Abstract
We used a ribozyme loss-of-function approach to demonstrate that the protein product of a cDNA encoding a multifunctional membrane-associated protein binds the seco-steroid 1,25(OH)2D3 and transduces its stimulatory effects on phosphate uptake. These results are paralleled by studies in which the ability of the hormone to stimulate phosphate uptake in isolated chick intestinal epithelial cells is abolished by preincubation with Ab099 directed against the amino terminus of the protein. We now report the complete sequence of the cloned chicken cDNA for the 1,25D3-MARRS (membrane-associated, rapid-response steroid-binding) protein and reveal it to be identical to the multifunctional protein ERp57. Functional studies showed that active ribozyme, but not a scrambled control, decreased specific membrane-associated 1,25(OH)2D3 binding, but did not affect binding to the nuclear receptor for 1,25(OH)2D3. Seco-steroid-dependent stimulation of protein kinase C activity was diminished as 1,25D3-MARRS protein levels were reduced in the presence of the ribozyme, as judged by Western blot analyses. Phosphate uptake in isolated cells is an index of intestinal phosphate transport that occurs during growth and maturation. Whereas cells and perfused duodena robustly responded to 1,25(OH)2D3 in preparations from young birds, older animals no longer responded with stimulated phosphate uptake or transport. The age-related decline was accompanied by a decrease in 1,25D3-MARRS mRNA that was apparent up to 1 year of age. Together, these studies functionally link phosphate transport in the chick duodenum with the 1,25D3-MARRS protein and point to a previously uncharacterized role for this multifunctional protein class.
Steroid hormones mediate classic, long-term, transcription-dependent events, as well as rapid, membrane-initiated signaling phenomena (1-4). In some systems, membrane-initiated steroid signaling is conducted through receptors that are structurally similar to ligand-activated nuclear transcription factors (5), whereas others use recently identified serpentine receptors (6).
The seco-steroid hormone, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], was postulated to act through a membrane-associated receptor to account for the rapid stimulation of calcium uptake in isolated rat intestinal epithelial cells (7), as well as calcium (8-10) and phosphate transport in perfused chick duodenum (11-12). A candidate protein was isolated from basolateral membrane preparations of chicken intestinal epithelium (8), and the N terminus was sequenced. In this article we report the cDNA sequence for the 1,25(OH)2D3 membrane-associated, rapid-response steroid-binding (1,25D3-MARRS) protein (previously named ERp57) and, through the use of ribozyme knockdown studies, its functional connection to phosphate uptake in intestinal cells. We also report the results of analyses of age-related 1,25D3-MARRS expression in chicks under conditions when phosphate transport is high (7 weeks) and low (14, 28, and 58 weeks). Of interest, the 1,25D3-MARRS/ERp57 protein belongs to a superfamily of multifunctional glucose-regulated and redox-sensitive proteins that have previously been implicated in binding thyroid hormones and estrogens (13, 14), in glycoprotein biosynthesis (15), and in immune responses (16). We suggest that the regulation of this protein's activity by 1,25(OH)2D3 provides a functional link between the seco-steroid and energy and ion metabolism.
Materials and Methods
Animals and Surgical Procedures. All protocols were approved by the Institutional Animal Care and Use Committee. White Leghorn chicks were obtained on the day of hatch and raised on standard diets (9, 10) for 7, 14, 28, or 58 weeks. On the day of experimentation, birds were anesthetized with chloropent (0.3 ml per 100 g of body weight). Detailed procedures for perfusion through the celiac artery with Gey's balanced salt solution (GBSS) containing vehicle or hormone, perfusion through the lumen with GBSS containing 2 μCi/ml (1 Ci = 37 GBq) H332PO4, and collection of the venous effluent to determine transport have been published (11).
For the preparation of isolated intestinal epithelial cells, the citrate chelation protocol was used (12, 17), with the exception that the media were adjusted to pH 5.0 to retain viability and morphology for extended periods (18).
