Abstract
Hematopoietic stem cell transplant (HSCT) recipients are at significant risk for BKV reactivation, hemorrhagic cystitis (HC) and renal dysfunction. We prospectively monitored 98 HSCT by serial BKV PCR in the urine through Day (D) +100 to analyze the relationship between BKV viruria and HC, serum creatinine (Cr) and creatinine clearance (CrCl) through D +180 or death. Patients, median age 52 years, range 20-73, received T-cell depleted (50%) or cord blood allografts (21%). Median pre-HSCT BKV IgG titers were 1:10,240. Incremental increase in BKV IgG titers correlated with developing BKV viruria ≥ 107 copies/mL. By D +100, 53 (54%) patients had BKV viruria. BKV viral load in the urine increased at engraftment and persisted throughout D +100. HC developed in 10 patients (10%); 7/10 with BKV viruria. In competing risk analyses, BKV viruria ≥ 107 copies/mL, older age, CMV reactivation and foscarnet use were risk factors for HC. Cr and CrCl at 2, 3 and 6 months post-HSCT were similar between patients with and without BKV viruria.
INTRODUCTION
BK virus (BKV) is acquired in childhood and establishes latency in the urothelium. Approximately 90% of adults have antibodies against BKV [1]. Transient, asymptomatic BKV shedding in the urine (viruria) may occur in up to 5% of healthy individuals and up to 60% of immunocompromised patients [2]. In renal transplant recipients, BKV associated neprhopathy is a recognized cause of allograft loss [3, 4]. An association between high titers of BKV IgG and BKV nephropathy has also been reported in renal transplant recipients [5]. In hematopoeietic stem cell transplant recipients (HSCT), BKV viruria is mainly associated with hemorrhagic cystitis (HC) with reported incidence ranging from 7% to 40% depending on HSCT type and HC severity [6-11]. The prognostic significance of the magnitude of viral load in the urine has not been establisehd in HSCT. In a prospective study, De Silva et al. did not demonstrate an association between the level of BKV viral load in the urine and HC [6]. In contrast, Leung et al. showed that a rise in BKV viral load in the urine preceded HC, yet values of BKV viral load varied widely and often overlapped between patients with and without HC [7]. Wong et al. using a laboratory-developed-test for BKV IgG, showed a correlation between high titers of recipient BKV IgG and rising BKV viral load in the urine post-HSCT [9]. Recent studies suggest that BKV viremia is associated with renal dysfunction after HSCT [12, 13]. Tissue proven BKV nephropathy has been rarely reported in HSCT [12-14]. It is plausible however that BKV nephropathy is under-recognized in HSCT due to a paucity of kidney biopsies or autopsies in HSCT compared to renal allograft recipients [15].
In the present study, we measured pre-transplant serum BKV IgG titers and monitored prospectively BKV viral load in the urine by a quantitative PCR in a cohort of 98 adult allogeneic HSCT recipients. Our objectives were to: 1) describe the natural history of BKV infection in urine in our patient population; 2) examine the relationship between serum BKV IgG titers and BKV viruria; 3) assess the impact of BKV viruria on HC and renal function post-HSCT.
METHODS
Patients
The study was reviewed by the Memorial Sloan-Kettering Cancer Center (MSKCC) institutional review board (IRB) and granted a Waiver of Authorization. The cohort consists of 98 consecutive adult patients who received allogeneic HSCT at MSKCC from April 2010 through September 2010 and from January 2011 through October 2011. Serum creatinine (Cr) values, creatinine clearance (CrCl) and data on HC were collected through July 31, 2012 or death, whichever occurred first. Minimum follow-up on survivors was 9 months. Clinical characteristics, laboratory and pharmacy data were extracted from a computerized database and medical chart review.
Supportive care
The procedure for T-cell depletion (TCD), grading and management of graft versus host disease (GVHD) has been previously described [16-18]. Recipients who were cytomegalovirus (CMV) seropositive or had a CMV seropositive donor were routinely monitored by CMV PCR at least weekly through day (D) +100 and as clinically indicated thereafter. For the present study, CMV reactivation is defined as ≥ 1 PCR of > 500 copies/ml. During the study period, there was no routine surveillance for other viral pathogens.
Recipients of cord blood (CB) allografts received routine antibacterial prophylaxis with ciprofloxacin starting on D −2 until engraftment or initiation of broad spectrum antibiotics whichever occurred first. Thirty patients received keratinocyte growth factor (Palifermin) 60mcg/kg intravenously (IV) for 3 consecutive days prior to conditioning, on D 0 (6 hours after stem cell infusion) and on D +1 and +2. Two patients were enrolled in double-blinded, placebo controlled, dose-escalation study of the safety, tolerability and ability of CMX001 to prevent or control Cytomegalovirus (CMV) infection (clinicaltrials.gov, ID:NCT00942305). Fourteen patients received open label CMX001. Twelve patients were enrolled in a multicenter, open-label study of CMX001 for the treatment of serious diseases or conditions caused by dsDNA viruses (ClinicalTrials.gov, ID:NCT01143181); and 2 patients under emergency investigational drug application (EIND) provisions. The primary indication for CMX001 was cytomegalovirus (CMV) in 13 patients and herpes simplex virus in 1 patient.
