Abstract
Chronic graft-versus-host disease (cGVHD) is an autoimmune-like syndrome, and donor B cells play important roles in augmenting its pathogenesis. B cell-depleting anti-CD20 mAb has been administered before or after cGVHD onset for preventing or treating cGVHD in clinic. Although administration before onset appeared to be more effective, the effect is variable and sometimes minimal. Here, we used two mouse cGVHD models to evaluate the preventive and therapeutic effect of anti-CD20 mAb. With the model of DBA/2 donor to MHC-matched BALB/c recipient, one intravenous injection of anti-CD20 mAb (40 mg/kg) the following day or on day 7 after HCT when serum autoantibodies were undetectable effectively prevented induction of cGVHD and preserved strong graft-versus-leukemia (GVL) effect. The separation of GVL effect from GVHD was associated with a significant reduction of donor CD4+ T cell proliferation and expansion, and protection of host thymic medullary epithelial cells. Anti-CD20 mAb administration also prevented expansion of donor T cells and induction of cGVHD in another mouse model of C57BL/6 donor to MHC-mismatched BALB/c recipients. In contrast, administration of anti-CD20 mAb after GVHD onset was not able to effectively deplete donor B cells or ameliorate cGVHD in either model. These results indicate that administration of anti-CD20 mAb prior to signs of cGVHD can prevent induction of autoimmune-like cGVHD while preserving GVL effect; there is little effect if administered after cGVHD onset. This provides new insights into clinical prevention and therapy of cGVHD with B cell-depleting reagents.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a curative therapy for hematological malignancies such as leukemia and lymphoma [1]. While donor T cells including CD4+ and CD8+ in transplants play a critical role in mediating graft-versus-leukemia/lymphoma (GVL) effects and preventing tumor relapse, alloreactive T cells also mediate a severe side effect called graft-versus-host disease (GVHD), a major obstacle for widespread application of allogeneic HCT [2–6]. While both CD4+ and CD8+ T cells can induce GVHD, CD8+ T cells are more potent than CD4+ T cells in mediating GVL effect [7–15].
GVHD is initiated by alloreactive T cell infiltration of GVHD target tissues (i.e. gut, skin, liver, lung, and thymus) in recipients conditioned with total body irradiation (TBI) or high dose chemotherapy [16]. The conditioning procedure causes local tissue inflammation and attracts alloreactive T cell infiltration [17]. GVHD can be divided into acute and chronic ones. Acute GVHD (aGVHD) is characterized by severe infiltration of lymphocytes and other mononuclear cells and tissue cell apoptosis [18, 19]. Chronic GVHD (cGVHD) usually follows aGVHD and has overlapping target organs with aGVHD, but some cGVHD can occur with little prior aGVHD, and has prototypical target organs such as the salivary gland [20–22]. Chronic GVHD is a systemic lupus- and multiple scleroderma-like autoimmune syndrome characterized with chronic inflammation as well as autoantibody and collagen tissue deposition [20, 23–26]. While current immunosuppressive therapy can effectively prevent aGVHD, these drugs have little effect in preventing cGVHD, and cGVHD remains the major cause of morbidity and mortality in long-term survivors after allogeneic HCT [19, 27–29].
Recent studies by our group and others have demonstrated that autoimmune-like cGVHD is mediated by both donor CD4+ T and B cells [10, 21, 22, 26, 30], which can derive from mature CD4+ T and B cells in transplants or from de novo development in a GVHD-damaged thymus deficient in proper negative selection [21, 22, 26, 31–33]. We recently showed that the pathogenic CD4+ T and B cells in cGVHD recipients mediate mutual activation and expansion [22]. Donor B cells can be an effective APC that mediate autoreactive CD4+ T cell clonal expansion, as depletion of donor B cells in transplants prevent the expansion of autoreactive CD4+ T cells that mediate persistent inflammation in GVHD target tissues. Once expanded, autoreactive CD4+ T cells can mediate chronic GVHD pathogenesis in the absence of donor B cells [22]. It has also been reported that lymphopenia in cGVHD recipients leads to unbalanced ratio of BAFF versus B cell numbers and expansion of autoreactive B cells [34]. In addition, allo- and autoantibody production and tissue deposition is associated with cGVHD pathogenesis [35, 36].
Thymic damage in cGVHD recipients is usually thought to be an outcome of aGVHD-mediated by alloreactive T cells in transplants, and the alloreactive T cell damage of the thymus has been shown to be dependent on Fas/FasL and TRAIL/DR5 pathways but not the Perforin/Granzyme pathway [14, 37, 38], which is in contrast to the GVL effect which was shown to be more dependent on Perforin/Granzyme pathway [14, 15, 38, 39]. Our recent studies showed that besides donor T cells in transplants, de novo-developed donor-type CD4+ T cells but not CD8+ T cells in GVHD recipients early after HCT contribute to perpetuation of thymus damage [26].
Anti-CD20 mAbs (such as Rituximab) can deplete CD20+ normal B cells and B cell lymphoma cells [40–47], and the mechanisms of depletion include monocyte-mediated antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity [48–52]. Administration of B cell-depleting anti-CD20 mAb was reported to prevent a variety of autoimmune diseases such as type 1 diabetes, arthritis, and thyroiditis [53–56], as depletion of B cells by anti-CD20 mAb inhibited expansion of antigen-specific autoreactive CD4+ T cells [57]. Administration of anti-CD20 mAb after disease onset was reported to be ineffective in reversal of autoimmune T1D, due to infiltrating B cells down-regulating their expression of CD20 [54], although anti-CD20 mAb was effective in treating ongoing autoimmune thyroiditis [53].
Rituximab has been used to treat corticosteroid-dependent cGVHD as a second line-of-treatment with varying efficacy [58–61]. More recent reports showed that administration of Rituximab at 2–3 months after HCT before the onset of cGVHD, appeared to be more effective than after onset [62, 63]; the treatment was able to deplete B cells and either prevented cGVHD or patients developed less severe cGVHD [62]. Administration of Rituximab prior to cGVHD onset was safe and did not increase tumor relapse rates or incidence of infection [62]. These trials suggest that earlier administration of Rituximab is likely to be more effective than later; however, it remains unclear how to clinically define the appropriate time of administration in order to obtain optimal prevention of cGVHD.
We have developed two separate models of cGVHD. In the first model, transplantation of high-dose DBA/2 donor spleen cells into MHC-matched BALB/c recipients, donor CD4+ T and B cells in transplants play an important role in mediating cGVHD [21, 22, 30, 64]; in the second model, transplantation of low-dose donor C57BL/6 spleen cells into MHC-mismatched BALB/c recipients, de novo-developed donor CD4+ T and B cells play an important role in mediating cGVHD [26]. Pathogenic CD4+ T and B cells can derive from mature T and B cells in transplants or arise from de novo development in a GVHD-damaged thymus. Additionally, B cells are capable of down-regulating CD20 expression [54] and pathogenic CD4+ T cells can mediate cGVHD pathogenesis in the absence of B cells after prior expansion by B cells [22, 26]. Therefore, we tested whether administration of anti-CD20 mAb the following day after HCT or “early” after HCT (prior to clinical manifestations) could prevent induction of cGVHD, and also whether administration of anti-CD20 mAb at cGVHD onset could ameliorate ongoing cGVHD in our two cGVHD models. We found that administration of anti-CD20 mAb the following day after HCT, or prior to appearance of serum autoantibodies, was able to effectively deplete donor B cells, protect the host thymus, and prevent induction of cGVHD in both models, and that anti-CD20 mAb treatment did not interfere with the GVL effect. On the other hand, administration of anti-CD20 mAb at the time of GVHD onset was less effective in depleting donor B cells and did not ameliorate ongoing GVHD. These studies indicate that administration of anti-CD20 mAb prior to autoreactive CD4+ T and B cell expansion and autoantibody production is required for effective prevention of cGVHD.
Materials and Methods
Mice
C57BL/6, DBA/2 and BALB/c mice were purchased from the National Cancer Institute animal production program (Frederick, MD). Rag-2−/− BALB/c mice were purchased from Taconic Farms (Germantown, NY). Rag-2−/− DBA/2 mice were developed in the City of Hope Animal Resource Center (Duarte, CA). Igμ−/− C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were maintained in a pathogen-free room in the City of Hope Animal Resource Center. All animal protocols were approved by the City of Hope Institutional Animal Care and Use Committee.
Statistical analysis
Clinical cutaneous damage scoring and survival in different groups were compared by using the rank sum test or log-rank test (Prism, version 5.0; GraphPad Software, San Diego, CA). Comparison of two means was analyzed using an unpaired two-tailed Student t-test.
Antibody, flow cytometry analysis, and cell sorting
PE CD4 (GK1.5), FITC CD5.1 (H11-86.1), FITC B220 (RA3-6B2), FITC H-2Kb (AF6-88.5) and Pacific Blue CD45R were purchased from BD Pharmingen (San Diego, CA). APC-CD8a (53-6.7), APC-eFluor 780 TCRβ (H57-597), PE-Cy7 TCRβ (H57-597) were purchased from eBioscience (San Diego, CA). Alexa Fluor® 488 Goat Anti-Mouse IgG (H+L), Streptavidin Alexa Fluor® 555 Alexa Fluor® 488 Donkey Anti-Rat IgG (H+L) were purchased from Life Technologies (Grand Island, NY). DAPI dilactate was purchased from Sigma (St. Louis, MO). Biotinylated Ulex europaeus agglutinin (UEA) I was purchased from Vector Laboratories (Burlingame, CA). Aqua fluorescent reactive dye for viability analysis was purchased from Invitrogen (Carlsbad, CA). APC CD20 (18B12) was the kind gift of David Serreze (Jackson Laboratory, Bar Harbor, ME) [54]. Flow cytometry experiments were performed and run on a CyAn Immunocytometry system (DAKO Cytomation, Fort Collins, CO) and the resulting data were analyzed with FlowJo software (Tree Star, Ashland, OR) [21, 22, 64]. Purified anti-CD20 mAb (isotype IgG2a, 5D2) was the kind gift of Genentech (San Francisco, CA).