Identification and Cloning of the 1,25D3-MARRS cDNA. The 1,25D3-MARRS protein was purified to homogeneity as described in ref. 8, by using specific [3H]1,25(OH)2D3 binding as the identification criterion. The N-terminal sequence obtained from the purified protein (SDVVELSDADFESGLAERPG) was back-translated with correction for chicken codon bias and compared to clones sequenced at the University of Delaware Technology Park [now deposited in the University of Delaware Chicken EST Project (www.chickest.udel.edu)]. A full-length cDNA, identical to that previously described as glucose-regulated thiol oxidoreductase protein precursor (GenBank accession no. AY122886/BI065250), was found.
RT-PCR of 1,25D3-MARRS mRNA. Duodenal mucosae from three birds per age group were collected by scraping and freezing in liquid nitrogen. On the day of use, total RNA was isolated with the TRIzol reagent (Sigma). For RT-PCR, 1 μg of total RNA was used with Ready-To-Go-PCR Beads (Amersham Biosciences). The primer for the 208-bp product in the forward direction was an 18-mer (5′-ACGAGGCCGAAGAGGGAG), and for the reverse direction 5′-AGAACTCCACGAGCACCAGT, a 20-mer was used. The forward and reverse primers for GAPDH (500-bp product) used were, respectively, 5′-ACGTGCAGCAGGAACACTA and 5′-CCTCTGTCATCTCTCCACA. Products were separated on 1.5% (wt/vol) agarose gels and stained with ethidium bromide. Band density was determined with an Alpha Imager (Alpha Innotech, San Leandro, CA).
Ribozyme Production. Sense and antisense single-stranded DNA coding for the active and scrambled ribozymes were manufactured (Sigma Genosys, The Woodlands, TX) with a 5′ SP6 promoter. The complementary single strands were annealed and transcribed in vitro, and ribozyme activity was analyzed against a synthetic 1,25D3-MARRS target as described (19). After demonstration of in vitro activity (data not shown), complementary sense and antisense strands of single stranded ribozyme DNA were manufactured (Sigma Genosys) with 5′ EcoRI and 3′ SpeI overhangs and without an SP6 promoter. The complementary single strands were annealed into a double-stranded oligo-nucleotide insert that was then ligated into the pU1ZeoEcoSpe vector plasmid by using EcoRI and SpeI directional ligation (19). Insert ligation was confirmed by using restriction enzyme mapping, PCR, and sequencing.
Tissue Culture. Cells from a single duodenum were collected by centrifugation (500 × g, 5 min) and resuspended in 40 ml of GBSS, and 0.2 ml was added to 35-mm plastic Petri dishes (Falcon, Fisher Scientific) with 3 ml of RPMI medium 1640, 100 units of penicillin, and 100 μg of streptomycin (Invitrogen-GIBCO) per ml of medium (18). The primary cultures were incubated overnight (37°C, 5% CO2/95% air) to allow adherence. The following morning, media were aspirated and each culture to be transfected was treated with 1 ml of medium containing 6 μl of Lipofectamine (Invitrogen) and 1 μg of the appropriate plasmid containing ribozyme cDNA. A complete description of ribozyme construction is given in Liu et al. (19). Five hours after transfection, an additional 4 ml of RPMI with 10% FBS, antibiotics, and 1.5 mM EDTA/25 mM Hepes (final concentration; ref. 18) was added to each dish. Incubation was continued for 22 h, and the cultures were assayed for phosphate uptake, specific binding of steroid hormone, protein levels by Western analyses, and protein kinase C (PKC) activity as described below.
Phosphate Uptake. Antiserum against the N-terminal sequence of the 1,25D3-MARRS protein (Ab 099) was generated by the multiple antigenic peptide format and extensively characterized (20). For inhibition studies with Ab 099, primary cultures were preincubated for 5 min (23°C) in 1 ml of GBSS/0.1% (wt/vol) BSA without or with antibody diluted 1:500. An additional ml of GBSS/0.1% BSA containing 4 μCi of H332PO4 (New England Nuclear) was then added, the incubation was continued for 7 min (12), and the adherent cells were rinsed with three 4-ml aliquots of ice-cold GBSS, followed by the addition of 0.5 ml of lysis buffer [0.1% (vol/vol) Triton X-100 in 10 mM Tris/1.5 mM EDTA/2 mM DTT, pH 7.4 (TED)] to each plate. Cells were collected by scraping and aspiration for determination of radioactivity and protein with the Bradford reagent (Bio-Rad). For studies with ribozymes, the preincubation step was omitted.
