Abstract
NK cell efficacy correlates with in vivo proliferation and we hypothesize that NK cell product manipulations may optimize this endpoint. Xenotransplantation was used to compare GMP grade freshly activated NK cells (FA-NK) and ex vivo expanded NK cells (Ex-NK). Cells were infused into NSG mice followed by IL-2, IL-15, or no cytokines. Evaluation of blood, spleen and marrow showed that persistence and expansion was cytokine dependent, IL-15 being superior to IL-2. Cryopreservation and immediate infusion resulted in less cytotoxicity and fewer NK cells in vivo and this could be rescued in FA-NK by overnight culture and testing the next day. Marked differences in the kinetics and homing of FA-NK versus Ex-NK were apparent: FA-NK cells preferentially homed to spleen, and persisted longer after cytokine withdrawal. These data would suggest that cryopreservation of FA-NK and Ex-NK is detrimental and that culture conditions profoundly affect homing, persistence and expansion of NK cells in vivo. The NSG mouse model is an adjuvant to in vitro assays prior to clinical testing.
Introduction
Natural Killer (NK) cells recognize targets altered by malignant transformation or infection. The first trials in humans to harness the anti-tumor properties of NK cells focused on the use of in vivo IL-2 to activate autologous NK cells. Ex vivo IL-2 activation of NK cells prior to infusion resulted in enhanced recovery of NK cell cytotoxicity in vivo compared to post-infusion IL-2 administration alone, but efficacy was probably limited by: 1) competition with the recipient’s lymphocytes for cytokines and ‘space’, 2) inhibition of autologous NK cells by self MHC, and 3) chronic immunosuppression induced by the tumor on host immunity. As inhibitory KIR and their ligands were further characterized, the next approach to utilizing NK cells as immunotherapy focused on allogeneic NK cells from healthy related donors. In this setting, allogeneic NK cells avoid tumor-induced suppression and have the advantage of being educated and fully functional.
The first trial of this approach was published in 2005 from the University of Minnesota [1]. Forty-three patients with metastatic melanoma, metastatic renal cell carcinoma or poor prognosis AML were enrolled. Peripheral blood was collected by apheresis from haploidentical related donors and NK cells were enriched before being incubated overnight in high dose IL-2. Prior to NK cell infusion, patients underwent one of three chemotherapy preparative regimens: high cyclophosphamide and fludarabine (Hi-Cy/Flu) that was potently lymphodepleting, or a lower intensity regimen of either low dose cyclophosphamide and methylprednisone, or fludarabine alone. Following infusion patients received IL-2 daily for 14 days. NK cell persistence was only observed in patients receiving the lymphodepleting preparatory regimen of Hi-Cy/Flu given to AML patients. On this initial protocol 30% of poor prognosis AML patients achieved a complete remission, which correlated with the presence of donor NK cells 7 and 14 days after infusion. Based on this, goals to improve NK cell based immunotherapy have focused on in vivo expansion as a surrogate biomarker to enhance efficacy. Cytokine choice may play a role in NK cell expansion. Although in vivo NK cell expansion is enhanced by cytokines, IL-2 can also stimulate regulatory T-cells (Treg) [2, 3], which can be avoided by use of IL-15 [4, 5].
In an alternative approach to enhance in vivo expansion, Lapteva et al have developed ex vivo GMP compatible NK cell expansion strategies [6] based on the use of K562 feeders transduced with membrane bound IL-15 and 41BB-ligand initially described by the Campana group [7]. It is unknown whether freshly isolated NK cells followed by post infusion cytokines (in vivo NK cell expansion) or ex vivo expansion strategies or both are the best to achieve efficacy, the goal of clinical trials. In vitro functional assays are of limited use to address this endpoint. Therefore, the goal of this study was to use a xenogeneic adoptive transfer model to examine the impact of clinical NK cell production methods and post-infusion cytokine administration on in vivo NK cell expansion. It is hoped that these results will guide the design of effective cancer therapies utilizing NK cells.
