Abstract
Human immunodeficiency virus -1 (HIV-1) enters the brain early during infection and leads to severe neuronal damage and central nervous system impairment. HIV-1 envelope glycoprotein 120 (gp120), a neurotoxin, undergoes intracellular trafficking and transport across neurons; however mechanisms of gp120 trafficking in neurons are unclear. Our results show that mannose binding lectin (MBL) that binds to the N-linked mannose residues on gp120, participates in intravesicular packaging of gp120 in neuronal subcellular organelles and also in subcellular trafficking of these vesicles in neuronal cells. Perinuclear MBL:gp120 vesicular complexes were observed and MBL facilitated the subcellular trafficking of gp120 via the endoplasmic reticulum (ER) and Golgi vesicles. The functional carbohydrate recognition domain of MBL was required for perinuclear organization, distribution and subcellular trafficking of MBL:gp120 vesicular complexes. Nocodazole, an agent that depolymerizes the microtubule network, abolished the trafficking of MBL:gp120 vesicles, suggesting that these vesicular complexes were transported along the microtubule network. Live cell imaging confirmed the association of the MBL:gp120 complexes with dynamic subcellular vesicles that underwent trafficking in neuronal soma and along the neurites. Thus, our findings suggest that intracellular MBL mediates subcellular trafficking and transport of viral glycoproteins in a microtubule-dependent mechanism in the neurons.
Keywords: Mannose binding lectin, Subcellular trafficking, HIV-1 gp120, Microtubule associated protein-2, Neuronal transport
Introduction
Human mannose binding lectin (MBL) is a C-type (Ca 2+ dependent) mammalian lectin of the innate immune system, coded by the MBL2 gene and specifically synthesized in the liver in two forms, serum MBL (S-MBL) and intracellular MBL (I-MBL), encoded by a single mRNA (Ma, Y et al., 1997, Kilpatrick D.C., 2002, Garred P. et al., 2003; Turner M.W. et al., 2003). Both forms exist as homo-oligomers of 32 kDa units and three subunits form a “structural unit” (Kurata H., et al., 1994). Each subunit possesses an NH2-terminal region containing cysteines involved in interchain disulfide bond formation; a collagen-like domain rich in hydroxyproline; a neck region; and a carbohydrate recognition domain (CRD) with an amino acid sequence highly homologous to those of other C-type lectins ( Hoppe, H.K., et al., 1994). The N-terminus of MBL mediates the higher oligomerization of the subunits and is necessary for the interaction with the MBL-associated serine proteases (MASPs) (Thielens, N.M. et al., 2001; Wallis, R. et al., 1999). In addition, the N-terminus contains a signal peptide responsible for vesicle sorting. S-MBL activates complement through interaction with three novel MASPs (lectin pathway) (Fujita, T., 2002) and also mediates cytotoxicity (Ma,Y. et.al., 1997; Ma.Y.et al.,1999). I-MBL functions as a carrier lectin facilitating endoplasmic reticulum (ER)-to- Golgi Apparatus (GA) glycoprotein trafficking in human hepatoma cells (Nonaka, M. et al., 2007). The ER and GA comprise the first two steps in protein secretion, and vesicular carriers mediate a continuous flux of proteins and lipids between these compartments. The recognition of N-linked glycans by intracellular lectins plays an important role in folding, sorting and targeting of glycoproteins through the secretory pathway to various subcellular compartments (Helenius A. et al., 2004; J.D. Schrag J.D. et. al., 2003; Sitia, R. et al., 2003; Ohtsubo et al., 2006; Aebi M. et al., 2010); Morgan G.W. et al., 2013). Previous studies showed that MBL binds glycoprotein 120 (gp120), the envelope glycoprotein of human immunodeficiency virus-1 (HIV-1). Gp120 is extensively glycosylated, with N-linked complex and high mannose carbohydrates (Saifuddin M., et al., 2000; Hart M.L. et al., 2002, Nonaka M., 2011). The envelope plays a critical role in HIV-1 infection because it mediates HIV binding to the target cell surface and subsequent fusion of the cellular and viral membranes.
Although, the neurons do not undergo active HIV-1 infection, several studies have shown that gp120 is internalized in neurons via chemokine receptors such as CXCR4, and gp120 is neurotoxic and induces neuronal apoptosis (Bachis A., et al., 2003; Bachis A et al., 2006).
Our lab has shown an increased MBL expression in the HIV-1 infected brain, specifically in the neuronal axons of HIV encephalitis brain suggesting that MBL could cause neuronal injury via the MBL complement activation pathway (Singh K.K. et al., 2011). In the current manuscript, we show that MBL bound gp120 complexes (MBL:gp120) were associated with perinuclear vesicles that underwent trafficking from ER-to-GA in neuronal cells. A functional CRD was essential for the subcellular localization and trafficking of MBL:gp120 vesicular complexes along neurites in a microtubule-dependent fashion using microtubule associated protein-2 (MAP2). These studies were further extended to human primary neurons where depolymerization of microtubules also impaired vesicular transport along neurites. Finally, live cell imaging showed that MBL:gp120 complexes were associated with subcellular vesicles and the dynamic vesicles that underwent trafficking along neurites.
Materials and methods
Antibodies and reagents
Polyclonal rabbit anti-MBL2 antibody was obtained from Sigma-Aldrich (St. Louis, MI). Goat polyclonal antibodies specific for gp120 were obtained from Santa-Cruz Inc. (Dallas, TX) and Fitzgerald Industries International (Acton, MA). Mouse monoclonal antibodies against p230 trans Golgi and Sec 31A were purchased from BD Biosciences (San Jose, CA). Rabbit polyclonal Sec31A antibody was from Novus Biologicals (Littleton, CO). Rabbit polyclonal ERp57 antibody was from Genetex (Irvine, CA). Sec 13 rabbit polyclonal antibody was purchased from Proteintech Group Inc. (Chicago, IL). GFP antibody was purchased from Cell Signaling Technologies (Boston, MA) and Clontech (Mountain View, CA). Monoclonal antibodies against MAP2 and TAU were obtained from Covance (San Diego, CA) and Millipore (Billerica, MA), respectively. All Alexa-conjugated secondary antibodies were purchased from Life Technologies (Carlsbad, CA). HIV-1 IIIB Gp120 (Reagent #11784) and mouse monoclonal antibodies to HIV-1 gp120 (Reagent #2343) (Dickey, C et al.2000; Gomen-Roman, VR et al., 2002) were obtained through NIH AIDS Research and Reference Reagent Program (Germantown, MD).
Plasmids
WT MBL-EGFP plasmid was kindly provided by Toshisuke Kawasaki, Japan. CS2 (C236S/C244S) MBL-EGFP was constructed using Site Direct Mutagenesis kit by mutating WT MBL-EGFP. pSyn JRFL gp120 plasmid (Reagent #4598) (Andre S et al., 1998; Haas J, 1996) was obtained from the NIH AIDS Research and Reference Reagent Program (Germantown, MD).