Specific Binding of [3H]1,25(OH)2D3. One hundred-microliter aliquots of the cell lysates used in the phosphate uptake studies were incubated in TED with 1 nM [3H]1,25(OH)2D3 (New England Nuclear) in the absence (total binding, performed in triplicate) or presence of a 200-fold molar excess of unlabeled 1,25(OH)2D3 (nonspecific binding, performed in triplicate) overnight on ice. For studies on membrane-associated binding (1,25D3-MARRS protein), bound and free ligand were separated by the perchloric acid precipitation method (9, 10), whereas for binding to the classic VDR, separation was by the hydroxylapatite method (9, 10).
PKC Determinations. After the transfection protocol, primary cell cultures were incubated with or without 130 pM 1,25(OH)2D3 for 1 min, the media were aspirated, and the cells were scraped and homogenized in 175 μl of double-distilled, deionized water. Enzyme activity was determined with 10 μl of the lysates and kits from Upstate Biotechnology (Lake Placid, NY) according to the manufacturer's instructions. In brief, lysates were incubated (30°C for 15 min) in the presence of peptide substrate (QKRPSQRSKYL), [γ-32]ATP, inhibitors of PKA and calmodulin kinase, calcium, phosphatidyl serine, diacylglycerol, and magnesium. Reaction mixture was spotted onto phosphocellulose paper and washed with phosphoric acid and acetone, and radioactivity was determined by liquid scintillation spectrophotometry. Activity was calculated relative to an aliquot of the substrate mixture.
Western Analyses. Aliquots of cell lysates (30 μg of protein per lane) from the PKC studies were separated on 8% (wt/vol) SDS-polyacrylamide gels and transferred to poly(vinylidene difluoride) membranes (Immobilon-P, Fisher Scientific). Western analyses with Ab 099 as primary antibody were performed as described (9, 10, 20).
Results and Discussion
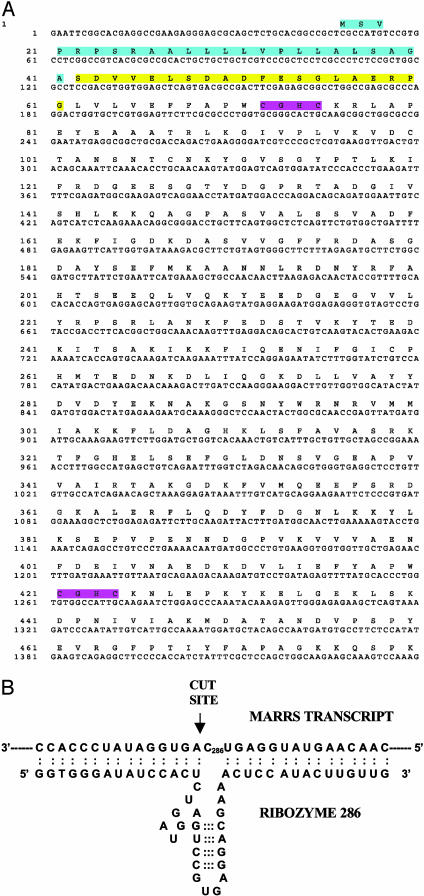
Fig. 1A provides the sequence of the chicken cDNA containing the 1,25-MARRS protein N-terminal sequence and its translated product. The sequence corresponds to a protein that has been alternatively identified as phospholipase C (PLC) α, but was later shown to have no phospholipase activity (21), or ERp57 (22). This, or a similar protein, has been implicated by others in estrogen action (14). Additionally, sequence similarities have been reported between PLCα and endoplasmic reticulum proteins [Erp60 (23, 24)], protein disulfide isomerase (25-27), which binds thyroid hormone (13), glucose-regulated protein (22), glycosylation site binding protein (15), and the scaffold protein, RACK1 (28). The full-length sequenced chicken clone was compared by using BLAST to sequences in the NCBI and Swiss-Prot databases and found to correspond to the 505-aa, 58-kDa, glucose-regulated protein GRP 58 (NCBI OMIM link *602046). It is surprisingly conserved among species, enough so that the antibody prepared against the N-terminal peptide in chick readily cross-reacts with the same protein in rat, mouse, and human cells. Transcription-translation of the chicken clone in vitro also produced a protein that is recognized by Ab 099, confirming the identity of the clone as ERp57, and thus antibody recognition cannot be attributed to a contaminant. Internal sequence from this clone was used to prepare the ribozyme. A complete discussion of bioinformatic analyses of the sequence and protein superfamily relationships will be the subject of a separate manuscript; however, it is of interest to note the presence of a signal peptide, two thioredoxin folds, and both nuclear (KAKKSKKK) and endoplasmic reticulum (KEDL) retention motifs. The highly conserved thioredoxin folds endow 1,25D3-MARRS with the unique ability to interact with a broad range of proteins by a redox mechanism that is conserved in numerous other multifunctional proteins. An example is protein disulfide isomerase, which also functions as the beta subunit of prolyl 4-hydroxylase, as a component of oligosaccharyl transferase, as thyroxine deiodinase, as glutathione-insulin transhydrogenase, and as a thyroid hormone binding protein (ExPASy, PROSITE, PDOC00172).