Materials and Methods
NK Cell isolation, processing and functional testing
All studies were in accordance with the Declaration of Helsinki and guidelines approved by the Committees on the Use of Human Subjects and Animals in Research. Non-mobilized apheresis products were collected from the University of Minnesota and Baylor College of Medicine (BCM). For production of FA-NK, NK cells were enriched from mononuclear cells (MNCs) by CD3+ and CD19+ cell depletion (Miltenyi Biotec, Bergisch Gladbach, Germany) followed by overnight IL-2 (Proleukin, 1000 U/ml; Prometheus, San Diego, California) incubation under cGMP [8]. Ex-NK were generated by culture of buffy coat MNCs with K562 cells expressing membrane bound IL-15 and 41BB-L and 10 U/ml IL-2 in G-Rex (Wilson Wolf, Minneapolis, MN) chambers for 10 days. On the day prior to shipping, contaminating CD3+ cells were removed [6]. NK cells were split and either infused directly or cryopreserved in 10% DMSO, then thawed, recounted and injected. NK cells were tested for function using a Cr51 release assay with K562 targets or by flow cytometry [9, 10].
Xenogeneic NK cell adoptive transfer
NOD/IL-2Rγc/Rag (NSG) mice were given 250 cGy total body irradiation by x-ray, and infused IV with 1–2 million fresh or frozen NK cells. Mice were left untreated, or were given 5 or 10 mcg of huIL-2 (Novartis), or 5 mcg huIL-15 (NCI-Frederick) in 6 ip injections over 2 weeks. PB, spleen and marrow were evaluated after adoptive transfer. Human NK cell counts were converted to absolute cell numbers per indicated volume of blood or the number of cells recovered per spleen or after flushing both femurs. For comparisons, a Student’s T-test was used with paired analysis where appropriate.
Flow cytometry
Blood or tissue were stained according to standard protocols. Antibodies used were: CD45 Horizon V500, CD56 PE-Cy7, and CD158b FITC (clone CH-L) from BD Biosciences; CD3 PE-Texas Red from Life Technologies; NKG2A Allophycocyanin from Beckman Coulter; CD158a PerCP-Cy5.5 (clone HP-MA4) from eBioscience; NKG2C Phycoerythrin from R&D Systems; CD57 Pacific Blue and CD158e1 Alexa Fluor 700 (clone DX9) from Biolegend.
Results
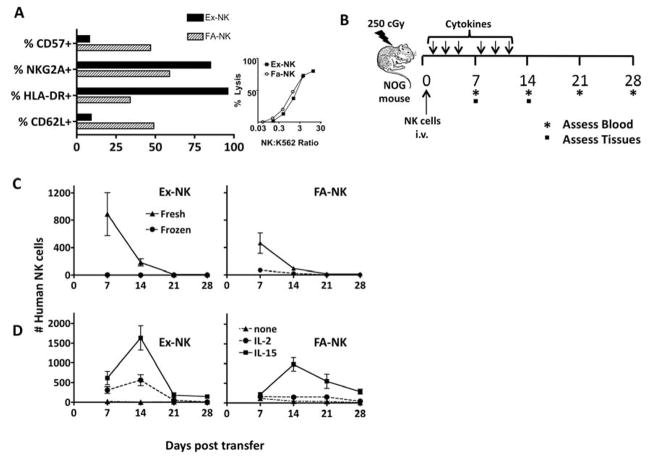
We propose that NK cell product manipulations may optimize in vivo proliferation to provide better therapy for treatment of cancer. Two cGMP-grade NK cells products produced by different Production Assistance for Cellular Therapies (PACT) facilities were evaluated: FA-NK used by the Minnesota group for the past 10 years, and Ex-NK developed as a cGMP product by the BCM group [6]. Ex-NK contained a higher proportion of CD56+/CD3− NK cells compared to FA-NK cells, which contained monocytes, but when Effector:Target ratios in the Cr51 release killing assay were normalized to NK cell frequency, the killing at each E:T ratio was similar (Fig. 1A). Phenotypically there were differences between the cell products (Fig 1A). Ex-NK had a lower proportion of the homing receptor CD62L, possibly related to activation induced shedding of CD62L [11], and a lower proportion of the mature CD57+ cells, likely explained by the higher proliferative rate of less mature CD57 negative cells [12, 13]. Compared to FA-NK, Ex-NK also had higher levels of the NKG2A inhibitory receptor, as well as HLA-DR, likely due to a longer culture period.
