Abstract
The effect of high dose isoflurane on cerebral blood flow (CBF) was investigated in adult macaque monkeys receiving 1% to 2% isoflurane with the pseudo continuous arterial-spin-labeling (pCASL) MRI technique. High concentration (2%) of isoflurane resulted in significant increase in the mean CBF of the global, cortical, subcortical regions and the regional CBF in all subcortical structures and most cortical structures (such as motor cortex, anterior cingulate cortex, but not media prefrontal cortex). In addition, the changes of regional CBF in the affected regions correlated linearly with increasing isoflurane concentrations. The study demonstrates region specific CBF abnormal increase in adult macaque monkeys under high dose (2%) isoflurane and suggests the brain functionality in corresponding structures may be affected and need to be taken consideration in either human or non-human primate neuroimaging studies.
Keywords: Dose-dependent effect, Isoflurane, Cerebral blood flow (CBF), autoregulation, non-human primate, pseudo continuous arterial-spin-labeling (pCASL)
Introduction
Isoflurane is an inhalational anesthetic and generally utilized in humans and animals [1, 2]. This popular anesthetic agent is found to interfere with normal physiology of subjects, causing cerebral vasodilatation[3], cerebral metabolism decrease [4, 5], functional activity reduction [6], mean arterial pressure (MAP) decline and cerebral blood flow (CBF) increase [7-10], and CBF autoregulation disruption [5, 11, 12]. In general preclinical and clinical studies, the maintenance dose (~1%) of isoflurane is normally used for sedation purpose [1, 2]. High dose isoflurane (2% or above) is usually used for rapid induction or surgery [13]. The dose-dependent influence of isoflurane on CBF, autoregulation, brain metabolites, brain functional performance, et al, are observed in various animal and human studies. It has been demonstrated abnormal CBF increase under mild or high dose isoflurane in non-human primates, and humans [5, 9-11, 14]. In addition, high isoflurane doses could abolish the coupling between CBF and cerebral metabolites and impair CBF autoregulation in primate and human [5, 11, 12].
Previous CBF measurements in human are mainly conducted with the Xenon-133 SPECT technique [5, 15-17]. Because of the limited spatial resolution of the Xenon-133 technique, the dosage effect of isoflurane on regional CBF of different brain structures is poorly understood. Due to the tight coupling between local CBF and brain neural activity, the functionality of affected brain structures can be misinterpreted due to the region-specific dose-dependence effect of isoflurane. The Arterial Spin Labeling (ASL) MRI technique is a non-invasive approach to measure CBF quantitatively by using intrinsic blood water as a freely diffusible tracer[18]. Continuous ASL (CASL) technique with separate labeling coil is an optimal setting for CBF measurements in preclinical research scanners and has been implemented successfully in clinic scanners [19, 20]. However, the CASL technique with separate labeling coil is not accessible in most clinical scanners as it requires additional RF hardware. In contrast, the pseudo-continuous Arterial Spin Labeling (pCASL) MRI technique allows measuring CBF with a standard clinical setting without requirement of any additional hardware [21-23]. Accordingly, the pCASL technique provides a robust means to measure CBF in a normal clinical scanner. Non-human primates (NHPs) resemble most aspects of humans in brain anatomical and vascular structures and functionality and are widely used in cerebral neural system (CNS) related disorder studies [24, 25]. In the present study, the region-specific effect of high dose isoflurane on CBF of rhesus monkeys was examined with the pCASL technique on a clinical 3T scanner.
Methods
Animal preparation
Adult female rhesus monkeys (n=4, 7-11 years old) were employed in this study. The animals were initially anesthetized with ketamine (5-10 mg/kg, IM), then orally intubated. An IV catheter was placed for delivering lactated ringers solution (3.5-10 ml/kg/hr). The anesthetized and spontaneously breathing animals were immobilized with a custom-made head holder and immobilized in the “supine” position during MRI scanning. The physiological parameters such as Et-CO2, inhaled CO2, and respiration rate were monitored with an anesthesia machine (GE Datex-ohmeda Cardiocap/5), O2 saturation and heart rate with a Nonin pulse oximeter (Nonin Medical, MN, USA), the systolic blood pressure (SBP) and diastolic blood pressure (DBP) (recorded every 5 minutes) with a SurgiVet non-invasive blood pressure monitor (Smiths Medical ASD Inc, Ohio, USA), and body temperature with Digi-Sense Temperature controller (Cole-Parmer, IL , USA). Those parameters were maintained in normal ranges [9]. The animals were given three different isoflurane doses with random order: 1.0%, 1.5 % and 2.0% (or 0.8. 1.2 and 1.6 minimum alveolar concentration (MAC) (end-tidal), respectively), mixed with ambient air. Fifteen-minute or more transition time was applied during each dose change. All procedures followed the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University in accordance with the NIH Guide for Care and Use of Laboratory Animals.