Fig. 1.
(A) Sequence of the full-length cDNA and translated polypeptide. Amino acids highlighted in blue correspond to the predicted signal peptide, and amino acids highlighted in yellow correspond to the N terminus of the purified protein recognized by antibody 099 and used for back translation and database searching. Consensus thioredoxin motifs are highlighted in purple. The transcript contains a long 3′ UTR after the KEDL termination sequence. (B) Schematic of targeted portion of MARRS transcript and site of ribozyme cleavage by MARRS 6 ribozyme.
Fig. 1B presents the structures of the catalytic sites and annealing arms for the hammerhead ribozyme, MARRS 6, used in these studies. MARRS 6 was shown in vitro to actively cleave the appropriate 1,25-MARRS sequence in a synthetic expressed target containing the target GUC sequence, whereas MARRS 8, containing a scrambled sequence, did not (data not shown).
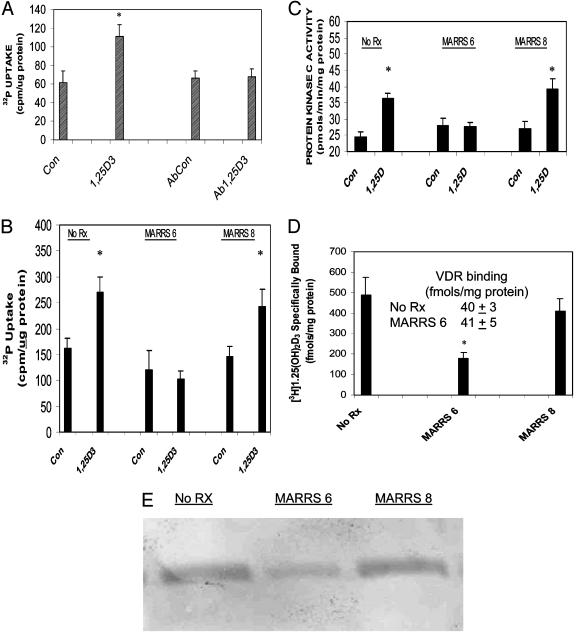
In the next series of experiments, we tested the functional involvement of 1,25D3-MARRS protein in mediating phosphate uptake by using two independent approaches. Fig. 2A depicts the effects of preincubating isolated enterocytes with Ab 099 before initiating phosphate uptake in the absence or presence of 130 pM 1,25(OH)2D3. After 7 min of incubation, the seco-steroid stimulated 32P accumulation to 182% of controls (P < 0.05). Whereas preincubation with Ab 099 had no effect on radionuclide uptake in controls, parallel incubations resulted in inhibition of 1,25(OH)2D3-stimulated uptake (Fig. 2 A). The Ab 099 dilution used in these experiments was previously shown to also inhibit steroid-mediated stimulation of PKC, whereas antibody 9A7, directed against the nuclear VDR, did not (20).
Fig. 2.