Figure 1. cGMP grade ex vivo expanded NK (Ex-NK) cells and fresh activated NK (FA-NK) cells differ in phenotype and their ability to expand in mice in vivo.
(A) The Ex-NK cell product contained an average of 88% NK cells while the FA-NK cell product contained 40% NK cells after CD3 and CD19 depletion with the remaining cells being CD14+ monocytes (not shown). Inset shows percent of Cr51 labeled K562 cells lysed when incubated for 4h with Ex-NK or FA-NK cells at the indicated ratios. (B) NSG mice were treated with 250 cGy and then Ex-NK or FA-NK cells (1–2 million absolute NK cells were held constant within each experiment) were infused intravenously. Cytokines were administered after cell infusion as indicated. Blood was sampled weekly and tissues collected as indicated. (C) The number of human CD45+ NK cells in 100 μL of blood collected at the various times after transfer of Ex-NK or FA-NK cells obtained either directly from in vitro cultures (fresh) or thawed after cryopreservation in 10% DMSO (frozen). All mice were given IL-2 (6 doses, 5 mcg per dose). Data are means ± SEM (n=4) (D) The number of human CD45+ NK cells in 100 μL of blood collected at the indicated times after transfer of Ex-NK or FA-NK from fresh cultures. Mice were given cytokines as indicated; IL-2 was given in 6 doses of 10 mcg/dose; IL-15 was given in 6 doses of 5 mcg/dose. Data are means ± SEM (n=6–16, depending on time point and group, as selected mice were sacrificed 8 or 15 days after cell infusion).
We first transferred fresh and frozen Ex-NK and FA-NK cells followed by IL-2 administration (5 mcg dose) and determined the kinetics of NK cell expansion and persistence. Given the differences in the composition of the cell products, each product was adjusted so that the same absolute number of CD56+CD3− NK cells was infused. We evaluated the cytokine dependent expansion phase (days 0–14) and a cytokine withdrawal phase (days 14–28) mimicking the proposed dosing strategy used in patients [2], as indicated in the schema in Fig 1B. NK cells were present at day 7 after adoptive transfer for both fresh Ex-NK and FA-NK cells but there was no in vivo expansion for either product between days 7 and 14 with IL-2 administration (Fig. 1C). Despite immediate rate-controlled freezing using clinical methods and high viability post-thaw (Ex-NK were 93% viable post-thaw; FA-NK were 92% viable post-thaw), cryopreserved NK cells that were thawed and immediately infused were found in much smaller numbers in vivo than fresh cells from both products, suggesting that they are sensitive to stress immediately after thawing.
We then compared the cytokine dependence of each NK cell product, giving 5 mcg (not shown) or 10 mcg IL-2, 5 mcg of IL-15 or no cytokines in each of the 6 doses (Fig. 1D). Seven days after adoptive transfer, there were significantly more NK cells recovered from mice given the Ex-NK vs FA-NK product and IL-15 (comparing day 7 Ex-NK to FA-NK: p=0.03). IL-15, but not IL-2, resulted in a significant increase in NK cells between days 7 and 14 in mice given either NK product (comparing day 7 to day 14 for each product in mice given IL-15, Ex-NK: p=0.004; FA-NK: p=0.00004). These results demonstrate the dependence of NK cell persistence and expansion on exogenous cytokine administration with IL-15 being more potent than IL-2. Importantly, NK cells recovered on day 14 from the blood of mice given IL-15 and both products were functional, as indicated by their ability to degranulate and produce interferon-γ after exposure to K562 targets (not shown). Mice sampled on day 21, one week after the final cytokine dose, showed a loss of NK cells in PB. Ex-NK cells from mice given IL-15 showed a 90% decrease in numbers, while FA-NK cells decreased by only 45%. One explanation for this difference may be greater cytokine “addiction” of the Ex-NK cells [14, 15]. Alternatively, different migration patterns may explain these differences.
