Abstract
INTRODUCTION
Multimodality therapy with chemotherapy and surgical resection is recommended for patients with locoregional pancreatic cancer, but is not received by many patients.
OBJECTIVE
To evaluate patterns in the use and timing of chemotherapy and resection and factors associated with receipt of multimodality therapy in older patients with locoregional pancreatic cancer.
METHODS
We used Surveillance, Epidemiology, and End Results (SEER)-linked Medicare data (1992–2007) to identify patients with locoregional pancreatic adenocarcinoma. Multimodality therapy was defined as receipt of both chemotherapy and pancreatic resection. Logistic regression was used to determine factors independently associated with receipt of multimodality therapy. Log-rank tests were used to identify differences in survival for patients stratified by type and timing of treatment.
RESULTS
We identified 10,505 patients with pancreatic adenocarcinoma. 5,358 patients (51.0%) received either chemotherapy or surgery, with 1,166 patients (11.1%) receiving both modalities. Resection alone was performed in 1,138 patients (10.8%) and chemotherapy alone was given to 3,054 (29.1%) patients. In patients undergoing resection as the initial treatment modality, 49.4% never received chemotherapy. 97.4% of patients who underwent chemotherapy as the initial treatment modality never underwent resection. The use of multimodality therapy increased from 7.4% of patients in 1992–1995 to 13.8% of patients in 2004–2007 (p<0.0001). 2-year survival was 41.0% for patients receiving multimodality therapy, 25.1% with resection alone, and 12.5% with chemotherapy alone (p<0.0001). Of the patients receiving multimodality therapy, chemotherapy was delivered in the adjuvant setting in 93.1% and in the neoadjuvant setting in 6.9%, with similar 2-year survival with either approach (neoadjuvant vs. adjuvant, 46.9% vs. 40.6%, p=0.16). Year of diagnosis, white race, less comorbidity, and no vascular invasion were independently associated with receipt of multimodality therapy.
CONCLUSION
Only half of older patients with locoregional pancreatic cancer receive any treatment, and less than a quarter of treated patients receive multimodality therapy. Nearly all patients receiving chemotherapy as the initial treatment modality did not undergo resection, while half of those undergoing resection first received chemotherapy. When multimodality therapy is used, the vast majority of patients had chemotherapy in the adjuvant setting and survival was similar regardless of approach.
Keywords: neoadjuvant therapy, adjuvant therapy, survival, pancreatic cancer
INTRODUCTION
Only 20% of patients with pancreatic cancer present with disease that is amenable to surgical resection.1 While surgical resection is the only potentially curative treatment option, most patients experience distant, extrapancreatic recurrence even after an R0 resection. This suggests that microscopic tumor spread may have already occurred at the time of presentation.2–4
Multiple prospective randomized controlled trials have documented improved disease-free survival when chemotherapy was administered in addition to pancreatic resection.5–11 Based on these factors, the 2013 National Comprehensive Cancer Network guidelines recommend systemic chemotherapy in conjunction with curative-intent surgery as the standard of care for resectable pancreatic adenocarcinoma.12 Despite these recommendations, prior population-based studies have illustrated that multimodality therapy is rarely administered.13, 14
While previous studies have evaluated the receipt of resection in patients with locoregional pancreatic cancer,15 the receipt of multimodality therapy in resected patients,14 and trends in multimodality therapy use,13 few studies have assessed population-based trends in the receipt and relative timing of chemotherapy and/or resection of the primary tumor. In addition, controversy exists regarding the optimal timing of chemotherapy (adjuvant vs. neoadjuvant).16 Most studies assessing the impact of neoadjuvant vs. adjuvant therapy approaches on survival in patients with resectable pancreatic cancer have focused on only those patients who have undergone resection. As such, the number of patients who receive chemotherapy but never undergo resection due to disease progression or declining performance status are often not reported and not considered when comparing survival with the two approaches.
We used the Surveillance, Epidemiology and End Results-linked Medicare database (1992–2007) to evaluate population-based patterns in the receipt of chemotherapy and/or surgical resection. We identified the initial treatment modality used (chemotherapy or resection) and the proportion of treated patients who received the second modality. In patients who received multimodality therapy (chemotherapy and resection) we evaluated the approach (neoadjuvant vs. adjuvant therapy) and the association between neoadjuvant vs. adjuvant therapy and long-term survival. Finally, we identified the factors independently associated with receipt of multimodality therapy for these patients.
METHODS
The Institutional Review Board at the University of Texas Medical Branch determined this study to be exempt from review.
Data Source
The Surveillance, Epidemiology, and End Results (SEER) database is a prospectively collected database of incident cancer cases for patients in select regions designed to be representative of the U.S. population, accounting for approximately 28% of the population. Ninety-three percent of patients in the SEER database can be linked with patients who have Medicare claims files.17 Medicare claims data used included the Denominator File, the Medicare Provider Analysis and Review file (MEDPAR), the Carrier claims file, and the Outpatient Standard Analytic File.18
Cohort Selection (Figure 1)
Figure 1.