The 1,25D3-MARRS protein mediates phosphate uptake and PKC activation. Isolated intestinal epithelial cells were cultured overnight without serum. The following day (A), media were replaced with GBSS/0.1% BSA without or with Ab 099 (1:500 dilution) and incubated for 5 min (23°C). Each dish then received 1 ml of GBSS containing 4 μCi of H332PO4 and vehicle (Con and cells pretreated with antibody, AbCon) or 130 pM 1,25(OH)2D3 (final concentration; 1,25D3 and cells pretreated with antibody, Ab1,25D3) and incubated for an additional 7 min. (B-E) Cells were either not transfected (No Rx) or transfected with MARRS 6 or MARRS 8 ribozymes for 5 h before addition of serum and were assayed 22 h later. (B) Cells were incubated for 7 min for 32P uptake as in A but without the antibody preincubation. (C) Cells were incubated for 1 min with or without hormone in GBSS and then collected for PKC analyses. (D) Cell lysates were incubated with [3H]1,25(OH)2D3 in the absence or presence of excess unlabeled hormone. Bound and free steroid were separated by perchloric acid precipitation to determine 1,25D3-MARRS-protein-specific binding (D) or by hydroxylapatite to determine specific VDR binding (Inset). (E) Cell lysates (30 μg per lane) were separated on SDS/PAGE and blotted onto poly(vinylidene difluoride) membranes, and Western blot analyses were performed with Ab 099 (against the N terminus of the 1,25D3-MARRS protein) as primary antibody. Values are presented as mean ± SEM for 6-11 independent samples. *, P < 0.02, relative to corresponding controls.
In primary cultures of intestinal epithelial cells, the 1,25(OH)2D3 hormone elicited a stimulation in phosphate uptake that was 166% of controls (P < 0.01; Fig. 2B), and in cells transfected with MARRS 8 (control ribozyme), 1,25(OH)2D3 stimulated 32P uptake to 165% of corresponding controls (P < 0.02; Fig. 2B). In contrast, 1,25(OH)2D3 failed to stimulate phosphate uptake in cells transfected with active ribozyme, MARRS 6 (Fig. 2B). We have previously reported that PKC almost certainly mediates enhanced phosphate uptake and transport as judged by studies with phorbol ester (12) and dose-response analyses (10). As indicated in Fig. 2C, 1,25D3-MARRS protein mediates PKC stimulation. A 1-min treatment with 130 pM 1,25(OH)2D3 of cultured control cells or cells transfected with inactive MARRS 8 ribozyme, produced a 150% and 146% stimulation, respectively, of enzyme activity, which was abolished in cells transfected with MARRS 6 ribozyme (Fig. 2C). Fig. 2D compares results of experiments on whole-cell lysates performed to assess specific binding to either the 1,25D3-MARRS protein (bars) or the VDR (Inset). Transfection of cells with MARRS 6 ribozyme resulted in a significant (40%) decrease in specific binding relative to either untransfected cells or cells transfected with MARRS 8 ribozyme (P < 0.01 for both). In contrast, transfection with MARRS 6 ribozyme had no effect on specific binding to the VDR (Fig. 2D Inset). We have previously reported (9) that the affinity of 1,25D3-MARRS for ligand is similar to that of the VDR (Kd = 0.6 nM vs. 0.4 nM, respectively), although the membrane-associated protein is 6-10 times more abundant than the nuclear receptor. Fig. 2E depicts a representative Western blot analysis that illustrates the reduction in Ab 099 reactivity in cells transfected with MARRS 6 ribozyme compared to untreated cells and cultures transfected with MARRS 8 ribozyme.