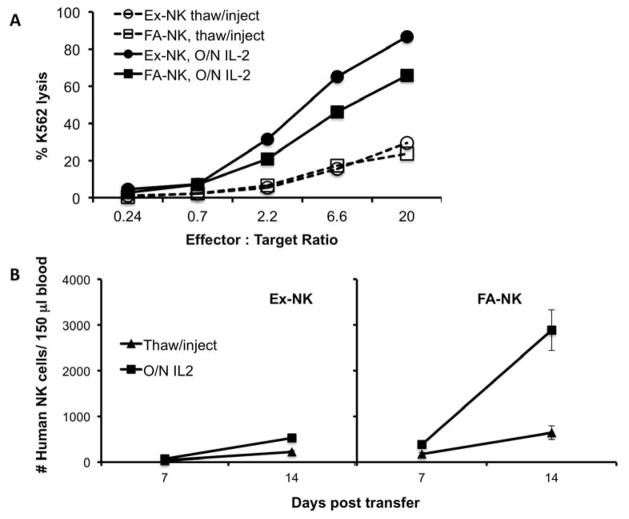
Given the potent effect of in vivo IL-15, we next examined the ability of thawed NK cells to expand in vivo with IL-15. We tested Ex-NK and FA-NK that were infused immediately after thawing or after overnight culture with IL-2, in an attempt to improve in vivo survival of cryopreserved cells. We evaluated cytotoxicity against K562 targets immediately after the thaw and after the overnight culture (Fig. 2A). Both Ex-NK and FA-NK were weakly cytotoxic immediately after thaw and both recovered cytotoxic activity after culture with overnight IL-2. Viabilities of both products were high after thawing (Ex-NK: 89%; FA-NK: 90%), however only 20% of Ex-NK cells remained after overnight culture, while 73% of FA-NK cells survived. Equal numbers of viable Ex-NK and FA-NK cells, both immediately post-thaw and after overnight culture, were injected into NSG mice. Mice were given IL-15 (6 doses of 5 mcg per dose) and PB was evaluated on day 7 and day 14 for the presence of human NK cells (Fig. 2B). NK cells that were injected immediately after thaw were found in slightly higher numbers in mice given IL-15 (Fig. 2B) than in mice given IL-2 (Fig. 1D). Overnight culture of thawed Ex-NK cells before transfer resulted in only a slight increase in the number of human NK cells found in the blood on days 7 or 14. This was not surprising since most of the Ex-NK cells did not survive the overnight culture therefore further optimization of rescue culture conditions for this product are needed to improve subsequent survival in vivo. In contrast, thawed and cultured FA-NK cells expanded to a similar extent as fresh FA-NK cells in mice given IL-15 (Fig. 1D).
Figure 2. Thawed cGMP grade Ex-NK and FA-NK cells expand in vivo when cultured overnight with IL-2 before adoptive transfer.
Ex-NK and FA-NK products were thawed after cryopreservation in 10% DMSO and transferred to NSG mice either immediately (Thaw/inject) or after overnight culture with IL-2 (O/N IL-2). All mice were subsequently given 6 doses of IL-15 at 5 mcg/dose. (A) Cytotoxic activity of each product is shown as the percent of Cr51 labeled K562 cells lysed when incubated for 4h with each product at the indicated ratios. (B) The number of human CD45+ NK cells in 150 μl of blood collected on day 7 and day 14 after transfer. Results shown are the means ± SEM, n=6 mice per group.