Induction and assessment of GVHD
Mice were exposed to 850 cGy total body irradiation with the use of a [137Cs] source 8 hours before HCT. Recipients were injected i.v. with either DBA/2 CD25+-cell depleted spleen cells and whole bone marrow (BM) cells or C57BL/6 whole spleen cell and T and B cell-depleted BM (TBCD-BM). CD25 depletion in the spleen, and T and B cell depletion in the BM was accomplished using biotin-conjugated anti-CD25 (spleen) and biotin-conjugated anti-CD3, anti-CD4, anti-CD8, anti-B220 and anti-CD19 mAb (BM) and anti-biotin micromagnetic beads, followed by passage through an autoMACS cell sorter (Miltenyi Biotec). The purity of depletion was >99%. The assessment and scoring of clinical cutaneous GVHD was described previously [21, 22, 65]. Assessment and scoring of total GVHD was as follows: hair loss (0–2), proteinuria (0–2), activity (0–2), posture (0–2) body weight loss (if no proteinuria occurred) (0–2).
Tissue collection for cellular analysis
Mice were euthanized using CO2 asphyxiation, and their spleens and thymuses were collected for analysis. Single cell suspensions were prepared by mashing tissues through a 70-mm filter.
Thymic epithelial cell staining and serum autoantibody staining of Rag-2−/− mouse skin, salivary gland, and lung tissues
Thymic epithelial staining was performed on cryosections of recipients’ thymuses at various time points after HCT. Serum autoantibodies were measured by staining thymuses of Rag-2−/−-BALB/c and -DBA/2 mouse skin, salivary gland and lung tissues, respectively as previously described [26, 66]. In brief, cryosections from thymuses of recipients, Rag-2−/− BALB/c or Rag-2−/− DBA/2 mice were prepared by soaking tissues in 4% paraformaldehyde for >1 h followed by dehydration in a solution of 30% sucrose in PBS. Tissue was then frozen in OCT gel and cryosectioned. Cryosections were placed in acetone at −20°C for 30 min and then rehydrated in PBS containing Mg2+. Tissues were blocked with 10% FBS in PBS for 2 h. Tissues were then incubated overnight with either Biotin UEA-1 (thymic epithelial cell staining) or diluted serum (autoantibody staining) and washed again. Tissues were then stained with either streptavidin-Alexa Fluor 555 (thymic epithelial cell staining) or anti-mouse IgG Alexa Fluor 488 (autoantibody staining) and DAPI for 2 h and washed. Representative photographs were taken of each tissue at x100 with the use of an Olympus BX51 and a Pixera (600CL) cooled charge-coupled device camera (Pixera). Quantification of antibody staining was done using Image-Pro Premier 9.1 (Rockville, MD). Serum autoantibody staining was quantified using Integrated Optical Density (OD) of Alexa Fluor 488 luminosity per pixel of each slide. UEA-1 staining of thymic epithelia was quantified using sum luminosity of Alexa Fluor 555 of each slide.
Bioluminescent imaging
Mice were injected with luciferase+ BCL1 cells (BCL1/Luc+) i.p. at time of transplantation and monitored for expansion of those cells using bioluminescent imaging. In vivo imaging of tumor growth was previously described [67]. Mice were injected with 200 μl firefly luciferin i.p. (Caliper Life Sciences, Hopkinton, MA), anesthetized, and imaged using an IVIS100 charge-coupled device imaging system (Xenogen). Data were analyzed using Igor Pro 4.0 software purchased from WaveMetrics (Lake Oswego, OR).
Results
Administration of anti-CD20 mAb effectively prevented induction of autoimmune-like cGVHD in BALB/c recipients given MHC-matched donor DBA/2 bone marrow transplants
We recently reported that depletion of donor B cells in transplants effectively prevented induction of autoimmune-like chronic GVHD in BALB/c recipients given MHC–matched donor DBA/2 transplants [22]. Thus, we tested whether administration of anti-CD20 mAb at the time of HCT could prevent induction of autoimmune-like cGVHD.
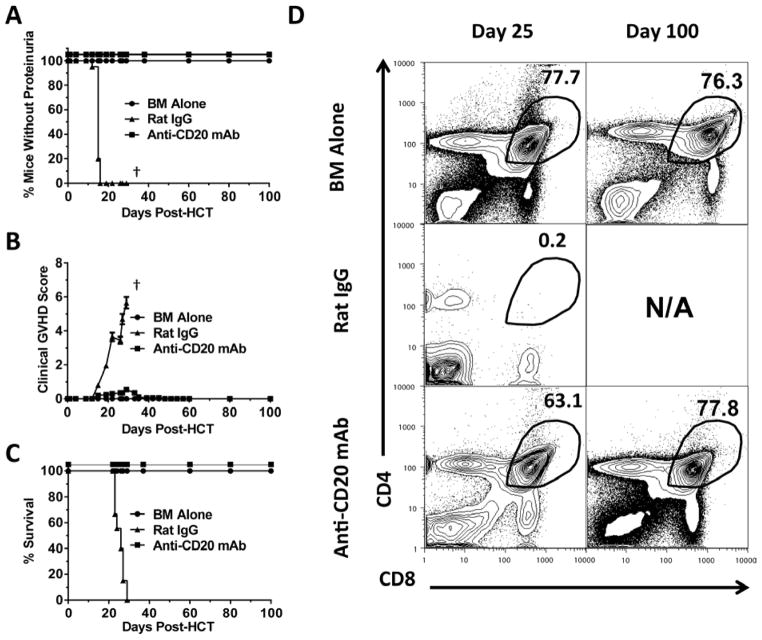
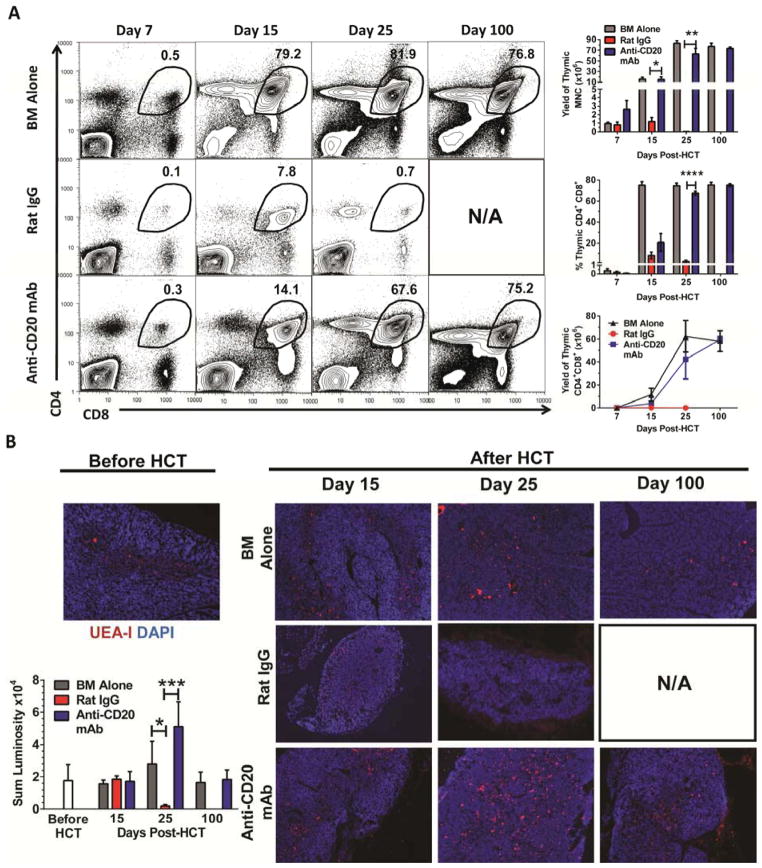
Accordingly, lethally irradiated BALB/c recipients (850 cGy, TBI) were injected with CD25+ T cell-depleted spleen (CD25−-SPL) cells (75×106) and BM cells (2.5×106) from DBA/2 donors. The following day after transplant, recipients were given one injection of anti-CD20 mAb (40 mg/Kg, clone 5D2, IgG2a). Injection of this anti-CD20 mAb was reported to begin depleting splenic B cells within 16 hours of injection, and the depletion could last for ~ 2 months, similar to another anti-CD20 mAb (clone MB20-11, IgG2c) [57, 68]. Consistently, we observed that one injection of this anti-CD20 mAb the following day after HCT effectively depleted B220+ B cells (>95%) for more than 25 days (Fig. S1). Percentage of B cells recovered and was similar to control recipients given BM alone by ~60 days after HCT (data not shown) and remained similar 100 days after HCT (Fig. S1). While all (12/12) recipients treated with rat IgG developed proteinuria, signs of clinical GVHD, and died approximately 25 days after HCT, all (12/12) recipients treated with anti-CD20 mAb showed no proteinuria and little clinical signs of GVHD, and all survived for more than 100 days (P<0.01, Fig. 1A–C). The anti-CD20 mAb-treated recipients looked similar to control recipients given BM alone (Fig. 1A–C). Twenty-five days after HCT, while all rat IgG-treated recipients had very small thymuses with few CD4+CD8+ thymocytes, anti-CD20 mAb-treated recipients had a similar percentage and yield of CD4+CD8+ thymocytes to that of GVHD-free control recipients given BM alone and this remained similar 100 days after HCT (Fig. 1D). The anti-CD20 mAb-treated long-term survivors showed no tissue damage in cGVHD target tissues such as skin, lung, and salivary gland [data not shown]. These results indicate that administration of anti-CD20 mAb at the beginning of HCT is able to effectively prevent induction of autoimmune-like cGVHD.