MRI examination
MRI was performed on a Siemens 3T Trio whole body scanner (Siemens Medical Solutions, Inc., PA, USA) with an 8-channel transceiver array knee coil (Invivo, Inc., FL, USA). Animal heads were placed in the supine position with the AC-PC line of animals kept almost perpendicular to the B0 field in each scan. The single-shot, gradient-echo planar imaging (EPI) was applied for CBF measurement with the pCASL technique [21]. The MRI parameters were: TR/TE= 4000ms / 25 ms, FOV= 96 mm × 96 mm, data matrix = 64 × 64, 16 slice with slice thickness = 1.5 mm, labeling-offset = 55mm, post-labeling-delay = 0.8 s, Labeling duration= 2.0 s. 80 pairs of control and labeling images were acquired and repeated 3 times at each dosage. Corresponding T2 weighted images also were acquired with the same slice positions by using fast spin-echo sequences with TR/TE= 5900ms/125ms, FOV = 96 mm × 96 mm, matrix = 128 × 128, slice thickness = 1.5mm, 16 slices, 2 averages. The CBF measurement was started at least 30 minutes later after the animal was moved into the scanner.
Data analysis
The procedure for CBF calculation and data analyses was basically as same as reported previously [9]. However, the labeling efficiency coefficient was modified accordingly to adapt to the current pCASL technique [22]. Motor cortex, medial frontal cortex (mPFC), anterior cingulated cortex (ACC), caudate, thalamus, cerebellum, global, cortical region and sub-cortical region were identified on the raw EPI images (not shown) and corresponding T2-weighted structural images by referring to a macaque monkey atlas [26] and used for Region of Interest (ROI) analysis (Fig. 1). For each animal, CBF of each ROI was normalized to its mean CBF value of the three-dose levels to reduce the inter-subject variation. Meanwhile, MAP was calculated based upon a standard formular (MAP = (SBP+2*DBP)/3). The MAP data at each dose had at least two records and were averaged. The mean MAP readings of each animal at different isoflurane doses were normalized to reduce the inter-subject variation. Repeated ANOVA was performed to analyze the CBF differences statistically across the different doses. Spearmen correlation analysis was used to study the dose-dependence effects on CBF. SPSS 20.0 was used for statistical analysis. P-values less than 0.05 were considered statistically significant.
Fig 1.
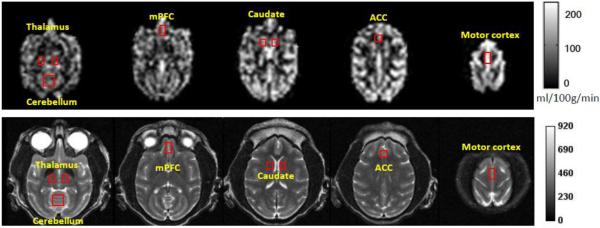
CBF maps of an adult macaque monkey acquired with the pseudo continuous ASL (pCASL) technique at 3T. Regions of Interest (ROIs) for data analysis are illustrated on the CBF maps (top) and corresponding T2-weighted structural images (bottom). mPFC: medial frontal cortex; ACC: anterior cingulated cortex.