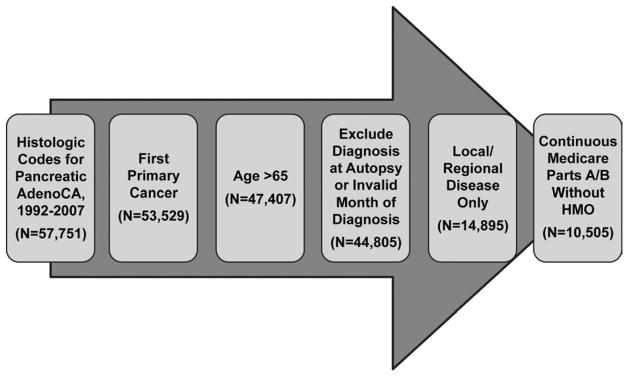
Cohort selection criteria. Only patients with histologically confirmed, locoregional pancreatic adenocarcinoma as their first primary cancer diagnosis were included. Patients without Medicare Parts A and B for 6 months before and after diagnosis, or until death, were excluded. All patients were followed for two years. N=10,505.
We included patients diagnosed with pancreatic adenocarcinoma from 1992–2007. SEER data were used to identify the primary tumor site codes for pancreas and International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) histology codes consistent with adenocarcinoma. Only patients with pancreatic adenocarcinoma as their first primary diagnosis and those aged 66 years or older were included. Patients whose diagnosis was only confirmed at autopsy or death were excluded. Only patients with locoregional disease were included. Finally, patients without Medicare Parts A and B for 6 months before and after diagnosis, or until death, were excluded. All patients were followed for two years after diagnosis, or until death.
Covariates
Patient baseline demographic and clinical characteristics included age, sex, marital status, race/ethnicity, education, income, area of residence (rural vs. urban), SEER region, presence of vascular invasion, radiation, and year of diagnosis. Charlson comorbidity index19 was used as a measure for patient comorbidity. Percent of residents with at least a 12th grade education and quartile of income were determined at the zip code level and categorized into quartiles. For education and income quartiles, quartile 1 is coded as the least educated/lowest income and quartile 4, the most educated/highest income. SEER Extent of Disease Coding (1992–2003) and Derived AJCC Tumor T Stage Codes (2004–2007) were used to identify vascular invasion. Patients with codes 54 (pancreatic head, blood vessel(s) (major): gastroduodenal artery, hepatic artery, pancreaticoduodenal artery, portal vein, superior mesenteric vein), 56 (body and tail, blood vessel(s): hepatic artery, portal vein, splenic artery/vein, superior mesenteric vein) and Derived AJCC 6th Edition Tumor T Stage Code 40 (Stage T4: tumor involves celiac axis or superior mesenteric artery) were classified as having vascular invasion.
Outcome Variable: Multimodality Therapy
Multimodality therapy was defined as receipt of both surgery and any instance of chemotherapy before surgery or within 6 months after surgery. Patients were considered not to have undergone multimodality therapy if they had resection only, received chemotherapy only, received chemotherapy more than 6 months after surgery, or had no treatment. Codes used to identify chemotherapy, surgery, and radiation are shown in Table 1. Chemotherapy and radiation were identified from the Medicare claims (MEDPAR, carrier, outpatient SAF) using Healthcare Common Procedure Coding System (HCPCS) codes, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) procedure and diagnosis codes, J codes, and revenue center codes for administration of chemotherapy as defined by SEER-Medicare.20 ICD-9-CM and Current Procedural Terminology (CPT) codes used to identify pancreatic head resection are also listed in Table 1. In patients receiving multimodality therapy, those receiving any chemotherapy (with or without radiation) before resection were considered to have undergone neoadjuvant therapy; if chemotherapy was within 6 months after resection, it was classified as adjuvant.
Table 1.
International Classification of Diseases for Oncology, 3rd Edition codes, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) Codes and Current Procedural Terminology (CPT) Codes
| ICD-9-CM Codes | CPT codes | |
|---|---|---|
| Diagnosis | ||
| Pancreatic adenocarcinoma | 8000/3, 8010/3, 8020/3, 8021/3, 8022/3, 8140/3, 8141/3, 8211/3, 8230/3, 8500/3, 8521/3, 8050/3, 8260/3, 8441/3, 8450/3, 8453/3, 8470/3, 8471/3, 8472/3, 8473/3, 8480/3, 8481/3, 8503* | NA |
| Procedures/Treatment | ||
| Pancreatic head resection | 52.6, 52.7, 52.51 | 48150, 48152, 48153, 48154, 48155 |
| Biliary stent | 51.86, 51.87, 51.99 | 43267, 43268, 43269 |
| Chemotherapy | 99.25 | Q0083, Q0084, Q0085, J7150, J2353, J2354, J9000-J9999 |
| Radiation | 99.21–99.29 | 77520, 77523, G0256, G0261, 77401-77499 |
International Classification of Diseases for Oncology, 3rd Edition codes
Statistical Analysis
Summary statistics were calculated for the overall cohort. Demographic and tumor characteristics of patients who received multimodality therapy were compared to patients who did not receive multimodality therapy. Chi-square tests were used to test significance for categorical variables and t-tests were used for continuous variables.