Figure 1. Anti-CD20 mAb Prevents Induction of Chronic GVHD in BALB/c Recipients Given MHC-matched Donor DBA/2 Bone Marrow Transplants.
Lethally irradiated BALB/c recipients were transplanted with spleen cells (75×106) and BM cells (2.5 ×106) from DBA/2 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) the following day. Control recipients were given BM cells alone. Recipients were monitored for clinical GVHD, including hair loss, proteinuria and survival († indicates death of all recipients in a group). (A–C) Percentage of recipients without (A) proteinuria, (B) clinical cutaneous GVHD score, and (C) percentage of survival. Each group contained 20 recipients combined from five replicate experiments. (D) Twenty-five and one hundred days after HCT, recipients thymuses were measured for percentage of CD4+CD8+ thymocytes and a representative pattern is shown of 4 replicate experiments.
Administration of anti-CD20 mAb effectively preserved GVL effect in BALB/c recipients given MHC-matched donor DBA/2 bone marrow transplants while preventing induction of cGVHD
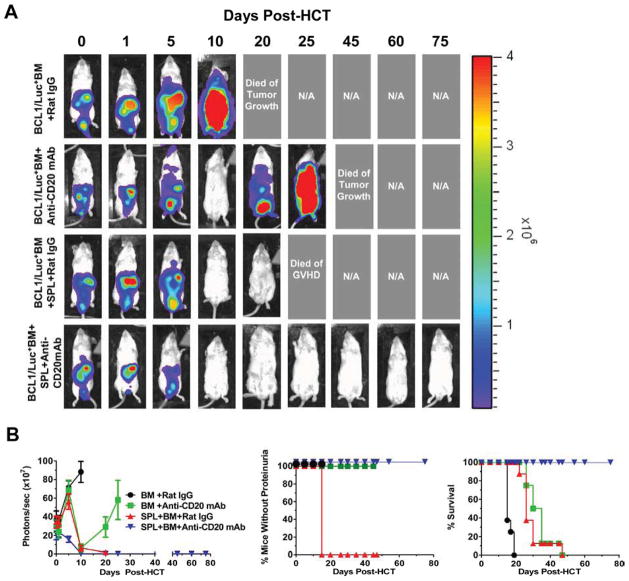
We evaluated anti-CD20 mAb administration’s impact on the GVL effect. Lethally TBI-conditioned BALB/c mice were injected with newly thawed luciferase-transfected BCL1 (BCL1/Luc+) leukemia/lymphoma cells (25×106) and transplanted with BM (2.5×106) alone or BM + CD25−-SPL cells (75×106) from DBA/2 donors. Anti-CD20 mAb (40mg/Kg) or rat IgG was administered the following day after HCT. Tumor cell growth was monitored with in vivo bioluminescent imaging (BLI) ~ every 5 days for up to 75 days. As shown in Fig. 2A and B, the BCL1/Luc+ tumor cells grew rapidly in recipients given BM alone plus control rat IgG and killed all (8/8) mice by approximately 20 days after tumor cell injection. The BCL1/Luc+ tumor cells gradually disappeared and became undetectable in mice injected with BM and anti-CD20 mAb by approximately 10 days after injection, but the tumor came back ~20 days and killed all (8/8) of the mice by ~ 25 days after tumor cell injection. The recipients injected with BM plus spleen cells and control rat IgG eliminated tumor cells by ~ 10 days after injection, but the mice developed proteinuria, and nearly all (7/8) died of GVHD by 25 days after HCT. One mouse did survive GVHD, but tumor also relapsed and the mouse died of tumor load ~45 days after HCT. In contrast, the recipients injected with BM plus spleen cells and anti-CD20 mAb eliminated the tumor cells, and all (8/8) survived for more than 75 days with no tumor or little signs of GVHD (P<0.01). These results indicate that anti-CD20 mAb treatment the following day after HCT preserves GVL effect while preventing GVHD.
Figure 2. Anti-CD20 mAb Prevents Induction of Chronic GVHD While Preserving GVL Effects in BALB/c Recipients Given MHC-matched Donor DBA/2 Bone Marrow Transplants.
Lethally irradiated BALB/c recipients were transplanted with spleen cells (75×106) and BM cells (2.5 ×106) from DBA/2 donors. Control recipients were given BM cells alone. Luciferase positive B-cell leukemia/lymphoma 1 cells (BCL1/Luc+) (25×106) were injected intraperitoneally at the same time when donor bone marrow (BM) and spleen cells were injected intravenously (i.v.). Twelve hours later mice were injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg). Recipients were monitored daily for signs of tumor and clinical GVHD, including hair loss, proteinuria and survival and in vivo imaging. (A) In vivo imaging shows progression of tumor over time. One representative image from each time point with at least 4 mice for time point is shown for each group. (B) Photons/sec (×107) of recipients in each group, percentage of recipients without proteinuria and survival. Each group is 6–8 mice combined from 3 replicate experiments.
Preventing induction of cGVHD by anti-CD20 mAb treatment was associated with prevention of production of antibodies against donor- and host-type GVHD target tissues
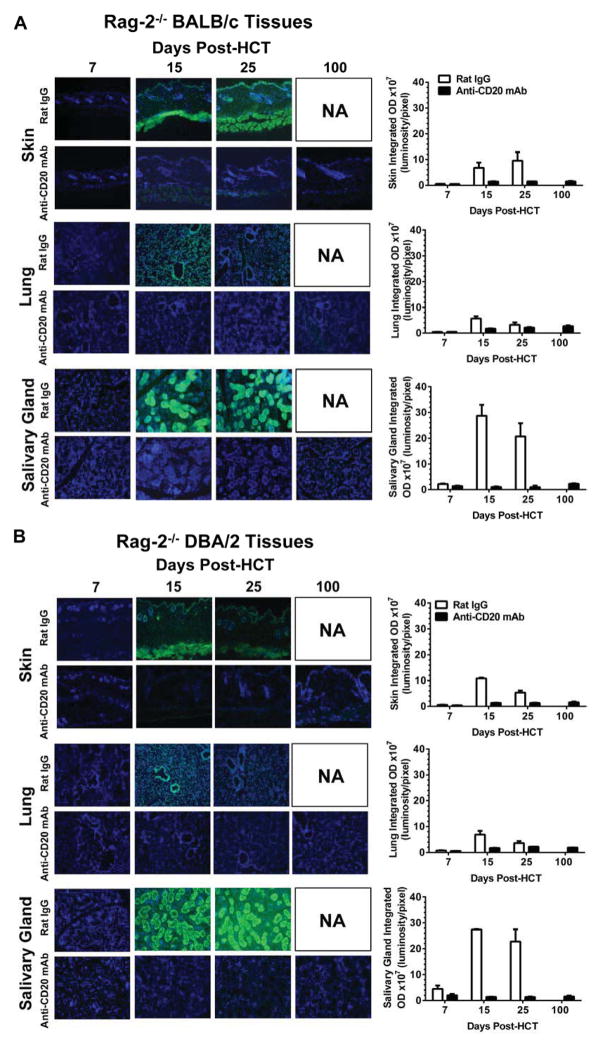
We recently reported that cGVHD recipients developed antibodies against both donor- and host-type GVHD target tissues [26]. Antibodies against host tissues were reported to contribute to cGVHD pathogenesis, especially in the lung [69]. We used skin, lung, and salivary gland of host-type Rag-2−/− BALB/c and donor-type Rag-2−/− DBA/2 as target tissues. As shown in Fig. 3A and B, sera from day 7 HCT recipients of all groups showed no staining of either donor- or host-type tissues. Sera from rat IgG-treated GVHD recipients on days 15 and 25 showed strong staining of the target tissues; no later time points were available due to GVHD death. At the same time, sera from anti-CD20 mAb-treated GVHD-free recipients showed little staining of GVHD target tissues, even at 100 days after HCT when the B cell population was totally recovered (Fig. 3). These results suggest that administration of anti-CD20 mAb the following day after HCT not only depletes pre-existing autoreactive B cells in transplants but also prevents de novo generation of autoreactive B cells, such that autoantibody production is completely prevented, and that this may contribute to preventing induction of cGVHD.
Figure 3. Anti-CD20 mAb Treatment Prevents Production of Serum Autoantibodies Against Skin, Salivary Gland, and Lung.
(A–B) Representative photographs (original magnification ×100) of (A) Rag-2−/− BALB/c and (B) Rag-2−/− DBA/2 skin, salivary gland, and lung tissues with GVHD recipient serum autoantibodies from days 7, 15, 25 and 100 after HCT are shown. DAPI staining is shown in blue, and autoantibody staining is shown in green. One representative staining is shown from four mice per group per time point. Average integrated optical density (OD) is shown of serum autoantibody staining from four mice per group per timepoint.