Results
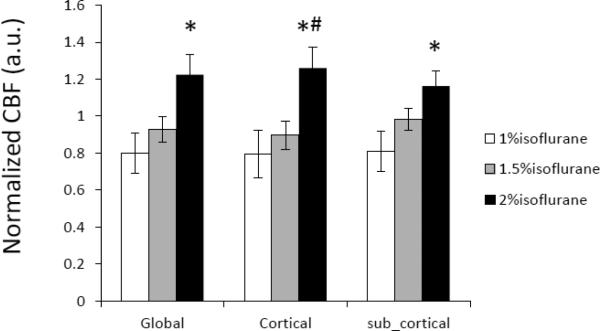
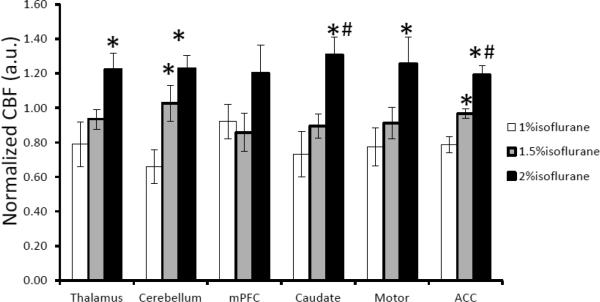
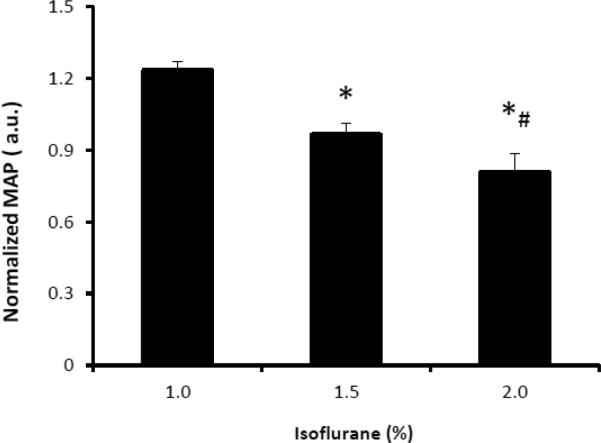
The dose-dependent effect of isoflurane on regional CBF is illustrated in Fig 2 and Fig 3. Mean CBF in the global, cortical and subcortical regions was increased significantly when the isoflurane concentration changed from 1% to 2% and correlated linearly (R2= ~0.5) with applied isoflurane doses (Fig 2 and 4). Regional CBF in thalamus, cerebellum, caudate, motor cortex, and ACC was increased 55%, 86%, 79%, 62%, 52%, respectively (Fig 3), and correlated linearly (R2= 0.5 – 0.8) with the isoflurane doses (1.0%, 1.5%, 2.0%) (Fig 3 and 5). The MAP (mean±SD) at 1%, 1.5%, and 2% isoflurane was 67.0±13.4 mm Hg, 52.3±10.4 mm Hg, and 43.3±6.8 mm Hg, respectively. Significant blood pressure decreases at 1.5% and 2% isoflurane were seen in the normalized MAP readings (Fig 6).
Fig 2.
Dose-dependence effect of isoflurane on mean CBF in global, cortical and subcortical regions of anesthetized macaque monkeys (mean ± SD). CBF in each region is normalized to its mean CBF value of three dose levels: 1.0%, 1.5% and 2.0% (end-tidal). *, #: p < 0.05, compared with 1.0% and 1.5% respectively.
Fig 3.
Dose-dependence effect of isoflurane on regional CBF in different brain structures of anesthetized macaque monkeys (mean ± SD). CBF in each ROI is normalized to its mean CBF value of three dose levels: 1%, 1.5%, 2% (end-tidal), respectively. *and #, p < 0.05, in comparison with CBF under 1% and 1.5% isoflurane.
Fig 4.
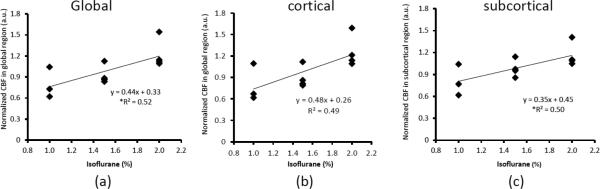
The correlation between isoflurane dosages and mean CBF changes in: a) the global, b) cortical, and c) subcortical regions. *: p<0.05
Fig 5.
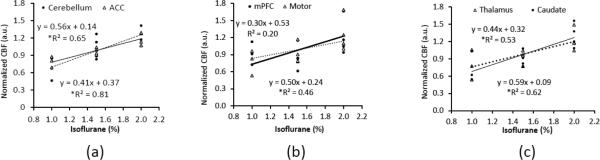
The correlation between isoflurane dosages and regional CBF changes in selected structures. a) Cerebellum, ACC; b) mPFC, motor cortex; c) Thalamus, caudate. *: p<0.05
Fig.6.
The normalized mean arterial pressure (MAP) of adult macaque monkeys at different isoflurane doses (mean ± SD). *, #:p < 0.05, in comparison with 1% and 1.5% isoflurane respectively.
Discussion and conclusion
The region specific effect of high dose isoflurane on CBF of macaque monkeys were revealed by the pseudo-CASL MRI technique. In comparison with the results of regular maintenance dosage (~1.0%), the 2% isoflurane resulted in significant increase in the mean global, cortical, and subcortical CBF, and regional CBF in some cortical structures, and all selected subcortical structures. Also, the present study demonstrates that the regions with abnormal CBF increase expands from subcortical structures (mainly affected at ~1% isoflurane doses) into more cortical structures of adult macaque brains, when isoflurane concentration is increased from the regular maintenance dose (~1%) to high dose (2% or more).
