Abstract
Organization of the central visual pathway is generally studied from a perspective of feedforward processes. However, there are horizontal connections and also strong feedback from extra striate to visual cortex. Here, we use visual stimuli designed to maximize relative differential involvements of these three main types of connections. The approach relies on differences between stimulation within the classical receptive field (CRF) and that of the surround region. Although previous studies have used similar approaches, they were limited primarily to spatial segregation of neural connections. Our experimental design provides clear segregation of fast and slow components of surround modulation. We assume these are mediated by feedback and horizontal connections, respectively, but other factors may be involved. Our results imply that both horizontal and feedback connections contribute to integration of visual information outside the CRF and provide suppressive or facilitative modulation. For a given cell, modulation may change in strength and sign from suppression to facilitation or the reverse depending on surround parameters. Sub-threshold input from the CRF surround increases local field potential (LFP) power in distinct frequency ranges which differ for suppression and facilitation. Horizontal connections have delayed CRF-surround modulation and are sensitive to position changes in the surround. Therefore, surround information beyond the CRF is initially processed by fast connections which we consider to be feedback, whereas spatially tuned mechanisms are relatively slow and presumably mediated by horizontal connections. Overall, results suggest that convergent fast (feedforward) inputs determine size and structure of the CRFs of recipient cells in visual cortex. And fast connections from extra striate regions (feedback) plus slow tuned connections (horizontal) within visual cortex contribute to spatial influences of CRF surround activation.
Keywords: visual cortex; feedforward, feedback, horizontal connections; classical receptive field; extra- classical receptive field
1. Introduction
The classical RF (CRF) of the visual system refers to spatial territory within which, appropriate stimulation can generate spike activity from a single neuron. Stimulation outside the CRF cannot independently activate the neuron, but it can influence output from the cell. CRF organization changes from early to central visual pathways. Most neurophysiological studies assume a hierarchical processing model such that information is encoded sequentially along the pathway (Hubel and Wiesel, 1962).
However, along with serial processing, parallel information flow occurs within a feedforward mechanism (Livingstone and Hubel, 1988; Nassi and Callaway, 2009). Anatomical studies demonstrate two additional major types of intercellular connections. One is feedback from extra striate regions (Peters et al., 1994; Sherman and Guillery, 1996; Budd, 1998; Galuske et al., 2002). The other is a horizontal pathway between adjacent cells in visual cortex (Rockland and Lund, 1983; Hirsch and Gilbert, 1991; McGuire et al., 1991; Bosking et al., 1997; Kisvárday et al., 1997). Feedback and horizontal connections share some similar characteristics. They do not exhibit retinotopic alignment as in the feedforward system (Alonso, 2002; Angelucci and Bullier, 2003). They represent large visual areas. They have many synaptic connections which are relatively weak as shown by inactivation of feedback which has minimal effects on spiking activity of cortical cells (Hupé et al., 1998; Bullier et al., 2001). Feedback and horizontal input do not appear to affect spike generation unless there is simultaneous feedforward activation (Toth et al., 1996; Bringuier et al., 1999).
Considered together, the three main neural connection types appear to have different functions. Feedforward processing consists of clear input to retinotopically aligned target cells. Non-feedforward connections may integrate visual information from outside the CRF which may be used to modulate CRF activity (Walker et al., 1999; Cavanaugh et al., 2002a, 2002b; Angelucci and Bullier, 2003; Seriès et al., 2003). The relative roles of feedback and horizontal connections are not clear but conduction velocities may provide clues. Onset times of surround suppression in V1 have been reported to be nearly constant over wide areas outside the CRF (Bair et al., 2003). However, the method used to reach this conclusion did not provide isolation of temporal parameters of horizontal transmission. Our current protocol is designed specifically to incorporate this important feature (see Experimental procedures and Results sections).
We use visual stimulation patterns intended to separate functional activity of the three major visual connection types. Two sets of stimuli are designed to differentially activate CRF and non-CRF regions in order to provide activity that emphasizes feedforward, feedback, or horizontal connections. Although we cannot confirm that we have exclusively isolated these three types of connections, our findings are consistent with their selective activation. Results show that activation outside the CRF can result in suppression or facilitation which can change depending on surround (non-CRF) parameters. The amount of response modulation of the CRF region varies with surround position. We find that excitatory and inhibitory inputs from surround areas are associated with different local field potential (LFP) frequency ranges. There are also temporal response modulation changes dependent on stimulus configurations. Overall, our results identify and imply some important functional differences in visual processing of feedforward, feedback, and horizontal connections.
2. Experimental procedures
Experiments were conducted using anesthetized and paralyzed cats (2.4~3.5kg, 12 female). All procedures followed the guidelines by NIH and by the Animal Care and Use Committee at the University of California, Berkeley.
2.1. Surgical preparation
Initial anesthesia was induced with isoflurane (3%). After venous catheters were inserted, anesthesia was continued with intravenous infusion of propofol (20mg/kg·hr) combined with fentanyl (10μg/kg·hr). A tracheotomy was performed, a tracheal cannula was inserted and the animal was artificially ventilated (25% O2 & 75% N2O). A craniotomy was then made in both hemispheres at 4mm posterior and 2mm lateral to Horsley-Clarke zero. The dura was incised carefully and reflected, then the cortical surface was covered with agar and wax. After the surgery, propofol and fentanyl infusion rates were reduced to an appropriate level for stabilized anesthesia (propofol: ~6-8mg/kg·hr, fentanyl: 4μg/kg·hr) which was determined individually for each animal. After stabilization, a continuous intravenous infusion of pancuronium (0.2mg/kg·hr) was initiated to block eye movements.
2.2. Recording procedures
Neural activity was recorded with two-channel tungsten microelectrodes. The signals from each electrode were amplified, bifurcated and then differentially filtered to extract single unit activity (500Hz~8MHz, digitized at 25kHz) and local field potentials (0.7~170Hz, digitized at 500Hz). Electrode penetrations were made down the medial bank of the postlateral gyrus to a depth of 5~6mm. Cells were encountered in multiple layers at RF eccentricities within the central 15° of the visual field (DeAngelis et al., 1993). RF eccentricity information for individual neurons was not recorded for this study. Once a unit was identified by spike waveform, optimal RF parameters were measured using drifting sinusoidal grating stimuli in the following sequence: orientation → spatial frequency → temporal frequency → binocular phase (for binocular cell) → size. RF dimension was determined as the peak of a size tuning curve for which response of a neuron ceases to increase. For cells that didn't show clear peaks in size tuning curves, we used the smallest inner diameter at which a cell stopped responding to an annulus grating stimulus as in a previous study (Cavanaugh et al., 2002a).
2.3. Design of visual stimuli
A crucial part of these experiments is the use of carefully selected visual stimuli that permit maximized separation of the three types of neural connections noted above. Anatomical findings show that feedforward connections cover a small visual space that is limited to the projection of the CRF region (Alonso et al., 2001; Angelucci and Bressloff, 2006). Therefore, the role of non-feedforward connections can be investigated by comparisons of visual responses to CRF activation versus those for which stimulation includes both CRF and adjacent non-CRF regions.
Since non-feedforward includes both feedback and horizontal connections, we require stimuli to separate them. For this, we note different characteristics for these two types of connections in spatial and temporal domains. Anatomical studies with use of retrograde tracers show that feedback connections can convey information to V1 from a much larger visual space than that for horizontal connections (Salin et al., 1989, 1992; Angelucci et al., 2002; Angelucci and Bressloff, 2006). In this case, the spatial extent of horizontal connections is approximately matched to the size of a low contrast summation field. This implies that beyond the low contrast summation field, feedback connections may dominate in surround suppression. In previous studies, surround suppression for spatial locations close to and far from the CRF was used to investigate horizontal connections (Hashemi-Nezhad and Lyon, 2012; Shushruth et al., 2013). However, the distribution of labeled neurons in V1 only covers monosynaptic connections, so the complete spatial extent of a horizontal pathway is not clear. Surround input is probably also transmitted via polysynaptic horizontal connections which will cause an enlargement of the spatial extent.
Besides a difference in spatial extent, another variation between horizontal and feedback connections is conduction velocity. Axons of horizontal connections are thin and unmyelinated with slow conduction velocities (Grinvald et al., 1994; Salami et al., 2003). Feedback and feedforward connections between macaque V1 and V2 have similar conduction velocities, which are about ten times faster than those of a horizontal type within V1 (Girard et al., 2001). Hence, if visual information of a non-CRF stimulus is conveyed through horizontal connections with slow conduction velocities, its arrival time should be more delayed as the non-CRF stimulus is placed further away from the CRF (Bringuier et al., 1999). In contrast, if it is conveyed through feedback connections with fast conduction velocities, arrival time delay, independent of center-surround distance, will be negligible. However, data suggest that surround information is conveyed by both types of neural connections so that the initial part of surround modulation is mediated by feedback (Hupé et al., 2001; Bair et al., 2003) and the later part by horizontal connections (Liu et al., 2013).
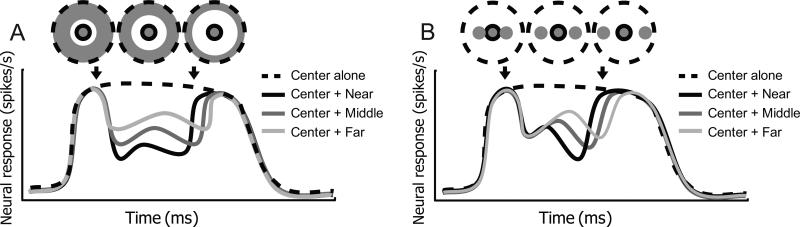
Comparisons of surround modulation time courses between stimulation of near and far distance conditions from the border of the CRF allow us to separate horizontal and feedback components. We have devised two sets of stimulus patterns by which center-surround distance is systematically varied as depicted in Figure 1.
Figure 1.