Cell culture, transfection and imaging
Human neuroblastoma SK-N-SH (HTB-11) and SH-SY5Y (CRL-2266) cell lines and EMEM or EMEM /Ham’s F12 (1:1) medium supplemented with 10% fetal bovine serum were purchased from ATCC (Manassas, VA). Neuroblastoma cell lines purchased from ATCC were used because their rate of transfection was greater than primary neurons and also because SH-SY5Y is considered a human derived cell line and an in vitro neuronal model. For human primary neurons (HPNs), forebrain fetal tissue was acquired from Advanced Bioscience Resources (Alameda, CA) according to the University of California San Diego Internal Review Board guidelines. HPNs were used to confirm that results obtained in neuroblastoma SK-N-SH and SH-SY5Y cells were relevant for human brain. The tissue was processed as previously described (Avramut et al., 2001). Cells were seeded at106 cells per well on glass coverslips coated with poly-ornithine and laminin in 24-well plates and maintained in neurobasal media supplemented with B27, 2mM Glutamax, 10 μg/mL gentamycin sulphate (all purchased from Life Technologies Inc., Carlsbad, CA) for 4 weeks with media-change every 3 days. Cells were fixed with 4% paraformaldehyde with sucrose in PBS and permeabilized with 0.25% Triton X-100. SK-N-SH and SH-SY5Y cells were transfected with Mirus Trans IT@-LT1 (Mirus Bio LLC, Madison, WI) and Lipofectamine LTX and plus reagent (Life Technologies Inc., Carlsbad, CA). For immunofluorescence experiments, cells were fixed and incubated with the appropriate primary antibodies followed by Alexa Fluor-conjugated secondary antibodies. Images were obtained using Perkin Elmer Ultra VIEW Vox-Spinning Disk Confocal laser-scanning microscope equipped with a 60X oil-immersion objective. All experiments were replicated three times.
Immunoprecipitation and western blot analysis
For immunoprecipitation and immunobloting, confluent cells were solubilized in 1% Nonidet P-40 buffer supplemented with 5 nM CaCl2. Cell extracts were clarified by centrifugation for 10 min at 12 000 rpm, and the protein concentrations in the supernatants were determined using the Bradford Assay (Bio-Rad Laboratories, Hercules, CA). The extracts were incubated over-night at 4°C with primary antibodies, followed by 1h incubation with magnetic beads A/G (Pierce Classic Magnetic IP/CO-IP kit, Thermo Fisher Scientific, Rockford, IL). The resultant immunoprecipitates were washed with the immunoprecipitation buffer, separated on SDS-PAGE and transferred to nitrocellulose membranes using I-Blot Western Blotting System (Life Technologies Inc., Carlsbad, CA). The membranes were blocked with 5% BSA in PBS with 0.1% Tween 20. Subsequently, the membranes were probed with primary antibodies followed by a Novex Western Breeze Immunodetection kit (Life Technologies Inc., Carlsbad, CA).
Silencing of MBL2 expression in SK-N-SH cell line
To down-regulate MBL2 expression in SK-N-SH cell line, cells were grown at confluence of 70-80% before transduction with MISSION shRNA Lentiviral Transduction Particles (Sigma-Aldrich, St. Louis, MI). As a control, MISSION sh RNA Non-target Control Transduction Particles were used.
Time lapse live cell imaging
For live cell imaging studies, instead of SK-N-SH cells, we used differentiated SH-SY5Y cells as these are considered a good model for neuronal studies in human brain (Aqholme L. et al., 2010). SH-SY5Y cells were plated on ibidi optical dishes (ibidi LLC., Verona, WI) and after 3 days in culture, the media were supplemented with 1 μg/mL retinoic acid (Sigma-Aldrich, St. Louis, MI) for 5-7 days to induce differentiation. As soon as the neurite network was observed, cells were transfected with WT MBL EGFP or CS2 MBL EGFP for 36 hrs. Cells were imaged post transfection in Live Cell Imaging media (Life Technologies Inc., Carlsbad, CA) and maintained at 37°C. Imaging was performed on a Perkin Elmer UltraView Spinning Disk Confocal microscope, with image acquisition by Volocity Software. Time lapse microscopy was acquired at a rate of 1 time point per 3 seconds for 10 min.
Results
Subcellular localization of neuronal MBL at perinuclear vesicular sites requires a functional carbohydrate recognition domain (CRD)
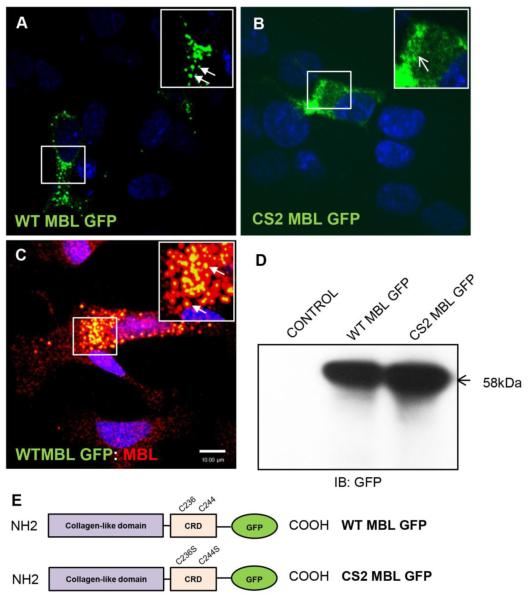
To study the distribution of MBL in neuronal cells, fluorescent MBL-fusion proteins, WT MBL-EGFP and mutant CS2 MBL-EGFP were used. Using a neuroblastoma cell line as a neuronal cell model (SK-N-SH) and confocal microscopy, transient expression of WT MBLEGFP revealed a punctate perinuclear vesicular localization (Figure 1A). In contrast, expression of a double mutant CS2 MBL-EGFP (C236S/C244S) that lacks the ability to recognize carbohydrate revealed a diffuse cytoplasmic localization, indicating that a functional CRD was required for the native subcellular perinuclear localization of MBL in neurons (Figure 1B). In order to test if exogenously expressed MBL-EGFP was functional and localized in the same way as endogenous MBL, we transfected the cells with WT MBL-EGFP plasmid and also stained the cells for endogenous MBL. The confocal laser scanning microscopy showed that indeed the WT MBL-EGFP displayed similar localization as the endogenous MBL suggesting that exogenously expressed protein showed similar localization and was functional in neuronal cells (Figure 1C). Because intracellular vesicles may contain multiple proteases, it was investigated whether the WT MBL-EGFP and CS2 MBL-EGFP remained intact in these vesicles by western blot analysis. As expected, anti-GFP antibody recognized a 58 kDa band equivalent to the size of MBL monomer (32 kDa) merged with EGFP (26 kDa) (Figure 1D).
Figure 1. Subcellular localization of neuronal MBL at perinuclear vesicles requires functional carbohydrate recognition domain.