Study design
Recipients’ BKV IgG serum titers were measured within ±2 days prior to conditioning. Serial urine specimens for quantitation of BKV were collected at baseline (± 2 days from conditioning), on D 0 ± 2 days (day of stem cell infusion), on the day of neutrophil engraftment ± 2 days, and 1, 2, 4 and 8 weeks post engraftment (± 3 days). Results of BKV and BKV IgG testing were not available to the clinicians.
Definitions
BKV viruria was defined as ≥ 1 BKV PCR ≥ 500 copies/mL by week 8 after neutrophil engraftment. “High BKV viruria” was defined as ≥ 1 BKV PCR ≥ 1x107 copies/mL [19].
HC was graded as previously described [20] (grade 1, microscopic hematuria; grade 2, macroscopic hematuria; grade 3, macroscopic hematuria with clots; grade 4, macroscopic hematuria with impaired renal function secondary to urinary tract obstruction). Grade 3 and 4 HC is defined as “severe.” Patients with HC grade ≥ 2 were treated as events in the analyses. Diagnostic procedures and management of cystitis were done as per standard care at MSKCC. Urine viral culture was ordered by clinicians’ discretion. Symptomatic patients with positive urine bacterial cultures were excluded from the study.
Laboratory methods
Serum and urine testing for BKV were performed by Viracor-IBT Laboratories (Lee's Summit, MO, USA). Serum BKV IgG titers were determined by an indirect-linked immunosorbent assay (ELISA) to the VP1 viral capsid protein of BKV. Serum was serially-diluted four-fold from 1:40 through 1:655,360. BKV IgG antibody titer is reported as the specimen dilution that produces signal greater than the assay background level. Currently there is no established clinical cut-off for this assay. Five patients received IV Immunoglobulin G within 30 days prior to measuring BKV IgG titers.
BKV in the urine was determined by a quantitative polymerase chain reaction (PCR) assay (linear range of quantitation 500 – > 1×1010 copies/mL). CMV in whole blood was monitored by a laboratory developed quantitative PCR assay using the Roche ASR. The lower limit of quantitation is ≥ 500 copies/mL. Specimens were batched prior to testing.
Creatinine clearance (CrCl) was estimated using the Cockcroft-Gault equation [21].
Statistical analyses
Patient and transplant characteristics were summarized as percentage for categorical variables and median (range) for continuous variables. Wilcoxon signed rank test was used to compare BKV viruria measured at every pair of neighbor time points, and Bonferroni adjusted P value was calculated.
We estimated the cumulative incidence rate (CIR) for BKV viruria (BKV viral load ≥ 500 copies/mL) and high BKV viruria (BKV viral load ≥ 1.0×107 copies/mL) at D +100, treating death and 2nd HSCT as the competing risk. Grey's test was used to examine risk factors for HC and high BKV viruria post-HSCT, also treating death and 2nd HSCT as the competing risk. Factors with univariate P-value less than 0.1 were further examined in multivariate analysis for high BKV viruria post-HSCT. Due to the small number of events, no multivariate analysis was performed for HC. Factors observed post-HSCT were treated as time-dependent variables in cause-specific Cox regression. The effect of HC on overall survival (OS) was also assessed using time-dependent cause-specific Cox regression.
In order to evaluate association between BKV status and Cr/CrCl values recorded at 2, 3 and 6 months, mixed effects models with subject random effects were used to take into account multiple measurements per patient. The fixed effects included time, BKV status, and interaction between BKV status and time.
Statistical analyses were performed with software package (SAS 9.2; SAS Institute Inc., Cary, NC, USA). A P value of ≤.05 was considered statistically significant.
RESULTS
Kinetics of BKV Replication Post-transplant
Table 1 shows the baseline characteristics of the patients. Forty nine (50%) patients received TCD allografts and 21 (21%) patients received double umbilical cord blood (CB) grafts.
Table 1.