Two sets of center-surround (CRF-outside CRF) stimuli: annulus surround (left) and small patch surround (right). With both sets, distance between center and surround stimuli is systematically varied. In annulus surround pattern (A), annuli of different widths are used so that increments of center-surround distances are accompanied with decreases of total stimulated area. In small patch surround pattern (B), change of center-surround distance doesn't cause increase or decrease of total area stimulated. (C) Sequence of a trial. Optimal sinusoidal moving gratings are used to stimulate CRF and surround regions of a cell under study. The center (CRF) stimulus (2000ms duration, 50% contrast) is presented first, followed by surround stimuli (500ms duration, 100% contrast) after a 500ms temporal interval. (D) Small patch surround stimuli and annulus surround are tested in separate blocks. For small patch surround blocks, two patch surround stimuli are positioned symmetrically with respect to the center stimulus along the axis of preferred orientation. Inter-patch distances (white arrows) are chosen randomly as one out of four values (0.5~3.5deg, 1deg step) for each trial. For annulus surround blocks, the outer diameter of the annulus is fixed at 30deg. Therefore, four levels of center-surround distance are controlled by the inner diameter of the annulus. In addition to four “center + surround” and four “surround alone” conditions, a “center alone” presentation is tested as a control.
Distances are defined as visual angles between edges of center and surround stimuli. In the annulus surround pattern (Figure 1A), annuli of different widths are used outside the CRF. In the small patch surround pattern (Figure 1B), two patches of CRF size are presented symmetrically along the axis of preferred orientation with different inter-patch distances. For both stimuli sets, increments of center-surround distance are expected to cause analogous effects on the horizontal component of surround modulation. First, the modulation onset should be delayed because of slow conduction velocity. There should also be a decrease of surround modulation magnitude. Horizontal connections between neighboring neurons in V1 are denser than those among distant cells (Bosking et al., 1997; Kisvárday et al., 1997). Therefore, as center-surround distance increases, surround modulation caused by horizontal connections should get weaker and slower.
We next consider the feedback component of surround modulation for variation of the center-surround distance. For the annulus surround pattern, an increase of center-surround distance should be accompanied by a decrease in activation of feedback connections since the amount of visual input to extrastriate cortex is reduced. So a difference between near and far surround conditions should affect both feedback and horizontal connections. Because of this, a previous study in which annulus surround stimuli were used to compare near vs. far surround conditions (Bair et al., 2003), did not isolate horizontal connections. In contrast, the small patch surround pattern we have used here permits selective control of horizontal connections without substantial change in activation of feedback. This follows from the observation that the visual space covered by feedback connections is much larger than the CRF size of a V1 cell (Salin et al., 1989, 1992; Henry et al., 1991; Angelucci and Bullier, 2003; Angelucci and Bressloff, 2006). Therefore, spatial resolution of feedback connections is worse than that for the horizontal type. Based on these factors, the stimulus patterns we use here are expected to provide data for center-surround distance effects on surround modulation. Details of our stimulus procedures with temporal and spatial parameters are depicted in Figure 1 C,D.
2.4. Data Analysis
2.4.1. Spike density function
To observe the time course of surround modulation, spike density functions for “center alone” and “center+surround” conditions are compared. Spike trains were digitized at 25kHz and resampled at 1kHz. They were convoluted with a kernel which resembles a post-synaptic potential (Thompson et al., 1996). The kernel is expressed by the following equation.
| (1) |
where R(t) is rate as a function of time. R(t) is computed with two time constants for the growth phase (τg =1ms) and the decay phase (τd =20ms).
2.4.2. Z-scored LFP spectrogram
The spectrograms of the local field potential (LFP) signals (digitized at 500Hz) were computed using the Chronux toolbox in Matlab (500ms sliding window with 10ms step size, frequency range 10~100Hz). The resultant time-frequency LFP power matrix follows a 1/f2 relationship. Since we are interested in relative rather than absolute power change for each frequency band depending on an event (e.g., onset of surround stimulus), the raw LFP power matrix is transformed to a z-score based on mean and standard deviation values during baseline period (>250ms before stimulus onset & >750ms after stimulus offset) for each frequency band.
| (2) |
where Pzscore(f ,t) and P(f ,t) are z-scored and raw LFP power at a frequency f and time t, μ(P(f ,tbaseline)) σ(P( f ,tbaseline)) are the mean and standard deviation of raw LFP power computed during baseline period at a given frequency f .
3. Results
We studied 89 cells from 12 animals. There were 127 recording sessions of which 92 employed both annulus and small patch surround stimuli for given cells yielding direct comparisons. For 24 cells, we tested different spatial phases of the center stimulus.
Drifting sinusoidal gratings are used to stimulate the CRF and non-CRF surround regions of recorded cortical cells. To prevent saturation of neural response and to maximize inputs from surround regions, contrast values for CRF and non-CRF stimuli were differently set at 50% and 100%, respectively. Optimal parameters of grating stimuli are used as determined by CRF mapping procedures. Optimal sizes of RF centers may vary depending on mapping methods or stimulus contrast (Sceniak et al., 1999; Seriès et al., 2003). Here, we use a summation RF method with 50% stimulus contrast (Cavanaugh et al., 2002a). Size of the RF center is defined as the peak or asymptote of the size tuning curve for which response of a neuron ceases to increase. The CRF measured with a high contrast stimulus (high contrast summation RF: hsRF) is generally smaller than that measured with low contrast (low contrast summation RF: lsRF). However, hsRF is not simply an underestimation of CRF center. Feedforward connections from LGN integrate signals within the hsRF of visual cortical neurons. The size of lsRF is approximately matched to the spatial extent of horizontal connections (Angelucci and Bressloff, 2006). The goal of the current study is to understand different functions of horizontal and feedback connections in visual information processing. In order to manipulate both horizontal and feedback components of surround modulation, we have chosen hsRF as a better choice than lsRF.
Each trial begins with onset of a center stimulus (duration = 2000ms). Surround stimuli, which have a shorter duration (500ms), follow the center stimulus with 500ms onset delay, i.e., the center stimulus is presented earlier and lasts longer than that for the surround. This is important because it enables observation of both beginning and end points of surround modulation. For each trial, distance between center and surround stimuli is randomly chosen among four predetermined values. For the nearest distance condition, edges of center and surround stimuli are separated by 0.5 degree visual angle. For the other three conditions, center and surround stimuli are separated further by progressive 1 degree steps. A “center alone” and four “surround alone” conditions are also included in the test sequence as control conditions. For most cells, nine separate stimulus conditions are tested and repeated 20~70 times.
3.1. Magnitude of center-surround modulation varies linearly with distance between center and surround stimuli
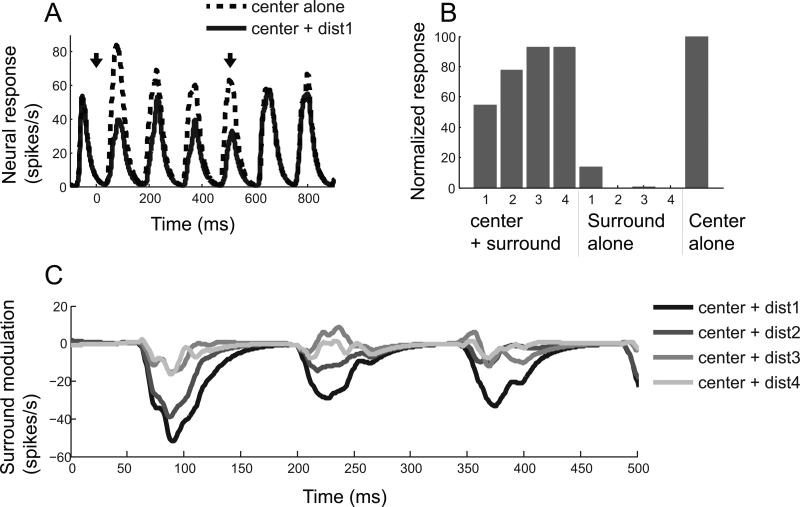
Figure 2 shows neural responses of a representative cell for which small patch surround stimuli induced suppressive modulation. This simple cell has an f1/f0 > 1, and its spike density function has periodic peaks as determined by temporal frequency (7 cycles/second) (Figure 2A). Dashed and solid curves indicate spike density functions computed for “center alone” and “center + the nearest (dist1) surround” conditions, respectively. Both curves are aligned at onset time of the surround (0 on time axis). Two downward arrows at the top of the curves indicate onset and offset times of the surround. Note that suppressive modulation (lower amplitude of solid curve) is apparent only during the 0~500ms time period during which surround regions of the CRF are co-stimulated with that of the center.
Figure 2.
A representative cell showing suppressive center-surround modulation. (A) Dashed and solid curves are spike density functions computed for “center alone” and “center + surround (dist1)” conditions, respectively. Time 0 indicates onset of surround stimulus whose duration is 500ms. Onset and offset of surround stimulus are indicated by two downward arrows. Note that the magnitude of the solid curve is lower than that of the dashed only from the 0 to 500ms interval, demonstrating that the neural response to the center stimulus is suppressed by the surround. (B) For each of 9 stimulus conditions, mean spike count during the 0~500ms period is computed and then normalized with the value computed for “center alone” condition. The smaller numbers for the x-axis represent the nearer center-surround distances. In this case, strength of surround suppression gets weaker as center-surround distance increases. (C) Each curve is created by subtracting the spike density function for “center alone” condition from that of each “center + surround” condition. Nearer center-surround distances are depicted in darker shades. For efficient comparisons between the four distances, curves are truncated to the interval from 0 to 500ms.
We quantified the magnitude of surround modulation by counting the number of spikes generated during the 0~500ms time period noted above. The values computed for 9 stimulus conditions (four “center + surround”, four “surround alone” & one “center alone”) are normalized to the value of the “center alone” condition. A value smaller (or larger) than 100 means suppressive (or facilitative) surround modulation. These data are shown in the histogram of Figure 2B. For the “center + dist1” condition, the neural response is suppressed by 50%. As expected, the suppressive modulation magnitude is gradually decreased as center-surround distance increases. This linearly decreasing pattern of suppressive modulation is shown in Figure 2C which gives the time course of surround modulation. Each curve here is created by subtraction of the spike density function of “center alone” from that of each of the four “center + surround” conditions. In the “center+dist1” condition, surround suppression begins to arise at around 60ms after surround onset time, and it is very slightly delayed or unchanged in subsequent distance conditions. Note that the latency of surround modulation may be less than 60ms, and that there is no spike between 0 and 60ms.