A, B, C, Confocal laser scanning microscopy analysis of SK-N-SH cells transfected with WT MBL-EGFP (A) or CS2 MBL-EGFP plasmid (B) or simultaneous expressing endogenous MBL and exogenously transfected WT MBL-EGFP (C). A, WT MBL-EGFP is localized at perinuclear vesicles (closed arrows). B, CS2 MBL-EGFP is diffusely distributed in the cytoplasm (open arrows). C, Functional co-localization of endogenous MBL with exogenously expressed WT MBL-EGFP (closed arrows, yellow indicates co-localization). SK-N-SH cells were immunostained for endogenous MBL (red) followed by corresponding Alexa Fluor secondary antibodies. GFP (green) was observed by its own fluorescence. DAPI (blue) stained nuclei. D, Western blot analysis of untransfected control cells and WT and CS2 MBL-EGFP expressing SK-N-SH cell lysates. E, Schematic representation of the domain structures of WT MBL-EGFP and CS2 MBL-EGFP: Collagen-Like Domain; Carbohydrate Recognition Domain (CRD). The two cysteine residues C236 and C244 in CRD domain were subjected to mutagenesis (WT MBL-EGFP panel). Double point mutation is displayed in the CRD of MBL as C236S and C244S (CS2 MBL-EGFP panel). Scale bar, 10 μm.
MBL interaction with HIV-1 gp120 and localization at perinuclear vesicles requires an active CRD
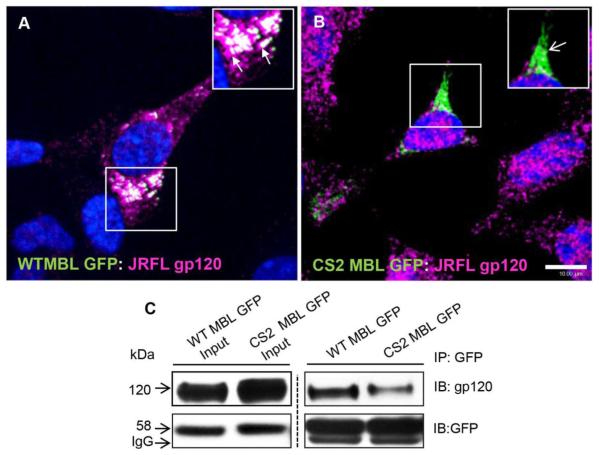
After determining the neuronal expression and subcellular localization of MBL in neurons, we checked if MBL:HIV-1 gp120 complex was processed likewise. SK-N-SH cells were transfected with WT/CS2 MBL-EGFP and JRFL gp120 plasmids as described above. We found that WT MBL-EGFP (Figure 2A), but not the mutant CS2 MBL-EGFP (Figure 2B) co-localized with gp120 at perinuclear vesicles, suggesting that the loss of active CRD impaired their subcellular localization.
Figure 2. MBL interacts and co-localizes with JRFL gp120 in perinuclear vesicles.
Confocal laser scan microscopy analysis of subcellular localization of WT MBL-EGFP (A) or Mut CS2 MBL-EGFP (B) with JRFL gp120. SK-N-SH cells for gp120 (magenta) followed by corresponding secondary antibodies and MBL WT and CS2 constructs were visualized by fluorescence of GFP (green). WT MBL-EGFP (closed arrows), (A) but not Mut CS2 MBL EGFP (open arrows) (B) co-localized with JRFL gp120 at perinuclear vesicles. White indicates co-localization. DAPI (blue) stained the nuclei. Scale bar, 10 μm. C, CRD-dependent subcellular interaction of MBL with gp120. SK-N-SH cells were co-transfected with WT MBL-EGFP or CS2 MBL-EGFP and JRFL gp120 plasmids with Mirus Trans IT-LT1. 36h post-transfection cells were solubilized with 1% NP40 lysis buffer in the presence of Ca2+ and immunoprecipitated with anti-GFP polyclonal antibody. The immunoblots were probed with antibodies against gp120 and GFP respectively.
Furthermore, to assess whether MBL binds to the HIV-1 gp120 via N-glycan mannose residues, and if this MBL:gp120 interaction is dependent on functional CRD, we co-transfected HEK 293T cells (that produce large amounts of proteins) with WT/CS2 MBL-EGFP and JRFL gp120 plasmids and confirmed their complex formation by immunoprecipitation and immunoblot (Figure 2C). These results showed that WT MBL EGFP was associated with clearly defined subcellular perinuclear vesicles; however mutant MBL with non-functional CRD displayed diffuse distribution in the cytoplasm. So, the presence of a functional MBL facilitated subcellular packaging of gp120 while a mutant MBL with non-functional CRD, impaired that function.
MBL:gp120 complexes are localized at subcellular perinuclear compartments in the ER and Golgi Apparatus
Subsequent to understanding the role of functional CRD in MBL:gp120 interaction and localization in perinuclear vesicles, we characterized these subcellular vesicles.
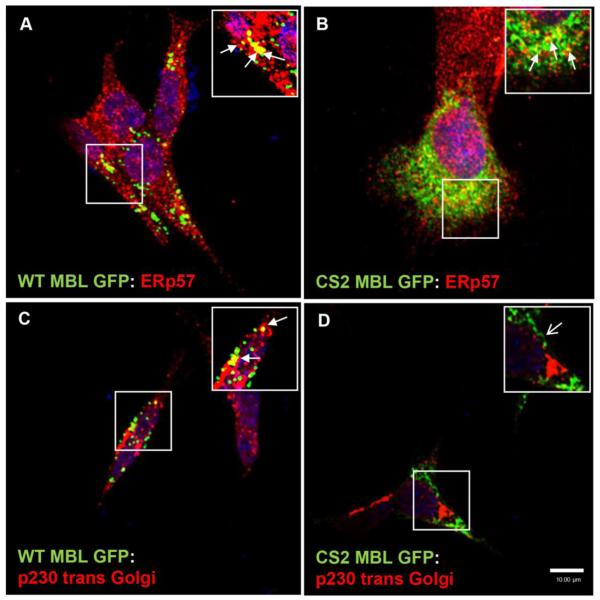
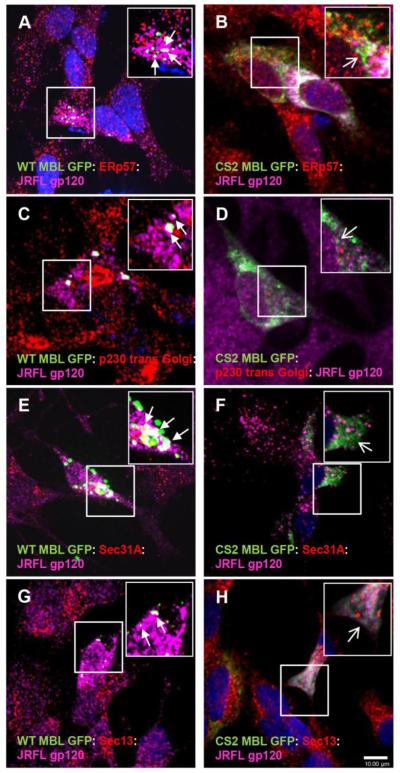
First, we found that WT MBL-EGFP was localized with both ER (ERp57) and Golgi (p230 trans-Golgi) markers (Figure 3A, C). In contrast, CS2 MBL EGFP was only detected in ER (Figure 3B) but not in Golgi (Figure 3D). After the co-transfection of SK-N-SH neuroblastoma cells with JRFL gp120 and WT-MBL-EGFP or CS2 MBL-EGFP plasmids we observed that WT MBL-EGFP (Figure 4A, C) but not CS2 MBL-EGFP (Figure 4B, D) co-localized with gp120 in ER and Golgi suggesting that MBL may function as a lectin carrier for intracellular trafficking of gp120 cargo in neurons. A small fraction of CS2 MBL-EGFP appears to co-localize with JRFL gp120 in ER and this could account for the weak CS2 MBL-EGFP interaction with gp120 that was seen by co-immunoprecipitation (Figure 2C). Lectins have been shown earlier to transport neuronal proteins based on their sugar binding activity (Borges L.F., 1982; Yamashitha K. et al., 1999). Moreover, in an early study in HIV-infected cells, the majority of gp160 protein (precursor protein for gp120 and gp41) was found in ER (Willey, R.L. et al., 1988; Fennie, C. et al., 1989; Hallenberger, S. et al., 1993). Therefore, we postulated that the intracellular function of MBL as “ER resident” may include the intracellular transport of glycoproteins, such as gp120.