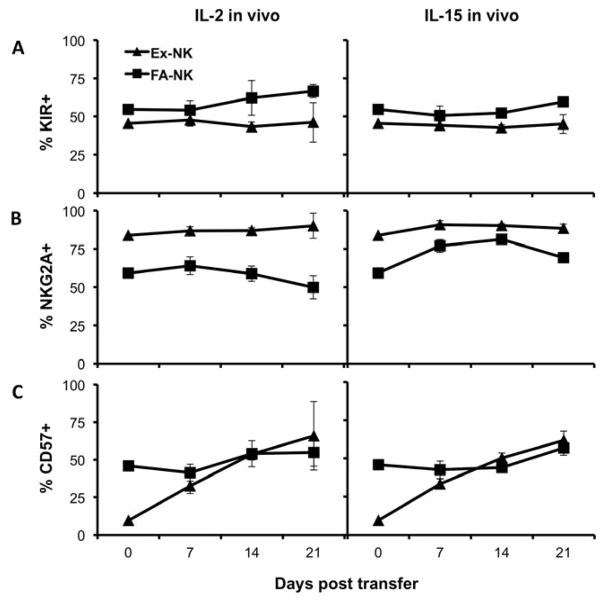
Since there were differences in the phenotype of the two NK products prior to adoptive transfer, we examined the phenotype of human NK cells in PB to determine if it would change over time or with different cytokine conditions. We found that the fraction of NK cells that expressed any of three KIR receptors remained stable (Fig. 3A) as well as the fraction expressing each individual KIR (data not shown). The percent of Ex-NK cells that expressed NKG2A was high before transfer and remained high while either IL-2 or IL-15 was being given and one week after cytokine withdrawal (Fig. 3B). NKG2A expression was less on FA-NK cells than on Ex-NK cells before adoptive transfer. In mice given FA-NK cells followed by IL-2, the average percent of NKG2A expression remained constant during the period of cytokine delivery. In contrast, in mice given FA-NK cells and IL-15 the percent of NK cells that were NKG2A+ increased by day 7 in all 16 mice, and continued to increase through day 14 (p=0.017 by paired t test). The average frequency of NKG2A+ cells decreased one week after withdrawal of either cytokine in mice given FA-NK cells, although this was only significant in mice given IL-15 (p=0.1 for mice given IL-2; p<0.001 for mice given IL-15 by paired t test). These results indicate that high NKG2A expression is a characteristic of the ex vivo expanded product and remains stable after adoptive transfer and different cytokine conditions, while NKG2A expression on FA-NK cells is more responsive to IL-15. CD57 is a marker of terminal maturation on NK cells [12, 13], and was expressed on a much smaller percentage of Ex-NK cells than FA-NK cells (9.4% and 45.8% CD57+, respectively) prior to adoptive transfer. We measured CD57 expression on the transferred NK cells in PB over time and found that expression on FA-NK cells was unchanged in mice given either IL-2 or IL-15, but increased significantly on Ex-NK cells in response to either cytokine (Fig. 3C). Since Ex-NK cells are highly cytokine dependent we were only able to evaluate CD57 expression in the absence of exogenous cytokine on day 7 after transfer (data not shown). At that time the frequency of CD57+ NK cells was higher in 12 of the 16 mice compared with the pre-transfer percentage, with an average of 18%, while all of the mice given either IL-2 or IL-15 had a higher CD57+ percentage compared with the pre-transfer value, and an average of 33% CD57+ in each group. These results suggest that while in vivo transfer alone may have some effect on CD57 expression, the addition of cytokines drives additional increases in CD57 expression on Ex-NK cells in vivo.
Figure 3. Phenotype of cGMP grade Ex-NK and FA-NK cells in the blood varies with NK cell source and cytokine conditions.
Cells and mice were as described in Fig 1D. (A) Percent of human NK cells expressing any of three KIRs (CD158a, CD158b, or CD158e1) (B) Percent of human NK cells expressing NKG2A. (C) Percent of human NK cells expressing CD57. Results shown are the means ± SD, n= 7–16, depending on time point and group).