Clinical characteristics of the 98 HSCT recipients.
| Characteristic | N (%) |
|---|---|
| Age, median (range), years | 52.1 (19.6 – 73) |
| Gender | |
| Male | 60 (61%) |
| Female | 38 (39%) |
| Underlying disease | |
| Acute leukemia | 44 (45%) |
| Lymphoma/CLL | 22 (23%) |
| Myelodysplastic syndrome/MPD | 21 (21%) |
| Multiple myeloma | 9 (9%) |
| Nonmalignant hematologic disorders | 2 (2%) |
| Stem cell source | |
| Peripheral blood | 74 (76%) |
| Bone marrow | 3 (3%) |
| Cord blood | 21 (21%) |
| Graft type | |
| T-cell depleted | 49 (50%) |
| Conventional | 49 (50%) |
| Donor type | |
| Matched related donor | 26 (26%) |
| Matched unrelated donor | 32 (33%) |
| Mismatched unrelated donor | 40 (41%) |
| Type of conditioning | |
| Myeloablative (MA) | 50 (51%) |
| Reduced intensity (RIC) | 38 (39%) |
| Nonmyeloablative (NMA) | 10 (10%) |
| Conditioning regimen | |
| Total body irradiation containing | |
| High dose (>1300 Gy) | 17 (17%) |
| Low dose (≤400 Gy) | 57 (58%) |
| All chemotherapy | 24 (25%) |
| Acute GVHD | |
| Grade 0-1 | 64 (65%) |
| Grade 2-3 | 32 (33%) |
| Not evaluable | 2 (2%) |
| Keratinocyte growth factor (Palifermin) | |
| Yes | 30 (31%) |
| No | 68 (69%) |
| Ciprofloxacin within 14 days of stem cell infusion | |
| Yes | 35 (36%) |
| No | 63 (64%) |
| Recipient serum BKV IgG titers | |
| >1:640 | 98 (100%) |
| CMV serology | |
| R+/D+ | 28 (28%) |
| R+/D− | 33 (34%) |
| R−/D+ | 8 (8%) |
| R−/D− | 29 (30%) |
| CMV reactivation | 37 (38%) |
Abbreviations: HSCT, hematopoietic stem cell transplantation; GVHD, graft versus host disease; TBI, total body irradiation; CMV, cytomegalovirus; R+, recipient positive; R−, recipient negative; D+, donor positive; D−, donor negative
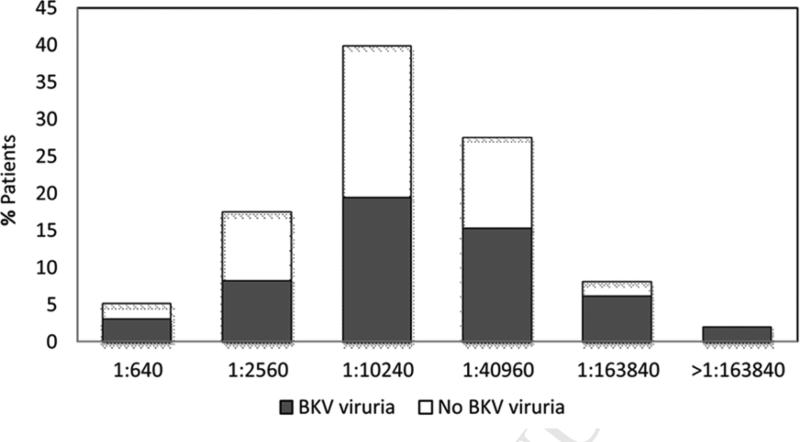
All (100%) patients had serum BKV IgG titer > 1:640. The distribution of BKV IgG titers is shown in Figure 1. At the last follow-up, 35 patients died among 98 patients. The median follow-up on survivors was 14.2 months (range: 9.4–27.6 months) and the median overall survival was not reached (95%CI: 15.6 months–not reached).
Figure 1.
Serum BKV IgG titers and BKV viruria.
X-axis: Pre-transplant serum BKV IgG titers. Y-axis: Percentage of patients.
During the study period, 53 (54%) patients had BKV viruria. Seven patients had low level BKV viruria [median BKV viral load 1,900 copies/mL (range 600–6,800)]. Forty-three (44%) patients had high BKV viruria (≥ 1×107 copies/mL) post-HSCT. Of those, 21 had BKV viruria prior to stem-cell infusion and 22 developed BKV viruria after HSCT.
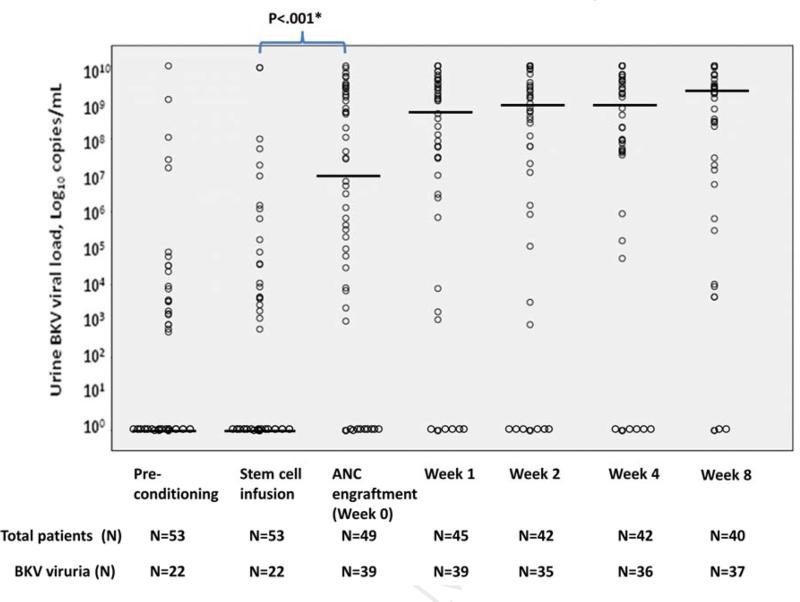
Figure 2 shows the BKV viral loads of patients with BKV viruria from pre-conditioning to 8 weeks. The median BKV viral load was higher at engraftment [median 6.8×106 copies/mL (range < 500-> 1.0×1010 copies/mL)] compared to day 0 [median of < 500 copies/mL (range < 500-9×109 copies/mL)] (P<.001). Median BKV viral loads were not different between all other neighboring time-points.
