Abstract
The Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) and Treatment Units for Research on Neurocognition and Schizophrenia projects were designed to facilitate the development of new drugs for the treatment of cognitive impairments in people with schizophrenia. The MATRICS project identified three drug mechanisms of particular interest: dopaminergic, cholinergic, and glutamatergic. As a group, while people with schizophrenia have moderate cognitive impairment, it is the best predictor of long-term outcome. Unfortunately, there are no approved medications for cognitive impairment in this population. Hence, the development of new pharmacological approaches is critical for reducing illness-related disability. The combination of an acetylcholinesterase inhibitor (AChEI) and memantine is more effective than either medication alone to improve cognition in Alzheimer’s dementia. Galantamine is not only an AChEI, but also a positive allosteric modulator of the α4β2 and α7 nicotinic receptors. Hypofunction of N-methyl-D-aspartate (NMDA) receptors has been implicated in the pathophysiology of cognitive symptoms in schizophrenia and hence memantine may positively impact cognition. Memantine decreases the tonic NMDA current and galantamine enhances the action potential mediated by a postsynaptic NMDA current. This results in an increased signal transmission; therefore, a greater signal-to-noise ratio occurs with the combination than memantine alone. Galantamine improves the α-amino-3-hydroxy-5-methyl-4-isoxazol-propionate (AMPA)-mediated signaling which could be neuroprotective and may improve memory coding. The combination of galantamine and memantine may be particularly effective in schizophrenia in order to increase the selective cognition enhancement produced by either medication alone. In the future, multitarget-directed ligands may play a role in the treatment of complex diseases like schizophrenia.
Keywords: Schizophrenia, cognitive impairments, galantamine, memantine
Introduction
Cognitive impairments are core features of schizophrenia. The degree of cognitive impairment in individuals with schizophrenia is the best predictor of their long-term outcome. Unfortunately, there are no effective approved medications for this major public health problem. Hence, the development of new pharmacological approaches is critical for reducing illness-related disability. The National Institute of Mental Health (NIMH) funded Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) and Treatment Units for Research on Neurocognition and Schizophrenia (TURNS) projects were designed to facilitate the development of new drugs for the treatment of cognitive impairments in people with schizophrenia. The MATRICS project identified three drug mechanisms of particular interest: dopaminergic, cholinergic, and glutamatergic.
The combination of an acetylcholinesterase inhibitor (AChEI) and memantine is more effective than either medication alone to improve cognition in Alzheimer’s dementia (Tariot et al., 2004; Atri et al., 2008; Parsons et al., 2013; Atri et al., 2013). The purpose of this paper is to review evidence suggesting a potential role of the combination of galantamine and memantine to improve cognitive impairments in schizophrenia.
Cholinergic system and cognition in schizophrenia
The cholinergic system is associated with attention, memory, processing speed, and sensory gating (Furey et al., 2000), processes that are impaired in schizophrenia. Postmortem studies have demonstrated alterations in muscarinic receptor and nicotinic receptor availability or expression (Lee et al., 2001). Serum anticholinergic activity in schizophrenia showed a significant association with impaired performance in the MATRICS-based measures of verbal working memory and verbal learning (Vinogradov et al., 2009). The FDA has approved AChEIs for the treatment of Alzheimer’s disease. Several studies have been done to test the efficacy of AChEIs to improve cognitive impairment in schizophrenia.
Donepezil and Rivastigmine are not effective for cognitive dysfunction in schizophrenia
Four Randomized Controlled Trials (RCTs) failed to observe significant cognitive effects of donepezil in this population (Friedman et al., 2002; Tugal et al., 2004; Freudenreich et al., 2005; Fagerlund et al., 2007). Similarly, in another 24-week RCT (N=21) in stable patients with schizophrenia (Sharma et al., 2006), rivastigmine failed to improve cognitive impairment. Finally, in the largest (N=245) RCT to date, donepezil failed to demonstrate any beneficial effects on a comprehensive neuropsychological battery (Keefe et al., 2008).