Preventing induction of cGVHD by administration of anti-CD20 mAb the following day after HCT is associated with reduction of donor CD4+ but not CD8+ T cell proliferation and expansion
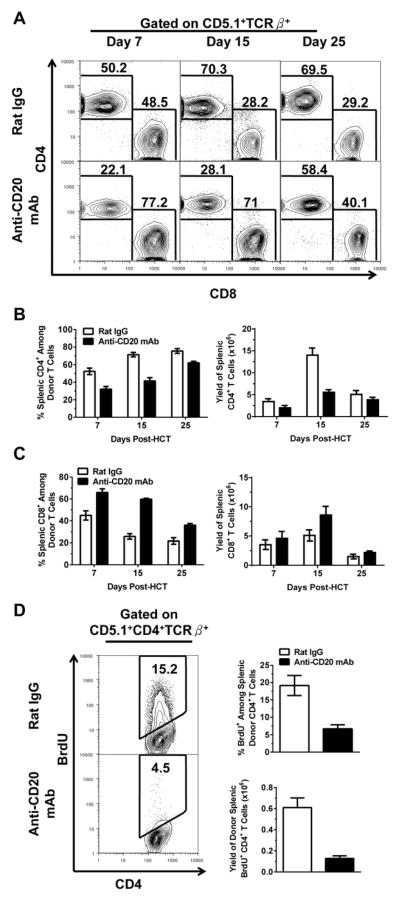
We compared the percentage and yield of total donor-type T cells as well as CD4+ and CD8+ subsets in recipients treated with anti-CD20 mAb or control rat IgG the following day after HCT. As compared with rat IgG treatment, anti-CD20 mAb treatment did not consistently change the percentage or yield of total donor-type T cells in the spleen of recipients at days 7, 15, and 25 after HCT (Fig. S2). However, anti-CD20 mAb-treatment decreased the percentage and yield of donor-type CD4+ T cells at days 7, 15, and 25 after HCT, especially 15 days after HCT (P<0.01, Fig. 4A & B). Conversely, anti-CD20 mAb-treatment increased the percentage of donor-type CD8+ T cells (P<0.01, Fig. 4A & C). This relative increase resulted from the decrease of donor-type CD4+ T cells. Anti-CD20 mAb-treatment significantly decreased donor-type CD4+ T proliferation as judged by in vivo BrdU-labeling (P<0.01, Fig. 4D). Anti-CD20 mAb-treatment did not change the activation status (as judged by CD62L and CD44 staining), did not change the apoptosis rate (as judged by Annexin V staining), and did not change the percentage of IFN-γ–, IL-17-, or IL-4-producing cells among donor-type CD4+ T cells (as judged by intracellular staining) [data not shown]. Anti-CD20 mAb treatment did not significantly change the percentage of Treg cells among donor-type CD4+ T cells or the percentage of IL-10-producing regulatory B cells among residual donor B cells [data not shown]. These results indicate that anti-CD20 mAb-treatment reduced donor-type CD4+ T cell proliferation and expansion but did not change differentiation or apoptosis rate of donor-type CD4+ T cells early after HCT; in addition, anti-CD20 mAb-treatment had little negative impact on donor CD8+ T cell expansion.
Figure 4. Anti-CD20 mAb Reduces Donor CD4+ T Proliferation and Expansion.
Lethally irradiated BALB/c recipients were transplanted with spleen cells (75×106) and BM cells (2.5 ×106) from DBA/2 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg). (A) Percentage of splenic CD4+ and CD8+ donor T cells (CD5.1+ TCRβ+) was measured on 7, 15 and 25 days after HCT. A representative pattern from each group at each time point is shown. Mean ± SE of percentage of (B) splenic CD4+ and (C) CD8+ donor T cells (CD5.1+ TCRβ+) was measured on 7, 15 and 25 days after HCT with 5–8 recipients at each time point from each group combined from four replicate experiments. (D) Mice were injected with 2.5 mg of BrdU 24 hours prior to sacrifice on day 7. Left- A representative staining pattern showing splenic donor Brdu+ CD4+ T cells gated on CD5.1+ TCRβ+. Right- Mean ± SE of percentage and yield of splenic CD5.1+ TCRβ+ Brdu+ CD4+ T cells (n=4).
Preventing induction of cGVHD by administration of anti-CD20 mAb the following day after HCT is associated with protection of thymic epithelial cells and thymocyte production
Our recent studies showed that damage of thymic medullary epithelial cells was associated with production of autoreactive CD4+ T cells that caused persistent thymic damage and development of cGVHD [26]. Percentage and yield of CD4+CD8+ thymocytes reflect the thymic regeneration capacity [70]. Thus, we evaluated the impact of anti-CD20 mAb-treatment on thymic production of CD4+CD8+ (double positive, DP) thymocytes and thymic medullary epithelial cell (mTEC) recovery at days 7, 15, 25, and 100 after HCT. The percentage of DP thymocytes in control recipients given donor BM only was ~0.5% at day 7 after HCT, but it rapidly increased and reached more than 75% by 15 days after HCT and was stable for up to 100 days (Fig. 5A, top row). The percentage of DP thymocytes of rat IgG-treated GVHD recipients was ~0.1% at day 7, ~8% at day 15, and dropped back to ~0.7% at day 25. No recipients in this group survived for more than 30 days. In contrast, the percentage of DP thymocytes of anti-CD20 mAb-treated GVHD-free recipients was ~0.3% at day 7, ~14% at day 15, ~68% at day 25, and ~75% at day 100. The yield of CD4+CD8+ thymocytes of rat IgG-treated GVHD mice was ~ 40 fold lower as compared to anti-CD20 mAb-treated recipients 25 days after HCT (P<0.01), although no significant difference was observed between anti-CD20 mAb-treated and control recipients given BM alone on days 7, 15, 25, and 100 days after HCT (Fig 5A).
Figure 5. Anti-CD20 mAb Protects Thymic Epithelial Cells and Thymocyte Regeneration.
Lethally irradiated BALB/c recipients were transplanted with spleen cells (75×106) and BM cells (2.5 ×106) from DBA/2 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg). Control recipients were given BM cells alone. (A) 7, 15, 25 and 100 days after HCT, recipients were measured for percentage and yield of CD4+CD8+ thymocytes. A representative staining pattern is shown from 4–8 mice for each group at each time point from 3 replicate experiments. (B) One representative photograph (original magnification x100) of the kinetic changes of thymic epithelial structure is shown 15, 25 and 100 days after HCT from four sections per mouse with four mice per time point. Thymic medullary epithelial cells are shown with UEA-1 (red) as well as DAPI (blue). Average sum luminosity is shown of UEA-1 staining (red) of thymic epithelia from four mice per group per timepoint.
Fifteen days after HCT, the thymus of rat IgG-treated GVHD recipients was much smaller than GVHD-free recipients given donor BM alone or anti-CD20 mAb treatment, although the latter two visibly appeared to be similar in size [data not shown] as well as yield (Fig. 5A). Immunofluorescent staining showed that UEA-I+ mTECs were detectable in the medulla of all three recipient types 15 days after HCT (Fig. 5B, left column). However, 25 days after HCT the UEA-1+ mTEC cells became undetectable in rat IgG-treated GVHD recipients, but appeared to have an expansion in the thymus of anti-CD20 mAb-treated GVHD-free recipients and BM alone recipients (Fig. 5B, middle column). One hundred days after HCT, the mTEC staining patterns in the thymus of GVHD-free control recipients and anti-CD20 mAb-treated recipients appeared similar (Fig. 5B, right column). These results indicate that further thymic damage in GVHD recipients can occur after emergence of de novo-developed donor-type T cells; and that anti-CD20 mAb-treatment prevents further thymic damage and allows for rapid recovery of thymic mTECs and de novo generation of thymocytes.
Administration of anti-CD20 mAb prior to but not after GVHD onset is effective in depleting B cells, protecting thymus, and preventing cGVHD
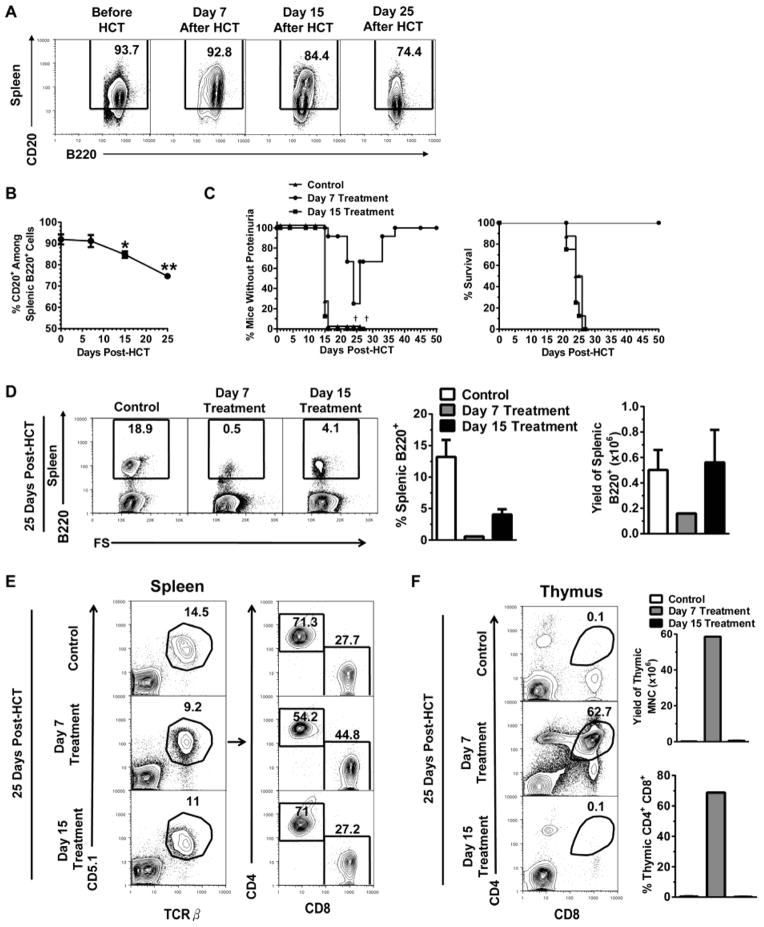
Administration of anti-CD20 mAb after the presence of autoantibodies in serum was reported to be ineffective in depleting B cells or preventing autoimmune type 1 diabetes onset in NOD mice due to B cell down-regulation of CD20 in inflammatory tissues [54]. We also observed that sera from GVHD recipients after day 15 but not day 7 showed allo- and autoreactivity against host- and donor-type GVHD target tissues (Fig. 3). Additionally, donor-type B cells gradually increased at Days 7, 15, and 25 in the spleen after HCT (Fig. S1), and their CD20 expression levels trended downwards by days 15 and 25, resulting in a significant reduction in the percentage of CD20+ B cells in the Control non-treated mice (P<0.05), although there was no difference between day 7 and before HCT (Fig. 6A and B). Thus, we compared the effect of administration of anti-CD20 mAb starting on day 7 or 15 after HCT in preventing or ameliorating cGVHD.