Figure 2.
BKV viral load among the 53 patients with BKV viruria.
Dots represent individual patients. Horizontal bars indicate median BKV viral load.
Abbreviations: ANC, absolute neutrophil count; Week 0, at ANC (absolute neutrophil count) engraftment; Week 1, at 1 week post-engraftment; Week 2, at 2 weeks post-engraftment; Week 4, at 4 weeks post-engraftment; Week 8, at 8 weeks post-engraftment. ANC engraftment is defined as the first of day of 3 three consecutive measurements of ANC > 500/mm3.
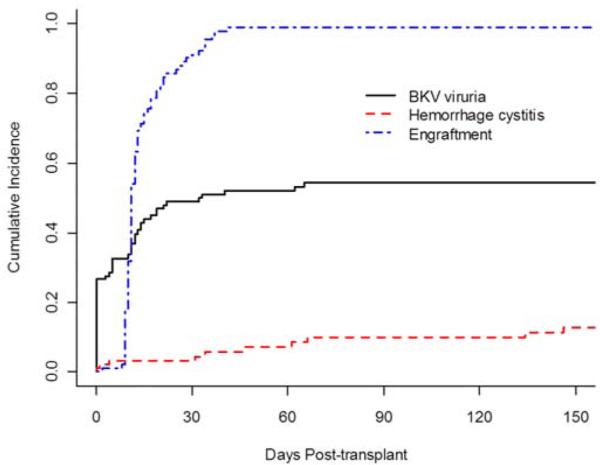
Figure 3 shows the cumulative incidence for BKV viruria, neutrophil engraftment and HC. Fifty-four percent of patients (95%CI: 44%-64%) had BKV viruria by D +100. Cumulative incidence of BKV viruria increased at the time of neutrophil engraftment and remained stable after D +30.
Figure 3.
Cumulative incidence rates for neutrophil engraftment, BKV viruria, and hemorrhagic cystitis.
Risk Factors for High BKV Viruria Post-HSCT
To identify risk factors for high level BKV viruria post-transplant, patient demographics, transplant characteristics, BKV IgG titers, and BKV viruria pre-transplant were examined in univariate and multivariate analyses (Table 2). Titers of BKV IgG, high BKV viruria pre-HSCT and myeloablative conditioning regimen were important in univariate analyses. Titers of BKV IgG (P=.013) and high BKV viruria pre-HSCT (P<.001) remained significant in multivariate analysis. Peri-transplant ciprofloxacin was not associated with high BKV viruria post-HSCT (P=.731)
Table 2.
Risk factors for high BKV viruria (≥ 107 copies/mL) post-HSCT.
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | ||
| Age (years) | 1 year increment | 1.00 (0.98,1.03) | .828 | ||
| Female | ref: male | 0.61 (0.32,1.14) | .123 | ||
| Keratinocyte growth factor | ref: no | 0.74 (0.37,1.46) | .383 | ||
| Titers of BKV IgG | 4-fold increment | 1.30 (1.00, 1.70) | .054 | 1.43 (1.08, 1.89) | .013 |
| Mismatched donor | ref: matcheda | 1.01 (0.56,1.83) | .963 | ||
| T-cell depletion (TCD) | ref: non-TCD | 1.04 (0.58,1.87) | .903 | ||
| Cord blood | ref: peripheral blood stem cell | 1.13 (0.61, 2.12) | .692 | ||
| Ciprofloxacin use | ref: no | 0.90 (0.49, 1.64) | .731 | ||
| Pre-HSCT BKV ≥ 1.0×107 | ref: < 1.0×107 | 7.00 (2.80, 17.46) | <.001 | 7.26 (2.24, 23.48) | <.001 |
| Myeloablative or reduced intensity conditioning | ref: NMA | 0.41 (0.21,0.81) | .011 | 0.54 (0.25, 1.15) | .110 |
| Total body irradiation (TBI) | ref: no TBI | 0.83 (0.46,1.49) | .526 | ||
| CMV seropositive recipient | ref: seronegative | 1.31 (0.7,2.46) | .405 | ||
Abbreviations: HSCT, hematopoietic stem cell transplantation; KGF, keratinocyte growth factor; TCD, T-cell depletion; MA, myeloablative; RIC, reduced intensity; NMA, nonmyeloablative; TBI, total body irradiation; CMV, cytomegalovirus; ref, reference
Matched donor included matched related donor and matched unrelated donor.