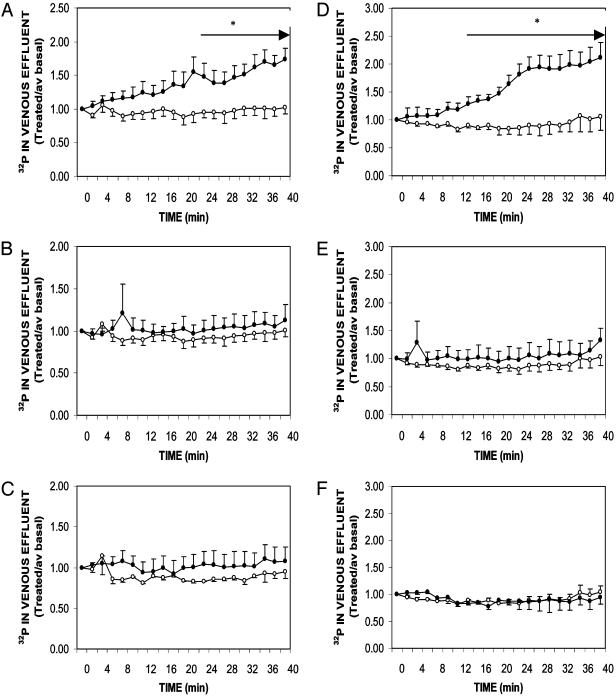
We have previously reported (12) that isolated intestinal epithelial cells respond to 130 pM 1,25(OH)2D3 with enhanced 32P uptake within 5-10 min, relative to corresponding controls. Moreover, hormone responsiveness was evident in cells prepared from young, growing chicks (7 weeks of age) but absent at 14 and 28 weeks of age (12). To validate that 32P uptake in isolated cells reflects transport, perfusion studies were undertaken with duodena from comparably aged male and female birds. As illustrated in Fig. 1, both 7-week-old males (Fig. 3A) and females (Fig. 3D) responded to arterial perfusion with hormone as indicated by enhanced phosphate transport that became significant in males at T = 22 min (P < 0.05, relative to corresponding controls) and in females at T = 12 min (P < 0.05, relative to corresponding controls). After 40 min of perfusion, steroid-stimulated phosphate transport achieved levels that were 170% of controls in males (Fig. 3A) and 200% of controls in females (Fig. 3D). Equivalent studies performed in 14- and 28-week-old males (Fig. 3 B and C, respectively) and 14- and 28-week-old females (Fig. 3 E and F, respectively) demonstrated a loss of 1,25(OH)2D3 responsiveness. Thus, uptake in isolated cells (12) reflects transport in the whole organ and validates the use of enterocytes in transfection studies with ribozymes directed against the 1,25D3-MARRS mRNA. Additionally, the observation that the steroid hormone rapidly stimulates phosphate transport in young animals, compared to mature animals, indicates that this is a physiologically important pathway during growth that supports bone mineralization. Thus, this membrane-associated protein (1,25D3-MARRS) and its signaling pathways may in turn present targets for therapeutic intervention in osteoporosis.
Fig. 3.
Effect of age and gender on the rapid, 1,25(OH)2D3-mediated stimulation of phosphate transport in perfused duodenal loops. Chickens were raised on a normal, vitamin D-replete diet for 7, 14, or 28 weeks before experimentation. Arterial perfusion with GBSS containing 0.125% (wt/vol) BSA and the vehicle ethanol [0.005% (vol/vol), final concentration] proceeded for a 20-min basal period, and samples were collected during the latter 10 min. At T = 0, arterial perfusion either continued with control media (open circles) or 130 pM 1,25(OH)2D3 (filled circles). The lumen was perfused with GBSS lacking bicarbonate and containing 2 μCi/ml of H332PO4. Transport was measured by the amount of radioactivity appearing in the venous effluent. Results are presented as mean ± SEM (or range) for 7-week-old males (A) (n = 4 controls and 4 treated), 14-week-old males (B) (n = 5 controls and 3 treated), 28-week-old males (C) (n = 2 controls and 4 treated), 7-week-old females (D)(n = 2 controls and 5 treated), 14-week-old females (E)(n = 3 controls and 4 treated), and 28-week-old females (F) (n = 2 controls and 5 treated). *, P < 0.05, relative to corresponding controls for times indicated by arrows.
By using the sequence information presented in Fig. 1, RT-PCR primers were designed to yield a 208-base product (see Materials and Methods) and used to assess the effects of aging on 1,25D3-MARRS mRNA levels (Fig. 4). When expressed as a ratio of MARRS:GAPDH mRNA levels, values of 1.28 ± 0.07, 0.91, and 0.95 ± 0.07 were observed in RNA prepared from 7-, 14-, and 58-week-old males, respectively, and values of 1.28 ± 0.07, 1.06 ± 0.06, 1.25 ± 0.1, and 1.12 ± 0.16 were observed in preparations from 7-, 14-, 28-, and 58-week-old females, respectively. In earlier work (9, 10) we found that 1,25D3-MARRS protein levels gradually declined with age (following the same trend as the message) but that the affinity abruptly decreased for both genders between 7 and 14 weeks of age. In contrast, no change in affinity was observed for the classic VDR in either males or females, and receptor levels remained constant in males with age but increased in females (9, 10).