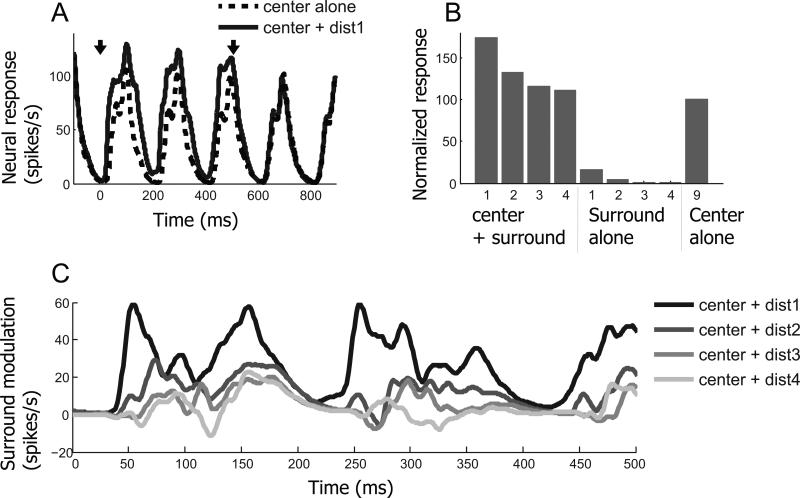
Results from another cell are presented in Figure 3 for which the same conditions are considered as in the previous figure. But this cell exhibits facilitative instead of suppressive modulation. In the “center + dist1” condition, neural response is about 1.6 times stronger than that for the “center alone” condition. And the strength of facilitative modulation exhibits a linearly decreasing pattern with increments of center-surround distance. Surround alone elicits weak spiking activity (5th and 6th bars in Figure 3B), indicating possible minimal overlap with the CRF. However, low spiking activity for the surround alone condition is not likely to be a direct cause of the relatively strong facilitative effect. The magnitude of the facilitative effect is much stronger than the value expected from linear summation of spiking activity of “center alone” and “surround alone” conditions, indicating that additional sub-threshold facilitative inputs from the surround must be involved. In addition, the observation of spiking activity for the surround alone condition is not limited to the facilitation case. As shown in Figure 2B, spiking activity in the surround alone condition (the 5th bar) is often associated with strong suppressive modulation (the 1st bar).
Figure 3.
An example cell which exhibits facilitative center-surround modulation. The same conventions are used as in Figure 2. (A) Spike density functions computed for “center alone (dashed)” and “center + dist1 (solid)” conditions. Arrows indicate times at which the surround is presented (first arrow) and when it is turned off (second arrow). (B) Normalized responses for 9 stimulus conditions. Strength of surround facilitation becomes weaker as center-surround distance is increased. (C) Time course of surround modulation. Surround facilitation tends to be diminished and delayed as surround distance from the center (CRF) is increased.
An interesting feature of this example is that surround modulation latency varies systematically depending on center-surround distance. In the “center + dist1” condition, facilitative modulation begins around 40ms after surround onset. It is delayed gradually as center-surround distance increases. This time-distance relationship of center-surround modulation may be explained as follows. Surround signals are transmitted through horizontal connections with slow conduction velocities. The initial portion of surround modulation may be mediated by fast feedback connections. But feedback connections from extra striate areas may require relatively strong visual input along with large CRFs. Also, surround modulation may require an integration process for activation. This idea is supported by a previous finding that the latency of surround suppression is negatively correlated with its strength (Bair et al., 2003). In this case, strong surround input can trigger immediate modulation, but if it is weak, time is required for it to be effective.
The above two representative results illustrate that our small patch surround stimuli can induce either suppressive or facilitative modulation of the response. In both cases, effective strength of surround modulation decreases as center-surround distance increases.
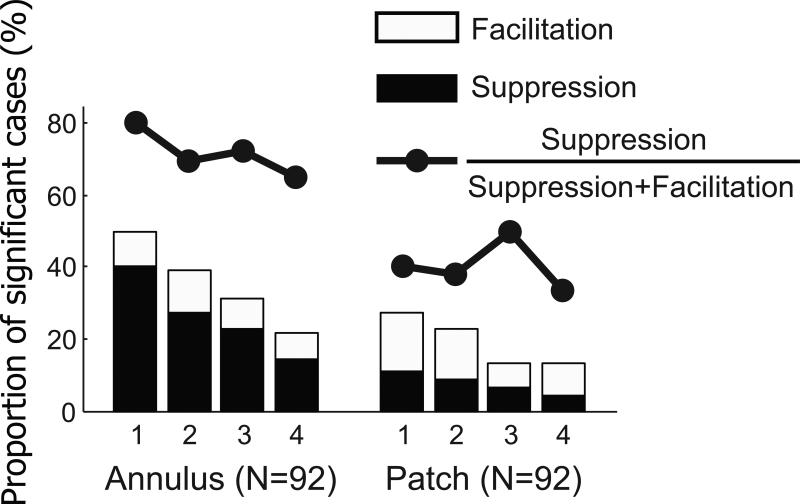
Next, we consider the annulus surround stimulus. For 92 recordings, both annulus and small patch surround stimuli were tested with the same cell. For these cases, Figure 4 shows proportions of significant (Two-sided Mann-Whitney U-test (p<0.05)) modulation cases as a function of center-surround distance for both sets of surround stimuli, (left: annulus surround, right: small patch surround). Filled and unfilled bars represent suppressive and facilitative modulation, respectively. For the “center + dist1” condition of annulus surround, about 50% of the population data show significant suppressive or facilitative modulation. The proportions of significant modulation cases decrease as center-surround distance increases. In the “center + dist4” condition, less than 30% of the data show significant modulation. This decreasing pattern is also clearly seen for small patch surrounds, but with much smaller proportions. To summarize, at a given distance, the annulus surround is a more effective way to modulate neural response. It seems clear that the stronger effect of an annulus surround is due to a larger amount of visual input compared with that for the small patch condition.
Figure 4.
Proportions of significant modulation for annulus and small patch surround conditions (Two-sided Mann-Whitney U-test (p<0.05)). Although, for both conditions, proportions of significant modulation cases (filled and unfilled bar areas) decreases as center-surround distance increases, bar heights for annulus conditions are nearly twice as tall as those for small patch application at corresponding center-surround distances. This demonstrates that the annulus surround is more effective for the induction of significant surround modulation. In addition, the dominant sign of surround modulation is suppression for the annulus pattern, but it is facilitation for the small patch. Furthermore, for the annulus pattern, relative ratios of suppression (filled circles) diminish with increasing center-surround distance. This suggests that suppression requires stronger surround input than facilitation.
Another interesting result is that the dominant sign of surround modulation is changed from suppression to facilitation when the annulus stimulus is replaced by a small patch. Neurons with inhibitory or facilitatory regions beyond the CRFs tend to be grouped in clusters of facilitation or inhibition (Yao and Li, 2002). If annulus and small patch surround stimuli are tested for different neural populations, there may be biased sampling. But in the current study, we can rule out this factor, because the two different types of surround stimuli are tested using the same population of cells.
We should also consider the possibility that the annulus stimulus may cover several zones of facilitation and suppression. There is a report of a spatial arrangement of opposing contextual interactions with collinear (end-zone) facilitation and lateral (side) inhibition (Kapadia et al., 2000). Our small patch surround stimuli are always presented at both end-zones of a preferred orientation axis. Thus, if a cell receives facilitative input from the end-zones, and its magnitude is weaker than suppressive areas from other regions in the annulus, it could cause a result like that of Figure 4. In this context, note that surround suppression can originate from a localized region and effective areas are sometimes spatially asymmetric (Walker et al., 1999). But, the most effective suppressive surround regions are end-zones (Walker et al., 1999). Therefore, it is likely that most of our results may be attributed to end-zone effects.
To examine this question more closely, consider the data curves above the histograms in Figure 4. Each point indicates a relative ratio (RR) of bar height data in black (RRfilled) as follows.
| (3) |
where Hfilled(Stim,Dist) and Hunfilled(Stim,Dist) are heights of filled and unfilled bars respectively for a given stimulus pattern (Stim: annulus or patch) and center-surround distance (Dist: dist1~dist4). For the annulus surround, suppression is dominant compared to that for facilitation for the “center + dist1” condition. This dominance is gradually lost as center-surround distance is increased along with the decreasing amount of visual input (80% in dist1 → 65% in dist4). This suggests that suppressive modulation requires stronger input from the surround compared to that for facilitation, and we consider this in what follows.
3.2. Suppressive modulation requires stronger input from surround region than that for facilitation
The finding that suppressive modulation requires stronger surround input than that for facilitation implies that inhibitory interneurons have higher activation thresholds than those for excitation. This idea has been postulated in computational models for integration of surround inputs (Somers et al., 1998; Schwabe et al., 2006). If this is correct and both excitatory and inhibitory interneurons are involved in center-surround modulation of neural activity, then the process of increased surround strength should be as follows.
1) No modulation → 2) Weak facilitation → 3) Strong facilitation → 4) Weak facilitation → 5) Weak suppression → 6) Strong suppression
Excitatory interneurons with low activation thresholds are relatively easily activated even by weak surround input, and this gives rise to facilitation (i.e., 1) No modulation → 2) Weak facilitation). The magnitude of facilitation increases as surround input gets stronger (i.e., 2) Weak facilitation → 3) Strong facilitation). But once surround input exceeds the activation threshold of inhibitory interneurons, facilitation begins to decrease and change to strong suppression (i.e., 3) Strong facilitation → 4) Weak facilitation → 5) Weak suppression → 6) Strong suppression). A decrease of surround input will cause changes in the opposite direction. If neural response is suppressed by an annulus surround, suppression may be maintained (but weakened) or changed to facilitation with a small patch surround. Alternatively, if the neural response of a cell is facilitated by an annulus surround, the same effect should occur for a small patch. The magnitude of facilitation might vary. Either stronger or weaker facilitation for a small patch can occur as in the changes from points 4) to 3), or from points 3) to 2).