Figure 6. Administration of Anti-CD20 mAb at Different Time Points After HCT is Associated with Differential B Cell Depletion, Donor CD4+ T Cell Expansion, Thymus Protection, and GVHD.
Lethally irradiated BALB/c recipients were transplanted with spleen cells (75×106) and BM cells (2.5 ×106) from DBA/2 donors and injected i.v. with anti-CD20 mAb (40 mg/Kg) on days 7, 15 and 20 (Day 7 Treatment) or 15 and 20 (Day 15 Treatment) or nothing (Control). (A–B) Some Control (no treatment) recipients were sacrificed on day 7, 15 and 25 after HCT and splenic B220+CD20+ percentage and yields were measured and a representative pattern is shown (n=4 before, Day 7, Day 15; n=3 Day 25). (C) Recipients were monitored for clinical GVHD, including proteinuria and survival († indicates death of all recipients in a group) (n=8). (D) Four recipients from each group were sacrificed on day 25 and percentage and yield of splenic B220+ cells were measured (right) and a representative staining pattern from each group is shown (left). (E) Four recipients from each group were sacrificed on day 25 and a representative splenic staining pattern gated on CD5.1+ TCRβ+ (left) of CD4+ and CD8+ donor T cells (right) is shown. (F) Twenty-five days after HCT, four recipients from each group were measured for percentage and yield of CD4+CD8+ thymocytes (right) and a representative pattern is shown (left).
Accordingly, one group of recipients was administered anti-CD20 mAb on days 7, 15, and 20, referred to as day 7 treatment, and the other group was administered anti-CD20 mAb on days 15 and 20, referred to as day 15 treatment. Additionally, a no treatment group was used as a control. Control recipients all developed proteinuria by day 16 and died by day 26 after HCT. Recipients given day 15 treatment showed similar proteinuria and survival curve to the control recipients. However, recipients given day 7 treatment showed delayed onset of proteinuria with reduced severity and recovery by 35 days after HCT; all of these recipients survived for more than 50 days without further development of signs of GVHD; this is a marked difference from the group given day 15 treatment (P<0.01, Fig. 6C).
Additionally, HCT recipients from the three groups were analyzed for percentage of B220+ B cells and CD4+ and CD8+ T cells in the spleen, as well as percentage and yield of CD4+CD8+ thymocytes, on day 25 after HCT. Day 7 treatment effectively depleted B cells and the percentage of residual B cells was only ~0.5%, a more than 30 fold reduction as compared to control recipients, and the yield of B cells was also reduced by ~ 5 fold (P<0.01, Fig. 6D). This was similar to treatment starting the following day after HCT (Fig. 6D; Supp. Fig. 1). In contrast, day 15 treatment was less effective in depleting B cells, the percentage of residual B cells was ~4.1%, which was ~8-fold higher than day 7 treatment (P<0.01). Day 15 treatment did not significantly reduce the yield of spleen B cells as compared to control recipients (Fig. 6D).
Day 7 treatment significantly reduced the percentage of donor CD4+ T cells but increased the percentage of CD8+ T cells in the spleen 25 days after HCT, as compared to control mice (P<0.01, Fig. 6E). However, Day 15 treatment did not make significant changes to these populations and appeared to be similar to control recipients (Fig. 6E). On the other hand, day 7 treatment markedly increased the percentage and yield of CD4+CD8+ thymocytes as compared to control mice (P<0.01, Fig. 6F), while day 15 treatment produced no difference compared to control mice (Fig 6E). These results indicate that administration of anti-CD20 mAb before but not after presence of autoantibodies or GVHD onset is able to effectively deplete donor B cells, reduce donor CD4+ T cell expansion, protect the thymus, and prevent development of cGVHD.
Administration of anti-CD20 mAb prevented induction of cGVHD in BALB/c recipients given low-dose MHC-mismatched C57BL/6 donor transplants
We recently reported that transplantation of high-dose (5×106) of C57BL/6 donor spleen cells and BM cells (2.5×106) into myeloablative TBI-conditioned MHC-mismatched BALB/c recipients caused lethal acute GVHD, but transplantation of low-dose (1.25×106) donor cells induced severe autoimmune-like cGVHD [26]. We also observed that de novo-developed donor B cells markedly augmented cGVHD in this model, as BALB/c recipients given low-dose donor C57BL/6 CD8+ T cells (0.5×106) induced severe cGVHD in recipients given WT donor BM that produced de novo-developed B cells; but the CD8+ T cells induced little signs of cGVHD in recipients given Igμ−/− donor BM that did not produce de novo-developed B cells (Fig. S3). Thus, we tested how administration of anti-CD20 mAb impacts the induction of acute and chronic GVHD.
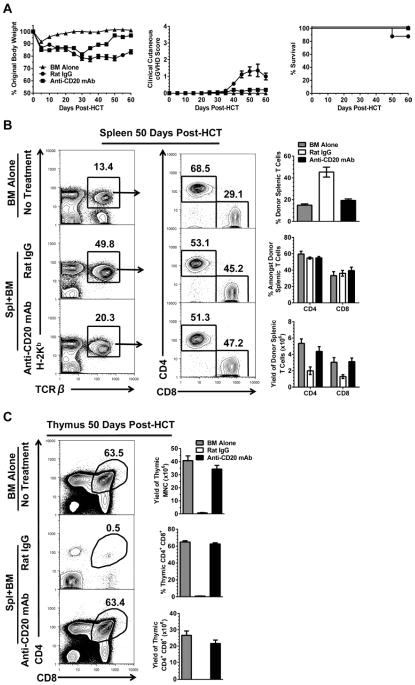
We observed that administration of anti-CD20 mAb (40mg/Kg) or rat IgG the following day after HCT of high-dose (5×106) spleen cells plus TBCD BM (2.5×106) from C57BL/6 donors did not prevent induction of acute GVHD, as 8/8 of recipients in both anti-CD20 mAb- and rat IgG- treated groups developed severe body weight loss, clinical signs of GVHD, and died by 7 days after HCT, and there was no difference between the two groups (Fig S4). In contrast, administration of anti-CD20 mAb the following day after HCT effectively prevented induction of cGVHD in recipients given low-dose (1.25×106) donor spleen cells as judged by prevention of body weight loss and hair loss 60 days after HCT (P<0.01, Fig. 7A), although almost all recipients in each group survived for more than 60 days after HCT. Compared with GVHD-free recipients given donor BM alone, 50 days after HCT the rat IgG-treated GVHD recipients showed a marked increase of percentage of donor-type T cells but a marked decrease of the donor-type T cell yield, although there was no significant changes in ratio of CD4+ versus CD8+ T cells (P<0.01, Fig. 7B). In contrast, anti-CD20 mAb-treated GVHD-free recipients appeared to be similar to recipients given BM alone in the percentage and yield of donor-type T cells (Fig. 7B).
Figure 7. Anti-CD20 mAb Prevents Induction of cGVHD in BALB/c Recipients Given MHC-mismatched C57BL/6 Donor T Cells.
Lethally irradiated BALB/c recipients were injected with 1.25×106 whole spleen cells and 2.5 ×106 TBCD-BM cells from C57BL/6 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) the following day. Recipients given TBCD-BM alone were used as controls. (A) Recipients were monitored for clinical GVHD, including body weight change, clinical cutaneous cGVHD score and survival. (n=12). (B–C) Recipients from each group were sacrificed on day 50 and (B) percentage and yield of splenic CD4+ and CD8+ donor T cells (H2Kb+TCRβ+) percentage and (C) yield of CD4+CD8+ thymocytes were measured. A representative staining pattern from each group at each time point is shown from four mice from two replicate experiments.
Administration of anti-CD20 mAb the following day after HCT also protected the thymus. While rat IgG-treated GVHD recipients had little CD4+CD8+ (DP) thymocytes in percentage or yield 50 days after HCT, anti-CD20 mAb-treated GVHD-free recipients had a percentage and yield of DP thymocytes similar to GVHD-free recipients given BM alone (Fig. 7C). In addition, administration of anti-CD20 mAb starting 45 days after HCT upon GVHD development, did not ameliorate the disease as judged by equally severe body weight loss and hair loss (Fig. S5). These results indicate that administration of anti-CD20 mAb the following day after HCT is able to prevent induction of cGVHD in MHC-mismatched recipients that do not have severe aGVHD. Administration of anti-CD20 mAb does not prevent aGVHD or ameliorate cGVHD after its onset.
Discussion
With MHC-matched and mismatched cGVHD models, we have observed the following: 1) administration of anti-CD20 mAb within 7 days of HCT when serum autoantibodies were not detectable was very effective in preventing induction of cGVHD; 2) the cGVHD-preventative effect was associated with effective depletion of donor B cells, and delayed administration after clinical signs of GVHD already appeared (proteinuria and presence of serum autoantibodies) failed to effectively deplete donor B cells or reverse GVHD; and 3) effective prevention of induction of cGVHD is associated with the preservation of strong GVL effects.