*acute graft versus host disease, forcarnet treatment, and CMX001 treatment were not included since BK viruria occurred before these events.
Risk Factors for Hemorrhagic Cystitis
During the study period, 10 patients (10%) developed HC. HC developed prior to neutrophil engraftment in 3 and after neutrophil engraftment in 7 patients. Four patients had CMV viremia, 3 had adenovirus viremia (2 with concomitant adenovirus viruria) and 3 had human herpes virus-6 (HHV-6) viremia. Five patients received IV foscarnet at the time of HC (3 patients for CMV and 2 for HHV-6).
Seven (7% of total) patients had BKV viruria at the time of diagnosis of HC. Three patients received TCD, 3 CB, and 1 patient conventional peripheral stem cell allograft. Two patients had concomitant adenovirus in the urine. Urine adenovirus was not checked in the remaining 5 patients. In univariate analyses, older age (P=.003), high BKV viruria (P=.001), CMV reactivation (P=.028), and treatment with foscarnet (P<.001) were risk factors for HC. Peri-transplant ciprofloxacin use was not associated with hemorrhagic cystitis (P=.094) (Table 3).
Table 3.
Risk factors for hemorrhagic cystitis.
| Factor | Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|
| Age (years) | 1 year increment | 1.07 (1.02,1.12) | .003 |
| Female | ref: male | 0.36 (0.08,1.62) | .185 |
| KGF | ref: no | 0.23 (0.03,1.88) | .170 |
| Serum BKV IgG titer | 4 fold increment | 1.06 (0.72,1.55) | .779 |
| Mismatched donor | ref: matcheda | 1.04 (0.30,3.63) | .956 |
| T-cell depletion (TCD) | ref: non-TCD | 0.58 (0.17,2.01) | .387 |
| Stem cell source Cord blood | ref: Peripheral blood | 2.22 (0.56,8.84) | .259 |
| aGVHD grade 2-4* | ref: aGVHD grade 0-1 | 3.46 (0.63, 19.02) | .153 |
| Ciprofloxacin use | ref=no | 0.18 (0.02,1.34) | .094 |
| Pre-HSCT urine BKV ≥ 1.0×107 copies/mL | ref < 1.0×107 | 9.01 (2.61,31.08) | .001 |
| Post-HSCT urine BKV ≥ 1.0×107 copies/mL* | ref < 1.0×107 | 9.07 (1.67, 49.16) | .011 |
| MA or RIC | ref: NMA | 1.51 (0.21, 10.80) | .680 |
| Total body irradiation | ref: no | 0.60 (0.16, 2.27) | .450 |
| CMX001 treatment* | ref: no | 2.96 (0.35, 25.09) | .316 |
| Foscarnet treatment* | ref: no | 20.20 (4.18, 97.66) | <.001 |
| CMV seropositive recipient | ref: seronegative | 1.43 (0.37, 5.52) | .600 |
| CMV reactivation* | ref: no | 6.36 (1.22, 33.09) | .028 |
Abbreviations: HSCT, hematopoietic stem cell transplantation; KGF, keratinocyte growth factor; TCD, T-cell depletion; aGVHD, acute graft versus host disease; MA, myeloablative; RIC, reduced intensity; NMA, nonmyeloablative; TBI, total body irradiation; CMV, cytomegalovirus; ref, reference
Matched donor included matched related donor and matched unrelated donor.
The post-transplant factor was examined as a time-dependent variable in cause-specific Cox regression.
Recurrence of HC
The clinical characteristics and outcomes of the 7 patients with HC and BKV viruria are shown in supplemental Table S1. Two patients developed recurrent HC (grade 3 at D +271 and grade 4 at D +204, respectively).
BKV Viruria and Renal Function Post-HSCT
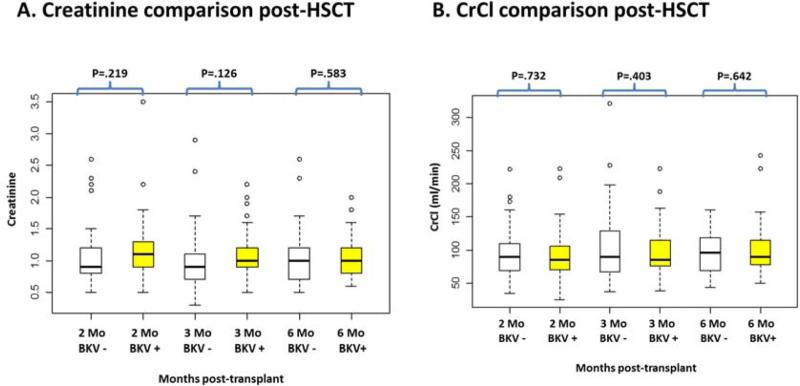
Creatinine (Cr) and CrCl values at 2, 3 and 6 months were not different between patients with BKV and without BKV viruria (Figure 4). Overall survival in patients survived at least 2 months were not different between BKV positive patients and BKV negative patients (P=.508).