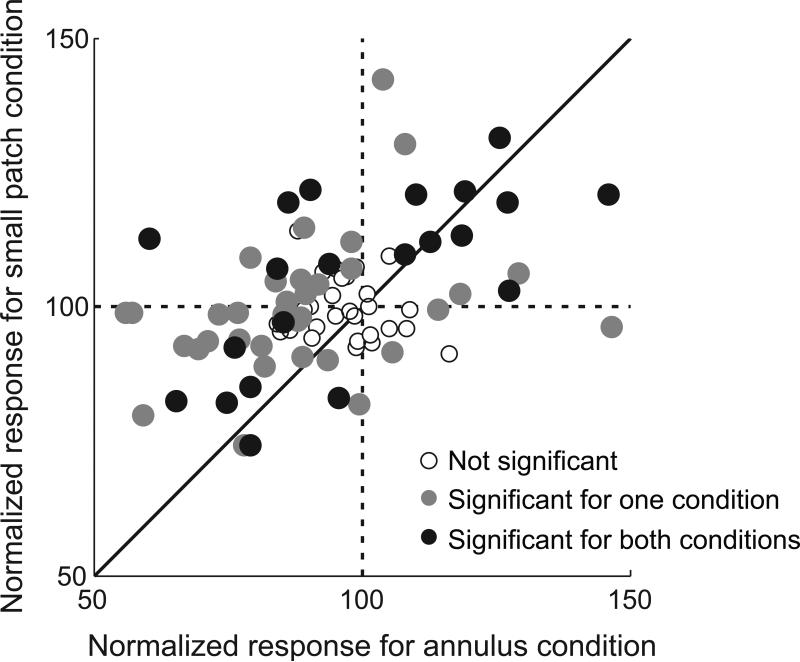
To test these predictions, we compare response magnitude for the annulus surround pattern with that for small patches. In Figure 5, each data point represents an individual cell. For each cell, neural responses of the four “center + surround” conditions are normalized (as in Figure 2B) and then averaged (annulus surround: abscissa, small patch surround: ordinate). Therefore, a value smaller (or larger) than 100 on each axis means suppressive (or facilitative) surround modulation. Statistical significance (two-sided Mann-Whitney U-test (p<0.05)) of surround modulation for each data point is expressed as different shading levels within circular data points (open circles: not significant for either condition, gray filled: significant for only one condition. black filled: significant for both conditions). The vertical dashed line divides suppressive modulation (left) from that for facilitation (right) for the annulus surround pattern. Similarly, the horizontal dashed line separates suppressive (below) from facilitative modulation (above) for the small patch surround stimuli.
Figure 5.
Modulation strength comparison: annulus vs. small patch pattern. Each circular symbol (N = 92) represents the mean value of normalized responses for four “center + surround” conditions (e.g., 1~4th bar in Figure3B). Abscissa values are for annulus conditions and ordinate levels are for small patch trials. Shading of circles convey statistical significance (two-sided Mann-Whitney U-test (p<0.05)) of center-surround modulation (open circles: not significant for either condition, gray filled: significant for only one condition. black filled: significant for both conditions). Symbols in left half of the graph mean that suppressive modulation is induced by annulus surround pattern. Almost all symbols in left half of the graph are positioned above the diagonal line (55 vs. 9). This means that neural responses to small patch surround patterns are stronger than those for the annulus. This follows because of surround facilitation (in 2nd quadrant, top left) or weakened surround suppression (in 3rd quadrant, bottom left). Symbols in the right half (facilitation cases for the annulus pattern) are positioned mainly in the 1st quadrant (top right), and rarely in the 4th quadrant (bottom right). Within the 1st quadrant, symbols are evenly distributed with respect to the diagonal line (9 vs. 9). This means that surround facilitation induced by the small patch surround can be either weaker or stronger than that caused by the annulus. These results support the idea that suppressive modulation requires stronger surround input than that for facilitation (see details in text).
The normalized response for annulus condition is positively correlated with that for small patch. (r=0.47, p<0.0001), indicating that there is a general trend of suppressive (facilitative) modulation from the annulus and the small patch. However, data points on the left half of Figure 5 are more numerous than those on the right (64 vs. 28, one-sample t-test, p<0.01), showing that the dominant sign of surround modulation for the annulus is suppression. Furthermore, most data in the left half fall above the diagonal line (55 vs. 9, paired-sample t-test, p<0.01). This means that the neural responses to small patch surrounds are stronger than those for the annulus condition (facilitation in the 2nd quadrant (top left) or suppression in the 3rd (bottom left)). For data points in the right half, nearly all are in the 1st quadrant (top right). The data points in the 4th quadrant (bottom right) do not exhibit significant (Two-sided Mann-Whitney U-test (p<0.05)) suppression for the small patch. Within the 1st quadrant, data points are evenly distributed with respect to the diagonal line (9 vs. 9, paired-sample t-test, p=0.74). This means that surround facilitation induced by small patch stimuli can be weaker or stronger than that induced by an annulus. These results are consistent with our predictions as outlined above.
3.3. Annulus surround stimulus increases LFP power spectra in the range of high gamma frequency
We establish here that center-surround modulation caused by an annulus differs from that for a small patch in magnitude and in sign. When an annulus or a small patch is presented without a center stimulus (i.e., “surround alone” condition), by definition, neither can evoke spiking activity. To make a difference in center-surround modulation, outside CRF stimuli must have different effects at sub-threshold levels.
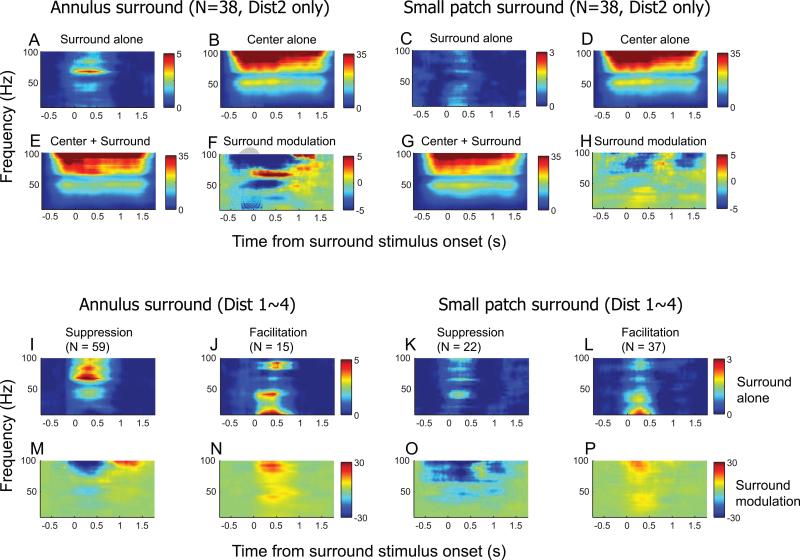
Local field potentials (LFPs) are believed to reflect mainly dendritic activity, i.e., neuronal input, including sub-threshold components (Mitzdorf, 1985; Logothetis, 2003). We computed z-scored LFP power spectrograms (see Experimental procedures section) for “surround alone” conditions and compared effects for annulus and small patch surround stimuli. For the nearest (dist1) “surround alone” condition, surround stimuli often partially invade the CRF and generate weak but significant spiking activity (see Figures 2 and 3). In order to exclude an effect of spiking activity on LFP signals in the “surround alone” condition, 38 recordings are used for which spiking activity in the “surround alone (dist2)” condition is zero.
For both surround stimulus patterns, the “surround alone” condition yields LFP power spectra as shown in Figure 6A & C. Response is clearly much bigger for the annulus surround compared to that for the small patch. In addition, for the annulus pattern, increased LFP power spectra is focused mainly in a high gamma frequency range (around 60~80Hz). In contrast, the small patch surround pattern yields LFP power increases in a lower frequency range (less than 40Hz).
Figure 6.
Population average z-scored LFP spectrograms. For a 10~100Hz frequency range, LFP power change from the baseline is plotted as a function of time. (Left two columns) Annulus surround pattern, (Right two columns) Small patch surround pattern. (A~H) To exclude effects of spiking activity on LFP spectrograms, 38 tests are used for which the “surround alone (dist2)” condition, for both surround patterns, does not evoke spiking activity. For the “surround alone” condition, the annulus causes a larger change in LFP power than that for the small patch (A vs. C), and the main change is focused on the high gamma frequency range (approximately 60~80Hz). This 60~80Hz frequency specific change in annulus surround alone result is also revealed in F, reflecting center-surround modulation of the LFP spectrogram. (I~P) Z-scored LFP spectrogram comparisons: surround suppression vs. surround facilitation. For each surround pattern, population data are divided into two groups: suppression vs. facilitation. Again, the tests included in this analysis do not evoke spiking responses for the “surround alone” condition so they are distinguishable only at subthreshold levels (I, J, K, & L). Note for the annulus surround, that increased LFP power in the 60~80Hz range (as shown in Figure 6A) is clear for suppression (I), but not for facilitation (J). Regardless of surround type, facilitation cases of the “surround alone” conditions (J & L) are similar in that LFP power change for the low frequency range is bigger than that for high frequencies. Depending on sign of modulation, center-surround effects in LFP spectrograms show the largest differences in the 80~100Hz frequency range (M vs. N or O vs. P). LFP power in this range decreases for suppression cases, but increases for facilitation.
We next created a center-surround modulation matrix of LFP spectrograms by subtraction of the “center alone” LFP spectrogram (Figure 6B & D) from that of the “center + surround” (Figure 6E & G). The resultant matrix (Figure 6F & H) is then compared with the “surround alone” LFP spectrogram (Figure 6A & C). During the 0~500ms interval, the data in Figure 6A & F share the property that LFP power is maximally increased in the 60~80Hz frequency range. Also, during the same interval, data in Figure 6C & H both have maximum LFP power increases in the <40Hz frequency range. The center-surround modulation of the LFP spectrogram also has features that are not consistent with linear summation. In Figure 6F & H, the blue coded component indicates that LFP power is lower for the “center + surround” condition compared to that for “center alone”. This is different from the data in Figure 6A & C, since the LFP spectrograms of “surround alone” conditions have only positive values.
3.4. In “surround alone” condition, increased LFP power spectra (high gamma range) is associated with suppressive modulation
We show above that a sub-threshold level difference between “surround alone” conditions for the two types of surround is reflected in the LFP signal. Although the LFP signal evoked only by an annulus surround shows stronger amplitude than that for a small patch over the entire frequency range (10~100Hz), the difference is most salient at high gamma levels (around 60~80Hz). Previous studies show that large gratings tend to produce stronger power in the gamma frequency range (Berens et al., 2008; Gieselmann and Thiele, 2008; Jia et al., 2011). However, our findings show that suppression is more prominent than facilitation for the annulus surround conditions (Figures 4 & 5). To pursue this in the local field potential domain, we examine increased LFP power in the 60~80 Hz frequency range to determine if it is associated with suppressive modulation and stimulus size. For each surround pattern, population data are divided into two groups based on differences of spike response between “center only” and “center + surround” conditions: one for suppressive and the other for facilitative modulation. For each annulus and small patch surround type, facilitation and suppression groups are taken from different neural populations. In this way, we do not have a center-surround distance limit for the creation of Figure 6I~J (Dist1~4 conditions are all considered). Cases that show spiking activity for “surround alone” conditions are not included in the analysis.