Fig. 4.
Effect of age and gender on mRNA levels for 1,25D3-MARRS. Duodenal mucosae were collected from each of the indicated age groups and frozen in liquid nitrogen. Total RNA was prepared with the TRIzol reagent, and 1 μg was used for RT-PCR with primers specific for 1,25D3-MARRS protein or GAPDH. (Upper) Males. (Lower) Females. The ratios for MARRS/GAPDH are provide underneath each panel.
The combined data strongly support the hypothesis that the 1,25D3-MARRS protein is a critical component of the machinery responsible for mediating the rapid actions of the steroid hormone 1,25(OH)2D3 on phosphate uptake and transport and PKC stimulation in the intestine. In fact, the ribozyme loss-of-function studies reported here indicate that the seco-steroid hormone, 1,25(OH)2D3, regulates an energy central, redox-regulated function that integrates rapid intracellular signaling and phosphate transport in the intestine. This finding is highly significant because it ties together a number of previously published observations about the multifunctional nature of members of this protein superfamily and indicates that they are much more than high-capacity hormone reservoirs as previously suggested (13). The multifunctional nature of 1,25-MARRS and other members of this family has generated significant debate in the literature about their “true function.” We suggest that, in fact, these family members are not limited to a single function but rather possess protein-protein interaction domains in the thioredoxin folds that allow them to modulate the activity of multiprotein complexes such as those involved in glycosylation, ion transport, energy metabolism, antigen processing, and possibly even gene transcription. The new observations reported here, that the activity of this protein is integrally linked to the rapid actions of 1,25(OH)2D3 in the initiation of phosphate transport, indicate that steroid hormones regulate the activity of these multifunctional protein complexes. Present studies are ongoing to identify the binding site for 1,25(OH)2D3 and to identify binding partners involved in ion transport in the intestine, conceivably including the classic nuclear receptor.
Acknowledgments
We thank Drs. Riting Liu, Julia Barsony, and Daniel Carson for many helpful discussions and insightful comments regarding this work. This work was supported by National Research Initiative-Cooperative State Research, Education, and Extension Service-U.S. Department of Agriculture Grant 2004-35206-14134 (to I.N.), National Institutes of Health Grant 1S11AR48554-01 (to S.E.S.), and in part by the Utah Agricultural Experiment Station. This paper has been approved as the Utah Agricultural Experiment Station jounal paper no. 7579.
Abbreviation: GBSS, Gey's balanced salt solution.
References
- 1.Pietras, R. J., Nemere, I. & Szego, C. M. (2001) Endocrine 14, 417-427. [DOI] [PubMed] [Google Scholar]
- 2.Nemere, I., Pietras, R. J. & Blackmore, P. F. (2003) J. Cell. Biochem. 88, 438-445. [DOI] [PubMed] [Google Scholar]
- 3.Farach-Carson, M. C. & Nemere, I. (2003) Curr. Drug Targets 4, 67-76. [DOI] [PubMed] [Google Scholar]
- 4.Li, X. & O'Malley, B. W. (2003) J. Biol. Chem. 278, 39261-39264. [DOI] [PubMed] [Google Scholar]