Three logistic regression models were performed to determine factors independently associated with receipt of multimodality therapy. The first model included the overall cohort and included age, race, sex, marital status, education, income, Charlson comorbidity index, year of diagnosis, SEER region, area of residence (rural vs. urban), vascular invasion, radiation, and biliary stent placement. The second model included only those patients without vascular invasion and included the same variables except for vascular invasion. Finally, a third logistic regression model was performed including all variables only for those patients who received treatment with surgery and/or chemotherapy.
Survival Analysis
Unadjusted Kaplan-Meier estimates of overall survival were obtained for patients who 1) underwent surgery and adjuvant chemotherapy, 2) underwent surgery alone, 3) underwent chemotherapy alone, and 4) received no treatment. For patients who received both modalities, Kaplan-Meier time-to-event analyses were performed for patients who underwent surgery and received neoadjuvant vs. adjuvant chemotherapy. Log-rank tests were used to determine statistically significant differences in survival between groups. A Cox proportional hazards model for survival at two years was developed only for those patients receiving multimodality therapy and included the variables age, sex, race, income, education, Charlson comorbidity, SEER region, radiation, endostent, vascular invasion, and timing of therapy (neoadjuvant v. adjuvant). Patients were censored when they were lost to follow-up or completed follow-up at two years.
RESULTS
We identified 10,505 patients who met our inclusion criteria. Table 2 illustrates baseline characteristics for these patients. The mean age was 77.1 ± 7.1 years, and the majority of patients were female, white, and married, from a large metropolitan area. Only 5,358 (51.0%) received surgery and/or chemotherapy at any time in their treatment course. Of these patients, 1,166 (11.1% of the overall cohort) received both chemotherapy and surgery, 1,138 (10.8%) received surgery alone, and 3,054 (29.0%) received chemotherapy alone. In the 1,166 patients receiving multimodality therapy, chemotherapy was delivered in the adjuvant setting in 93.1% and in the neoadjuvant setting in 6.9% (Figure 2A). Of the patients classified as having received adjuvant chemotherapy, 78.4% received chemotherapy within three months after the date of surgery (median=62 days, interquartile range 48, 88).
Table 2.
Summary Statistics for Overall Cohort and Proportion of Patients Receiving Multimodality Therapy for Each Factor
| Overall Cohort N (%) |
Patients Receiving Multimodality Therapy N (%) |
||
|---|---|---|---|
| Factor | N=10,505 | N= 1,166 | p value |
| Age (Mean ± SD) | 77.2 ± 7.1 | 72.8 ± 4.8 | <0.0001 |
| Female | 6,129 (58.3) | 633 (10.3) | 0.0029 |
| Race | <0.0001 | ||
| White | 8,631 (82.2) | 1,032 (12.0) | |
| Black | 979 (9.3) | 53 (5.4) | |
| Other | 895 (8.5) | 81 (9.1) | |
| Marital Status (N=10,210) | <0.0001 | ||
| Single | 1,457 (14.3) | 149 (10.2) | |
| Married | 5,369 (52.6) | 769 (14.3) | |
| Widowed | 3,384 (33.1) | 226 (6.7) | |
| Stage | <0.0001 | ||
| Localized | 2,782 (26.5) | 139 (5.0) | |
| Regional | 7,723 (73.5) | 1,027 (13.3) | |
| SEER Region | <0.0001 | ||
| Connecticut | 922 (8.8) | 124 (13.5) | |
| Louisiana | 570 (5.4) | 46 (8.1) | |
| New Jersey | 1,142 (10.9) | 192 (16.8) | |
| San Francisco | 557 (5.3) | 45 (8.1) | |
| San Jose | 343 (3.3) | 47 (13.7) | |
| Los Angeles | 972 (9.3) | 108 (11.1) | |
| Greater California | 1,296 (12.3) | 156 (12.0) | |