For the annulus surround pattern, LFP spectrograms in “surround alone” condition clearly differ with the sign of surround modulation. For the suppression group (Figure 6I), the largest increase of LFP power is observed in a >60Hz frequency range as shown above in Figure 6A. For the facilitation group (Figure 6J), an increase of LFP power is apparent in a <40Hz frequency range. These results show that coherent gamma rhythms for the annulus (Figure 6A vs. C) are not entirely due to different stimulus size. Furthermore, although differences between the two groups for the small patch surround are less clear than those for the annulus, a prominent dissimilarity is clear. An increase of LFP power in the <40Hz frequency range is much bigger for facilitation than for suppression (Figure 6K & L). The differences in center-surround modulation of LFP spectrograms between suppression and facilitation are reflected in the highest frequency range (>80Hz). Regardless of stimulus pattern, suppression (Figure 6M & O) and facilitation (Figure 6N & P) groups are characterized by decreased and increased LFP power, respectively. These results are consistent with a previous study that showed positive correlation between high gamma LFP and spiking activity (Belitski et al., 2008).
3.5. Time course of surround modulation
Surround modulation latency is the time when the spike density function of a “center + surround” condition begins to be differentiated from that of “center alone”. Since conduction velocities of feedback connections are on average 10 times faster than those of the horizontal type (Girard et al., 2001), observation of variation of surround modulation latency, depending on center-surround distance, is necessary in order to demonstrate that horizontal and feedback connections are separated in our experimental design.
We note above (Experimental procedures section) that the annulus surround pattern is not appropriate for selective control of the horizontal connection component of surround modulation, because both horizontal and feedback types are weakened as center-surround distance increases. Based on this, we make a simple prediction about surround modulation latency in Figure 7A. The prediction is that surround modulation consists of feedback and horizontal components. Interaction between the two components and the possible contribution of feedforward connections (Ozeki et al., 2004; Webb et al., 2005) may be involved but are not considered. The dashed curve represents neural response for the center alone condition and the other three solid curves in grayscale indicate those for center + near, middle, and far surround conditions in the order of darkness. Onset and offset times of surround stimuli are marked as two downward arrows at the top of the curves. Fast feedback connections are responsible for the initial part of surround modulation. Depending on center-surround distance, this feedback component varies in magnitude but not in latency. A slow horizontal component occurs in a later part of surround modulation. It varies in latency as well as magnitude for subsequent surround stimuli. Figure 7B shows a different prediction for a small patch surround. The same conventions are used as in Figure 7A. Since small patch surrounds are designed to maintain activation levels of feedback connections, regardless of center-surround distance, initial parts of surround modulation are constant both in magnitude and latency. The differences between near and far surround conditions are expected to be only revealed in a subsequent horizontal component participation.
Figure 7.
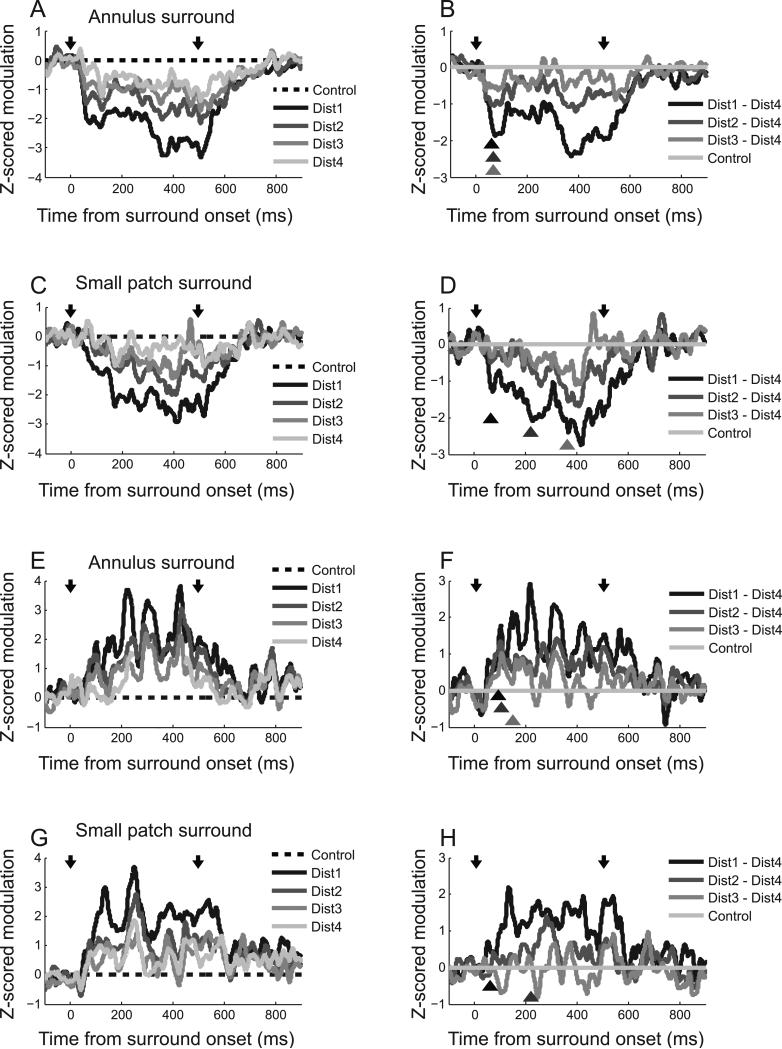
Predictions for time course of surround modulation. The first and second downward arrows represent onset and offset of surround stimuli, respectively. Therefore, the center stimulus is presented earlier and lasts longer than that for the surround. Time courses depicted in darker shades indicate nearer center-surround distances. The predictions are based on the following assumptions. 1) Surround modulation is mediated by both feedback and horizontal connections. 2) There is limited interaction between the two types of neural connections. 3) Given that conduction velocities of feedback connections are much faster than those for the horizontal type, the earliest part of surround modulation is mediated by feedback connections. 4) The onset of the feedback component of surround modulation is minimally affected by center-surround distance. 5) The onset of the horizontal component of surround modulation is increasingly delayed as center-surround distance increases. (A) Annulus surround pattern: Increasing center-surround distance causes decrease in both feedback and horizontal components of surround modulation. So, differences between middle and far conditions occur at the same time as those between near and far. (B) Small patch surround pattern: Increasing center-surround distance causes selective decrease of horizontal component of surround modulation. So, differences between middle and far conditions are delayed more than those between near and far.
As noted below, it is difficult to define surround modulation latency for most cells in our population (see Discussion). As an alternative, we use a population surround modulation function so that curves of individual neurons (e.g., Figure 2C or Figure 3C) are normalized and then averaged. The analysis is as follows. First, we choose 41 of 97 annulus surround tests for which neural activity is significantly suppressed (two-sided Mann-Whitney U-test, p<0.05) for the nearest (dist1) “center + surround” condition. Second, for each of the 41 tests, surround modulation curves of four “center + surround (dist 1-4)” conditions are transformed to z-scores using a baseline (from -500ms to 0ms before surround stimulus onset) mean and standard deviation. Third, the resulting baseline adjusted z-score surround modulation curves are averaged across the 41 tests. The same procedure is used for 16 small patch surround tests that show significant (two-sided Mann-Whitney U-test, p<0.05) surround suppression. The results are depicted in Figure 8A (annulus) and Figure 8C (small patch). Again, two downward arrows indicate onset and offset of surround stimuli. Different levels of center-surround distance are expressed as the degree of darkness of solid curves (The darker is the nearer). The main predictions for the four annulus and small patch conditions are as follows. In the annulus condition, differences depending on center-surround distances are reflected in both feedback and horizontal components. Therefore, stronger modulation for a closer surround location will be observable even from the earliest part of the surround modulation time course. Modulation onset delay for a far surround, which is supposed to appear in a later portion of the time course (horizontal component), is problematic because feedback and horizontal components are not entirely separated. For the small patch condition, however, only the horizontal component is affected by center-surround distance, so this can be segregated from the feedback role by subtracting the modulation time course of “center + dist4” from each of the “center + dist1,2,3” variables. Compared with the difference between “center + dist1” and “center + dist4”, those between “center + dist2 or 3” and “center + dist4” are not only smaller in magnitude but also temporally delayed. And these systematic variations are reflected in later rather than earlier parts of the modulation time course.
Figure 8.
Time course of surround modulation: Suppression (A, B: Annulus surround / C, D: Small patch surround), Facilitation (E, F: Annulus surround / G, H: Small patch surround). Two downward arrows indicate onset and offset of surround stimuli, respectively. Nearer center-surround distances are depicted in darker shades. (A, C, E, G): Differences between “center alone” control and each of four “center + surround” conditions are z-score normalized using mean and standard deviation of differences during baseline periods (from -500ms to 0ms before surround stimulus onset). The dashed line serves as a reference for comparison among conditions. (B, D, F, H): Dist4 curve is subtracted from each of four gray curves. The positions of three triangles indicate onset times of significant difference (two-sided Wilcoxon signed rank test in the 100ms sliding window, p<0.05) between each of Dist1~3 curves and control (Dist4). In (H), difference between Dist3 and Dist4 is not statistically significant, so only two triangles are drawn. For annulus surround pattern (B, F), differences between the resultant three curves appear from the initial part of the modulation without substantial difference in onset delay depending on center-surround distance. However, for the small patch surround pattern (D, H), difference between Dist2 or 3 and Dist4 appear later than those between Dist1 and Dist4.
The time course for surround modulation is illustrated In Figure 8. In Figure 8A, suppression begins around 50ms after annulus onset and remains while center and surround regions of the CRF are stimulated together (0~500ms). At the end of the surround period, suppression is diminished and disappears about 300ms later than surround offset. As predicted in Figure 7A, surround modulation curves are similar in shape and have varying effects depending on center-surround distance. In Figure 8B, modulation curve of “center + dist4” condition is subtracted from those of the other nearer conditions. Onset times of significant difference (two-sided Wilcoxon signed rank test in the 100ms sliding window, p<0.05) are marked as triangles below the curves. Differences between “center + middle (dist2 or 3)” and “center + far (dist4)” conditions are seen from the earliest part of the surround modulation time course. A similar pattern is seen for the difference between “center + near (dist1)” and “center + far (dist4)” conditions (Figure 8B).