Figure 4.
Comparison of serum creatinine and CrCl between patients with BKV and without BKV viruria post-HSCT at 2, 3, and 6 months. Panel A (left) shows creatinine comparison between patients with BKV and without BKV viruria at 2 months, 3 months and 6 months post-HSCT. Panel B (right) shows CrCl comparison between patients with BKV and without BKV viruria at 2 months, 3 months and 6 months post-HSCT. The central box represents the values from 25% to 75% and the middle line represents median. The dot represents outliers.
Abbreviations: CrCl, Creatinine Clearance; HSCT, hematopoietic stem cell transplantation; Mo, month
DISCUSSION
Hemorrhagic cystitis (HC) and renal dysfunction are well-recognized complications of HSCT. While several studies report an association between BKV and HC, a causative link has not been clearly established [6, 7, 22, 23]. Renal dysfunction is a late complication of HSCT. Factors implicated in the pathogenesis of renal dysfunction include toxicity of conditioning regimens, especially myeloablative regimens containing total body irradiation, calcineurin inhibitors, older age and viral infections [12, 13, 22, 24]. Recent studies in conventional HSCT suggest an association between BKV infection and renal dysfunction [12]. Recipients of TCD allografts do not receive calcineurin inhibitors in the absence of GVHD. Thus the relative contribution of viruses to renal dysfunction would be more pronounced in this patient population. Immunity to viral infections, including BKV, is dependent on the presence of virus-specific cytotoxic T cells (CTLs) transferred from the donor. Recipients of TCD or CB allografts would be expected to be at greater risk for BKV infection and BKV related complications due to the absence of BKV specific cells transferred through the allograft.
We monitored prospectively a cohort of 98 HSCT recipients comprising of 50% TCD and 21% cold blood allografts for BKV in the urine. All HSCT recipients had detectable BKV IgG pre-transplant. Incremental BKV IgG titers were associated with high BKV viruria. Our findings are in agreement with published studies in renal allografts and HSCT using laboratory developed tests for BKV IgG [5, 9]. BKV viruria was observed in approximately 20% of patients pre-transplant and 50% by neutrophil engraftment. Forty-seven percent of our cohort had BKV viral load > 1×107copies/mL. The cumulative incidence of BKV viruria was reached by D +30 and BKV viruria persisted for the duration of monitoring.
We observed an increase in the magnitude of BKV viral load at the time engraftment. This is a novel observation which merits further investigation. Leung et al. have previously reported that HC is preceded by an increase in BKV viral load and suggested that suppression of BKV viral load periengraftment may reduce the rate of early HC [25]. In our cohort, peri-engraftment HC was rare despite the observed increase in BKV viral load, suggesting that additional factors may be contributing to the pathogenesis of early HC.
Overall 10 (10%) patients developed HC. Seven patients had concomitant BKV viruria (7% of total study population). Our rate of HC is consistent with rates reported for conventional bone marrow or stem cell allografts [6]. Higher rates (up to 26%), however, have been reported in pediatric HSCT patients [8, 11]. During the study period, several changes in supportive care may have impacted rates of HC. Thirty (33%) patients received peri-transplant keratinocyte growth factor (KGF). In animal models, KGF showed a protective effect against cyclophosphamide-induced ulcerative hemorrhagic cystitis [26]. Successful treatment of HC with KGF has also been reported [27]. The impact of KGF on HC would be best addressed in a randomized clinical trial. During the study, 14 patients received brincidofovir for the treatment of Ds DNA viruses. While 8 patients received brincidofovir during the BKV monitoring, they all started after D +52. Since the cumulative incidence of BKV viruria was reached by D +30, it would be unlikely that brincidofovir had an impact on our observed rates of BK viruria. Only six patients received brincidofovir after the BKV monitoring period including 2 patients with BKV viruria and HC. CMX001 is active against BKV and may have impacted the risk of developing HC, but the number of patients who received brincidofovir was low [28]. Ongoing clinical trials of CMX001 in stem cell transplant recipient will address the role of CMX001 for prevention of BKV viruria and HC. Thirty-five patients received ciprofloxacin for routine peri-transplant bacterial prophylaxis or other indications. Ciprofloxacin has activity against BKV in vitro and prolonged use of ciprofloxacin has been shown to reduce rates of BKV viruria [25, 29]. Leung et al. however failed to demonstrate a protective effect of ciprofloxacin against HC in HSCT recipients [25]. In our univariate analyses, peri-transplant ciprofloxacin was not associated with high BKV viruria or hemorrhagic cystitis. The exposure to ciprofloxacin as antibacterial prophylaxis was brief, so it is unlikely to have had any significant impact on the rates of BKV viruria and HC.