Figure 3. MBL accumulates and localizes in ER and Golgi vesicles.
A and C, Confocal laser scanning microscopy analysis of the subcellular co-localization of WT MBL EGFP with ER marker (ERp57) and Golgi marker (p230 trans Golgi), (closed arrows; yellow indicates co-localization) in SK-N-SH cells. B, Confocal laser scanning microscopic analysis of subcellular co-localization of CS2 MBL EGFP with ER marker (closed arrows; yellow indicates co-localization). D, Diffused distribution of CS2 MBL EGFP in the cytoplasm and absence of CS2 MBL-EGFP localization in Golgi (open arrow). WT and CS2 MBL-EGFP (green) expressing cells were stained with an ER marker, Erp57 (red) or Golgi marker p230 trans Golgi (red), followed by corresponding Alexa Fluor secondary antibodies. DAPI (blue) stained nuclei. Scale bar, 10 μm.
Figure 4. Functional co-localization of MBL:gp120 complexes with COPII transport vesicles markers.
A,C,E,G Functional co-localization of WT MBL EGFP (green) and JRFL gp120 (magenta) with ER marker (red), Golgi marker (red) and COPII transport vesicles markers (red): Sec 31A and Sec 13 (closed arrows, white indicates co-localization of three markers) in SK-N-SH cells. B, Localization of CS2 MBL (green) and JRFL gp120 (magenta) in ER (red) (open arrows). D, F, H Localization of CS2 MBL EGFP (green) and JRFL gp120 (magenta) with COP II transport vesicles: Sec 31A or Sec 13 (red) (open arrows). DAPI (blue) stained nuclei. Scale bar, 10 μm.
Functional co-localization of MBL:gp120 complexes with intracellular transport vesicles in neuronal cells
Considering the subcellular perinuclear localization of MBL alone and in association with gp120 we speculated that MBL could serve as a carrier lectin for gp120 cargo from ER to Golgi using subcellular transport vesicles. COPI- and COPII-coated vesicles are transport intermediates of the secretory pathways. COPII vesicles emerge from the ER in order to export newly synthesized secretory proteins towards the Golgi (Nickel W.et al., 2002). Because COPII vesicles (Sec31A) and COPI vesicles (β-COP) are the main transport vesicles bearing anterograde and retrograde-targeted cargos respectively in the ER and Golgi compartments and considering that MBL is localized in a perinuclear compartment in vesicular like-structures, it seemed likely that MBL’s CRD function was associated with ER-to-Golgi transport of gp120. To test this, we co-transfected cells with WT/CS2 MBL-EGFP and JRFL gp120 plasmids and immunostained with markers for transport vesicles. Similar to above observations, MBL accumulated as distinctive puncta in the perinuclear region. A high degree of localization of MBL:gp120 with COPII transport vesicles Sec31A was observed (Figure 4E). Moreover, MBL:gp120 complexes were present along with Sec 13, an ER exit marker for COPII budding vesicles (Figure 4G). In contrast, CS2 MBL-EGFP not only displayed a diffuse cytoplasmic localization but also a lack of association with the transport or exit vesicle markers (Figure 4F, 4H). We also investigated the association of WT/CS2 MBL-EGFP and JRFL gp120 with COPI Golgi-ER retrograde vesicles such as β-COP but no co-localization was observed (data not shown). Taken together, these data suggested that MBL:gp120 complexes were present at the ER exit sites and in ER-to-Golgi COPII vesicles and that MBL was associated with the subcellular anterograde transport of gp120 from ER to Golgi but not the retrograde transport from Golgi to ER.
MBL:gp120 vesicular complexes are transported along neuronal microtubules
HIV-1 gp120 gets internalized and transported in anterograde and retrograde directions in the nervous system and exacerbates the pathogenesis of HIV-1 via axonal transport (Ahmed F. et al, 2009; Bachis A. et al., 2006). Interestingly, gp120 was observed around synaptic vesicles (Bachis et al. 2006) in association with dendrites and axonal microtubules; microtubules being essential for intracellular transport of pro-survival proteins in both axons and dendrites. In addition, axonal transport in neurons necessitates the most rapid and long mitochondrial movements along microtubules for maintaining local ATP levels at the synapse (Boldogh I.R. and Pon L.A., 2007; Chen H. and Chan D.C., 2009; Mocchetti I. et al., 2012). MAP2 (microtubule associated protein 2), expressed in neuronal soma and dendrites interacts with microtubules, neurofilaments and actin filaments and is involved in microtubule assembly. As such, it serves as a marker of the integrity of the neuronal dendritic network (Ellis R., 2007). An unresolved issue in the neuronal transport of internalized gp120 is the identity of the “carrier” molecule (s) that helps transporting gp120 along the microtubules.