On the other hand, when surround modulation is induced by small patch stimuli, overall shapes differ depending on center-surround distances (Figure 8C). For the “center + dist1” condition, suppression begins at around 50ms as it does for the annulus surround. But for the other three conditions (“center + dist2,3,4), it occurs tens of milliseconds later. We do not predict an earlier onset for the “center + dist1” condition. Feedforward connections may be involved in the earlier suppression onset. Previous studies suggest that feedforward connections participate in the initial part of surround suppression by withdrawal of excitation (Ozeki et al., 2004; Webb et al., 2005). However, feedforward connections to early surround suppression are spatially limited to very close surround areas (Angelucci and Bressloff, 2006). Therefore, if feedforward contributions occur in the “center + dist 1” condition but not in the other (further distance) configurations, a result like that illustrated in Figure 8C is likely.
Except for the “center + dist1” condition, the other three curves (“center + dist 2,3,4” conditions) of Figure 8C resemble those of Figure 7B in that relative differences among the three curves occur in a later part of the modulation time course (>200ms). Moreover, consistent with the prediction outlined above, the onset of differences between “center + middle (dist2 or 3)” and “center + far (dist4)” conditions is relatively delayed compared to those between “center + near (dist1)” and “center + far (dist4)” conditions (triangles in Figure 8D). Similar results occur for the time course of facilitative surround modulation (15 annulus surround tests & 26 small patch surround tests, Figure 8E~H). Considered together, these results suggest that differences depending on center-surround distances for small patch stimuli are mediated mainly by slow horizontal connections (but see Discussion 4.5 for limitations).
In the cat's visual cortex, the average cortical magnification factor for 1 degree in the central visual field is assumed to be 1 (1 mm2/deg2) and conduction velocities of horizontal connections range from 0.1 to 0.4m/s (Albus, 1975; Grinvald et al., 1994; Bringuier et al., 1999; Girard et al., 2001; Angelucci and Bullier, 2003). Previous studies have shown that sub-threshold input from visual stimuli presented outside the CRF is transmitted with a velocity which is well matched to conduction times in the horizontal connection system (Grinvald et al., 1994; Bringuier et al., 1999). When these values are considered in the current study, modulation onset delay between near and far surround conditions (temporal interval between adjacent triangles in Figure 8D) is expected to be as short as tens of milliseconds. However, we observe much longer modulation onset delay times (about 150ms) in our data. This suggests that unlike sub-threshold input, supra-threshold suppression or facilitation may require additional integration processes.
4. Discussion
We have designed visual stimuli to differentially activate three major routes of visual processing: feedforward, feedback, and horizontal connections in order to determine relative functional differences. To achieve this, stimuli are presented within and beyond the CRF. Two different surround stimulus patterns (annulus and small patch) are used and distances are varied between CRF stimuli and those in the surround. Both patterns cause response modulation that decreases as center-surround distance is increased. Annulus stimuli have mainly suppressive effects while small patch patterns more frequently cause facilitation. This difference is also expressed for different frequency bands of LFPs. As CRF-surround distance is varied, modulation time course changes as follows. For the annulus, differences between near, middle, and far surround positions occur from the initial phase of modulation. For the small patch, center-surround distance dependent changes occur at later phases of the modulation time course. Moreover, temporal delay between middle and far surround conditions is increased compared to that between near and far positions. This implies that center-surround distance dependent changes of response modulation are due mainly to activation levels of slow horizontal connections.
4.1. A comparison of annulus and small patch stimuli
In previous studies with these types of stimuli, center-surround distance was varied by either an annulus whose outer diameter was fixed and inner diameter systematically varied (Levitt and Lund, 2002; Bair et al., 2003; Ichida et al., 2007) or by small patches whose position was progressively moved away from the CRF (Bringuier et al., 1999; Mizobe et al., 2001; Kim et al., 2012). With both types of surround stimuli, the magnitude of modulation is gradually decreased with increasing distance between center and surround. Our current results are consistent with this. However, our stimulus design provides detailed comparisons for each neuron between annulus and small patch stimuli. This provides new insights about relative pathway functions.
One obvious difference between the two types of surround stimuli we have used here is that surround areas occupied by an annulus are much larger than those for small patches. For this reason, at a given center-surround distance, an annulus can induce modulation more effectively than that for a small patch. A larger stimulated area, i.e., a stronger surround input, also seems to be related to higher occurrence rates of suppressive (rather than facilitative) modulation for annulus compared to small patch conditions (Figure 4 & 5) as considered below.
Note that the small patch surround is different from the annulus type in that the former is independent of stimulus size variation. This is advantageous, because it allows activation levels of feedforward connections to be relatively stable. Therefore, in the small patch surround condition, differences in modulation that depend on center-surround distance are probably mediated by horizontal connections. In summary, we show here that time courses of surround modulation for small patch conditions are clearly distinguished from those for the annulus (Figure 8).
4.2. Surround modulation: suppression vs. facilitation
Previous investigations show that modulation is strong when center and surround stimuli share similar stimulus parameters (Blakemore and Tobin, 1972; DeAngelis et al., 1994; Cavanaugh et al., 2002b). In addition, surround regions separated by identical distances from the center do not necessarily produce the same modulation effects (Walker et al., 1999; Kapadia et al., 2000; Cavanaugh et al., 2002b).
Effective positions of surround stimuli are often asymmetric and an influential location is frequently one or both end-zones on the axis of preferred orientation. These characteristics may be mediated by long-range horizontal connections in cortical layer 2/3 (Rockland and Lund, 1983; Hirsch and Gilbert, 1991; McGuire et al., 1991; Malach et al., 1993; Chisum et al., 2003). Anatomically, they extend several millimeters parallel to the cortical surface (Bosking et al., 1997; Kisvárday et al., 1997).
Reports vary regarding frequencies of suppressive and facilitative modulation. Some find mainly suppressive modulation (DeAngelis et al., 1994; Grinvald et al., 1994; Walker et al., 1999; Cavanaugh et al., 2002b) while others report prevalent facilitation (Nelson and Frost, 1985; Kapadia et al., 1995; Toth et al., 1996). Cortical neurons with similar extra-CRF properties (inhibitory or excitatory) may be clustered (Yao and Li, 2002). This finding is consistent with our current data in that surround modulation induced by a given stimulus pattern may cause suppression or facilitation. However, we also find for a given cell, that the sign of modulation can change from suppression to facilitation as center-surround distance increases or size of the surround stimuli decreases. This finding is in accord with a previous result showing that facilitative surround modulation can be changed into suppression by increasing surround size or center contrast (Ichida et al., 2007). Considered together, suppressive modulation may require stronger input from the surround than that for facilitation.
Surround modulation has also been studied with visual stimuli of varying contrast levels (Toth et al., 1996; Levitt and Lund, 1997; Polat et al., 1998). We have not varied contrast in the current work but consider here how it may be relevant to our scheme. To encompass surround facilitation or suppression, both excitatory and inhibitory interneurons must be involved. Specific interneurons must receive input from the CRF and surrounding region. Inhibitory interneurons should have higher activation thresholds than excitatory types. When input to interneurons is not strong because of low stimulus contrast or long center-surround distances or small sized visual stimuli, excitatory interneurons (but not inhibitory) are activated so that surround facilitation can occur. If input is strong enough to activate inhibitory interneurons, surround facilitation will be canceled out and replaced by surround suppression. Our current data (see Figure 5) are consistent with this process.
4.3. Gamma frequency range of local field potential
The annulus surround is more effective for inducing modulation than the small patch. The dominant sign of modulation is also different for the two stimulus patterns. For the annulus, suppression dominates facilitation. For the small patch, facilitation occurs slightly more often (Figure 4). The CRF stimulus for the current experiments is always fixed as the optimal grating that evokes maximum neural response. By definition, the annulus and small patch surround stimuli cannot evoke any spike activity in the “surround alone” condition. However, with power spectral analysis of the LFP, we observe that surround stimuli are distinguishable at sub-threshold levels (Figure 6). In the “surround alone” condition, the annulus causes more change in LFP power than the small patch, and the main change is focused in the high gamma frequency range. The magnitude of gamma frequency LFP monotonically increases with size of the visual stimulus (Berens et al., 2008; Gieselmann and Thiele, 2008; Jia et al., 2011), and the maximum increase occurs when a stimulus overlaps the CRF surround (Gieselmann and Thiele, 2008). Another variable that can affect gamma frequency LFPs is stimulus contrast. High stimulus contrast apparently causes considerable LFP gamma oscillation, and the maximum increase of gamma frequency power occurs when single unit activity is saturated (Henrie and Shapley, 2005). The results above suggest that gamma frequency LFPs may reflect activation of inhibitory interneurons in addition to the responses to stimulus size or contrast levels. Our current data are consistent with this idea. Increased gamma frequency LFP power for the “annulus surround alone” condition appears only with the suppression group (Figure 6I) and not for facilitation (Figure 6J).
4.4. Surround modulation latency
Comparisons of surround modulation time courses between different center-surround conditions are required to determine if horizontal and feedback components are separated in our experimental design. If differences depending on center-surround distance arise only from the horizontal component, they should be apparent in relatively delayed phases of the modulation time course. Furthermore, differences between dist2 or 3 and dist4 conditions should appear at delayed times with weaker magnitudes compared with those between dist1 and dist4 conditions.
Ideally, surround modulation latency should be defined and compared for individual cells using each of the four center-surround distance conditions. Population data could be summarized in distributions. However, this is problematic because of response variability, occasional weak modulation, and the confounding of suppressive and facilitative patterns within single time courses. Furthermore, simple cells, whose neural activity oscillates at the temporal frequency of the moving grating, often obscures onset time of surround modulation. If we analyze only complex cells with strong modulation, this can result in a biased outcome. Instead, we use population surround modulation functions for latency comparisons. For this analysis, cells that exhibit significant modulation (two-sided Mann-Whitney U-test (p<0.05)) for the dist1 condition, are included. Surround modulation functions for individual cells are normalized before being averaged. These procedures minimize the possibility of a biased outcome.