Older age, high grade BKV viruria pre- or post-HSCT, CMV infection and foscarnet use were associated with HC. CMV is known to have immunomodulatory properties and may facilitate reactivation of other latent viruses. An association between CMV and BKV reactivation has been suggested in renal allograft recipients but was not confirmed in a prospective study [3, 30]. HC is a recognized side-effect of foscarnet with incidence up to 40% [31]. Thirty nine (39%) of our patients received treatment with foscarnet for CMV or HHV-6 infection. Our sample size precluded multivariate analyses. Although none of the first episodes of HC were severe (grade 3-4), 2 patients with recurrent HC developed late severe HC. Both patients were severely immunosuppressed and had concomitant viral infections, further supporting a multifactorial etiology for late HC.
Studies on the role of polyoma viruses [BKV and John Cunningham Virus (JCV)] in renal dysfunction following liver or kidney transplantation have yielded conflicting results. Kusne et al. reported that BKV viruria was not associated with renal dysfunction in kidney allograft recipients. In contrast JCV viruria was associated with renal dysfunction in liver transplant recipients [32]. Recent studies suggest an association of BKV viremia with renal dysfunction after conventional HSCT [12]. We found no difference in Cr or CrCL among patients with and without BKV viruria.
The limitations of our study are inherent to its observational nature, relatively small sample size and heterogeneity of our patient population with regards to underlying diagnoses, stem cell sources and conditioning regimens. Several supportive care modalities were implemented gradually during the study period may have affected the natural history of HC. Third, low rates of HC precluded multivariate analyses of risk factors. Fourth, we monitored exclusively for BKV viruria and not for additional viruses implicated in HC or renal dysfunction (adenovirus, CMV or JCV) [33].
In summary: 1) Rates of BKV viruria and HC in TCD and CB allograft recipients were comparable to rates in conventional allograft recipients; 2) High BKV IgG titers were associated with “high” BKV viruria; 3) BKV viral load increased at neutrophil engraftment and persisted through D +100; 4) While the cumulative incidence for BKV viruria was reached by D +30, severe BKV-associated HC occurred late post-transplant suggesting additional co-factors; 5) We could not demonstrate an association between BKV viruria and creatinine clearance in the first 6 months post-HSCT. Randomized clinical trials are needed to assess the effect of BKV suppression or keratinocyte growth factor on BKV viruria and related complications in T-cell depleted and cord blood HSCT.
Supplementary Material
Acknowledgements
The authors thank Cesar Figueroa for assisting with sample collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: Supported in part by National Institutes of Health grant P01 CA023766 immunobiology for Marrow Allografts for Leukemia.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: YJ.L. performed research, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript. J.Z. performed statistical analysis. Y.K. collected data. D.C. collected data. I.A. contributed to research design. T.S. collected data. K.C. collected data. J.H. contributed to study design, laboratory testing, data interpretation, and wrote the manuscript. S.A.G. wrote the manuscript. I.G.G. interpreted data and wrote the manuscript. A.A.J. designed the research, interpreted data, and wrote the manuscript. G.A.P. designed the research, analyzed and interpreted data, and wrote the manuscript.