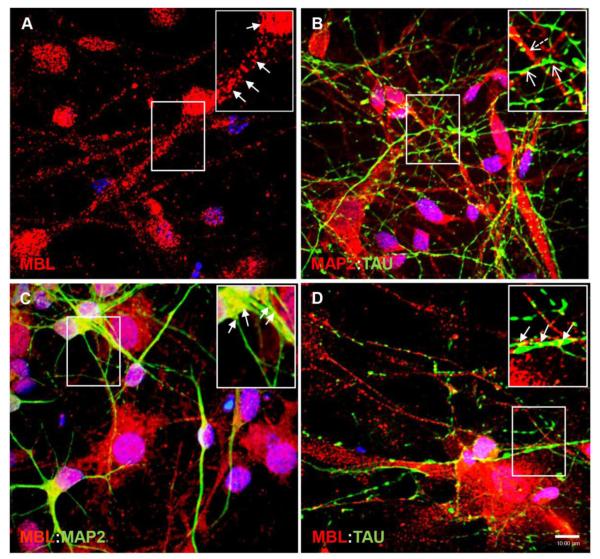
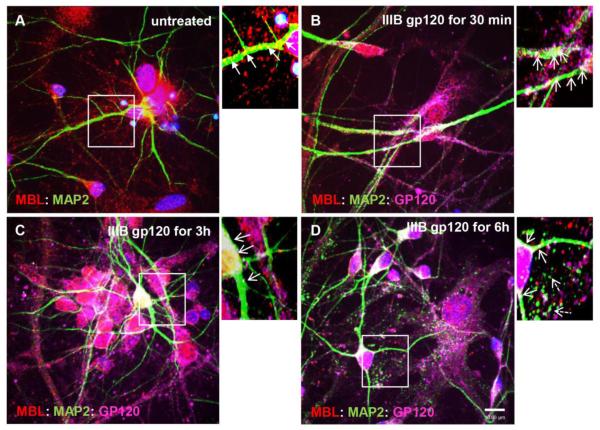
To validate neuroblastoma cell model in the human neurons, we investigated if MBL:gp120 vesicular complexes underwent similar trafficking in human primary neurons (HPNs). HPNs selected on neurobasal media from a primary neuroglial culture were kept in culture for two weeks to differentiate and were stained for MBL (Figure 5A) in combination with two neuronal makers: somatodendritic marker MAP2 (Figure 5C) and axonal marker TAU (Figure 5D). Similar to SK-N-SH neuroblastoma cells, endogenous MBL had vesicular perinuclear localization in the neuronal soma as well as a clear dendritic (Figure 5C) and axonal punctate localization along the neurites (Figure 5D). Co-staining with MAP2 and TAU confirmed that HPNs were highly homogenous containing more than 90% neurons (Figure 5B). Furthermore, to determine the association of MBL:gp120 complexes with microtubules, HPNs were exposed to IIIB gp120 (5 nM) for 30min to 6h and neurons were immunostained and visualized by confocal microscopy. Untreated neurons showed MBL association with MAP2 in neuronal soma and along the neurites (Figure 6A). On 30min IIIB gp120 treatment, MBL:MAP2 and newly formed MBL:gp120:MAP2 complexes were present in neuronal soma and neurites (Figure 6B) suggesting that MBL binds gp120 and travels along microtubules in HPNs. By 3h and 6h treatment of HPNs with IIIB gp120, MBL:gp120:MAP2 complexes were present more abundantly in neuronal soma than in neurites and ensuing degradation of the microtubule network was observed (Figure 6C, 6D). Considering that microtubules are essential for the intracellular transport in axons and dendrites, these results suggested a potential mechanism of MBL mediated gp120 neuronal transport and showed that gp120 mediates neuronal damage via MAP2 degradation.
Figure 5. MBL is associated with somatodendritic marker MAP2 and axonal marker TAU in human primary neurons.
A,B,C,D, Human primary neurons grown for two weeks in neurobasal selection media were stained for endogenous MBL (red) (A), MAP2 (green) (C), or TAU (green), (D). A, Localization of endogenous MBL in neuronal soma and neurites. B, Co-staining with MAP2 (Dashed open arrows) and TAU (open arrows). C, D, Endogenous MBL co-localizes with MAP2 and TAU (closed arrows; yellow indicates co-localization). DAPI (blue) stained nuclei. Scale bar, 10 μm.
Figure 6. IIIB gp120 co-localizes with MBL and MAP2 and is transported along the microtubules in human primary neurons.
Human primary neurons were untreated (A) or treated with IIIB gp120 (5 nM) for 30min (B), 3h (C) and 6h (D). Neurons were fixed and stained for MBL (red), MAP2 (green), gp120 (magenta) and DAPI for nuclei (blue). Localization of MBL:MAP2 (closed arrows; yellow indicates co-localization) and MBL:MAP2:gp120 complexes (open arrows; white indicates co-localization) in neuronal soma and neurites. Dashed open arrows indicate fragmentation of the microtubules (D). Scale bar, 10 μm.
Depolymerization of microtubules prevents MBL:gp120 vesicle trafficking along the neurites
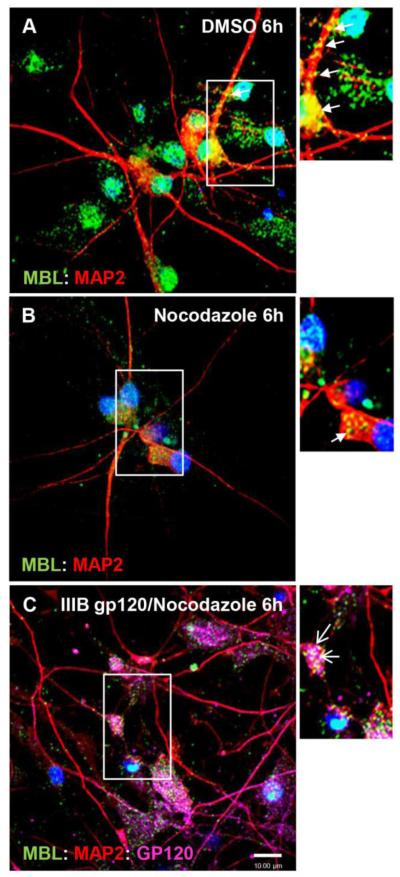
HIV-1 gp120-mediated neuronal degeneration can be prevented by nocodazole (Bachis et.al, 2006), an inhibitor of fast axonal transport (Samson et al., 1976) and an agent that depolymerizes the microtubule network (Cid-Arrequi et al.,1995) indicating that trafficking of gp120 along axons is crucial for its neurotoxic effects. In contrast, the gp120 mediated degeneration of dendrites occurred without significant apoptosis (Iskander et al., 2004). Here, we determined the effect of nocodazole treatment on MBL:gp120 vesicle transport hypothesizing that depolymerization of microtubule network will impair MBL:gp120 trafficking along the neurites. HPNs were exposed to IIIB gp120 (5 nM) alone for 6h or in combination with nocodazole (5 μg/ml). In control HPNs, untreated with nocodazole, MBL was co-localized with MAP2 in the neuronal soma and in the neurites (Figure 7A) while, in nocodazole treated cells for 6h, most of the MBL:MAP2 complexes were present in the neuronal soma around the nucleus (Figure 7B). Nocodazole mediated depolymerization of microtubules impaired the transport of MBL:gp120 vesicles along the neurites leading to their accumulation in the neuronal soma (Figure 7C).
Figure 7. Nocodazole blocks MBL:gp120 traffic in human primary neurons.
Human primary neurons were untreated (A) or treated with nocodazole (5 μg/ml) (B) or nocodazole plus IIIB gp120 (5 nM) for 6h. Nocodazole was added 15min before IIIB gp120 was added. Neurons were fixed, and stained for MBL (green), MAP2 (red), gp120 (magenta) and DAPI (blue). A,B, Localization of MBL:MAP2 complexes (closed arrows; yellow indicates co-localization) in neurites and neuronal soma. C, Localization of MBL:MAP2:gp120 complexes predominantly in neuronal soma only (open arrows; white indicates co-localization). Scale bar, 10 μm.