| Detroit | 1,218 (11.6) | 129 (10.6) | |
| Hawaii | 243 (2.3) | 32 (13.2) | |
| Iowa | 839 (8.0) | 81 (9.7) | |
| New Mexico | 315 (3.0) | 21 (6.7) | |
| Seattle | 803 (7.6) | 55 (6.9) | |
| Utah | 325 (3.1) | 28 (8.6) | |
| Atlanta | 396 (3.8) | 41 (10.4) | |
| Kentucky | 564 (5.4) | 61 (10.8) | |
| Charlson Comorbidity | <0.0001 | ||
| 0 | 5,066 (48.2) | 674 (13.3) | |
| 1 | 3,032 (28.9) | 332 (11.0) | |
| 2 | 1,325 (12.6) | 117 (8.8) | |
| ≥3 | 1,082 (10.3) | 43 (4.0) | |
| Education | <0.0001 | ||
| Quartile 1 (lowest) | 2,607(25.0) | 211 (8.1) | |
| Quartile 2 | 2,607 (25.0) | 252 (9.7) | |
| Quartile 3 | 2,607 (25.0) | 321 (12.3) | |
| Quartile 4 | 2,605 (25.0) | 374 (14.4) | |
| Income | <0.0001 | ||
| Quartile 1 (lowest) | 2,606 (25.0) | 178 (6.8) | |
| Quartile 2 | 2,607 (25.0) | 259 (9.9) | |
| Quartile 3 | 2,606 (25.0) | 307 (11.8) | |
| Quartile 4 | 2,607 (25.0) | 414 (15.9) | |
| Diagnosis Year | <0.0001 | ||
| 1992–1995 | 1,596 (15.2) | 118 (7.4) | |
| 1996–1999 | 1,576 (15.0) | 133 (8.4) | |
| 2000–2003 | 3,471 (33.0) | 382 (11.0) | |
| 2004+ | 3,862 (36.8) | 533 (13.8) | |
| No Vascular Invasion (N=10,013) | 8,253 (78.6) | 1,078 (13.1) | <0.0001 |
| Radiation | 3,794(36.1) | 932 (24.6) | <0.0001 |
| Adjuvant | NA | 844 (73.1) | |
| Neoadjuvant | NA | 77 (24.8) | |
| Unknown | NA | 11 (2.1) | |
| Biliary Stent | 4,031 (38.4) | 560 (13.9) | <0.0001 |
| Therapy | <0.0001 | ||
| Both Surgery and Chemotherapy | 1,166 (11.1) | 100% | |
| Surgery Only | 1,138 (10.8) | NA | |
| Chemotherapy Only | 3,054 (29.1) | NA | |
| None | 5,147 (49.0) | NA | |
| Initial Treatment Modality (N=5,358) | <0.0001 | ||
| Surgery | 2,223 (41.5) | 1,085 (48.8) | |
| Chemotherapy | 3,135 (58.5) | 81 (2.6) |
Figure 2.
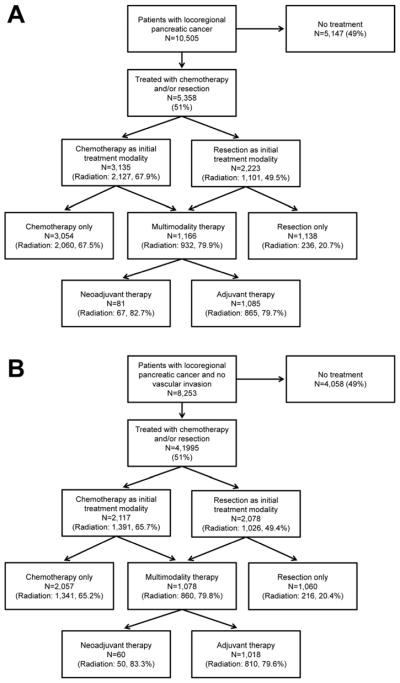
Figure 2A. Management of older patients with locoregional pancreatic cancer. Nearly half of all patients received no treatment. Only 11.1% of the overall cohort received multimodality therapy. Over 97% of patients receiving chemotherapy as the initial treatment modality did not receive surgery, while 51% of patients who received surgery as the initial treatment modality did not receive chemotherapy. The proportion of patients receiving radiation therapy for each group is also listed.
Figure 2B. Management of older patients with locoregional pancreatic cancer and no vascular invasion. Of these patients with potentially treatable pancreatic cancer (N=8,253), only 51% received any treatment, and only 13.1% received multimodality therapy. Over 97% of patients receiving chemotherapy as the initial treatment modality did not receive surgery, while 51% of patients who received surgery as the initial treatment modality did not receive chemotherapy. The proportion of patients receiving radiation therapy for each group is also listed.
In the 5,358 treated patients, chemotherapy was the initial treatment modality in 3,135 patients (58.5%) and resection was the initial treatment modality in 2,223 (41.5%). In patients undergoing resection as the initial treatment modality, 51.2% never received chemotherapy. In patients undergoing chemotherapy as the initial treatment modality 97.4% never underwent resection (Figure 2A). In patients receiving chemotherapy as the initial treatment modality, 67.9% received radiation, compared to only 49.5% of those undergoing surgical resection first. In patients receiving multimodality therapy, 82.7% of patients who underwent neoadjuvant therapy underwent neoadjuvant radiation and 79.7% of patients who had adjuvant chemotherapy also received adjuvant radiation.