Galantamine is better than donepezil and rivastigmine
In contrast to other donepezil and rivastigmine, galantamine has some unique pharmacological properties. Galantamine is not only an AChEI, but also a positive allosteric modulator of the α4β2 and α7 nicotinic receptors. The allosteric properties of galantamine could lead directly to increased release of acetylcholine and activation of postsynaptic nicotinic receptors (Samochocki et al., 2003), or act indirectly through its effects on the release of other neurotransmitters, especially glutamate and dopamine Galantamine increases dopaminergic activity and release in the prefrontal cortex in a dose-dependent manner (Schilstrom et al., 2007). Galantamine increased dopamine release in the hippocampus and this effect was associated with improvement in cognition in a mouse model of Alzheimer’s disease (Wang and Moyzis, 2007).
Galantamine shows selective benefits for cognitive impairments
In an RCT with 24 participants with schizophrenia (Lee et al., 2007), galantamine improved performance on the Hopkins Verbal Learning Test recognition subtest, on the Stroop color subtest. These improvements are suggestive of selective benefit for visual recognition. In another RCT (Schubert et al., 2006), galantamine showed improvement in delayed memory and attention in participants with schizophrenia. In a 12-week RCT in 86 participants with schizophrenia, galantamine produced a significant benefit for processing speed and verbal memory. There was some evidence of galantamine improving the alogia subscale of the Scale for the Assessment of Negative Symptoms (Buchanan et al., 2008). Finally, in a 52-week RCT in 32 participants with schizophrenia, who were on long-acting injectable risperidone, social cognition was better in those who received adjunctive galantamine from month six to 12 compared to placebo (Lindenmayer and Khan, 2011). The above studies suggest a potential beneficial effect of galantamine in improving cognition in schizophrenia.
Glutamatergic system and cognitive impairments
A growing body of evidence supports a link between the glutamatergic neurotransmission and schizophrenia (Javitt and Zukin, 1991; Bressan and Pilowsky, 2000; Carlsson et al., 2000; Egerton et al., 2005; Yang and Chen, 2005). Memantine is an non competitive antagonist of N-methyl-D-aspartate (NMDA) glutamatergic receptors, an effect hypothesized to arise from its ability to block the excessive influx of calcium ions through the channel of the activated NMDA receptor (Parsons et al., 2007). Memantine is FDA approved for the treatment of moderate to severe Alzheimer’s dementia. The rationale for using memantine as an adjunctive therapy for people with schizophrenia comes from the circumstantial evidence that the glutamatergic system, and specifically hypofunction of NMDA receptors, is involved in the pathophysiology of positive, negative, and cognitive symptoms (Javitt and Zukin, 1991; Bressan and Pilowsky, 2000; Carlsson et al., 2000; Egerton et al., 2005; Yang and Chen, 2005). Several RCTs have shown improvement in people with schizophrenia receiving first and second generation antipsychotics, when treated with NMDA receptor allosteric agonists at the glycineB site (glycine, D-serine, D-cycloserine, sarcosine) (Coyle et al., 2002; Tsai and Coyle, 2002; Heresco-Levy et al., 2005). However, large multicenter RCTs have failed to demonstrate beneficial effects of glycine or D-cycloserine (glycine-site agonists of NMDA receptors) on negative symptoms or cognition (Buchanan et al., 2007). Paradoxically, NMDA receptor antagonists may also improve symptoms of schizophrenia, as glutamatergic neurons in the prefrontal cortex are inhibited by GABA interneurons acting through the NMDA receptor (Maccaferri and Dingledine, 2002; Homayoun and Moghaddam, 2007). NMDA receptor antagonists have been shown to release this inhibition, producing profound indirect excitation of cortical pyramidal neurons and increased glutamatergic outflow in the cortex (Moghaddam et al., 1997; Homayoun and Moghaddam, 2007).
Memantine for cognitive impairments in schizophrenia
In schizophrenia, an RCT (N=138) of memantine failed to improve cognition (Lieberman et al., 2009). However, this study focused on residual psychopathology (Brief Psychiatric Rating Scale total score ≥26 and individual item ≥4) rather than impaired cognition, used the Brief Assessment of Cognition in Schizophrenia instead of the MATRICS Consensus Cognitive Battery (MCCB) to assess change in cognition, and was a short trial of eight weeks. In a 12-week RCT, memantine did not improve cognition in 26 participants with chronic schizophrenia (Lee et al., 2012). In another 12-week RCT (N=21), memantine as an augmentation treatment significantly (p<0.01) improved cognition in participants with schizophrenia on clozapine (de Lucena et al., 2009). The last two studies used Mini-Mental State Examination to assess cognition. The FDA-NIMH-MATRICS workshop has recommended the MCCB to measure cognition in clinical trials (Buchanan et al., 2005; Green et al., 2008; Kern et al., 2008; Nuechterlein et al., 2008; Kern et al., 2011). Finally, in a meta-analysis of the three RCTs described above (N=186), memantine significantly (p=0.002) improved some cognitive functioning in people with schizophrenia (Kishi and Iwata, 2013).