Silencing of MBL impairs trafficking of MBL:gp120 vesicular complexes along microtubules
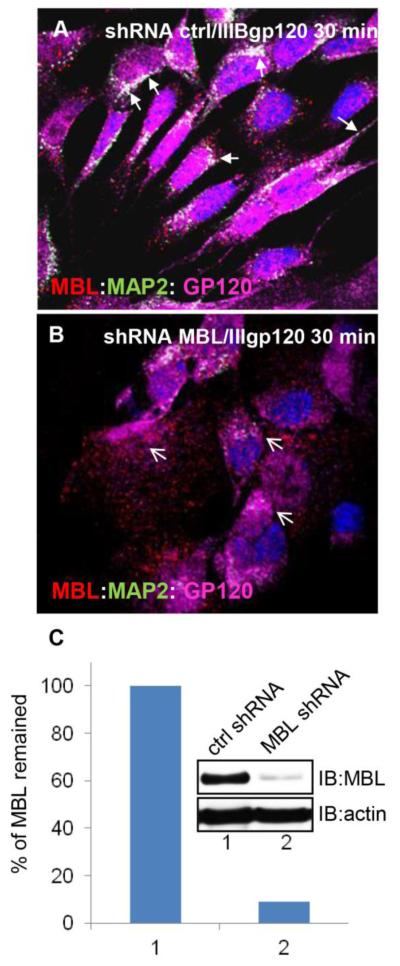
To further assess the role of MBL in the gp120 trafficking along the microtubules, we used MBL specific MISSION transduction particles (Sigma-Aldrich, St. Louis, MI) to silence endogenous MBL expression in SK-N-SH cells. In cells treated with 5 nM IIIB gp120 for 30min in the presence of control shRNA, MBL co-localized and associated with gp120 and MAP2 (Figure 8A). In contrast, in cells treated with 5nM IIIB gp120 and MBL shRNA, the association of MBL with gp120 and MAP2 was impaired (Figure 8B). MBL2 shRNA inhibited about 90% of MBL expression in SK-N-SH cells (Figure 8C).
Figure 8. Silencing of MBL expression impaired gp120 trafficking along the neurites.
SKN-SH cells treated with shRNA control (ctrl) (A) or shRNA MBL (B) in the presence of IIIB gp120 for 30min, were fixed and analyzed with laser scanning confocal microscopy. A, Localization and presence of MBL (red):MAP2 (green):gp120 (magenta) complexes in soma and processes of SK-N-SH cells (closed arrows; white indicates co-localization). B, Loss of MBL:MAP2:gp120 complex formation (open arrows). DAPI (blue) stains nuclei. Scale bar, 10 μm. C, Percentage of MBL remained in SK-N-SH cells based on the western blot analyses after MBL2 silencing.
Live cell imaging shows dynamic MBL:gp120 vesicle trafficking along neurites
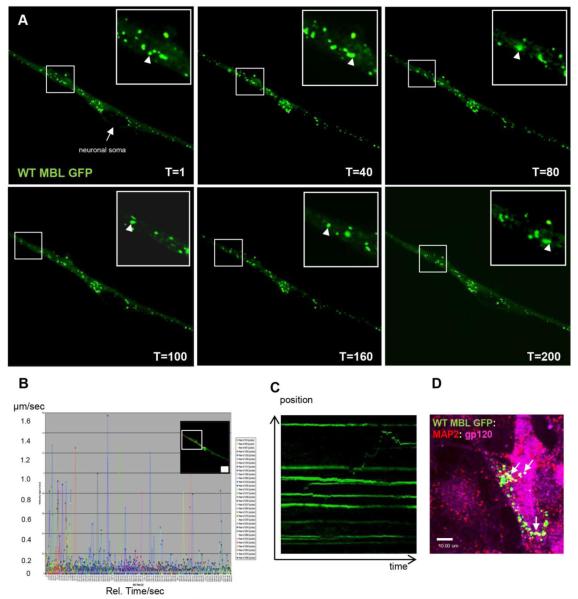
To determine if MBL was associated with dynamic intracellular vesicles and played a role in intracellular trafficking and the transport along the neurites, we used SH-SY5Y cells that were differentiated with retinoic acid for 5-7 days until neuronal network was observed. Cells were transfected with WT MBL-EGFP for 36h, treated for 30min with 5 nM IIIB gp120 soluble protein followed by live cell imaging. Using time lapse confocal microscopy, cells were visualized every 3 seconds for 10min (Figure 9A). Acquired snapshots of specific time points of time lapse microscopy showed specific anterograde vesicle movement away from neuronal soma (T=1 to T=100) followed by retrograde vesicle movement toward the neuronal soma (T=100 to T=200) as indicated by arrowheads. A more detailed analysis was conducted to detect individual vesicle velocity by live cell tracking using Volocity software (Perkin Elmer) analysis for all time points. A histogram was generated (Figure 9B) for all the frames that were acquired showing that a significant population of MBL:gp120 associated intracellular vesicles moved with a velocity of 0.4 - 1.6 μm/sec which is defined as a dynamic vesicle category or fast vesicle transport; another group of vesicles moved with a velocity of 0.01- 0.1 μm/sec that are designated slow vesicle transport (Lasek et al., 1984). An automatically generated kymograph showed the vesicle movement in both anterograde and retrograde directions (Figure 9C). Similar to the neuronal immunofluorescence data, we observed that MBL not only localized in variable sized subcellular vesicles in the perinuclear area but also along the neurites. A population of these cells was fixed and immunostained with specific antibodies to confirm the immunoreactivity and co-localization of MBL:gp120 complexes in the studied vesicles (Figure 9D).
Figure 9. Time lapse live cell imaging shows MBL:gp120 trafficking in subcellular dynamic vesicles.
A, Time lapse microscopy of differentiated SH-SY5Y cells transfected with WT MBLEGFP (green) and treated with 5nM IIIB gp120 for 30min. Time lapse was acquired as one frame every 3 seconds for 10min. Arrowhead indicates anterograde (away from neuronal soma) (T=1 to T=100) and retrograde (towards neuronal soma) vesicle movement (T=100 to T=200). B, Automated vesicle tracking of a specific neurite area indicating vesicle velocities as μm/second on y-axis and relative time (seconds) on x-axis. C, Kymograph analyses of vesicle movement in the specific neurite area indicating anterograde and retrograde movement of a vesicle expressing WT MBL-EGFP and IIIB gp120. D, Co-localization of WT MBL- EGFP (green) with MAP2 (red) and gp120 (magenta) in SH-SY5Y cells (closed arrow; white indicates co-localization). DAPI (blue) stains nuclei. Scale bar, 10 μm.
Discussion
Lectins have long been shown to transport neuronal proteins associated with their ability to specifically recognize such proteins via the sugar binding activity (Borges L.F. et al., 1982; Yamashitha K. et al., 1999). HIV-1 gp120 is internalized in neurons primarily by a CXCR4 mediated mechanism (Bachis A. et al, 2003) and is further transported retrograde and anterograde across the neurons leading to neuropathological changes as observed in HIV associated dementia (Ahmed, F. et al., 2009); however cellular mechanisms underlying subcellular trafficking of gp120 along neurites are poorly understood.
Mannose binding lectin is an innate immune response protein that binds to highly glycosylated HIV-1 gp120 (Saifuddin M., et al., 2000; Hart M.L. et al., 2002, Nonaka M., 2011) and leads to activation of lectin complement pathway (Xin Ji et al., 2005, Eisen S. et al., 2011). Our earlier studies showed that MBL is expressed in the major cell types of HIV-1 infected brain such as neurons, astrocytes, microglia, oligodendrocytes and its expression is increased in neurons in HIV encephalitis (Singh K.K. et al., 2011).