Based on SEER extent of disease codes, 8,253 (78.6%) of patients had no vascular invasion and were potentially eligible for neoadjuvant therapy plus resection or resection plus adjuvant therapy. Their trajectory is shown in Figure 2B. Vascular invasion was more common in patients undergoing chemotherapy as the initial treatment modality compared to patients receiving surgery initially (28.4% vs. 5.3%, p<0.0001). 4,058 patients (49.2%) received no treatment. Of the 4,195 who were treated, 2,117 received chemotherapy as the initial treatment modality and 2,078 underwent resection as the initial treatment modality. Overall, 1,078 (13.1%) received multimodality therapy, 2,057 (24.9%) received chemotherapy alone, and 1,060 (12.8%) received surgery alone.
Trends in the use of multimodality therapy are illustrated in Figure 3. Multimodality therapy use increased over time from 7.4% in 1992–1995 to 13.8% in 2004–2007 (p<0.0001). There was also an increase in the use of neoadjuvant therapy over time, from 2.5% of patients in 1992–1995 to 9.4% of patients in 2004–2007 (p=0.0095). In patients without vascular invasion (N=8,253), multimodality therapy use increased from 7.7% in 1992–1995 to 17.4% of patients in 2004–2007 (p<0.0001); for these patients, neoadjuvant therapy use increased from 1.0 % to 8.4% over the same time period (p=0.001).
Figure 3.
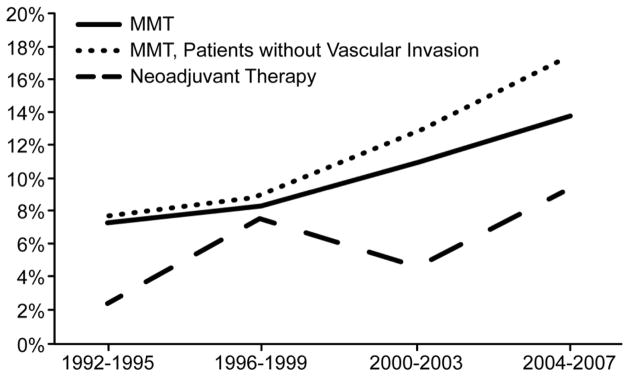
Trends in the use of multimodality therapy in older patients with locoregional pancreatic adenocarcinoma, 1992–2007. The use of multimodality therapy increased over time from 7.4% of patients in 1992–1995 compared to 13.8% of patients in 2004–2007 (p<0.0001); this increase was more pronounced for patients without vascular invasion (7.7% to 17.4%, p<0.0001). Neoadjuvant therapy use also increased over this same time period (2.5% vs. 9.4%, p=0.0095).
Patient demographic, tumor, and treatment characteristics differed significantly between the multimodality therapy and no multimodality therapy groups (Table 2). Patients who received multimodality therapy were younger, more likely to be female, white, married, have less comorbidity, live in higher socioeconomic areas, have received radiation therapy, and have no evidence for vascular invasion. The use of multimodality therapy was variable by SEER region.
In a logistic regression analysis, year of diagnosis was independently associated with receipt of multimodality therapy (OR 1.09, 95% CI 1.06–1.11, Table 3) reflecting evolving recommendations over this time period. Other factors independently associated with receipt of multimodality therapy included younger age, white race, higher education quartile, lower Charlson comorbidity score, absence of vascular invasion, treatment with radiation, and biliary stent placement (Table 3). The strongest effects were observed for no vascular invasion (OR 5.78, 95% CI 4.5–7.4) and radiation treatment (OR 6.82, 95% CI 5.8–8.0).
Table 3.