Isoflurane interferes with normal physiology of animals or humans. Previous study in the baboon has reported that isoflurane resulted in dose-related MAP decrease, cerebral metabolism depression, and biphasic CBF response (CBF decreases at low isoflurane doses (<1%) but increase in high doses (~1.4%)) [11]. Evident CBF increase was observed in thalamus and basal ganglia and pons of humans anesthetized with 1 MAC isoflurane but not in cortex [10, 14]. With high dose (2.0% or more) isoflurane, previous study in dogs has found that under 2.8% isoflurane, CBF was increased significantly in cerebrum, medulla, cerebellum, caudate and CBF autoregulation was eliminated compared to 1.4% [27]. Also, Tetrault et al demonstrated that high dose isoflurane (3%) broke down the blood-brain barrier (BBB) in the cortex and thalamus of cats while only BBB in thalamus was affected under 1% isoflurane [28]. Olsen and colleagues found that CBF was increased and cerebral metabolic rate for oxygen (CMRO2) was decreased when increasing isoflurane from 1 to 2 MAC, and CBF autoregulation was disrupted at 2 MAC in human by using Xenon-133 technique [5]. To date, the dose-dependence effect of high dose isoflurane in CBF of different brain structures is poorly understood in human or primates. In the present study, our findings in adult macaque monkeys demonstrate that high dose isoflurane (2%) results in abnormal CBF increase in the global, cortical, and subcortical, and selected ROIs in subcortex and cortex, indicating the affected regions expanded from subcortical structures (mainly affected at ~1% isoflurane) to most cortical structures (except mPFC), in comparison with those previously reported at regular maintenance doses [9]. The results of high dose isoflurane effect in the present study are expected and in agreement with those seen in man and primate previously [5, 9, 11].
Elevated isoflurane concentration is usually companied with declining MAP due to systemic vasodilation effect [7, 11]. Impaired CBF autoregulation has been observed in previous human and primate studies with higher isoflurane doses (>1.0%) [5, 11, 12]. In particular, the prior baboon study demonstrated that ~1.4% isoflurane caused ~50% MAP reduction and ~16.33% CBF increase (1.4% vs baseline (0%)) and impaired CBF autoregulation [11]. In the present study, the blood pressure readings exhibited significantly decrease when isoflurane dose increased from 1 to 2% (Fig 6). In comparison with reported blood pressure readings (99±10.3 mmHg, mean±SD) of conscious adult rhesus monkeys [29], the blood pressure reduction at 1.5% and 2.0% isoflurane was about 47% and 56% respectively. In addition, the abnormal CBF increases in most ROIs were correlated linearly with the isoflurane dose changes from 1% – 2%, indicating disrupted coupling between CBF and brain metabolism as isoflurane causes CMRO2 decreased or abolished at higher doses [5, 11]. Compared with the findings of previous baboon study by Van Aken et al and also the human study by Olsen et al, it could be suggested that CBF autoregulation in the affected regions of adult macaque monkeys might be impaired under 2% (1.6 MAC) isoflurane.
As seen in the present study, the high dose isoflurane causes widespread CBF alteration in the subcortical structures and some cortical structures in macaque monkeys, consistent with Manohar et al’ results in pigs [30] with invasive blood flow measurement by radionuclide labelled microspheres. Interestingly, a prior rat study demonstrated that the CBF in lateral cortex, posterior cortex, pons, and medulla reduced significantly (2% vs 0% isoflurane) but no significant changes were seen in other regions such as anterior cortex, basal ganglia, cerebellum, et al [31]. The discrepancy of isoflurane dosage effects on CBF between rats and monkeys mostly is due to the species difference of anesthetic effects as reported in other mammals including rats, dogs, pigs, cats, rabbits [11, 27, 32-36].
In conclusion, the present study demonstrates that CBF is increased significantly in most brain regions in macaque monkeys when isoflurane concentration is changed from general maintenance dosage (~1%) to high dose (2%). Also, the high dose isoflurane results suggest that the affected regions expand from subcortical structures into more cortical structures of adult macaque monkeys with increasing isoflurane doses. The dose-dependent effect of 2% isoflurane anesthesia on CBF is still region-specific and should be taken into consideration in related function and physiology studies of NHP models and in humans as well.
