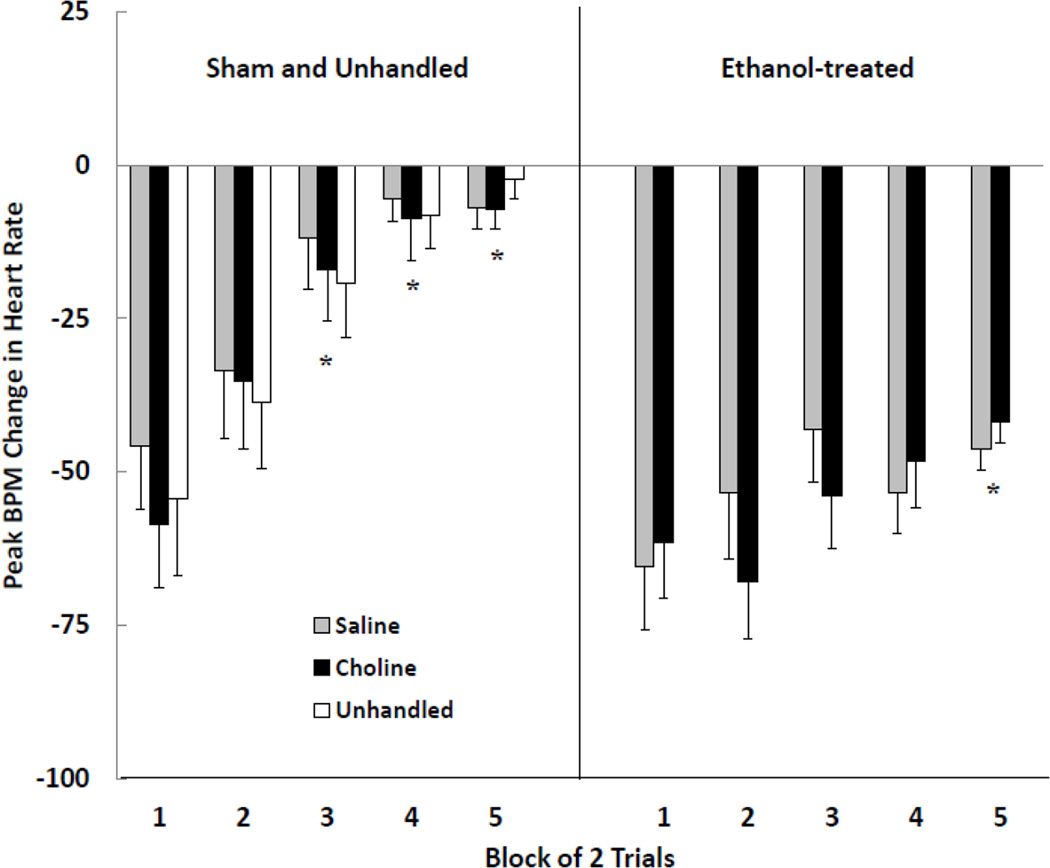
The peak changes in heart rate averaged across blocks of 2 trials were subjected to a 2 (ethanol treatment) × 2 (choline supplementation) × 5 (trial block) mixed-factor ANOVA. Significant main effects of ethanol treatment [F (1, 32) = 44.43, p < .001] and trial block [F (4, 128) = 11.98, p < .001] were obtained. The Ethanol Treatment × Trial Block interaction approached significance [F (4,128) = 2.38, p = .055]. There was no effect of choline supplementation. Subjects in the EtOH groups responded with large magnitude heart rate responses throughout the test session. This is in contrast to subjects in the Sham groups which exhibited complete habituation by block 3. Choline had no effect on rate of habituation in either the EtOH or Sham subjects. Comparison of habituation in sham and unhandled controls revealed no significant difference, F < 1. These data are shown in Figure 2.
Figure 2.
Peak beats-per-minute (BPM) change in heart rate (M +/− SEM) averaged for blocks of two trials during the test session. Subjects were given sham intubations (left panel) or were administered 5.0 g/kg/day ethanol (right panel) on postnatal days (PD) 4–9, and received choline or saline injections once daily on PD 4–20. Unhandled subjects (left panel) were given no treatment prior to the day of testing. Postnatal ethanol resulted in impaired response habituation, and supplemental choline administration had no effect. * denotes that the response is significantly smaller than that recorded on the first trial block.
4 Discussion
The present results replicate previous work from our lab showing that neonatal ethanol exposure produces a profound deficit in habituation of the heart rate orienting response to a novel olfactory stimulus. The time-course and magnitude of the orienting response in the Sham and EtOH treated subjects was highly similar to our previous findings [27] and those from other labs [18]. The impairment is thus not in altered stimulus detection or the expression of a stimulus-evoked change in heart rate, but rather in some other aspect of information processing such as memory formation or response inhibition. Further, it was shown that supplemental choline was ineffective in mitigating this ethanol impairment. While supplementation with choline during this period of development can attenuate many effects of neonatal ethanol exposure, the persistent orienting response observed in ethanol-treated animals was not improved.