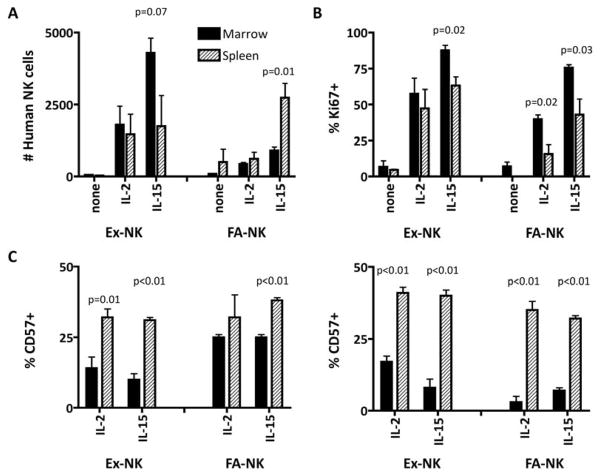
For immune cells to be efficacious, they must achieve favorable biodistribution into sites of tumor. Thus, we evaluated tissue recovery of human NK cells in groups of mice at day 8 (Fig. 4A) and day 15 (data not shown) after adoptive transfer. In the absence of cytokine administration, NK cells were barely detectable in blood or tissues. Mice given Ex-NK cells and IL-15 had higher numbers of NK cells in BM than in mice given Ex-NK cells and IL-2; conversely, mice that received FA-NK cells had more NK cells in the spleen when given IL-15, showing dramatic homing differences between the two cell products. After adoptive transfer, more Ki-67+ proliferating NK cells were found in BM than spleen; the differences were significant for both products in IL-15 treated mice (Fig. 4B). Lastly, CD57 was expressed by more NK cells recovered from spleen than BM on day 8 for the Ex-NK product (Fig. 4C, left) and the preferential accumulation in the spleen of terminally differentiated CD57+ NK cells, presumed to exhibit maximal effector function, was further enhanced on day 15 for both products (Fig. 4C, right). Expression of CD57 in the spleen mirrored closely the expression on NK cells in the blood (Fig 3C). The preferential homing of Ex-NK cells to bone marrow, and the lower expression of CD57 on Ex-NK cells, suggest that CD57 expression could be a predictor of in vivo trafficking preference, at least for spleen compared with bone marrow.
Figure 4. cGMP grade Ex-NK and FA-NK cells have different biodistribution in bone marrow and spleen.
(A) The number of human NK cells in bone marrow and spleen of mice given Ex-NK or FA-NK cells and cytokines as shown in Figure 1E. (B) The percent of proliferating (as evidenced by co-expression of Ki-67 on CD56+/CD3− cells) human NK cells 8 days after adoptive transfer of Ex-NK or FA-NK cells in bone marrow or spleen, respectively. (C) The proportion of CD57+ human NK cells 8 (left panel) and 15 (right panel) days after adoptive transfer of Ex-NK or FA-NK cells in bone marrow or spleen, respectively. Data are mean ± SE for all analyses (n=4). Where indicated, P values were determined using an unpaired t test to compare results for bone marrow and spleen within a treatment group.