Acknowledgements
The authors are thankful to Wendy Williamson Coyne and Ruth Connelly for animal handling. The project was funded by the National Center for Research Resources P51RR000165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD011132, This project was also supported by NIH R01-MH080892; R01-NS081077, and R01-EB014922 (DW), and by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources (XZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors have no conflict of interest in this work.
References
- 1.Dohoo SE. Isoflurane as an inhalational anesthetic agent in clinical practice. Can Vet J. 1990;31:847–50. [PMC free article] [PubMed] [Google Scholar]
- 2.Hildebrandt IJ, Su H, Weber WA. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J. 2008;49:17–26. doi: 10.1093/ilar.49.1.17. [DOI] [PubMed] [Google Scholar]
- 3.Iida H, Ohata H, Iida M, Watanabe Y, Dohi S. Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiology. 1998;89:954–60. doi: 10.1097/00000542-199810000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Newberg LA, Milde JH, Michenfelder JD. The cerebral metabolic effects of isoflurane at and above concentrations that suppress cortical electrical activity. Anesthesiology. 1983;59:23–8. doi: 10.1097/00000542-198307000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Olsen KS, Henriksen L, Owen-Falkenberg A, Dige-Petersen H, Rosenorn J, Chraemmer-Jorgensen B. Effect of 1 or 2 MAC isoflurane with or without ketanserin on cerebral blood flow autoregulation in man. Br J Anaesth. 1994;72:66–71. doi: 10.1093/bja/72.1.66. [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 7.Grundmann U, Muller M, Kleinschmidt S, Larsen B, Larsen R. Cardiovascular effects of desflurane and isoflurane in patients with coronary artery disease. Acta Anaesthesiol Scand. 1996;40:1101–7. doi: 10.1111/j.1399-6576.1996.tb05571.x. [DOI] [PubMed] [Google Scholar]
- 8.Schlunzen L, Cold GE, Rasmussen M, Vafaee MS. Effects of dose-dependent levels of isoflurane on cerebral blood flow in healthy subjects studied using positron emission tomography. Acta Anaesthesiol Scand. 2006;50:306–12. doi: 10.1111/j.1399-6576.2006.00954.x. [DOI] [PubMed] [Google Scholar]
- 9.Li CX, Patel S, Auerbach EJ, Zhang X. Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci Lett. 2013;541:58–62. doi: 10.1016/j.neulet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinstrup P, Ryding E, Algotsson L, Berntman L, Uski T. Regional cerebral blood flow (SPECT) during anaesthesia with isoflurane and nitrous oxide in humans. Br J Anaesth. 1997;78:407–11. doi: 10.1093/bja/78.4.407. [DOI] [PubMed] [Google Scholar]
- 11.Van Aken H, Fitch W, Graham DI, Brussel T, Themann H. Cardiovascular and cerebrovascular effects of isoflurane-induced hypotension in the baboon. Anesth Analg. 1986;65:565–74. [PubMed] [Google Scholar]
- 12.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–9. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman WE, Edelman G. Enhancement of brain tissue oxygenation during high dose isoflurane anesthesia in the dog. J Neurosurg Anesthesiol. 2000;12:95–8. doi: 10.1097/00008506-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Reinstrup P, Ryding E, Algotsson L, Messeter K, Asgeirsson B, Uski T. Distribution of cerebral blood flow during anesthesia with isoflurane or halothane in humans. Anesthesiology. 1995;82:359–66. doi: 10.1097/00000542-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Algotsson L, Messeter K, Nordstrom CH, Ryding E. Cerebral blood flow and oxygen consumption during isoflurane and halothane anesthesia in man. Acta Anaesthesiol Scand. 1988;32:15–20. doi: 10.1111/j.1399-6576.1988.tb02678.x. [DOI] [PubMed] [Google Scholar]
- 16.Madsen JB, Cold GE, Hansen ES, Bardrum B. Cerebral blood flow, cerebral metabolic rate of oxygen and relative CO2-reactivity during craniotomy for supratentorial cerebral tumours in halothane anaesthesia. A dose-response study. Acta Anaesthesiol Scand. 1987;31:454–7. doi: 10.1111/j.1399-6576.1987.tb02602.x. [DOI] [PubMed] [Google Scholar]