Our observations suggest that the timing of administration of anti-CD20 mAb is critical, and the sooner the administration of anti-CD20 mAb, the better the cGVHD preventative effect. We observed that administration of anti-CD20 mAb the following day after HCT prevented induction of cGVHD in 100% of recipients; administration of anti-CD20 mAb starting 7 days after HCT when serum autoantibodies were undetectable could still effectively prevent induction of cGVHD, although some recipients developed transient and reversible proteinuria. However, administration of anti-CD20 mAb after cGVHD onset did not have any effect in either MHC-matched or MHC-mismatched models. The ineffectiveness of cGVHD prevention by delayed administration of anti-CD20 mAb was associated with a reduced depletion of donor B cells. We observed that administration of anti-CD20 mAb the following day after HCT reduced the percentage of B220+ B cells on day 25 from ~12% to ~0.2%, administration at 7 days after HCT still reduced it to <1%, and administration at 15 days after HCT reduced it to 4.1% with no reduction in total spleen B cell yield. In addition, the inability to reverse ongoing GVHD 15 days after HCT might also be associated with preexisting pathogenic CD4+ T cells that were expanded by donor B cells prior to the anti-CD20 mAb depletion. Our recent report indicates that after an initial expansion of pathogenic CD4+ T cells by donor B cells, B cells are no longer required for continued GVHD pathogenesis [22]
Our observations with mouse models are consistent with clinical studies. Administration of anti-CD20 mAb (Rituximab) to patients with refractory cGVHD achieved variable and minimal effect in ameliorating the disease [59, 60, 71], and administration of anti-CD20 mAb 2–3 months after HCT, that is, before cGVHD onset, significantly reduced severity of cGVHD as judged by a significant reduction in corticosteroid-requiring cGVHD and non-relapse-related mortality, although patients still developed oral cGVHD [62]. Since administration of anti-CD20 mAb did not appear to increase relapse rate or infection incidence [62], it would be of interest to test whether administration of anti-CD20 mAb earlier than 2 months after HCT would be more effective in preventing induction of cGVHD. It would also be of interest to test whether occurrence of autoantibodies in the serum could serve as a prognostic biomarker for predicting the effect of anti-CD20 mAb therapy for prevention of cGVHD, since current studies showed that administration of anti-CD20 mAb prior but not after presence of autoantibodies in the serum could effectively prevent induction of cGVHD in a mouse model. This thought is consistent with recent reports that increased BAFF/B cell ratio after HCT could be a biomarker for predicting cGVHD development, and anti-CD20 mAb treatment that effectively ameliorated cGVHD reduced BAFF/B cell ratio [72, 73].
Autoantibodies can be produced by donor B cells in transplants and de novo-developed B cells in GVHD recipients. Our previous reports indicate that early alloimmune response after HCT leads to activation of donor CD4+ T cells that possess both donor- and host-reactivity, and those CD4+ T cells interact with autoreactive B cells in transplants to induce autoantibody production and mediate cGVHD pathogenesis [21, 22]. Supplemental data in our current report indicate that cGVHD induced by T cells in transplants is augmented by de novo-developed B cells.
We observed that delayed administration of anti-CD20 mAb was associated with less effective depletion of donor B cells. Consistent with previous reports that B cell lymphoma cells [42, 74] and activated B cells in the pancreas of type 1 diabetes NOD mice became resistant to anti-CD20 mAb depletion via down-regulation of CD20 expression [54], we also observed that the CD20 expression levels on B cells in the spleen trended down after HCT, although the drop in CD20 expression was quite small. This can contribute to ineffective depletion of B cells by delayed administration of anti-CD20 mAb, but other factors can also be of importance for HCT recipients. We observed that HCT recipients often appeared to be mixed chimeric 7 days after HCT but became complete chimeric by 15 days after HCT. A recent report showed that the primary mechanism of depletion by anti-CD20 mAb requires the presence of liver Kupffer cells in order to mediate antibody-dependent phagocytosis [68]. The same anti-CD20 antibody is used in the current studies. Therefore, it is likely that the ineffective depletion of B cells by anti-CD20 mAb 15 days after HCT is due to the lack of liver Kupffer cells, as at that time point, host-type Kupffer cells have been ablated in the liver, and donor-type Kupffer cells have yet to take up residence. This warrants further investigation. In addition, activated B cells can up-regulate expression of FcγRIIb and become more resistance to anti-CD20 mAb induced apoptosis [75]; however, we observed little difference in FcγRIIb levels on donor B cells after HCT (data not shown).
It is of interest that administration of anti-CD20 mAb after HCT was able to protect recipient thymus and prevent induction of cGVHD in BALB/c recipients transplanted with MHC-matched DBA/2 or MHC-mismatched C57BL/6 donor spleen cells. As we know, chronic GVHD can be mediated by autoreactive CD4+ T and B cells in transplants or de novo-developed autoreactive CD4+ T and B cells after acute GVHD [21, 26]. The cGVHD model of DBA/2 donor to BALB/c recipient is one model that has an amplified role of donor CD4+ T and B cells in transplants in the induction of cGVHD, as a large amount of DBA/2 donor spleen cells (~50×106) induced weak acute GVHD, but strong cGVHD [21, 22, 30]. The cGVHD model of C57BL/6 to BALB/c is one model with an amplified role of de novo-developed donor CD4+ T and B cells in mediating cGVHD, as donor C57BL/6 spleen cells generally induced severe lethal acute GVHD, but a small dose (<1.25 ×106) of donor spleen cells allowed for the induction of cGVHD after thymic damage [17].
In both models, we found that depletion of donor B cells by anti-CD20 mAb was able to augment thymic epithelial cell regeneration and prevent aGVHD’s transition into cGVHD. It is not yet clear how depletion of donor B cells lead to protection of thymic epithelial cells. Since we found that de novo-developed donor-type autoreactive CD4+ T cells early after HCT were responsible for persistent thymic damage mediated by alloreactive T cells in transplants [26], and since it was reported that B cells mediate expansion of autoreactive CD4+ T cells that recognize low levels of autoantigen [76], we hypothesize that depletion of donor B cells prevents the expansion of autoreactive CD4+ T cells derived from both mature CD4+ T cells in transplants and de novo-generated CD4+ T cells from an acute GVHD-damaged thymus. The prevention of autoreactive CD4+ T cell expansion can prevent thymic epithelial damage, since our recent publication showed that autoreactive CD4+ T cells can perpetuate thymus damage initiated by alloreactive T cells from the transplants [26]. This protection allows for survival of radiation-resistant IL-22-producing lymphoid inducer cells [77] to augment regeneration of thymic epithelial cells. Future studies will test this hypothesis.
It is also of interest that early administration of anti-CD20 mAb after HCT prevented induction of cGVHD while preserving strong GVL effects. We found that anti-CD20 mAb treatment did not change the activation status but significantly reduced the proliferation and yield of donor-type CD4+ T cells. In contrast, anti-CD20 mAb treatment did not change the donor CD8+ T cell activation status, proliferation or yield. Previous reports proposed that B cells mediated expansion of CD4+ T cells responsive to low levels of autoantigen [76], thus, depletion of donor B cells by anti-CD20 mAb may reduce the expansion of donor-type CD4+ T cells that mediate cGVHD pathogenesis, that is, CD4+ T cells that possess both donor- and host-reactivity and recognize the non-polymorphic antigens expressed by both donor and host tissues, as proposed by our and other previous publications [21, 22, 78]. Additionally, donor CD4+ T cells were reported to use FasL/Fas pathway to kill target cells [37, 38], and FasL/Fas and TRAIL/DR5 pathways were shown to play more important roles in thymic damage than the perforin/granzyme pathway [37]. On the other hand, alloreactive donor CD8+ T cells play a more important role than CD4+ T cells in mediating GVL effect via the perforin/granzyme pathway [15, 38, 39]. Therefore, it is conceivable that although depletion of donor B cells by anti-CD20 mAb can inhibit donor CD4+ T cell expansion, prevent persistent thymic damage, and prevent induction of cGVHD, depletion of B cells does not interfere with donor CD8+ T cell activation, expansion or their GVL effects. Our recent report indicates that donor CD8+ T cells do not induce cGVHD in the absence of help from pathogenic CD4+ T cells [26].
In addition, although administration of anti-CD20 at the following day after HCT did not have an obvious impact on induction of acute GVHD in MHC-mismatched HCT of C57BL/6 donor to BALB/c recipient in the current studies, administration of B cell-depleting anti-CD20 mAb (Rituximab) pre- and peri-transplantation was reported to reduce aGVHD in HCT patients given MHC-matched donor transplants in some cases, although it was not consistently effective in prevention of cGVHD [79–83]. Acute GVHD is mediated mainly by alloreactive T cells. In MHC-mismatched recipients, alloreactive T cells receive enough stimulation that the contribution of donor B cells to their activation and expansion is negligible. The reduction of aGVHD by depletion of donor B cells in MHC-matched patient recipients may result from the reduction of expansion of alloreactive T cells. We reported that in MHC-matched HCT recipients, donor B cells can expand donor T cells that have both allo- and autoreactivity [21]. The lack of prevention of cGVHD may be due to the short duration of B cell depletion. We have observed that donor B cells in transplants or de novo-developed after HCT could augment induction of cGVHD. Thus, injection of depleting anti-CD20 mAb following HCT for a long enough time period, that is, depletion of de novo-developed donor B cells for a certain time period, may be required for preventing induction of cGVHD.