4.5. Fast feedback and slow horizontal connections
Previous studies of different functions of feedback and horizontal connections in center-surround modulation rely largely on spatial domain analysis. (Angelucci and Bressloff, 2006; Hashemi-Nezhad and Lyon, 2012; Shushruth et al., 2013). This assumes that a surround which is distant from the CRF is out of range of cortical horizontal connections. The assumption is that comparisons between near and far surround conditions will elucidate the role of cortical horizontal connections in surround suppression. The finding that orientation tuning of surround suppression is apparent in near (but not far) surround conditions (Hashemi-Nezhad and Lyon, 2012; Shushruth et al., 2013), suggests that feedback circuits are less orientation biased than horizontal types.
Attempts to segregate feedback and horizontal connections based on differences in the temporal domain are clearly limited. The conduction velocity of horizontal connections is 10 times slower than that of feedback types (Girard et al., 2001). Therefore, surround modulation mediated by horizontal connections must be delayed and more sensitive to position change in the surround compared with those of a feedback type. Orientation-tuned surround suppression is reported to be temporally delayed compared with orientation-unturned surround suppression (Xing et al., 2005; Henry et al., 2013; Liu et al., 2013). However, there appears to be no evidence that the onset of surround modulation is systematically delayed as distance between center and surround stimuli increases. In a previous study of the time-distance relationship for surround suppression in the visual cortex, onset time was reported to be relatively constant as distance between center and surround stimuli was varied (Bair et al., 2003). If surround suppression is mediated by thin unmyelinated horizontal connections from adjacent cortical neurons, the latency of suppression for a distant surround stimulus should be substantially delayed compared to that for a nearby surround. So the finding noted above of non-delayed rapid suppression is probably caused by fast feedback connections from higher visual areas (Hupé et al., 2001; Bair et al., 2003).
In the current study, we use two sets of surround stimulus patterns which demonstrate that the annulus type used in a previous study (Bair et al., 2003) does not permit isolation of temporal parameters of horizontal connection transmission. With our annulus, center-surround distance is controlled by annuli of different widths whose outer diameter is fixed. Increments of center-surround distance are accompanied by decreases of stimulated surround areas (see Figure 1A). This causes decreased activation of feedback connections from extra striate cortex and horizontal branches from nearby cortical cells. Therefore, differences of modulation depending on center-surround distance occur from the beginning of the modulation time course which is mediated by fast feedback connections. Differences due to slow horizontal connections are reflected in a later part of the modulation time course. However, differences at later times are also mediated by feedback connections. Hence, we cannot separate contributions of feedback and horizontal connections with the annulus surround. In contrast, the small patch surround allows relatively stable activation levels of feedback connections regardless of center-surround distance. Differences depending on center-surround distance are initially very small but become more obvious at later times in the modulation time course. Also, differences between middle and far surround positions are delayed relatively more than those of near and far, suggesting involvement of slow horizontal connections.
We have shown that the Bair et al. (2003) finding obtained in monkey V1, that surround modulation onset is fast and rarely affected by the inner diameter of an annulus surround, can be replicated in the cat's visual cortex. Additionally, using small patch surround stimuli, we have isolated a slow component of surround modulation which is systematically delayed depending on center-surround distance. However, our findings have limitations. First, our stimulus design and hypothesis are based on a parsimonious model that surround modulation is explained by linear summation of feedback and horizontal components. Interaction between two components and possible contribution of feedforward connections are not fully examined. Therefore, we do not have direct evidence that only horizontal connections mediate the slow component of surround modulation. Second, we do not have precise receptive field (RF) eccentricity information for individual neurons. Visual angles on the retina are mapped in visual cortex with different cortical distances depending on eccentricity. With detailed eccentricity information about individual neurons, it is possible to provide relatively precise estimations of conduction velocity using cortical coordinates of center and surround stimuli. Third, for the slow component of surround modulation which we segregated here, the difference of onset between near and far surround conditions is longer than the temporal delay predicted from well-known conduction velocity estimates. Previous studies suggest that sub-threshold input from the CRF surround is transmitted with conduction velocities which are matched with those of horizontal connections (Grinvald et al., 1994; Bringuier et al., 1999). Our results are different in that we observe supra-threshold modulation, so an additional input integration process may be involved which results in longer temporal delays. These issues may be best approached by use of intracellular recordings.
5. Conclusions
Identical CRF stimuli are processed differently depending on overall context. Although previous studies have utilized CRF-surround modulation to elucidate distinctive features of horizontal and feedback connections, the current work provides the following novel findings. First, our results show clearly that activation of inhibitory interneurons requires stronger input from the CRF surround compared with that of excitatory types. Second, sub-threshold inputs from the CRF surround are reflected in different frequency bands of LFP power spectra depending on whether they are suppressive or facilitative. And our current protocol provides a critical isolation of fast and slow components of surround modulation. Furthermore, we show that unlike fast components, slow types are delayed as distance between CRF and surround stimuli increases. Surround information that is not spatially tuned may be processed earlier by fast feedback connections. Spatially tuned surround stimuli presented at specific positions may be processed later by slow horizontal connections. This type of mechanism may apply, e.g., to spatial coarse-to-fine processing found for single neurons in the visual pathway (Bredfeldt and Ringach, 2002; Mazer et al., 2002; Menz and Freeman, 2003; Frazor et al., 2004; Allen and Freeman, 2006; Malone et al., 2007). The results presented here have an important application because they concern areas outside the CRF which by definition cannot initiate spike activity. Manipulations of timing and spatial arrangements of CRF and surround stimuli may enable determinations of the participation of fast feedback and slow horizontal connections for processing global and local visual information.
Highlights.
Feedforward connections provide clear inputs to retinotopically aligned target cells.
Horizontal and feedback connections are thought to integrate visual information outside the CRF.
Suppressive modulation requires stronger input from CRF surround than facilitation.
Excitatory and inhibitory inputs from CRF surround are reflected differently in LFPs.
Findings are consistent with relatively slow spatially tuned horizontal connections.
Acknowledgement:
This research is supported by NIH grant EY01175.
Abbreviations list
- CRF
classical receptive field
- RF
receptive field
- LFP
local field potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Both authors contributed to conception and design of the experiments. Taekjun Kim conducted data collection and analysis. Both authors wrote the manuscript and approved the final version.
Conflict of Interest: None
References
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. I. The precision of the topography. Exp Brain Res. 1975;24:159–179. doi: 10.1007/BF00234061. [DOI] [PubMed] [Google Scholar]
- Allen EA, Freeman RD. Dynamic spatial processing originates in early visual pathways. J Neurosci. 2006;26:11763–11774. doi: 10.1523/JNEUROSCI.3297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM. Neural connections and receptive field properties in the primary visual cortex. Neuroscientist. 2002;8:443–456. doi: 10.1177/107385802236967. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. Rules of connectivity between geniculate cells and simple cells in cat primary visual cortex. J Neurosci. 2001;21:4002–4015. doi: 10.1523/JNEUROSCI.21-11-04002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res. 2006;154:93–120. doi: 10.1016/S0079-6123(06)54005-1. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Bullier J. Reaching beyond the classical receptive field of V1 neurons: Horizontal or feedback axons? J Physiol Paris. 2003;97:141–154. doi: 10.1016/j.jphysparis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Levitt JB, Walton EJS, Hupe J-M, Bullier J, Lund JS. Circuits for local and global signal integration in primary visual cortex. J Neurosci. 2002;22:8633–8646. doi: 10.1523/JNEUROSCI.22-19-08633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair W, Cavanaugh JR, Movshon JA. Time course and time-distance relationships for surround suppression in macaque V1 neurons. J Neurosci. 2003;23:7690–7701. doi: 10.1523/JNEUROSCI.23-20-07690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitski A, Gretton A, Magri C, Murayama Y, Montemurro MA, Logothetis NK, Panzeri S. Low-frequency local field potentials and spikes in primary visual cortex convey independent visual information. J Neurosci. 2008;28:5696–5709. doi: 10.1523/JNEUROSCI.0009-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P, Keliris GA, Ecker AS, Logothetis NK, Tolias AS. Feature selectivity of the gamma-band of the local field potential in primate primary visual cortex. Front Neurosci. 2008;2:199–207. doi: 10.3389/neuro.01.037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Tobin EA. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15:439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Bosking WH, Zhang Y, Schofield B, Fitzpatrick D. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J Neurosci. 1997;17:2112–2127. doi: 10.1523/JNEUROSCI.17-06-02112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredfeldt CE, Ringach DL. Dynamics of spatial frequency tuning in macaque V1. J Neurosci. 2002;22:1976–1984. doi: 10.1523/JNEUROSCI.22-05-01976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringuier V, Chavane F, Glaeser L, Frégnac Y. Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science. 1999;283:695–699. doi: 10.1126/science.283.5402.695. [DOI] [PubMed] [Google Scholar]
- Budd JM. Extrastriate feedback to primary visual cortex in primates: a quantitative analysis of connectivity. Proc Biol Sci. 1998;265:1037–1044. doi: 10.1098/rspb.1998.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullier J, Hupé JM, James AC, Girard P. The role of feedback connections in shaping the responses of visual cortical neurons. Progress in Brain Research. 2001:193–204. doi: 10.1016/s0079-6123(01)34014-1. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J Neurophysiol. 2002a;88:2530–2546. doi: 10.1152/jn.00692.2001. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Selectivity and spatial distribution of signals from the receptive field surround in macaque V1 neurons. J Neurophysiol. 2002b;88:2547–2556. doi: 10.1152/jn.00693.2001. [DOI] [PubMed] [Google Scholar]