Logistic Regression Models, Factors Associated with Receipt of Multimodality Therapy* for Overall Cohort, for Patients without Vascular Invasion, and for Treated Patients
| Multimodality Therapy** | ||||||
|---|---|---|---|---|---|---|
| Factor (Ref) | Overall Cohort (N=10,505) | Patients without Vascular Invasion (N=8,253) | Patients treated with Surgery or Chemotherapy (N=5,358) | |||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Year of Diagnosis | 1.09 | 1.06–1.11 | 1.10 | 1.08–1.12 | 1.08 | 1.05–1.09 |
| Age (years) | 0.90 | 0.89–0.91 | 0.90 | 0.88–0.91 | 0.92 | 0.91–0.94 |
| Married (Single) | 1.22 | 0.99–1.5 | 1.31 | 1.04–1.65 | 1.0 | 0.81–1.26 |
| Widowed (Single) | 1.00 | 0.78–1.29 | 1.07 | 0.82–1.40 | 0.94 | 0.72–1.22 |
| Male (Female) | 1.00 | 0.78–1.29 | 1.01 | 0.86–1.18 | 0.98 | 0.85–1.15 |
| White (Black) | 1.90 | 1.36–2.65 | 1.91 | 1.34–2.71 | 1.67 | 1.19–2.35 |
| Other (Black) | 1.16 | 0.75–1.80 | 1.16 | 0.73–1.84 | 1.01 | 0.65–1.59 |
| Education Q2 (Q1) | 1.15 | 0.90–1.46 | 1.11 | 0.86–1.44 | 1.14 | 0.88–1.46 |
| Education Q3 (Q1) | 1.33 | 1.02–1.74 | 1.18 | 0.89–1.57 | 1.31 | 0.99–1.72 |
| Education Q4 (Q1) | 1.39 | 1.03–1.88 | 1.26 | 0.92–1.74 | 1.32 | 0.97–1.81 |
| Charlson Comorbidity 0 (3) | 3.18 | 2.26–4.49 | 3.51 | 2.43–5.07 | 2.48 | 1.74–3.53 |
| Charlson Comorbidity 1 (3) | 2.58 | 1.81–3.68 | 2.85 | 1.96–4.16 | 2.02 | 1.40–2.90 |
| Charlson Comorbidity 2 (3) | 2.17 | 1.47–3.21 | 2.34 | 1.54–3.54 | 1.84 | 1.23–2.76 |
| Vascular Invasion (Yes) | 5.78 | 4.49–7.44 | N/A | N/A | 5.63 | 4.37–7.26 |
| Radiation (No) | 6.82 | 5.79–8.02 | 7.10 | 6.00–8.41 | 3.55 | 3.00–4.21 |
| Stent (No) | 1.38 | 1.19–1.59 | 1.34 | 1.15–1.56 | 1.37 | 1.18–1.59 |
Defined as any course of chemotherapy and surgery, N=1,166
SEER Region, quartile of income, and location of residence were also controlled for in the model and were not significant
In the subset of patients without vascular invasion (N=8,253), younger age, white race, lower Charlson comorbidity score, treatment with radiation, and biliary stent placement continued to be associated with receipt of multimodality therapy. Similar findings were observed for treated patients (Table 3).
Survival Analysis (Figure 4)
Figure 4.
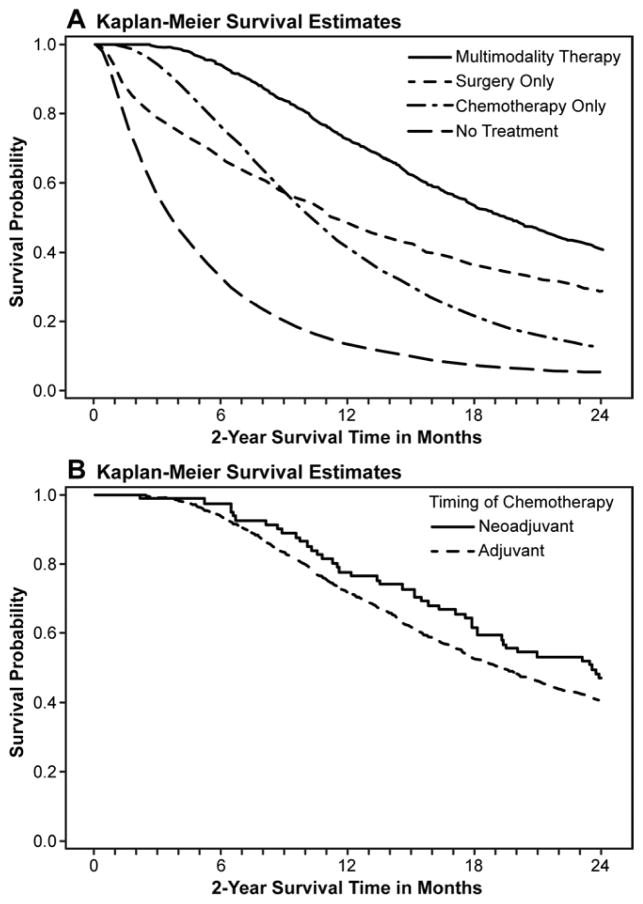
Figure 4A. Kaplan-Meier two-year survival curves in patients with locoregional pancreatic adenocarcinoma (N =10,505) by treatment group: multimodality therapy, surgery alone, chemotherapy alone, and no treatment. Survival improved with multimodality therapy compared to the other groups (41.0% vs. 29.0% vs. 12.5% vs. 5.1%, p<0.0001). Patients were censored at loss of follow-up and at two years.
Figure 4B. Kaplan-Meier estimate of two-year survival for patients who received multimodality therapy (N=1,166). There were no differences in survival between a neoadjuvant and adjuvant approach to chemotherapy (46.9% vs. 40.6%, p=0.16). Patients were censored at loss of follow-up and at two years.