In summary, administration of anti-CD20 mAb one to seven days after HCT (prior to the appearance of autoantibodies) effectively prevented induction of cGVHD and preserved the GVL effect. Administration starting at day 15 (upon GHVD onset and autoantibody appearance) did not ameliorate ongoing GVHD. These observations in combination with others’ reports suggest that 1) in order to obtain the optimal effect in prevention of aGVHD and cGVHD development, anti-CD20 mAb should be administered before and after HCT for a period of time beyond the usual time point for aGVHD onset. Once aGVHD occurs, administration of anti-CD20 mAb alone may no longer be effective, as by that time point, donor B cells may have already down-regulated CD20, and autoreactive CD4+ T cells may have already been expanded. Thus, after aGVHD onset, the effective approach for ameliorating aGVHD and preventing chronic GVHD should be depleting the activated donor B and CD4+ T cells by administration of anti-CD20 mAb and other B cell-depleting antibodies such anti-CD22/calicheamicin-conjugate mAb in combination with anti-CD4 mAb or ATG to deplete T cells. Anti-CD22 calicheamicin-conjugate mAb has been reported to be an effective reagent for depleting mature B cells [84, 85]. Addition of ATG and anti-CD20 mAb to conditioning regimen was reported to be more effective in the prevention of GVHD [82]. Whether administration of depleting anti-CD20 and anti-CD4 mAb can yield optimal prevention of acute and chronic GVHD while preserving GVL effects need to be tested in future studies, as anti-CD4 mAb can spare donor CD8+ T cells that mediate GVL effects.
Supplementary Material
Lethally irradiated BALB/c recipients were transplanted with spleen cells (75×106) and BM cells (2.5 ×106) from DBA/2 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) the following day after HCT. Control recipients were given BM alone. Recipients were sacrificed on day 7, 15, 25 and 100 and percentage and yield of splenic B220+ cells were measured. A representative pattern from each group at each time point is shown from 6 mice from four replicate experiments.
Lethally irradiated BALB/c recipients were transplanted with spleen cells (75×106) and BM cells (2.5 ×106) from DBA/2 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) the following day after HCT. Recipients were sacrificed 7, 15 and 25 days after HCT. Percentage and yield as well as a representative pattern of splenic CD5.1+ TCRβ+ T cells is shown from 4 mice each group per time point.
Lethally irradiated BALB/c recipients were given a low dose of donor C57BL/6 CD8+ T cells (0.5×106) and either WT donor BM or Igμ−/− donor BM (2.5×106). Recipients were monitored for clinical GVHD, including (A) body weight loss, (B) clinical cutaneous cGVHD score and (D) survival. A representative photograph taken at day 60 is shown (n=4).
Lethally irradiated BALB/c recipients were injected with 5×106 whole spleen cells and 2.5 ×106 TBCD-BM cells from C57BL/6 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) the following day after HCT. Recipients given TBCD-BM alone were used as controls. Recipients were monitored for clinical GVHD, including body weight change, clinical GVHD score, and survival (n=8 from two replicate experiments).
Lethally irradiated BALB/c recipients were injected with 1.25×106 whole spleen cells and 2.5 ×106 TBCD-BM cells from C57BL/6 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) starting on day 45 after disease onset, with follow-up injections on day 50 and 55. Recipients were monitored for clinical GVHD, including body weight change, clinical cutaneous GVHD, and survival (n=4 from two replicate experiments).
Acknowledgments
This work was supported by Nesvig Lymphoma Foundation and NIH R01AI066008 (to D. Zeng).
We would like to thank Dr. Arthur Riggs for his support of this project, and thank Lucy Brown and her staff at the City of Hope (COH) Flow Cytometry Facility, Sofia Loera and her staff at the COH Anatomic Pathology Laboratory, Donna Isbell and her staff at the COH Animal Resource Center for their excellent technical assistance. We also thank Dr. David Serreze and his lab at the Jackson Laboratory (Bar Harbor, ME) for their kind gift of APC-anti-CD20 mAb (18B12) and Genentech (San Francisco, CA) for their kind gift of purified anti-CD20 mAb mAb (5D2). Dr. Andrew Chan is an employee of Genentech, the company that makes and sells the anti-CD20 mAb used in this study.
Footnotes
All other authors declare no conflict of interest.
Author contributions:
H.F.J. designed experiments, performed research, analyzed data, wrote manuscript; Y.X. assisted in research, analyzed data; J.J.R. assisted in designing experiments, performing research, analyzing data, and writing manuscript. K.C. performed research, analyzed data. X.N. assisted in research. T. W. performed research, analyzed data; A.C. provided Anti-CD20, critically reviewed manuscript; S.F. supported research, critically reviewed manuscript; D.Z. directed research, designed experiments, wrote manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–9. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 2.Riddell SR. The graft-versus-leukemia effect--breaking the black box open. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14:2–3. doi: 10.1016/j.bbmt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Fefer A. Graft-vs.-Tumor Responses. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic cell transplantation. 3. Malden, MA: Blackwell Publishing; 2004. pp. 369–79. [Google Scholar]
- 4.Korngold R, Friedman TM. Murine Models of Graft versus-Host Disease and Graft-versus-Tumor Effect. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoeitic Cell Tranplantation. 4. Hoboken, NJ: John Wiley & Sons; 2009. pp. 176–87. [Google Scholar]
- 5.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nature medicine. 2005;11:1244–9. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 6.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110:9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin PJ. Donor CD8 cells prevent allogeneic marrow graft rejection in mice: potential implications for marrow transplantation in humans. J Exp Med. 1993;178:703–12. doi: 10.1084/jem.178.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin PJ, Rowley SD, Anasetti C, Chauncey TR, Gooley T, Petersdorf EW, et al. A phase I-II clinical trial to evaluate removal of CD4 cells and partial depletion of CD8 cells from donor marrow for HLA-mismatched unrelated recipients. Blood. 1999;94:2192–9. [PubMed] [Google Scholar]
- 9.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nature reviews Immunology. 2012;12:443–58. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo DJ, Stone R, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2006;12:1047–55. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, Garcia-Ojeda M, Sibley R, et al. Bone marrow NK1.1(−) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189:1073–81. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nature medicine. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Shizuru JA. Graft-vs.-lymphoma effect in an allogeneic hematopoietic stem cell transplantation model. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 1999;5:357–68. doi: 10.1016/s1083-8791(99)70012-1. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh MH, Patterson AE, Korngold R. T-cell subsets mediate graft-versus-myeloid leukemia responses via different cytotoxic mechanisms. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2000;6:231–40. doi: 10.1016/s1083-8791(00)70005-x. [DOI] [PubMed] [Google Scholar]
- 16.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. [PubMed] [Google Scholar]
- 17.Chakraverty R, Cote D, Buchli J, Cotter P, Hsu R, Zhao G, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–31. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler CAJH. Manifestations and Treatment of Acute Graft-versus-Host Disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4. Wiley-Blackwell; 2009. pp. 1287–303. [Google Scholar]
- 19.Korngold R, Antin JH. Biology and management of acute graft-versus-host disease. Cancer treatment and research. 2009;144:257–75. doi: 10.1007/978-0-387-78580-6_11. [DOI] [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhao D, Young JS, Chen YH, Shen E, Yi T, Todorov I, et al. Alloimmune response results in expansion of autoreactive donor CD4+ T cells in transplants that can mediate chronic graft-versus-host disease. Journal of immunology. 2011;186:856–68. doi: 10.4049/jimmunol.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young JS, Wu T, Chen Y, Zhao D, Liu H, Yi T, et al. Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune-like chronic graft-versus-host disease. Journal of immunology. 2012;189:222–33. doi: 10.4049/jimmunol.1200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavletic SZ, Vogelsang GB. Chronic Graft-versus-Host Disease: Clinical Manifestations and Therapy. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4. Hoboken, NJ: Blackwell Publishing; 2009. pp. 1304–24. [Google Scholar]
- 24.Arai S, Jagasia M, Storer B, Chai X, Pidala J, Cutler C, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118:4242–9. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz KR. Pathophysiology of Chronic Graft versus Host Disease. In: Vogelsang GB, Pavletic SZ, editors. Chronic Graft versus Host Disease: Interdisciplinary Management. New York, NY: Cambridge University Press; 2009. pp. 17–30. [Google Scholar]
- 26.Wu T, Young JS, Johnston H, Ni X, Deng R, Racine J, et al. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. Journal of immunology. 2013;191:488–99. doi: 10.4049/jimmunol.1300657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao NJ. Graft versus host disease following allogeneic bone marrow transplantation. Curr Opin Immunol. 1992;4:571–6. doi: 10.1016/0952-7915(92)90028-d. [DOI] [PubMed] [Google Scholar]
- 28.Koreth J, Antin JH. Current and future approaches for control of graft-versus-host disease. Expert review of hematology. 2008;1:111. doi: 10.1586/17474086.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler C, Antin JH. Chronic graft-versus-host disease. Current opinion in oncology. 2006;18:126–31. doi: 10.1097/01.cco.0000208784.07195.84. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Todorov I, Zhang Z, Liu Y, Kandeel F, Forman S, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T cells generated de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. Journal of immunology. 2007;179:3305–14. doi: 10.4049/jimmunol.179.5.3305. [DOI] [PubMed] [Google Scholar]
- 32.Teshima T, Reddy P, Liu C, Williams D, Cooke KR, Ferrara JL. Impaired thymic negative selection causes autoimmune graft-versus-host disease. Blood. 2003;102:429–35. doi: 10.1182/blood-2003-01-0266. [DOI] [PubMed] [Google Scholar]
- 33.van den Brink MR, Moore E, Ferrara JL, Burakoff SJ. Graft-versus-host-disease-associated thymic damage results in the appearance of T cell clones with anti-host reactivity. Transplantation. 2000;69:446–9. doi: 10.1097/00007890-200002150-00026. [DOI] [PubMed] [Google Scholar]
- 34.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–74. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–8. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2011;119:1570–80. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Na IK, Lu SX, Yim NL, Goldberg GL, Tsai J, Rao U, et al. The cytolytic molecules Fas ligand and TRAIL are required for murine thymic graft-versus-host disease. The Journal of clinical investigation. 2010;120:343–56. doi: 10.1172/JCI39395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsieh MH, Korngold R. Differential use of FasL- and perforin-mediated cytolytic mechanisms by T-cell subsets involved in graft-versus-myeloid leukemia responses. Blood. 2000;96:1047–55. [PubMed] [Google Scholar]