To summarize, the strategy of cholinergic enhancement and using NMDA antagonists demonstrated only selective benefits. This approach of using only one target has not completely resolved the issue of treating impaired cognition in schizophrenia.
Advantages combining galantamine and memantine
Galantamine increases glutamatergic activity, whereas memantine inhibits it (Santos et al., 2002; Rogawski and Wenk, 2003). Thus, at first glance, the two drugs appear to act in an opposing manner. However, a closer examination of the effects of both medications on the cholinergic and glutamatergic systems reveals that these medications work synergistically (Figure 1) to improve cognitive impairments in people with Alzheimer’s dementia. In addition, it is unlikely that the combination treatment would affect the metabolism of either drug because galantamine is metabolized by cytochrome P450 (CYP) 2D6 and CYP3A4, which are not affected by memantine (Micuda et al., 2004). The use of galantamine and memantine in combination is supported by pharmacodynamic and pharmacokinetic studies (Wenk et al., 2000; Yao et al., 2005; Grossberg et al., 2006). Through the presynaptic α-7 nicotinic acetylcholine receptor (nAChR), galantamine increases glutamate release. Memantine decreases the tonic NMDA current and galantamine enhances the action potential mediated by a postsynaptic NMDA current which results in an increased signal transmission. Therefore, a greater signal-to-noise ratio occurs with the combination than memantine alone. Galantamine improves the α-amino-3-hydroxy-5-methyl-4-isoxazol-propionate (AMPA)-mediated signaling which could be neuroprotective and may improve memory coding as shown in Figure 1 (Geerts and Grossberg, 2006). Finally, galantamine potentiates the neuroprotective effect of memantine against NMDA-induced excitotoxicity (Zhao et al., 2006; Lopes et al., 2013). Combination therapy with memantine and AChEIs may have synergistic efficacy in slowing the progression of Alzheimer’s dementia by modulating the glutamatergic and cholinergic neurotransmitter systems (Butterfield and Pocernich, 2003; Parsons et al., 2013).
Figure 1.
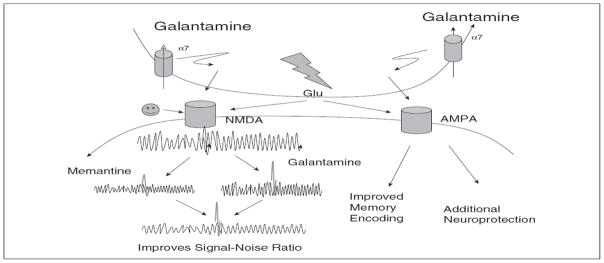
Galantamine may augment the noise suppression of memantine’s glutamatergic action. Galantamine potentiates glutamatergic neurotransmission mediated by α-amino-3-hydroxy-5-methyl-4-isoxazol-propionate (AMPA) and N-methyl-D-aspartate (NMDA) receptors.
Reproduced with permission.
Combination is effective in dementia
RCTs with the combination therapy of AChEIs and memantine in Alzheimer’s dementia slowed cognitive decline/improved cognition compared to cholinesterase inhibitor monotherapy (Tariot et al., 2004; Atri et al., 2008; Atri et al., 2013). Functioning improved significantly in the activities of daily living (ADL) (Tariot et al., 2004) and this finding may map on to people with schizophrenia. An effect size of 0.49 and 0.73 for cognition and ADL respectively were shown for combination therapy versus monotherapy at the end of year four (Atri et al., 2008). A clinically significant effect size of 0.2–0.4 were shown in all efficacy domains such as cognition, functioning and global outcome (Atri et al., 2013). In a 2-year, RCT, a combination of galantamine and memantine (compared to galantamine alone or placebo) modified cognitive function in 232 subjects with mild cognitive impairment (prodrome of Alzheimer’s dementia) (Peters et al., 2012). In addition to the pharmacological agents to modulate NMDA function, the NMDA antagonist in the central nervous system could also be a possible target to improve cognitive function. Kynurenic acid is the only NMDA receptor antagonist in the human central nervous system (Stone, 1993; Krystal et al., 1994).