The present study focuses on the potential role of MBL in subcellular localization, and trafficking of the vesicles containing MBL bound gp120 complexes along the neurites. We have shown that MBL mediates subcellular localization and intracellular trafficking of gp120 along neurites. Endogenous MBL as well as exogenously expressed MBL localized to perinuclear vesicles in both SK-N-SH cells and primary neurons, and led to the similar intracellular co-localization with gp120 envelope protein. The co-localization required a functional CRD domain which enables direct binding of MBL to gp120.
Significant advances have been made in understanding of intracellular processing and trafficking of glycoproteins via ER and Golgi apparatus (Lippincott-Schwartz, J. et al., 2000; Schrag J.D. et al., 2003). An important observation in the present study was that an interaction of MBL with HIV-1 gp120 envelope protein facilitated the localization of these complexes in the perinuclear ER and GA compartments in neurons, as well as their trafficking from ER to GA through COPII transport vesicles. In addition, this trafficking was mediated by a functional MBL because a mutant MBL without a functional CRD lost its lectin function and association with gp120 and dynamic transport vesicles. MBL:gp120 complexes were not only localized in the perinuclear compartment but also along the microtubules suggesting that these complexes are transported along the microtubules. Some of these vesicles may be processed and degraded through the late endosomal and lysosomal pathways (Akasaki K. et al., 1996); however we focused on understanding the trafficking of these vesicles in the neuronal soma and neurites that may allow transmission and dissemination of viral proteins during HIV-1 neuropathogenesis.
Differential targeting of neuronal proteins to either the axonal or somatodendritic compartment is essential for the establishment and maintenance of neuronal structure and function (Horton and Ehlers, 2003). During synthesis in the secretory pathways, the neuronal proteins are sorted in distinct vesicles at the trans-Golgi compartment and are transported within the somatodendritic compartment, although the mechanism by which such vesicles are confined to the somatodendritic compartment is poorly understood. We found that the treatment of human primary neurons with IIIB gp120 led to the formation of MBL:gp120 complexes not only in perinuclear vesicles but also along MAP2 in the somatodendritic compartment by 30min treatment of gp120. By 6h these complexes were present more in the neuronal soma and less in the neurites and MAP2 degradation was observed due to gp120 neurotoxicity. Consequently, pretreatment of human primary neurons with nocodazole (microtubule depolymerizing agent) followed by exposure of IIIB gp120, impaired the transport of MBL:gp120 complexes along the neurites. Additionally, when MBL expression was silenced, significantly fewer gp120:MAP2 complexes were observed across the neurites suggesting that MBL may be a key factor in stability and intracellular transport of gp120 in neurons. Taken together these findings suggest that functional MBL acts as a carrier for gp120 to dynamic vesicles which travel along the microtubules in neurons.
Furthermore, the time lapse imaging of vesicles carrying WT MBL-EGFP:IIIB gp120 confirmed that these vesicles not only had different sizes, but also different velocities and directions of movement. Vesicle tracking and kymograph analysis indicated a major vesicular population that displayed a fast vesicular transport as well as a significant vesicular population with a slow vesicular trafficking, and the movement was anterograde, retrograde or bidirectional. In neurons, vesicles are equally likely to enter the dendrites and the axons regardless of their contents (Al-Bassam S. et al., 2012). Time lapse imaging data also showed that some of the vesicle movements included stops (halts) followed by directional anterograde, retrograde or bidirectional movements that are probably due to their cargo composition. Earlier studies have also shown that different forces coordinate the movement of the vesicles based on their cargos, for example, vesicles carrying dendritic proteins versus vesicles carrying axonal specifically or non-specifically - localized proteins (Al-Bassam S. et al., 2012).
In summary, MBL was enriched in perinuclear compartments as well as in neurites where it co-localized with viral gp120. The functional CRD domain of MBL was crucial for subcellular localization and perinuclear distribution of MBL:gp120 vesicles. Also, live cell imaging confirmed that MBL:gp120 complexes were associated with subcellular dynamic vesicles and MBL acted as a lectin carrier for gp120 neuronal trafficking that required functional microtubules. Thus, these studies suggest that MBL mediates gp120 trafficking in subcellular compartments of neuronal cells. Our study reveals a novel function of MBL as a lectin carrier for the subcellular trafficking and potential transport of viral glycoproteins in neuronal cells. Glycoprotein 120 from both chemokine receptor CCR5 using HIV-1 JRFL strain and a dual tropic HIV-1 IIIB strain showed similar subcellular localization and trafficking in the neurons. Moreover, confirmation of results from human neuroblastoma cells in human primary neurons suggests importance of these findings for a better understanding of HIV-1 neuropathogenesis in humans. Further studies are required to determine the detailed mechanisms of how gp120 triggers MBL-mediated transport via the cellular machinery in neurons and possibly across synapses.
Highlights.
MBL bound gp120 complexes were localized at neuronal perinuclear vesicles.
MBL:gp120 subcellular trafficking required MBL carbohydrate recognition domain.
Subcellular trafficking of MBL:gp120 vesicles via ER and Golgi required MAP2.
MBL:gp120 dynamic subcellular vesicles underwent trafficking along the neurites.
Acknowledgements
The authors acknowledge the support by National Institute of Health (NIH) (R01MH085608 to KKS; R01MH087332, P50DA026306 to MK; and P30MH62512, P50DA26306 to CLA and BS). We thank Jennifer Santini, UCSD Neuroscience Core and Light Microscopy Facility (Grant P30, NS047101) for confocal microscopy technical assistance and Paul Miller, Perkin Elmer for help with processing live cell imaging data. We are thankful to Dr. Motohiro Nonaka and Dr. Toshisuke Kawasaki for providing WT-MBL-EGFP plasmid. The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: pSyn gp120 JR-FL from Dr. Eun-Chung Park and Dr. Brian Seed; and HIV-1 gp120 Monoclonal Antibody (ID6) from Dr. Kenneth Ugen and Dr. David Weiner. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This paper is subject to the NIH Public Access Policy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interest.