Higher baseline heart rates were observed in ethanol-exposed animals, compared with sham-intubated and unhandled controls, throughout the test session. This also parallels previous research [14, 17]. Baseline heart rates in-and-of-themselves do not necessarily correlate with larger magnitude heart rate orienting responses [12]. Indeed, a correlation evaluating baseline heart rate and peak change on trial 1 yielded no significant relationship, r (36) = −0.20, p = 0.23.
The mechanisms by which choline supplementation produces long-term positive effects on development remain unknown. Choline serves many biological functions [1]. For example, choline is a precursor of phosphatidylcholine and sphingomyelin, structural phospholipids found in all biological membranes. Choline is a precursor for two signaling lipids (sphingosylphosphocholine and platelet-activating factor) and is enzymatically oxidized to the methyl donor betaine. It is the precursor for acetylcholine, which not only serves as a neurotransmitter throughout life, but is also an important developmental growth factor [24]. The ability of supplemental choline to rescue brain systems from neurotoxicity likely involves many, if not all, of the above mechanisms. More specifically, early supplementation with choline may promote the tuning of cellular networks, especially cholinergic networks [25] and may strengthen, protect, and perhaps reverse ethanol effects via direct actions on central cholinergic systems (e.g. [4]). The neural systems responsible for olfactory processing and habituation are not well characterized, and the role of the cholinergic system in olfactory habituation is subject to debate [15, 22]. The observation that choline supplementation benefits a variety of outcome measures, some of which are non-cholinergic [25, 34], suggests that choline has wide-ranging effects on the developing brain that are not limited to cholinergic systems.
A variety of treatment approaches have been examined in animal models of fetal alcohol exposure. Many of these produce positive results, although there is no one treatment that can mitigate the variety of neural, physiological, behavioral and cognitive problems associated with alcohol exposure. For example, antioxidants were found to decrease hippocampal cell loss due to ethanol but there was no corresponding improvement in spatial learning abilities [23]. Neonatal handling can attenuate T maze reversal learning deficits due to alcohol exposure [21], but may not benefit spatial memory impairments [5]. Motor rehabilitation training can improve spatial learning and reverse hippocampal long-term potentiation deficits caused by neonatal alcohol exposure, but has no ameliorative effect on delay eyeblink conditioning [19]. Supplemental choline, as described above, can alleviate some of the behavioral, physiological and cognitive consequences of neonatal ethanol exposure, but is ineffective for treating motor coordination deficits [38], impaired delay eyeblink conditioning [39], or the lack of habituation of the orienting response [present results]. Given that a particular treatment may help with some, but not all, consequences of alcohol exposure, the most effective strategy for improving long-term outcome will likely be a combination of nutritional and other therapies [6, 8].
Highlights.
Neonatal ethanol exposure leads to impaired habituation of the orienting response to a novel olfactory stimulus in rats
Supplemental choline given during and after ethanol administration had no effect on habituation
Deficits in response habituation are not treated with supplemental choline, although choline does improve performance on other memory tasks
Acknowledgments
This research was supported by NIH grant AA015343 to PSH and a Howard Hughes Medical Institute Undergraduate Science Education Project grant awarded to the College of William & Mary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors have no conflicts to declare.
References
- 1.Blusztajn JK, Cermak JM, Holler T, Jackson DA. Imprinting of hippocampal metabolism of choline by its availability during gestation: Implications for cholinergic neurotransmission. J. Physiol. 1998;92:199–203. doi: 10.1016/s0928-4257(98)80010-7. [DOI] [PubMed] [Google Scholar]
- 2.Cohen-Kerem R, Koren G. Antioxidants and fetal protection against ethanol teratogenicity I. Review of the experimental data and implications to humans. Neurotoxicol. Teratol. 2003;25:1–9. doi: 10.1016/s0892-0362(02)00324-0. [DOI] [PubMed] [Google Scholar]
- 3.Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC. Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcohol., Clin. Exp. Res. 2010;34:897–906. doi: 10.1111/j.1530-0277.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa LG, Giordano G, Guizzetti M. Inhibition of cholinergic muscarinic signaling by ethanol: Potential mechanism of developmental neurotoxicity and biological plausibility for the beneficial effects of choline supplementation. Int. J. Alcohol Drug Res. 2013;2 [Google Scholar]
- 5.Gabriel KI, Johnston S, Weinberg J. Prenatal ethanol exposure and spatial navigation: Effects of postnatal handling and aging. Dev. Psychobiol. 2002;40:345–357. doi: 10.1002/dev.10023. [DOI] [PubMed] [Google Scholar]