Kynurenine abnormality in Schizophrenia
The kynurenine pathway regulates the synthesis of kynurenic acid (KYNA) by an enzymatic cascade, known as the kynurenine pathway, which is controlled by the immune system. The indoleamine dioxygenase (IDO) activity is associated with the type-1/type-2 immune response (Grohmann et al., 2003). Th-1 or type-1 cytokines, such as interferon (IFN)-γ and interleukin (IL)-2, stimulate the activity of IDO and tryptophan catabolism to kynurenine (Grohmann et al., 2003). Additional evidence supporting this hypothesis is molecular genetics of schizophrenia – genes involved in the glutamatergic system (Collier and Li, 2003). Increased levels of KYNA have been observed in the cerebrospinal fluid of 28 people with schizophrenia compared to 17 healthy controls (Erhardt et al., 2001). KYNA has been identified as a potent antagonist of NMDA receptor and the α-7 nicotinic receptor (Parsons et al., 1997; Hilmas et al., 2001). This antagonism may be associated with cognitive impairment. Stimulation of the cholinergic system down regulates the inflammatory immune response which is known as cholinergic anti-inflammatory pathway (van Westerloo and van der Poll, 2005). Therefore α-7 nicotinic receptor antagonism of KYNA may contribute to the inhibition of this cholinergic anti-inflammatory pathway. Early developmental elevations of brain KYNA was associated with cognitive impairments in adult rats which were reversed with galantamine (Alexander et al., 2013). There is a growing body of evidence that KYNA is associated with cognitive impairments in schizophrenia (Wonodi and Schwarcz, 2010). The combination of galantamine and memantine may decrease KYNA via the α-7 nicotinic and the NMDA receptors resulting in cognitive enhancement as shown in Figure 2 (Wonodi and Schwarcz, 2010).
Figure 2.
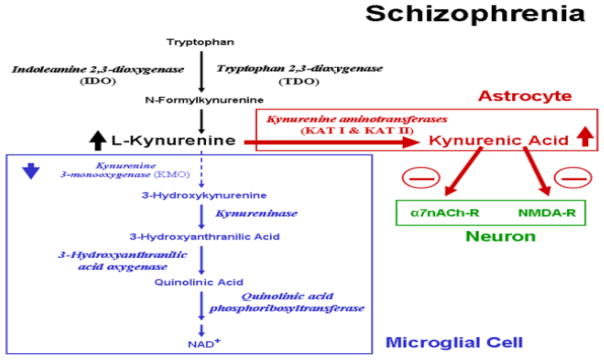
Excess Kynurenic acid (KYNA) is associated with cognitive impairments in schizophrenia. Galantamine and Memantine combination may target α7nACh-R and NMDA-R respectively to decrease KYNA thereby improving cognitive impairments.
Discussion
The common mechanisms of cognitive impairment in schizophrenia and Alzheimer’s dementia are the cholinergic, α-7 nicotinic receptor and glutamatergic systems (Palmer and Gershon, 1990; Nordberg, 1992; Buchanan et al., 2007). To our knowledge, there are no head-to-head clinical trials comparing donepezil or rivastigmine and memantine vs. galantamine and memantine in Alzheimer’s dementia. As mentioned above, in schizophrenia, galantamine, not donepezil or rivastigmine, has shown an efficacy signal for cognitive impairments.
Therapeutic doses of clozapine, olanzapine, and, to a lesser extent, quetiapine are associated with clinically relevant anticholinergic activity (Chew et al., 2006). These antipsychotics are likely to obscure potential cognitive enhancement. In addition, chronic and excessive use of benztropine may worsen cognition and show lack of response to the combination treatment with galantamine and memantine (Wijegunaratne et al., 2014).