- 39.Schmaltz C, Alpdogan O, Horndasch KJ, Muriglan SJ, Kappel BJ, Teshima T, et al. Differential use of Fas ligand and perforin cytotoxic pathways by donor T cells in graft-versus-host disease and graft-versus-leukemia effect. Blood. 2001;97:2886–95. doi: 10.1182/blood.v97.9.2886. [DOI] [PubMed] [Google Scholar]
- 40.Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. The New England journal of medicine. 2012;366:2008–16. doi: 10.1056/NEJMct1114348. [DOI] [PubMed] [Google Scholar]
- 41.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nature reviews Immunology. 2010;10:301–16. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 42.Davis TA, Czerwinski DK, Levy R. Therapy of B-cell lymphoma with anti-CD20 antibodies can result in the loss of CD20 antigen expression. Clinical cancer research: an official journal of the American Association for Cancer Research. 1999;5:611–5. [PubMed] [Google Scholar]
- 43.Grillo-Lopez AJ, White CA, Dallaire BK, Varns CL, Shen CD, Wei A, et al. Rituximab: the first monoclonal antibody approved for the treatment of lymphoma. Curr Pharm Biotechnol. 2000;1:1–9. doi: 10.2174/1389201003379059. [DOI] [PubMed] [Google Scholar]
- 44.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–20. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 45.Beers SA, Chan CH, James S, French RR, Attfield KE, Brennan CM, et al. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112:4170–7. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009;114:4002–8. doi: 10.1182/blood-2009-07-143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuehnle I, Huls MH, Liu Z, Semmelmann M, Krance RA, Brenner MK, et al. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood. 2000;95:1502–5. [PubMed] [Google Scholar]
- 48.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, et al. Complement activation determines the therapeutic activity of rituximab in vivo. Journal of immunology. 2003;171:1581–7. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 49.Golay J, Lazzari M, Facchinetti V, Bernasconi S, Borleri G, Barbui T, et al. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood. 2001;98:3383–9. doi: 10.1182/blood.v98.12.3383. [DOI] [PubMed] [Google Scholar]
- 50.Cragg MS, Morgan SM, Chan HT, Morgan BP, Filatov AV, Johnson PW, et al. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood. 2003;101:1045–52. doi: 10.1182/blood-2002-06-1761. [DOI] [PubMed] [Google Scholar]
- 51.Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:859–66. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 52.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nature medicine. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 53.Hong SH, Braley-Mullen H. Follicular B cells in thyroids of mice with spontaneous autoimmune thyroiditis contribute to disease pathogenesis and are targets of anti-CD20 antibody therapy. Journal of immunology. 2014;192:897–905. doi: 10.4049/jimmunol.1301628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serreze DV, Chapman HD, Niens M, Dunn R, Kehry MR, Driver JP, et al. Loss of intra-islet CD20 expression may complicate efficacy of B-cell-directed type 1 diabetes therapies. Diabetes. 2011;60:2914–21. doi: 10.2337/db11-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. The New England journal of medicine. 2009;361:2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. Journal of immunology. 2007;179:1369–80. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 57.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20878–83. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Bonin M, Oelschlagel U, Radke J, Stewart M, Ehninger G, Bornhauser M, et al. Treatment of chronic steroid-refractory graft-versus-host disease with low-dose rituximab. Transplantation. 2008;86:875–9. doi: 10.1097/TP.0b013e318183f662. [DOI] [PubMed] [Google Scholar]
- 59.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2009;15:1005–13. doi: 10.1016/j.bbmt.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Ratanatharathorn V, Ayash L, Reynolds C, Silver S, Reddy P, Becker M, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2003;9:505–11. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 61.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone marrow transplantation. 2001;28:121–9. doi: 10.1038/sj.bmt.1703111. [DOI] [PubMed] [Google Scholar]
- 62.Cutler C, Kim HT, Bindra B, Sarantopoulos S, Ho VT, Chen YB, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122:1510–7. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arai S, Sahaf B, Narasimhan B, Chen GL, Jones CD, Lowsky R, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–54. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112:2129–38. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft versus host disease. Blood. 2009;114:3101–12. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–59. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Lou J, Li N, Todorov I, Lin CL, Cao YA, et al. Donor CD8+ T cells mediate graft-versus-leukemia activity without clinical signs of graft-versus-host disease in recipients conditioned with anti-CD3 monoclonal antibody. Journal of immunology. 2007;178:838–50. doi: 10.4049/jimmunol.178.2.838. [DOI] [PubMed] [Google Scholar]
- 68.Montalvao F, Garcia Z, Celli S, Breart B, Deguine J, Van Rooijen N, et al. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. The Journal of clinical investigation. 2013;123:5098–103. doi: 10.1172/JCI70972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119:1570–80. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ladi E, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nature immunology. 2006;7:338–43. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- 71.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–62. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobson CA, Sun L, Kim HT, McDonough SM, Reynolds CG, Schowalter M, et al. Post-Transplantation B Cell Activating Factor and B Cell Recovery before Onset of Chronic Graft-versus-Host Disease. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2014 doi: 10.1016/j.bbmt.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarantopoulos S, Stevenson KE, Kim HT, Washel WS, Bhuiya NS, Cutler CS, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117:2275–83. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jilani I, O’Brien S, Manshuri T, Thomas DA, Thomazy VA, Imam M, et al. Transient down-modulation of CD20 by rituximab in patients with chronic lymphocytic leukemia. Blood. 2003;102:3514–20. doi: 10.1182/blood-2003-01-0055. [DOI] [PubMed] [Google Scholar]
- 75.Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HT, et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118:2530–40. doi: 10.1182/blood-2011-01-330357. [DOI] [PubMed] [Google Scholar]
- 76.Schultz KR, Paquet J, Bader S, HayGlass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone marrow transplantation. 1995;16:289–95. [PubMed] [Google Scholar]
- 77.Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–5. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rangarajan H, Yassai M, Subramanian H, Komorowski R, Whitaker M, Gorski J, et al. Emergence of T cells that recognize nonpolymorphic antigens during graft-versus- host disease. Blood. 2012;119:6354–64. doi: 10.1182/blood-2012-01-401596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crocchiolo R, Castagna L, El-Cheikh J, Helvig A, Furst S, Faucher C, et al. Prior rituximab administration is associated with reduced rate of acute GVHD after in vivo T-cell depleted transplantation in lymphoma patients. Experimental hematology. 2011;39:892–6. doi: 10.1016/j.exphem.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 80.Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–6. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glass B, Hasenkamp J, Gorlitz A, Dreger P, JS, Wulf G, et al. Rituximab for Graft-Versus-Host-Disease-Prophylaxis after Allogeneic Stem Cell Transplantation Given as Treatment of High Risk Relapse of Aggressive Lymphoma: Results of a Randomized Phase II Study. 50th ASH Annual Meeting and Exposition; 2008. [Google Scholar]
- 82.Christopeit M, Schutte V, Theurich S, Weber T, Grothe W, Behre G. Rituximab reduces the incidence of acute graft-versus-host disease. Blood. 2009;113:3130–1. doi: 10.1182/blood-2009-01-200527. [DOI] [PubMed] [Google Scholar]
- 83.Van Hoef ME. Towards a rational graft-versus-host disease (GVHD) prophylaxis: rituximab should not be forgotten. Haematologica. 2013;98:e40–1. doi: 10.3324/haematol.2012.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dunussi-Joannopoulos K, Hancock GE, Kunz A, Hegen M, Zhou XX, Sheppard BJ, et al. B-cell depletion inhibits arthritis in a collagen-induced arthritis (CIA) model, but does not adversely affect humoral responses in a respiratory syncytial virus (RSV) vaccination model. Blood. 2005;106:2235–43. doi: 10.1182/blood-2004-11-4547. [DOI] [PubMed] [Google Scholar]
- 85.Fiorina P, Vergani A, Dada S, Jurewicz M, Wong M, Law K, et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes. 2008;57:3013–24. doi: 10.2337/db08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lethally irradiated BALB/c recipients were transplanted with spleen cells (75×106) and BM cells (2.5 ×106) from DBA/2 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) the following day after HCT. Control recipients were given BM alone. Recipients were sacrificed on day 7, 15, 25 and 100 and percentage and yield of splenic B220+ cells were measured. A representative pattern from each group at each time point is shown from 6 mice from four replicate experiments.
Lethally irradiated BALB/c recipients were transplanted with spleen cells (75×106) and BM cells (2.5 ×106) from DBA/2 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) the following day after HCT. Recipients were sacrificed 7, 15 and 25 days after HCT. Percentage and yield as well as a representative pattern of splenic CD5.1+ TCRβ+ T cells is shown from 4 mice each group per time point.
Lethally irradiated BALB/c recipients were given a low dose of donor C57BL/6 CD8+ T cells (0.5×106) and either WT donor BM or Igμ−/− donor BM (2.5×106). Recipients were monitored for clinical GVHD, including (A) body weight loss, (B) clinical cutaneous cGVHD score and (D) survival. A representative photograph taken at day 60 is shown (n=4).
Lethally irradiated BALB/c recipients were injected with 5×106 whole spleen cells and 2.5 ×106 TBCD-BM cells from C57BL/6 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) the following day after HCT. Recipients given TBCD-BM alone were used as controls. Recipients were monitored for clinical GVHD, including body weight change, clinical GVHD score, and survival (n=8 from two replicate experiments).
Lethally irradiated BALB/c recipients were injected with 1.25×106 whole spleen cells and 2.5 ×106 TBCD-BM cells from C57BL/6 donors and injected i.v. with either rat IgG or anti-CD20 mAb (40 mg/Kg) starting on day 45 after disease onset, with follow-up injections on day 50 and 55. Recipients were monitored for clinical GVHD, including body weight change, clinical cutaneous GVHD, and survival (n=4 from two replicate experiments).