- 5.Wyckoff, M. H., Chambliss, K. L., Mineao, C., Yuhanna, I. S., Mendelsohn, M. E., Mumby, S. M. & Shaul, P. W. (2001) J. Biol. Chem. 276, 27071-27076. [DOI] [PubMed] [Google Scholar]
- 6.Zhu, Y., Rice, C. D., Pang, Y., Pace, M. & Thomas, P. (2003) Proc. Natl. Acad. Sci. USA 100, 2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemere, I. & Szego, C. M. (1981) Endocrinology 108, 1450-1462. [DOI] [PubMed] [Google Scholar]
- 8.Nemere, I., Dormanen, M. C., Hammond, M. W., Okamura, W. H. & Norman, A. W. (1994) J. Biol. Chem. 269, 23750-23756. [PubMed] [Google Scholar]
- 9.Larsson, B. & Nemere, I. (2003) Endocrinology 144, 1726-1735. [DOI] [PubMed] [Google Scholar]
- 10.Larsson, B. & Nemere, I. (2003) J. Cell. Biochem. 90, 901-913. [DOI] [PubMed] [Google Scholar]
- 11.Nemere, I. (1996) Endocrinology 137, 2254-2261. [DOI] [PubMed] [Google Scholar]
- 12.Zhao, B. & Nemere, I. (2002) J. Cell. Biochem. 86, 497-508. [DOI] [PubMed] [Google Scholar]
- 13.Primm, T. P. & Gilbert, H. F. (2001) J. Biol. Chem. 276, 281-286. [DOI] [PubMed] [Google Scholar]
- 14.Mobbs, C. V., Kaplitt, M., Kow, L.-M. & Pfaff, D. W. (1991) Mol. Cell. Endocrinol. 80, C187-C191. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, H., Noiva, R., Mizunaga, T., Yamauchi, K., Horiuchi, R., Cheng, S. Y. & Lennarz, W. J. (1990) Biochem. Biophys. Res. Commun. 170, 1319-1324. [DOI] [PubMed] [Google Scholar]
- 16.Bouvier, M. (2003) Mol. Immunol. 39, 697-706. [DOI] [PubMed] [Google Scholar]
- 17.Nemere, I. & Campbell, K. (2000) Steroids 65, 451-457. [DOI] [PubMed] [Google Scholar]
- 18.Caldwell, D. J., Droleskey, R. E., Elissalde, M. H., Kogut, M. H., DeLoach, J. R. & Hargis, B. M. (1993) J. Tissue Cult. Methods 15, 15-18. [Google Scholar]
- 19.Liu, R., Li, W., Karin, N. J., Bergh, J. J., Adler-Storthz, K. & Farach-Carson, M. C. (2000) J. Biol. Chem. 275, 8711-8718. [DOI] [PubMed] [Google Scholar]
- 20.Nemere, I., Ray, R. & McManus, W. (2000) Am. J. Physiol. 278, E1104-E1114. [DOI] [PubMed] [Google Scholar]
- 21.Hirano, N., Shibasaki, F., Kato, H., Sakai, R., Tanaka, T., Nishida, J., Yazaki, Y., Takenawa, T. & Hirai, H. (1994) Biochem. Biophys. Res. Commun. 204, 375-382. [DOI] [PubMed] [Google Scholar]
- 22.Hirano, N., Shibasaki, F., Sakai, R., Tanaka, T., Nishida, J., Yazai, Y., Takenawa, T. & Hirai, H. (1995) Eur. J. Biochem. 234, 336-342. [DOI] [PubMed] [Google Scholar]
- 23.Mazzarella, R. A., Marcus, N., Haugejorden, S. M., Balcarek, J. M., Baldassare, J. J., Roy, B., Li, L.-j., Lee, A. S. & Green, M. (1994) Arch. Biochem. Biophys. 308, 454-460. [DOI] [PubMed] [Google Scholar]
- 24.Urade, R., Nasu, M., Moriyama, T., Wada, K. & Kito, M. (1992) J. Biol. Chem. 267, 15152-15159. [PubMed] [Google Scholar]
- 25.Srivastava, S. P., Chen, N., Liu, Y. & Holtzman, J. L. (1991) J. Biol. Chem. 266, 20337-20344. [PubMed] [Google Scholar]
- 26.Bonfils, C. (1998) Eur. J. Biochem. 254, 420-427. [DOI] [PubMed] [Google Scholar]
- 27.Ferraro, A., Altieri, F., Coppari, S., Eufemi, M., Chichiarelli, S. & Turano, C. (1999) J. Cell. Biochem. 72, 528-539. [DOI] [PubMed] [Google Scholar]
- 28.Cox, E. A., Bennin, D., Doan, A. T., O'Toole, T. & Huttenlocher, A. (2003) Mol. Biol. Cell 14, 658-669. [DOI] [PMC free article] [PubMed] [Google Scholar]