The use of combination treatment is playing a major role in the treatment of several other complex pathological conditions such as depression in schizoaffective disorder, bipolar type, posttraumatic stress disorder (PTSD), unipolar and bipolar depression (Koola et al., 2011; Koola et al., 2014). There is evidence that naltrexone and prazosin combination may be effective for PTSD and alcohol use disorder (Qazi et al., in press). An alternative to drug combinations is the use of multitarget-directed ligands (MDTLs) that can hit multiple targets (Cavalli et al., 2008; Simoni et al., 2012). The MDTL of galantamine and memantine has been shown to be potentially useful in Alzheimer’s dementia (Simoni et al., 2012). The advantages of MDTLs include: clinical development is faster because it is easier to understand the pharmacokinetics of a single compound than the combination treatment, hitting multiple targets simultaneously for the synergistic effect leading to improved efficacy, less side effects with more safety and less drug-drug interactions, simplified medication regimen leading to better adherence (Zimmermann et al., 2007; Small and Dubois, 2007; Morphy and Rankovic, 2007; Cavalli et al., 2008; Hopkins, 2008).
Conclusions and Future Directions
To date, similar studies (Tariot et al., 2004; Atri et al., 2008; Peters et al., 2012; Atri et al., 2013) combining AChEI/galantamine and memantine have not been done in schizophrenia. The combination of galantamine and memantine may be particularly effective in schizophrenia to broaden the selective cognition enhancement produced by either medication alone. Development of an effective treatment for cognitive impairments in schizophrenia is a major public health need. The proposed novel dual receptor mechanism targeting cholinergic and glutamatergic systems simultaneously may be molecular targets that are clinically meaningful for the cognitive impairments in schizophrenia. Cognitive enhancement from treatment with the combination of galantamine and memantine may lead to improved functioning in people with schizophrenia. Future RCTs are warranted to integrate the pathophysiology of cognition in schizophrenia with the galantamine and memantine combination treatment. Because of lack of biomarkers, more research is warranted to shed light on the association of biomarkers and cognitive enhancement with treatment interventions.
Acknowledgments
Role of Funding Source
Faith Dickerson is funded by the Grant 07R-1690 from the Stanley Medical Research Institute.
Maju Koola was supported by the NIMH funded 5R13MH064074 to present part of this manuscript at the 2010 APA Research Colloquium for Junior Investigators in New Orleans, LA, May 22–26. We like to thank Robert Schwarcz, PhD for Figure 2 and Robert Freedman, MD for reviewing the manuscript.
Footnotes
Contributors
Maju Koola wrote part of this manuscript under the mentorships of Drs. Juan Bustillo and Robert W. Buchanan for the 2010 American Psychiatric Association (APA) Research Colloquium for Junior Investigators. Maju Koola wrote the first draft of the manuscript. Drs. Scott Aaronson, Katherine Aitchison, Robert Buchanan, Faith Dickerson, Anilkumar Pillai, Daniel Weinberger made significant intellectual contributions in the manuscript preparation.
Conflict of Interest
Dr. Buchanan has served as a DSMB member for Otsuka and Pfizer; has consulted with Abbott; Amgen; Bristol-Meyers Squibb; EnVivo; Omeros; Pfizer and have served on the following Advisory Boards: Amgen; EnVivo; Janssen Pharmaceutical, Inc; NuPathe, Inc.; Pfizer; Roche and Takeda. Other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander KS, Pocivavsek A, Wu HQ, Pershing ML, Schwarcz R, Bruno JP. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience. 2013;238:19–28. doi: 10.1016/j.neuroscience.2013.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atri A, Shaughnessy LW, Locascio JJ, Growdon JH. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209–21. doi: 10.1097/WAD.0b013e31816653bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atri A, Molinuevo JL, Lemming O, Wirth Y, Pulte I, Wilkinson D. Memantine in patients with Alzheimer’s disease receiving donepezil: new analyses of efficacy and safety for combination therapy. Alzheimers Res Ther. 2013;5(1):6. doi: 10.1186/alzrt160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan RA, Pilowsky LS. Imaging the glutamatergic system in vivo--relevance to schizophrenia. Eur J Nucl Med. 2000;27(11):1723–31. doi: 10.1007/s002590000372. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, Nuechterlein KH, Laughren T, Levin R, Stover E, Fenton W, Marder SR. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31(1):5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull. 2007;33(5):1120–30. doi: 10.1093/schbul/sbm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164(10):1593–602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Conley RR, Dickinson D, Ball MP, Feldman S, Gold JM, McMahon RP. Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am J Psychiatry. 2008;165(1):82–9. doi: 10.1176/appi.ajp.2007.07050724. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Pocernich CB. The glutamatergic system and Alzheimer’s disease: therapeutic implications. CNS Drugs. 2003;17(9):641–52. doi: 10.2165/00023210-200317090-00004. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Waters S, Carlsson ML. Network interactions in schizophrenia - therapeutic implications. Brain Res Brain Res Rev. 2000;31(2–3):342–9. doi: 10.1016/s0165-0173(99)00050-8. [DOI] [PubMed] [Google Scholar]
- Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51(3):347–72. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Kirshner MA, Bies RR, Kapur S, Gharabawi G. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr Res. 2006;88(1–3):63–72. doi: 10.1016/j.schres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Collier DA, Li T. The genetics of schizophrenia: glutamate not dopamine? Eur J Pharmacol. 2003;480(1–3):177–84. doi: 10.1016/j.ejphar.2003.08.105. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G, Goff DC. Ionotropic glutamate receptors as therapeutic targets in schizophrenia. Curr Drug Targets CNS Neurol Disord. 2002;1(2):183–9. doi: 10.2174/1568007024606212. [DOI] [PubMed] [Google Scholar]
- de Lucena D, Fernandes BS, Berk M, Dodd S, Medeiros DW, Pedrini M, Kunz M, Gomes FA, Giglio LF, Lobato MI, Belmonte-de-Abreu PS, Gama CS. Improvement of negative and positive symptoms in treatment-refractory schizophrenia: a double-blind, randomized, placebo-controlled trial with memantine as add-on therapy to clozapine. J Clin Psychiatry. 2009;70(10):1416–23. doi: 10.4088/JCP.08m04935gry. [DOI] [PubMed] [Google Scholar]
- Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA. Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia. Psychopharmacology (Berl) 2005;179(1):77–84. doi: 10.1007/s00213-004-2109-y. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313(1–2):96–8. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- Fagerlund B, Soholm B, Fink-Jensen A, Lublin H, Glenthoj BY. Effects of donepezil adjunctive treatment to ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin Neuropharmacol. 2007;30(1):3–12. doi: 10.1097/01.WNF.0000240940.67241.F6. [DOI] [PubMed] [Google Scholar]
- Freudenreich O, Herz L, Deckersbach T, Evins AE, Henderson DC, Cather C, Goff DC. Added donepezil for stable schizophrenia: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2005;181(2):358–63. doi: 10.1007/s00213-005-2235-1. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Adler DN, Howanitz E, Harvey PD, Brenner G, Temporini H, White L, Parrella M, Davis KL. A double blind placebo controlled trial of donepezil adjunctive treatment to risperidone for the cognitive impairment of schizophrenia. Biol Psychiatry. 2002;51(5):349–57. doi: 10.1016/s0006-3223(01)01342-7. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290(5500):2315–9. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- Geerts H, Grossberg GT. Pharmacology of acetylcholinesterase inhibitors and N-methyl-D-aspartate receptors for combination therapy in the treatment of Alzheimer’s disease. J Clin Pharmacol. 2006;46(7 Suppl 1):8S–16S. doi: 10.1177/0091270006288734. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Kern RS, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Seidman LJ, Stover E, Marder SR. Functional co-primary measures for clinical trials in schizophrenia: results from the MATRICS Psychometric and Standardization Study. Am J Psychiatry. 2008;165(2):221–8. doi: 10.1176/appi.ajp.2007.07010089. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24(5):242–8. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- Grossberg GT, Edwards KR, Zhao Q. Rationale for combination therapy with galantamine and memantine in Alzheimer’s disease. J Clin Pharmacol. 2006;46(7 Suppl 1):17S–26S. doi: 10.1177/0091270006288735. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, Catinari S, Ermilov M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57(6):577–85. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21(19):7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–90. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Malhotra AK, Meltzer HY, Kane JM, Buchanan RW, Murthy A, Sovel M, Li C, Goldman R. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology. 2008;33(6):1217–28. doi: 10.1038/sj.npp.1301499. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165(2):214–20. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, Keefe RS, Mesholam-Gately RI, Seidman LJ, Lee C, Sugar CA, Marder SR. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1–3):124–31. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Iwata N. NMDA receptor antagonists interventions in schizophrenia: Meta-analysis of randomized, placebo-controlled trials. J Psychiatr Res. 2013;47(9):1143–9. doi: 10.1016/j.jpsychires.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989–90. doi: 10.1097/NMD.0b013e3182392d8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koola MM, Varghese SP, Fawcett JA. High-dose prazosin for the treatment of post-traumatic stress disorder. Ther Adv Psychopharmacol. 2014;4(1):43–47. doi: 10.1177/2045125313500982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lee JG, Lee SW, Lee BJ, Park SW, Kim GM, Kim YH. Adjunctive memantine therapy for cognitive impairment in chronic schizophrenia: a placebo-controlled pilot study. Psychiatry Investig. 2012;9(2):166–73. doi: 10.4306/pi.2012.9.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Breese CR, Strook ML, Leonard S. The effect of nicotine and haloperidol co-treatment on nicotinic receptor levels in the rat brain. Brain Res Mol Brain Res. 2001;86(1–2):115–24. doi: 10.1016/s0169-328x(00)00274-6. [DOI] [PubMed] [Google Scholar]
- Lee SW, Lee JG, Lee BJ, Kim YH. A 12-week, double-blind, placebo-controlled trial of galantamine adjunctive treatment to conventional antipsychotics for the cognitive impairments in chronic schizophrenia. Int Clin Psychopharmacol. 2007;22(2):63–8. doi: 10.1097/YIC.0b013e3280117feb. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Papadakis K, Csernansky J, Litman R, Volavka J, Jia XD, Gage A. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology. 2009;34(5):1322–9. doi: 10.1038/npp.2008.200. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Khan A. Galantamine augmentation of long-acting injectable risperidone for cognitive impairments in chronic schizophrenia. Schizophr Res. 2011;125(2–3):267–77. doi: 10.1016/j.schres.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Lopes JP, Tarozzo G, Reggiani A, Piomelli D, Cavalli A. Galantamine potentiates the neuroprotective effect of memantine against NMDA-induced excitotoxicity. Brain Behav. 2013;3(2):67–74. doi: 10.1002/brb3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Dingledine R. Control of feedforward dendritic inhibition by NMDA receptor-dependent spike timing in hippocampal interneurons. J Neurosci. 2002;22(13):5462–72. doi: 10.1523/JNEUROSCI.22-13-05462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micuda S, Mundlova L, Anzenbacherova E, Anzenbacher P, Chladek J, Fuksa L, Martinkova J. Inhibitory effects of memantine on human cytochrome P450 activities: prediction of in vivo drug interactions. Eur J Clin Pharmacol. 2004;60(8):583–9. doi: 10.1007/s00228-004-0825-1. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphy R, Rankovic Z. Fragments, network biology and designing multiple ligands. Drug Discov Today. 2007;12(3–4):156–60. doi: 10.1016/j.drudis.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Nordberg A. Neuroreceptor changes in Alzheimer disease. Cerebrovasc Brain Metab Rev. 1992;4(4):303–28. [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–13. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Gershon S. Is the neuronal basis of Alzheimer’s disease cholinergic or glutamatergic? FASEB J. 1990;4(10):2745–52. doi: 10.1096/fasebj.4.10.2165009. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G, Hartmann S, Lorenz B, Wollenburg C, Baran L, Przegalinski E, Kostowski W, Krzascik P, Chizh B, Headley PM. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J Pharmacol Exp Ther. 1997;283(3):1264–75. [PubMed] [Google Scholar]
- Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Dekundy A, Pulte I. Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res. 2013;24(3):358–69. doi: 10.1007/s12640-013-9398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters O, Lorenz D, Fesche A, Schmidtke K, Hull M, Perneczky R, Ruther E, Moller HJ, Jessen F, Maier W, Kornhuber J, Jahn H, Luckhaus C, Gertz HJ, Schroder J, Pantel J, Teipel S, Wellek S, Frolich L, Heuser I. A combination of galantamine and memantine modifies cognitive function in subjects with amnestic MCI. J Nutr Health Aging. 2012;16(6):544–8. doi: 10.1007/s12603-012-0062-8. [DOI] [PubMed] [Google Scholar]