- 6.Goodlett CR. Fetal alcohol spectrum disorders: New perspectives on diagnosis and intervention. Alcohol. 2010;44:579–582. doi: 10.1016/j.alcohol.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: Timing and dose effects on place learning. Neurotoxicol. Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- 8.Guerri C, Pascual M, Garcia-Minguillan MC, Charness ME, Wilkemeyer MF, Klintsova AY, Goodlett CR, Greenough WT, Sakata-Haga H, Dominguez HD, Thomas JD. Fetal alcohol effects: Potential treatments from basic science. Alcohol., Clin. Exp. Res. 2005;29:1074–1079. [Google Scholar]
- 9.Guiraud JA, Kushnerenko E, Tomalski P, Davies K, Ribeiro H, Johnson MH. Differential habituation to repeated sounds in infants at high risk for autism. Neuroreport. 2011;22:845–849. doi: 10.1097/WNR.0b013e32834c0bec. [DOI] [PubMed] [Google Scholar]
- 10.Hannigan JH, O’Leary-Moore SK, Berman RF. Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neurosci. Biobehav. Rev. 2007;31:202–211. doi: 10.1016/j.neubiorev.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Hepper PG, Dornan JC, Lynch C. Fetal brain function in response to maternal alcohol consumption: Early evidence of damage. Alcohol., Clin. Exp. Res. 2012;36:2168–2175. doi: 10.1111/j.1530-0277.2012.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt PS, Hess MF, Campbell BA. Autonomic mediation of unconditioned and conditioned heart rate responses in the 16-day-old rat. Psychobiology. 1994;22:209–218. [Google Scholar]
- 13.Hunt PS, Morasch KC. Modality-specific impairments in orienting response habituation following neonatal binge ethanol exposure. Neurotoxicol. Teratol. 2004;26:451–459. doi: 10.1016/j.ntt.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Hunt PS, Phillips JS. Postnatal binge ethanol exposure affects habituation of the cardiac orienting response to an olfactory stimulus in preweanling rats. Alcohol., Clin. Exp. Res. 2004;28:123–130. doi: 10.1097/01.ALC.0000108650.02216.1A. [DOI] [PubMed] [Google Scholar]
- 15.Hunter AJ, Murray TK. Cholinergic mechanisms in a simple test of olfactory learning in the rat. Psychopharmacology. 1989;99:270–275. doi: 10.1007/BF00442821. [DOI] [PubMed] [Google Scholar]
- 16.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 17.Kelly SJ, Richards JE. Development of heart rate inter-beat interval variability in preweanling rats: Effects of exposure to alcohol and hypoxia. Physiol. Behav. 1997;61:231–241. doi: 10.1016/s0031-9384(96)00365-4. [DOI] [PubMed] [Google Scholar]
- 18.Kelly SJ, Richards JE. Heart rate orienting and respiratory sinus arrhythmia development in rats exposed to alcohol or hypoxia. Neurotoxicol. Teratol. 1998;20:193–202. doi: 10.1016/s0892-0362(97)00090-1. [DOI] [PubMed] [Google Scholar]
- 19.Klintsova AY, Goodlett CR, Napper RMA, Greenough WT. Therapeutic motor training ameliorates cerebellar effects of postnatal binge alcohol. Neurotoxicol. Teratol. 2000;22:125–132. doi: 10.1016/s0892-0362(99)00052-5. [DOI] [PubMed] [Google Scholar]
- 20.Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational properties. Mahwah, NJ: Erlbaum; 1997. [Google Scholar]
- 21.Lee MH, Rabe A. Infantile handling eliminates reversal learning deficit in rats prenatally exposed to alcohol. Alcohol. 1999;18:49–53. doi: 10.1016/s0741-8329(98)00067-6. [DOI] [PubMed] [Google Scholar]
- 22.Linster C, Menon AV, Singh CY, Wilson DA. Odor-specific habituation arises from interaction of afferent synaptic adaptation and intrinsic synaptic potentiation in olfactory cortex. Learn. Mem. 2009;16:452–459. doi: 10.1101/lm.1403509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. Int. J. Dev. Neurosci. 2004;22:363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 24.McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci. Biobehav. Rev. 2006;30:696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 26.Monk BR, Leslie FM, Thomas JD. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus. 2012;22:1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morasch KC, Hunt PS. Persistent deficits in orienting response habituation following neonatal binge ethanol exposure. Alcohol., Clin. Exp. Res. 2009;33:1596–1604. doi: 10.1111/j.1530-0277.2009.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: An overview. Neuropsychol. Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampson PD, Streissguth AP, Bookstein FL, Litte RE, Clarren SK, Dehaene P, Hanson JW, Graham JM. Incidence of fetal alcohol syndrome and prevalence of alcohol0related neurodevelopmental disorder. Teratol. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Sansavini A, Guarini A, Caselli MC. Preterm birth: Neuropsychological profiles and atypical developmental pathways. Dev. Disabil. Res. Rev. 2011;17:102–113. doi: 10.1002/ddrr.1105. [DOI] [PubMed] [Google Scholar]
- 32.Streissguth AP. Offspring effects of prenatal alcohol exposure from birth to 25 years: The Seattle Prospective Longitudinal Study. J. Clin. Psychol. Med. Settings. 2007;14:81–101. [Google Scholar]
- 33.Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton Scale. Child Dev. 1983;54:1109–1118. [PubMed] [Google Scholar]
- 34.Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol. Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav. Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- 36.Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Thomas JD, La Fiette MH, Quinn VRE, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol. Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 38.Thomas JD, O’Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2004;26:223–229. doi: 10.1016/j.ntt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22:619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: Reversal by choline. Behav. Neurosci. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]