Survival for the overall cohort was 13.8% at two years (median = 7.2 months). Figure 4A shows 2-year survival in patients who received multimodality therapy (41.0%), resection only (25.1%), chemotherapy only (12.5%), or no therapy (5.1%, p<0.0001). For patients who received multimodality therapy, there was no difference in survival when chemotherapy was administered in the neoadjuvant vs. adjuvant setting (Figure 4B, 46.9% vs. 40.6%, p=0.16). In a Cox proportional hazards model controlling for age, sex, race, income, education, Charlson comorbidity, SEER region, radiation, endostent, and vascular invasion, neoadjuvant therapy was not independently associated with improved survival relative to adjuvant therapy in patients receiving multimodality therapy (HR 0.76, 95% CI 0.55–1.06). The absence of vascular invasion was most strongly associated with improved survival for these patients (HR 0.54, 95% CI 0.40–0.72).
CONCLUSIONS
We observed that only 11% of older patients with locoregional pancreatic cancer in SEER regions received multimodality therapy. In addition, over half of older patients with potentially treatable pancreatic cancer did not receive any treatment at all. When treatment was instituted, over half of patients received chemotherapy as the initial treatment modality, but very few of these patients ultimately underwent surgical resection. Finally, we observed that when patients received multimodality therapy, there were no differences in survival between patients who received chemotherapy in the neoadjuvant versus adjuvant setting.
Our study is the first to illustrate general management trends in an older population with locoregional pancreatic cancer. While previous studies have focused on the timing of chemotherapy in only those patients undergoing resection14, 21, 22, we have identified treatment trends in all patients with potentially treatable pancreatic cancer. While the majority of patients who received treatment were treated with chemotherapy as the initial treatment modality, only 2% of these patients ultimately underwent surgery. There may be several reasons for this observation. Patients may have developed metastatic disease progression during neoadjuvant therapy and would not have benefited from surgical resection. Proponents of neoadjuvant therapy would argue that these patients would have been spared the morbidity of invasive surgery.16, 23 However, some patients may have experienced local disease progression without distant metastatic disease and missed their window for curative resection.24 It is also possible that patients may have developed toxicity related to neoadjuvant therapy with an associated decline in performance status and were no longer surgical candidates. Finally, it is possible that patients were treated with chemotherapy with palliative intent for locally unresectable disease and were never considered for resection. However, even in the group without vascular invasion, nearly all patients receiving chemotherapy first did not undergo resection.
Prior studies have suggested that neoadjuvant therapy may be associated with improved survival. 21, 25 However, in our population-based cohort, we observed no difference between neoadjuvant and adjuvant approaches in patients who received multimodality therapy. Furthermore, like the other studies, our survival comparison does not account for the patients who received chemotherapy with the intent to eventually undergo surgery but whose disease progressed in the interim. As a result, we have likely overestimated the survival benefit with a neoadjuvant approach. All of these survival comparisons, including our own, are limited by their retrospective nature but represent the best available evidence until large-scale prospective data are accumulated. Two previous recent single center reviews retrospectively compared patterns in care for patients with pancreatic adenocarcinoma who underwent a neoadjuvant-intent approach versus a surgery-first approach. Of the 167 patients in their study, Tzeng et al. identified improved receipt of multimodality therapy in those patients receiving a neoadjuvant-intent approach compared to patients receiving surgery first (95/155, 83% vs. 29/50, 50%, respectively, p<0.001), and no difference in survival between the two approaches.26 Another study by Papalezova et al. similarly identified no difference in median overall survival between the two approaches (15 months vs. 13 months, p=NS). While these studies provide insight into specific reasons for failure of therapy and survival outcomes, their single institution nature limits their generalizability. These studies are biased by institutional practices that may favor aggressive neoadjuvant approaches first in patients who are surgical candidates, and do not represent population-based practices; as a result, the external validity of these studies is limited.
Our study expands on previous findings13, 15 that treatment for pancreatic cancer is underutilized. In a specific population of patients older than 65, this disparity in pancreatic cancer care is even more pronounced. As has been suggested previously15 this may be due to nihilistic attitudes regarding pancreatic cancer care, particularly for older patients who may have limited life expectancy.
Finally, our data support previous observations13 that while patient and tumor characteristics largely determine receipt of multimodality therapy, administration of therapy can be improved. In an analysis of 301,033 patients with pancreatic adenocarcinoma from the National Cancer Data Base, Bilimoria et al. determined that patients at high volume centers, patients at NCCN/National Cancer Institute hospitals, and patients in metropolitan areas were all more likely to receive multimodality therapy.13 We observed that patients who received multimodality therapy were selected to do so based on favorable patient characteristics (younger age, less comorbidity) or favorable tumor biology (no vascular invasion). However, multimodality therapy increased over time and was also associated with higher education/income quartile, biliary stenting, and radiation use, factors that should not typically drive multimodality therapy use. In addition, the use of multimodality therapy varied widely by SEER region, from 6% to almost 17% of patients for each region. This may reflect treatment practices of physicians in these states, patient preferences regarding aggressiveness of care, or variable access to resources, but we cannot identify the precise reasons behind these observations with these data.