REFERENCES
- 1.Aebi M. N-linked glycosylation in ER. BBA. 2013;1833(11):2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed F. Retrograde and anterograde transport of HIV gp120 in the nervous system. Brain Behav Immun. 2009;23(3):335–364. doi: 10.1016/j.bbi.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akasaki K. Biosynthetic transport of a major lysosome-associated membrane glycoprotein 2, lamp-2: a significant fraction of newly synthesized lamp-2 is delivered to lysosomes by way of early endosomes. J Biochem. 1996;120(6):1088–1094. doi: 10.1093/oxfordjournals.jbchem.a021526. [DOI] [PubMed] [Google Scholar]
- 4.Al-Bassam S. Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. Cell Reports. 2012;2:89–100. doi: 10.1016/j.celrep.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andre S. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J Virol. 1998;72:1497–1503. doi: 10.1128/jvi.72.2.1497-1503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aqholme L. Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis. 2010;20(4):1069–1082. doi: 10.3233/JAD-2010-091363. [DOI] [PubMed] [Google Scholar]
- 7.Avramut M. The immunosuppressant drug FK506 is a potent throphic agent for human fetal neurons. Brain Res. Dev Brain Res. 2001;132:151–157. doi: 10.1016/s0165-3806(01)00307-8. [DOI] [PubMed] [Google Scholar]
- 8.Bachis A. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23(13):5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachis A. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J Neuroci. 2006;21(26):6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007;17(10):502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Borges LF. Axonal transport of lectins in the peripheral nevous system. The Journal of Neuroscience. 1982;2:647–653. doi: 10.1523/JNEUROSCI.02-05-00647.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Chan DC. Mitochondrial dynamics-fusion, fission, movement, and mitophagy-in neurodegenerative diseases. Hum. Mol. Genet. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cid-Arrequi A. Nocodazole-dependant transport and brefeldin A-sensitive processing and sorting of newly synthesized membrane proteins in cultured neurons. The Journal of Neuroscience. 1995;15(6):4259–4269. doi: 10.1523/JNEUROSCI.15-06-04259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickey C. Murine monoclonal antibodies biologically active against the amino region of HIV-1 gp120: Isolation and characterization. DNA and Cell Biology. 2000;19:243–252. doi: 10.1089/104454900314519. [DOI] [PubMed] [Google Scholar]
- 15.Eisen S. Mannose-binding lectin in HIV infection. Future Virol. 2008;3(3):225–233. doi: 10.2217/17460794.3.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis R. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Neuroscience. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 17.Fennie C, Lasky LA. Model for intracellular folding of the human immunodeficiency virus type1 gp120. J Virol. 1989;63:639–646. doi: 10.1128/jvi.63.2.639-646.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita T. Evolution of lectin-complement pathway and its role in innate immunity. Nature Rev. Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 19.Garred P. Mannose-binding lectin deficiency -revisited. Mol Immunol. 2003;40(2–4):73–84. doi: 10.1016/s0161-5890(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Roman VR. Phage-display mimotopes recognizing a biologically active anti-HIV-1 gp120 murine monoclonal antibody. J. Acquir. Immune Defic. Syndr. 2002;31:147–153. doi: 10.1097/00126334-200210010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Hallenberger S. Secretion of a truncated form of the human immunodeficiency virus type1 envelope glycoprotein. Virology. 1993;193:510–514. doi: 10.1006/viro.1993.1156. [DOI] [PubMed] [Google Scholar]
- 22.Hart ML. High mannose glycans and sialic acid on gp120 regulate binding of mannose-binding lectin (MBL) to HIV1 type1. AIDS Research and Human Retroviruses. 2002;18(17):1311–1317. doi: 10.1089/088922202320886352. [DOI] [PubMed] [Google Scholar]
- 23.Haas J. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996;6:315–324. doi: 10.1016/s0960-9822(02)00482-7. [DOI] [PubMed] [Google Scholar]
- 24.Helenius A, Aebi M. Intracellular functions of N-linked Glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 25.Hoppe HK, Reid KB. Collectins containing collagenous regions and lectin domains and their role in innate immunity. Protein Sci. 1994;3(8):1143–1158. doi: 10.1002/pro.5560030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40(2):277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- 27.Iskander S. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation. 2004;271(1):7. doi: 10.1186/1742-2094-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilpatrick DC. Mannan-binding lectin: clinical significance and applications. Biochem Biophys Acta. 2002;1572(2–3):401–413. doi: 10.1016/s0304-4165(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 29.Kurata H. Structure and function of mannan-binding proteins isolated from human liver and serum. J Biochem. 1994;114(6):1148–1154. doi: 10.1093/oxfordjournals.jbchem.a124471. [DOI] [PubMed] [Google Scholar]
- 30.Lasek RJ. Axonal transport of the cytoplasmic matrix. The Journal of Cell Biology. 1984;99(1):212–221. doi: 10.1083/jcb.99.1.212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippincott-Schwartz J. Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell Dev. Biol. 2000;16:557–589. doi: 10.1146/annurev.cellbio.16.1.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y. Functional expression of human mannan-binding proteins (MBPs) in human hepatoma cell lines infected by recombinant vaccinia virus: post-translational modifications, molecular assembly, and differentiation of serum and liver MBL. J Biochem. 1997;122:810–818. doi: 10.1093/oxfordjournals.jbchem.a021827. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y. Anti-tumor activity of mannan-binding protein in vivo as revealed by a virus expression system: Mannan-binding protein dependent cell-mediated cytotoxicity. PNAS. 1999;92:371–375. doi: 10.1073/pnas.96.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mochetti I. Neurotoxicity of human immunodeficiency virus-1 viral protein and axonal transport. Neurotox Res. 2012;12:79–89. doi: 10.1007/s12640-011-9279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan GW. Combined biochemical and cytological analysis of membrane trafficking using lectins. Anal Biochem. 2013;441:21–31. doi: 10.1016/j.ab.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 36.Nickel W. Vesicular transport: the core machinery of COPI recruitment and budding. Journal of Cell Science. 2002;115:3235–3240. doi: 10.1242/jcs.115.16.3235. [DOI] [PubMed] [Google Scholar]
- 37.Nonaka M. Subcellular localization and physiological significance of intracellular mannan binding protein. JBC. 2007;282:17908–17920. doi: 10.1074/jbc.M700992200. [DOI] [PubMed] [Google Scholar]
- 38.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Saifuddin M. Interaction of mannose-binding lectin with primary isolates of human immunodeficiency virus type 1. Journal of General Virology. 2000;81:949–955. doi: 10.1099/0022-1317-81-4-949. [DOI] [PubMed] [Google Scholar]
- 40.Samson F. Nocodazole action on tubulin assembly, axonal ultrastructure and fast axoplasmic transport. J Pharmacol Exp Ther. 1976;208(3):411–417. [PubMed] [Google Scholar]
- 41.Schrag JD. Lectin control of protein folding and sorting in the secretory pathway. Trends Biochem Sci. 2003;28(1):49–57. doi: 10.1016/s0968-0004(02)00004-x. [DOI] [PubMed] [Google Scholar]
- 42.Singh KK, et al. Expression of mannose binding lectin in HIV-1 infected brain: implications for HIV-related neuronal damage and neuroAIDS. Neurobehavioral HIV Medicine. 2011;3:41–52. doi: 10.2147/NBHIV.S19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sitia R. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426(6968):891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 44.Thielens NM. Interaction properties of human mannan-binding lectin (MBL)-associates serine proteases-1 and -2, MBL-associated protein 19, and MBL. J. Immunol. 2001;166:5068–5077. doi: 10.4049/jimmunol.166.8.5068. [DOI] [PubMed] [Google Scholar]
- 45.Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40(7):423–9. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 46.Wallis R. Molecular determinants of oligomer formation and complement fixation in mannose-binding proteins. J.Biol Chem. 1999;274:3580–3589. doi: 10.1074/jbc.274.6.3580. [DOI] [PubMed] [Google Scholar]
- 47.Willey RL. Biosynthesis, cleavage, and degradation of human immunodeficiency virus 1 envelope glycoprotein gp160. PNAS. 1998;85:9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamashitha K. Intracellular lectins associated with N-linked glycoprotein traffic. BBA. 1999;1473:147–160. doi: 10.1016/s0304-4165(99)00175-0. [DOI] [PubMed] [Google Scholar]
- 49.Ji X. Mannose Binding Lectin (MBL) and HIV. Molecular Immunology. 2005;42(2):145–152. doi: 10.1016/j.molimm.2004.06.015. [DOI] [PubMed] [Google Scholar]