Discussion
NK cells have potent anti-tumor activity when they are not constrained by class I-recognizing inhibitory receptors. They are particularly attractive as therapeutic effectors because they do not cause graft-versus-host disease (GvHD) and they can be selected therapeutically using available GMP reagents. One of the challenges of NK cell therapy is how to optimize the effector to target ratio dose that is critical for cytotoxicity and ultimately clinical efficacy. Two approaches have been tested to increase NK cell numbers in an attempt to promote anti-tumor efficacy. One strategy is aimed at in vivo expansion of adoptively transferred NK cells that can be obtained with a single apheresis product (1–2 × 107 NK cells/kg). The success of this strategy depends on a lymphodepleting chemotherapy regimen. One of the complexities of this therapy is that in vivo expansion is achieved in a low number of patients. It may be that cancer patients have the ability to reject rapidly adoptively transferred cells or that the cancer itself directly inhibits NK cell immunity. An alternative approach is to expand NK cells ex vivo to markedly increase the number of infused cells by use of K562 stimulators transduced with 41BB-ligand and IL-154 or IL-21 [16] or the use of lymphoblastoid cell line targets [17]. Compared with resting cells these expanded NK cells change receptors important in activation pathways needed for cytokine production and cytotoxicity. However, cytokine withdrawal may be detrimental to cell function. As one contemplates comparative studies between in vivo and ex vivo expansion approaches, several questions need to be answered. Cells that are activated may change shape and adhesion receptor profiles. How this affects homing and targeting to tumors is not known and cannot be tested in vitro. It is also unclear whether expansion of NK cells in vivo or ex vivo may lead to better survival and persistence, the goal of our study.
This xenogeneic model provides definitive readouts with distinct characteristics of FA-NK and Ex-NK cell products that may inform clinical efficacy. Common to both products is the profound negative impact of cryopreservation. This can be rescued in FA-NK by thawing and overnight culture with IL-2. However the same strategy used on Ex-NK was significantly less effective for that product suggesting that other methods are needed. There was a significant benefit of IL-15 to support in vivo proliferation of NK cells in vivo after adoptive transfer from both products. We find that better in vivo expansion with IL-15 leads to enhanced expression of NKG2A on NK cells in vivo, consistent with the activation dependent expression of this inhibitory receptor, which is also increased early after hematopoietic transplantation. For tumors that highly express HLA-E, high [18] expression of this inhibitory receptor may not be optimal but this needs to be tested clinically. These considerations are important as IL-15 is being developed clinically and is currently in phase I clinical trials. The rapid loss of Ex-NK cells between days 14 and 21 after IL-15 withdrawal compared to FA-NK cells emphasizes the importance of continued cytokine stimulation for ex vivo expanded cells. In addition to kinetic differences, we identify different homing properties after infusion for the two NK cell products. Our findings support the possibility that different properties of NK cell products may be exploited to treat cancer in the marrow or spleen but this will require comparative clinical trials. It is still unknown whether preferential homing has any relevance in eradicating tumor. Adapting this strategy in tumor bearing mice will be studied in the future to more definitely test these approaches.
Acknowledgments
This work was supported in part by NIH P30 CA77598 utilizing the following Masonic Cancer Center, University of Minnesota shared resources: Translational Cell Therapy and Flow Cytometry.
We would like to acknowledge Darin Sumstad and Diane Kadidlo for GMP processing at the Minnesota Molecular and Cellular Therapeutics Facility.
Grants and financial support: These studies were supported in part by P01 CA65493 (JSM, BRB, JT, JW, MRV), P01 CA111412 (JSM, MRV), and in part with Federal funds from the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contracts HHSN268201000008C (MN, JEW, JSM) and HHSN268201000007C (Baylor, CMR, NL).
Footnotes
Conflict of interest statement: We declare that there is no conflict of interest on behalf of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 2.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer immunology, immunotherapy: CII. 2010;59:1739–44. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–26. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 117:4787–95. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 14:1131–43. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenna DH, Jr, Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, et al. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–8. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 9.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189:5082–8. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118:2784–92. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 121:3599–608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 116:3853–64. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 108:14725–32. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heemskerk B, Liu K, Dudley ME, Johnson LA, Kaiser A, Downey S, et al. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum Gene Ther. 2008;19:496–510. doi: 10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-Bound IL-21 Promotes Sustained Ex Vivo Proliferation of Human Natural Killer Cells. PloS one. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg M, Childs R. Ex-vivo expansion of NK cells: what is the priority--high yield or high purity? Cytotherapy. 2010;12:969–70. doi: 10.3109/14653249.2010.536216. [DOI] [PubMed] [Google Scholar]
- 18.Cooley S, McCullar V, Wangen R, Bergemann TL, Spellman S, Weisdorf DJ, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–6. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]