- Chisum HJ, Mooser F, Fitzpatrick D. Emergent properties of layer 2/3 neurons reflect the collinear arrangement of horizontal connections in tree shrew visual cortex. J Neurosci. 2003;23:2947–2960. doi: 10.1523/JNEUROSCI.23-07-02947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat's primary visual cortex. J Neurophysiol. 1994;71:347–374. doi: 10.1152/jn.1994.71.1.347. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Ohzawa I, Freeman RD. Spatiotemporal organization of simple-cell receptive fields in the cat's striate cortex. I. General characteristics and postnatal development. J Neurophysiol. 1993;69:1091–1117. doi: 10.1152/jn.1993.69.4.1091. [DOI] [PubMed] [Google Scholar]
- Frazor RA, Albrecht DG, Geisler WS, Crane AM. Visual cortex neurons of monkeys and cats: temporal dynamics of the spatial frequency response function. J Neurophysiol. 2004;91:2607–2627. doi: 10.1152/jn.00858.2003. [DOI] [PubMed] [Google Scholar]
- Galuske RAW, Schmidt KE, Goebel R, Lomber SG, Payne BR. The role of feedback in shaping neural representations in cat visual cortex. Proc Natl Acad Sci U S A. 2002;99:17083–17088. doi: 10.1073/pnas.242399199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann MA, Thiele A. Comparison of spatial integration and surround suppression characteristics in spiking activity and the local field potential in macaque V1. Eur J Neurosci. 2008;28:447–459. doi: 10.1111/j.1460-9568.2008.06358.x. [DOI] [PubMed] [Google Scholar]
- Girard P, Hupé JM, Bullier J. Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities. J Neurophysiol. 2001;85:1328–1331. doi: 10.1152/jn.2001.85.3.1328. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke EE, Frostig RD, Hildesheim R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J Neurosci. 1994;14:2545–2568. doi: 10.1523/JNEUROSCI.14-05-02545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi-Nezhad M, Lyon DC. Orientation tuning of the suppressive extraclassical surround depends on intrinsic organization of V1. Cereb Cortex. 2012;22:308–326. doi: 10.1093/cercor/bhr105. [DOI] [PubMed] [Google Scholar]
- Henrie JA, Shapley R. LFP power spectra in V1 cortex: the graded effect of stimulus contrast. J Neurophysiol. 2005;94:479–490. doi: 10.1152/jn.00919.2004. [DOI] [PubMed] [Google Scholar]
- Henry CA, Joshi S, Xing D, Shapley RM, Hawken MJ. Functional characterization of the extraclassical receptive field in macaque V1: contrast, orientation, and temporal dynamics. J Neurosci. 2013;33:6230–6242. doi: 10.1523/JNEUROSCI.4155-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry GH, Salin P a., Bullier J. Projections from Areas 18 and 19 to Cat Striate Cortex: Divergence and Laminar Specificity. Eur J Neurosci. 1991;3:186–200. doi: 10.1111/j.1460-9568.1991.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Gilbert CD. Synaptic physiology of horizontal connections in the cat's visual cortex. J Neurosci. 1991;11:1800–1809. doi: 10.1523/JNEUROSCI.11-06-01800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL DH, WIESEL TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupé JM, James AC, Girard P, Lomber SG, Payne BR, Bullier J. Feedback connections act on the early part of the responses in monkey visual cortex. J Neurophysiol. 2001;85:134–145. doi: 10.1152/jn.2001.85.1.134. [DOI] [PubMed] [Google Scholar]
- Hupé JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J. Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature. 1998;394:784–787. doi: 10.1038/29537. [DOI] [PubMed] [Google Scholar]
- Ichida JM, Schwabe L, Bressloff PC, Angelucci A. Response facilitation from the “suppressive” receptive field surround of macaque V1 neurons. J Neurophysiol. 2007;98:2168–2181. doi: 10.1152/jn.00298.2007. [DOI] [PubMed] [Google Scholar]
- Jia X, Smith MA, Kohn A. Stimulus selectivity and spatial coherence of gamma components of the local field potential. J Neurosci. 2011;31:9390–9403. doi: 10.1523/JNEUROSCI.0645-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia MK, Ito M, Gilbert CD, Westheimer G. Improvement in visual sensitivity by changes in local context: parallel studies in human observers and in V1 of alert monkeys. Neuron. 1995;15:843–856. doi: 10.1016/0896-6273(95)90175-2. [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Westheimer G, Gilbert CD. Spatial distribution of contextual interactions in primary visual cortex and in visual perception. J Neurophysiol. 2000;84:2048–2062. doi: 10.1152/jn.2000.84.4.2048. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim HR, Kim K, Lee C. Modulation of V1 Spike Response by Temporal Interval of Spatiotemporal Stimulus Sequence. PLoS One. 2012:7. doi: 10.1371/journal.pone.0047543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisvárday ZF, Tóth E, Rausch M, Eysel UT. Orientation-specific relationship between populations of excitatory and inhibitory lateral connections in the visual cortex of the cat. Cereb Cortex. 1997;7:605–618. doi: 10.1093/cercor/7.7.605. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Lund JS. Contrast dependence of contextual effects in primate visual cortex. Nature. 1997;387:73–76. doi: 10.1038/387073a0. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Lund JS. The spatial extent over which neurons in macaque striate cortex pool visual signals. Vis Neurosci. 2002;19:439–452. doi: 10.1017/s0952523802194065. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Hashemi-Nezhad M, Lyon DC. Sharper orientation tuning of the extraclassical suppressive-surround due to a neuron's location in the V1 orientation map emerges late in time. Neuroscience. 2013;229:100–117. doi: 10.1016/j.neuroscience.2012.10.071. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Amir Y, Harel M, Grinvald A. Relationship between intrinsic connections and functional architecture revealed by optical imaging and in vivo targeted biocytin injections in primate striate cortex. Proc Natl Acad Sci U S A. 1993;90:10469–10473. doi: 10.1073/pnas.90.22.10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone BJ, Kumar VR, Ringach DL. Dynamics of receptive field size in primary visual cortex. J Neurophysiol. 2007;97:407–414. doi: 10.1152/jn.00830.2006. [DOI] [PubMed] [Google Scholar]
- Mazer JA, Vinje WE, McDermott J, Schiller PH, Gallant JL. Spatial frequency and orientation tuning dynamics in area V1. Proc Natl Acad Sci U S A. 2002;99:1645–1650. doi: 10.1073/pnas.022638499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Gilbert CD, Rivlin PK, Wiesel TN. Targets of horizontal connections in macaque primary visual cortex. J Comp Neurol. 1991;305:370–392. doi: 10.1002/cne.903050303. [DOI] [PubMed] [Google Scholar]
- Menz MD, Freeman RD. Stereoscopic depth processing in the visual cortex: a coarse-to-fine mechanism. Nat Neurosci. 2003;6:59–65. doi: 10.1038/nn986. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Mizobe K, Polat U, Pettet MW, Kasamatsu T. Facilitation and suppression of single striate-cell activity by spatially discrete pattern stimuli presented beyond the receptive field. Vis Neurosci. 18:377–391. doi: 10.1017/s0952523801183045. [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat Rev Neurosci. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JI, Frost BJ. Intracortical facilitation among co-oriented, co-axially aligned simple cells in cat striate cortex. Exp Brain Res. 1985;61:54–61. doi: 10.1007/BF00235620. [DOI] [PubMed] [Google Scholar]
- Ozeki H, Sadakane O, Akasaki T, Naito T, Shimegi S, Sato H. Relationship between excitation and inhibition underlying size tuning and contextual response modulation in the cat primary visual cortex. J Neurosci. 2004;24:1428–1438. doi: 10.1523/JNEUROSCI.3852-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Payne BR, Budd J. A numerical analysis of the geniculocortical input to striate cortex in the monkey. Cereb Cortex. 4:215–229. doi: 10.1093/cercor/4.3.215. [DOI] [PubMed] [Google Scholar]
- Polat U, Mizobe K, Pettet MW, Kasamatsu T, Norcia AM. Collinear stimuli regulate visual responses depending on cell's contrast threshold. Nature. 1998;391:580–584. doi: 10.1038/35372. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Lund JS. Intrinsic laminar lattice connections in primate visual cortex. J Comp Neurol. 1983;216:303–318. doi: 10.1002/cne.902160307. [DOI] [PubMed] [Google Scholar]
- Salami M, Itami C, Tsumoto T, Kimura F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc Natl Acad Sci U S A. 2003;100:6174–6179. doi: 10.1073/pnas.0937380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Bullier J, Kennedy H. Convergence and divergence in the afferent projections to cat area 17. J Comp Neurol. 1989;283:486–512. doi: 10.1002/cne.902830405. [DOI] [PubMed] [Google Scholar]
- Salin PA, Girard P, Kennedy H, Bullier J. Visuotopic organization of corticocortical connections in the visual system of the cat. J Comp Neurol. 1992;320:415–434. doi: 10.1002/cne.903200402. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast's effect on spatial summation by macaque V1 neurons. Nat Neurosci. 1999;2:733–739. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Obermayer K, Angelucci A, Bressloff PC. The role of feedback in shaping the extra-classical receptive field of cortical neurons: a recurrent network model. J Neurosci. 2006;26:9117–9129. doi: 10.1523/JNEUROSCI.1253-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seriès P, Lorenceau J, Frégnac Y. The “silent” surround of V1 receptive fields: Theory and experiments. Journal of Physiology Paris. 2003:453–474. doi: 10.1016/j.jphysparis.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Shushruth S, Nurminen L, Bijanzadeh M, Ichida JM, Vanni S, Angelucci A. Different orientation tuning of near- and far-surround suppression in macaque primary visual cortex mirrors their tuning in human perception. J Neurosci. 2013;33:106–119. doi: 10.1523/JNEUROSCI.2518-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DC, Todorov E V, Siapas AG, Toth LJ, Kim DS, Sur M. A local circuit approach to understanding integration of long-range inputs in primary visual cortex. Cereb Cortex. 8:204–217. doi: 10.1093/cercor/8.3.204. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Toth LJ, Rao SC, Kim DS, Somers D, Sur M. Subthreshold facilitation and suppression in primary visual cortex revealed by intrinsic signal imaging. Proc Natl Acad Sci U S A. 1996;93:9869–9874. doi: 10.1073/pnas.93.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Asymmetric suppression outside the classical receptive field of the visual cortex. J Neurosci. 1999;19:10536–10553. doi: 10.1523/JNEUROSCI.19-23-10536.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BS, Dhruv NT, Solomon SG, Tailby C, Lennie P. Early and late mechanisms of surround suppression in striate cortex of macaque. J Neurosci. 2005;25:11666–11675. doi: 10.1523/JNEUROSCI.3414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Shapley RM, Hawken MJ, Ringach DL. Effect of stimulus size on the dynamics of orientation selectivity in Macaque V1. J Neurophysiol. 2005;94:799–812. doi: 10.1152/jn.01139.2004. [DOI] [PubMed] [Google Scholar]
- Yao H, Li CY. Clustered organization of neurons with similar extra-receptive field properties in the primary visual cortex. Neuron. 2002;35:547–553. doi: 10.1016/s0896-6273(02)00782-1. [DOI] [PubMed] [Google Scholar]