Our study has several limitations. Confounding by treatment indication is a significant concern when using administrative data. We attempted to control for locally advanced/ unresectable disease using Extent of Disease Coding, but resectability may not have been captured accurately with our methodology, since 74% of patients without vascular invasion did not undergo resection. In addition, we do not know the intent of therapy so it would have been inappropriate to compare survival on an “intent-to-treat” basis in patients who received chemotherapy as an initial treatment modality to those who received surgery as an initial treatment. We limited this bias by only comparing survival between neoadjuvant and adjuvant therapy in those patients who ultimately received both surgery and chemotherapy. We also do not know if patients who received chemotherapy in the six months after surgery did so with adjuvant intent or for treatment of recurrent or advancing disease. As a result, we may have overestimated the use of multimodality therapy. However, nearly 80% of patients received chemotherapy within three months after surgery, making treatment for recurrent or advanced disease less likely. Previous population-based studies have also used similar methodology to identify adjuvant treatment.14 Finally, the sample size for patients receiving neoadjuvant and adjuvant therapies is still relatively small, and as a result, our study may be underpowered to detect significant survival differences between these groups. As a result, our survival analysis is limited and only a multicenter prospective randomized collaboration can definitively determine the efficacy of a neoadjuvant-intent approach vs. an adjuvant-intent approach.
We observed that for older patients undergoing treatment for pancreatic cancer, a chemotherapy-first approach is likely associated with a lower likelihood for receipt of multimodality therapy. Multimodality therapy is underutilized in older patients with pancreatic cancer. While patient and tumor characteristics are being used to guide treatment decisions, the variability in the administration of multimodality therapy and the increased use over time imply that improvements in the delivery of care can be made. Finally, our data suggest that there are no differences in survival between a neoadjuvant vs. adjuvant approach when multimodality therapy is administered. Multicenter prospective clinical trials are needed to determine the optimal timing of chemotherapy and surgery in patients with resectable pancreatic cancer.
Acknowledgments
Funding: Cancer Prevention Research Institute of Texas Grant # #RP101207-P03, UTMB Clinical and Translational Science Award #UL1TR000071, NIH T-32 Grant # T32DK007639, AHRQ Grant # 1R24HS022134.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should it be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicate database.
Footnotes
Presented at the 9th Annual Academic Surgical Congress in San Diego, CA, February 6, 2014
Author Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66(1):56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(4):836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smeenk HG, Tran TC, Erdmann J, et al. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg. 2005;390(2):94–103. doi: 10.1007/s00423-004-0476-9. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 6.Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101(6):908–15. doi: 10.1038/sj.bjc.6605256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 8.Bakkevold KE, Arnesjø B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater--results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993;29A(5):698–703. doi: 10.1016/s0959-8049(05)80349-1. [DOI] [PubMed] [Google Scholar]
- 9.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 10.Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308(2):147–56. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 11.Sperti C, Pasquali C, Piccoli A, et al. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21(2):195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 12.Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8(9):972–1017. doi: 10.6004/jnccn.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilimoria KY, Bentrem DJ, Ko CY, et al. Multimodality therapy for pancreatic cancer in the U.S: utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110(6):1227–34. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- 14.Simons JP, Ng SC, McDade TP, et al. Progress for resectable pancreatic [corrected] cancer?: a population-based assessment of US practices. Cancer. 2010;116(7):1681–90. doi: 10.1002/cncr.24918. [DOI] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246(2):173–80. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goff SL, Chabot JA. A neoadjuvant strategy for the management of nonmetastatic pancreatic cancer. Cancer J. 2012;18(6):602–8. doi: 10.1097/PPO.0b013e318279aade. [DOI] [PubMed] [Google Scholar]
- 17. [Accessed December, 2012];SEER-Medicare: Brief Description of the SEER-Medicare Database. 2009 Nov; Available at: http://healthservices.cancer.gov/seermedicare/overview/
- 18. [Accessed December, 2012];Research, Statistics, Data & Systems. Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Research-Statistics-Data-and-Systems.html.
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Procedure Codes for SEER-Medicare Analyses. SEER-Medicare Linked Database. web site. 2013 Oct 18; Available at: http://healthservices.cancer.gov/seermedicare/considerations/procedure_codes.html. 2013.
- 21.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8(2):123–32. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 22.Kooby DA, Gillespie TW, Liu Y, et al. Impact of adjuvant radiotherapy on survival after pancreatic cancer resection: an appraisal of data from the national cancer data base. Ann Surg Oncol. 2013;20(11):3634–42. doi: 10.1245/s10434-013-3047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496–502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 24.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Artinyan A, Anaya DA, McKenzie S, et al. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011;117(10):2044–9. doi: 10.1002/cncr.25763. [DOI] [PubMed] [Google Scholar]
- 26.Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18(1):16–25. doi: 10.1007/s11605-013-2412-1. [DOI] [PubMed] [Google Scholar]