- Qazi H, Wijegunaratne H, Savajiyani R, Koola MM. Naltrexone and Prazosin Combination for Posttraumatic Stress Disorder and Alcohol Use Disorder. The Primary Care Companion for CNS Disorders. doi: 10.4088/PCC.14l01638. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS Drug Rev. 2003;9(3):275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochocki M, Hoffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, Lubbert H, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305(3):1024–36. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Santos MD, Alkondon M, Pereira EF, Aracava Y, Eisenberg HM, Maelicke A, Albuquerque EX. The nicotinic allosteric potentiating ligand galantamine facilitates synaptic transmission in the mammalian central nervous system. Mol Pharmacol. 2002;61(5):1222–34. doi: 10.1124/mol.61.5.1222. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32(1):43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biol Psychiatry. 2006;60(6):530–3. doi: 10.1016/j.biopsych.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Sharma U, Morris C, Safranek S. What is the recommended approach to asymptomatic patients who develop a reactive PPD? J Fam Pract. 2006;55(2):163–5. discussion 163. [PubMed] [Google Scholar]
- Simoni E, Daniele S, Bottegoni G, Pizzirani D, Trincavelli ML, Goldoni L, Tarozzo G, Reggiani A, Martini C, Piomelli D, Melchiorre C, Rosini M, Cavalli A. Combining galantamine and memantine in multitargeted, new chemical entities potentially useful in Alzheimer’s disease. J Med Chem. 2012;55(22):9708–21. doi: 10.1021/jm3009458. [DOI] [PubMed] [Google Scholar]
- Small G, Dubois B. A review of compliance to treatment in Alzheimer’s disease: potential benefits of a transdermal patch. Curr Med Res Opin. 2007;23(11):2705–13. doi: 10.1185/030079907x233403. [DOI] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45(3):309–79. [PubMed] [Google Scholar]
- Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–24. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–79. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Tugal O, Yazici KM, Anil Yagcioglu AE, Gogus A. A double-blind, placebo controlled, cross-over trial of adjunctive donepezil for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):117–23. doi: 10.1017/S1461145703004024. [DOI] [PubMed] [Google Scholar]
- van Westerloo D, van der Poll T. Acute vagotomy activates the cholinergic anti-inflammatory pathway. Am J Physiol Heart Circ Physiol. 2005;288(2):H977–8. doi: 10.1152/ajpheart.00837.2004. author reply H978–9. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055–62. doi: 10.1176/appi.ajp.2009.09010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Moyzis RK. Genetic evidence for ongoing balanced selection at human DNA repair genes ERCC8, FANCC, and RAD51C. Mutat Res. 2007;616(1–2):165–74. doi: 10.1016/j.mrfmmm.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Quack G, Moebius HJ, Danysz W. No interaction of memantine with acetylcholinesterase inhibitors approved for clinical use. Life Sci. 2000;66(12):1079–83. doi: 10.1016/s0024-3205(00)00411-2. [DOI] [PubMed] [Google Scholar]
- Wijegunaratne H, Qazi H, Koola MM. Chronic and bedtime use of benztropine with antipsychotics: Is it necessary? Schizophr Res. 2014;153(1–3):248–9. doi: 10.1016/j.schres.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr Bull. 2010;36(2):211–8. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Chen L. Targeting prefrontal cortical dopamine D1 and N-methyl-D-aspartate receptor interactions in schizophrenia treatment. Neuroscientist. 2005;11(5):452–70. doi: 10.1177/1073858405279692. [DOI] [PubMed] [Google Scholar]
- Yao C, Raoufinia A, Gold M, Nye JS, Ramael S, Padmanabhan M, Walschap Y, Verhaeghe T, Zhao Q. Steady-state pharmacokinetics of galantamine are not affected by addition of memantine in healthy subjects. J Clin Pharmacol. 2005;45(5):519–28. doi: 10.1177/0091270005274551. [DOI] [PubMed] [Google Scholar]
- Zhao X, Marszalec W, Toth PT, Huang J, Yeh JZ, Narahashi T. In vitro galantamine-memantine co-application: mechanism of beneficial action. Neuropharmacology. 2006;51(7–8):1181–91. doi: 10.1016/j.neuropharm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Zimmermann GR, Lehar J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12(1–2):34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]