- 17.Madsen JB, Cold GE, Hansen ES, Bardrum B. The effect of isoflurane on cerebral blood flow and metabolism in humans during craniotomy for small supratentorial cerebral tumors. Anesthesiology. 1987;66:332–6. doi: 10.1097/00000542-198703000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Detre JA, Zhang W, Roberts DA, Silva AC, Williams DS, Grandis DJ, Koretsky AP, Leigh JS. Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed. 1994;7:75–82. doi: 10.1002/nbm.1940070112. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Nagaoka T, Auerbach EJ, Champion R, Zhou L, Hu X, Duong TQ. Quantitative basal CBF and CBF fMRI of rhesus monkeys using three-coil continuous arterial spin labeling. Neuroimage. 2007;34:1074–83. doi: 10.1016/j.neuroimage.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talagala SL, Ye FQ, Ledden PJ, Chesnick S. Whole-brain 3D perfusion MRI at 3.0 T using CASL with a separate labeling coil. Magn Reson Med. 2004;52:131–40. doi: 10.1002/mrm.20124. [DOI] [PubMed] [Google Scholar]
- 21.Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020–7. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- 22.Jain V, Duda J, Avants B, Giannetta M, Xie SX, Roberts T, Detre JA, Hurt H, Wehrli FW, Wang DJ. Longitudinal reproducibility and accuracy of pseudo-continuous arterial spin-labeled perfusion MR imaging in typically developing children. Radiology. 2012;263:527–36. doi: 10.1148/radiol.12111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–97. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perretta G. Non-Human Primate Models in Neuroscience Research. Scand J Lab Anim Sci. 2009;36:77–85. [Google Scholar]
- 25.Dudkin KN, Chueva IV, Makarov FN, Bich TG, Roher AE. Disorders of learning and memory processes in a monkey model of Alzheimer's disease: the role of the associative area of the cerebral cortex. Neurosci Behav Physiol. 2006;36:789–99. doi: 10.1007/s11055-006-0089-6. [DOI] [PubMed] [Google Scholar]
- 26.Logothetis KSSaNK. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. 2007:326. [Google Scholar]
- 27.McPherson RW, Traystman RJ. Effects of isoflurane on cerebral autoregulation in dogs. Anesthesiology. 1988;69:493–9. doi: 10.1097/00000542-198810000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Tetrault S, Chever O, Sik A, Amzica F. Opening of the blood-brain barrier during isoflurane anaesthesia. Eur J Neurosci. 2008;28:1330–41. doi: 10.1111/j.1460-9568.2008.06443.x. [DOI] [PubMed] [Google Scholar]
- 29.Hom GJ, Bach TJ, Carroll D, Forrest MJ, Mariano MA, Trainor CE, Wang PR, MacIntyre DE. Comparison of Cardiovascular Parameters and/or Serum Chemistry and Hematology Profiles in Conscious and Anesthetized Rhesus Monkeys (Macaca mulatta). Contemp Top Lab Anim Sci. 1999;38:60–4. [PubMed] [Google Scholar]
- 30.Manohar M, Parks C. Regional distribution of brain and myocardial perfusion in swine while awake and during 1.0 and 1.5 MAC isoflurane anaesthesia produced without or with 50% nitrous oxide. Cardiovasc Res. 1984;18:344–53. doi: 10.1093/cvr/18.6.344. [DOI] [PubMed] [Google Scholar]
- 31.Chi OZ, Anwar M, Sinha AK, Wei HM, Klein SL, Weiss HR. Effects of isoflurane on transport across the blood-brain barrier. Anesthesiology. 1992;76:426–31. doi: 10.1097/00000542-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Cucchiara RF, Theye RA, Michenfelder JD. The effects of isoflurane on canine cerebral metabolism and blood flow. Anesthesiology. 1974;40:571–4. doi: 10.1097/00000542-197406000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Todd MM, Weeks J. Comparative effects of propofol, pentobarbital, and isoflurane on cerebral blood flow and blood volume. J Neurosurg Anesthesiol. 1996;8:296–303. doi: 10.1097/00008506-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Kimme P, Ledin T, Sjoberg F. Dose effect of sevoflurane and isoflurane anesthetics on cortical blood flow during controlled hypotension in the pig. Acta Anaesthesiol Scand. 2007;51:607–13. doi: 10.1111/j.1399-6576.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- 35.Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and Analgesia in Laboratory Animals 2nd Edition Preface. Am Coll Lab. 2008:Xiii–Xiv. [Google Scholar]
- 36.Pan WJ, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. Broadband local field potentials correlate with spontaneous fluctuations in functional magnetic resonance imaging signals in the rat somatosensory cortex under isoflurane anesthesia. Brain Connect. 2011;1:119–31. doi: 10.1089/brain.2011.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]