REFERENCES
- 1.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HH. BK virus: opportunity makes a pathogen. Clin Infect Dis. 2005;41:354–360. doi: 10.1086/431488. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 4.Drachenberg CB, Papadimitriou JC, Hirsch HH, et al. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. Am J transplant. 2004;4:2082–2092. doi: 10.1046/j.1600-6143.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- 5.Bohl DL, Brennan DC, Ryschkewitsch C, Gaudreault-Keener M, Major EO, Storch GA. BK virus antibody titers and intensity of infections after renal transplantation. J Clin Virol. 2008;43:184–189. doi: 10.1016/j.jcv.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva Lde P, Patah PA, Saliba RM, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–1190. doi: 10.3324/haematol.2009.016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung AY, Suen CK, Lie AK, Liang RH, Yuen KY, Kwong YL. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood. 2001;98:1971–1978. doi: 10.1182/blood.v98.6.1971. [DOI] [PubMed] [Google Scholar]
- 8.Cesaro S, Facchin C, Tridello G, et al. A prospective study of BK-virus-associated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transpl. 2008;41:363–370. doi: 10.1038/sj.bmt.1705909. [DOI] [PubMed] [Google Scholar]
- 9.Wong AS, Chan KH, Cheng VC, Yuen KY, Kwong YL, Leung AY. Relationship of pretransplantation polyoma BK virus serologic findings and BK viral reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2007;44:830–837. doi: 10.1086/511863. [DOI] [PubMed] [Google Scholar]
- 10.Giraud G, Priftakis P, Bogdanovic G, et al. BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA mismatched grafts. Bone Marrow Transpl. 2008;41:737–742. doi: 10.1038/sj.bmt.1705962. [DOI] [PubMed] [Google Scholar]
- 11.Gorczynska E, Turkiewicz D, Rybka K, et al. Incidence, clinical outcome, and management of virus induced hemorrhagic cystitis in children and adolescents after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:797–804. doi: 10.1016/j.bbmt.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell PH, Swanson K, Josephson MA, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biol Blood Marrow Tr. 2009;15:1038–1048. e1. doi: 10.1016/j.bbmt.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haines HL, Laskin BL, Goebel J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Tr. 2011;17:1512–1519. doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Raval M, Gulbis A, Bollard C, et al. Evaluation and management of BK virus-associated nephropathy following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Tr. 2011;17:1589–1593. doi: 10.1016/j.bbmt.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papanicolaou G, Kolitsopoulos Y, Young J, et al. BK Virus-Associated Nephropathy (BKVN), an Under Recognized Cause of Renal Dysfunction in Severely Immunosuppressed Hematopoietic Stem Cell Transplant (HSCT) Patients: Report of 5 Cases of Bkvn and the Potential Role of CMX001 for Treatment. Biol Blood Marrow Tr. 2013;19:S302–S3. [Google Scholar]
- 16.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Tr. 2011;17:1335–1342. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57(2):267–276. [PubMed] [Google Scholar]
- 18.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Brit J Haemat. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 19.Zaia J, Baden L, Boeckh MJ, et al. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transpl. 2009;44:471–482. doi: 10.1038/bmt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedi A, Miller CB, Hanson JL, et al. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol. 1995;13:1103–1109. doi: 10.1200/JCO.1995.13.5.1103. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Glezerman IG, Jhaveri KD, Watson TH, et al. Chronic kidney disease, thrombotic microangiopathy, and hypertension following T cell-depleted hematopoietic stem cell transplantation. Biol Blood Marrow Tr. 2010;16:976–984. doi: 10.1016/j.bbmt.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Eng J Med. 1986;315:230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 24.Delgado J, Cooper N, Thomson K, et al. The importance of age, fludarabine, and total body irradiation in the incidence and severity of chronic renal failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Tr. 2006;12:75–83. doi: 10.1016/j.bbmt.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 25.Leung AY, Chan MT, Yuen KY, et al. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:528–537. doi: 10.1086/427291. [DOI] [PubMed] [Google Scholar]
- 26.Ulich TR, Whitcomb L, Tang W, et al. Keratinocyte growth factor ameliorates cyclophosphamide induced ulcerative hemorrhagic cystitis. Cancer Res. 1997;57:472–475. [PubMed] [Google Scholar]
- 27.Czibere A, Bruns I, Graef T, et al. Treatment of severe hemorrhagic cystitis after allogeneic stem cell transplantation with palifermin, a recombinant human keratinocyte growth factor. Biol Blood Marrow Tr. 2007;13:872–874. doi: 10.1016/j.bbmt.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Rinaldo CH, Gosert R, Bernhoff E, Finstad S, Hirsch HH. 1-O-hexadecyloxypropyl cidofovir (CMX001) effectively inhibits polyomavirus BK replication in primary human renal tubular epithelial cells. Antimicrob Agents Ch. 2010;54:4714–4722. doi: 10.1128/AAC.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller AN, Glode A, Hogan KR, et al. Efficacy and safety of ciprofloxacin for prophylaxis of polyomavirus BK virus-associated hemorrhagic cystitis in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Tr. 2011;17:1176–1181. doi: 10.1016/j.bbmt.2010.12.700. [DOI] [PubMed] [Google Scholar]
- 30.Barri YM, Ahmad I, Ketel BL, et al. Polyoma viral infection in renal transplantation: the role of immunosuppressive therapy. Clin Transplant. 2001;15:240–246. doi: 10.1034/j.1399-0012.2001.150404.x. [DOI] [PubMed] [Google Scholar]
- 31.Mancini M, Matozzo V, Previtali D, Ciceri F. Observational retrospective study on the incidence of haemorrhagic cystitis and genital lesions in allogenic THSC patients treated with foscarnet. Bone Marrow Transpl. 2011;46:S417–S. [Google Scholar]
- 32.Kusne S, Vilchez RA, Zanwar P, et al. Polyomavirus JC urinary shedding in kidney and liver transplant recipients associated with reduced creatinine clearance. J Infect Dis. 2012;206:875–880. doi: 10.1093/infdis/jis469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006;43:331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.