Abstract
Hemophagocytic lymphohistiocytosis (HLH) comprises a heterogeneous group of diseases which are characterized by a hyperinflammatory state due to uncontrolled T cell, macrophage and histiocyte activation, accompanied by excessive cytokine production. This rare condition is almost uniformly fatal unless promptly recognized and treated. Much progress has been made in the last two decades in our understanding of the mechanisms underlying familial, and to a lesser extent, acquired cases of HLH. Recurrent mutations in more than 10 different genes have now been identified, involving biological pathways converging on intracellular vesicle trafficking, and cytolytic granule exocytosis. Mechanisms underlying the majority of acquired HLH cases, however, remain elusive, hampering both diagnostic evaluation as well as therapeutic management of these patients. Given that the majority of intensive care unit (ICU) patients with sepsis or multiorgan failure share many features of HLH, it is especially critical for pediatric and adult intensivists to be able to recognize patients with bona fide HLH and initiate treatment without delay. In this article, we review our current understanding of the pathophysiology, clinical testing, diagnosis, and treatment of patients with HLH, especially as it pertains to the care of critically ill patients in pediatric and medical ICUs.
Keywords: HLH, hemophagocytic lymphohistiocytosis, ferritin, hemophagocytic syndrome, HPS
DEFINITION AND INCIDENCE
History of HLH
Hemophagocytic lymphohistiocytosis (HLH), sometimes referred to as familial hemophagocytic lymphohistiocytosis (FHL), familial erythrophagocytic lymphohistiocytosis (FEL) and virally associated hemophagocytic syndrome (VAHS), was initially described by James Farquhar and Albert Claireaux, two Scottish pediatricians at the University of Edinburgh in 1952(1). Farquhar and Claireaux reported a case of two 9-week old infants born one year apart to the same parents. They presented with fever, pancytopenia, and hepatosplenomegaly with jaundice, which evolved into a rapidly fatal clinical course that could not be arrested by antibiotics, antipyretics or adrenocorticotropic hormone (ACTH). Post-mortem analysis revealed histiocytic infiltration of the spleen, liver and lymph nodes, accompanied by hemophagocytosis; thus they termed this condition “familial haemophagocytic reticulosis.” HLH remains primarily a syndrome of infancy, although multiple cases in older children and adults have now been described. Many of these have been found to be associated with infectious, neoplastic and autoimmune diseases (reviewed in (2-4)).
Incidence and Classification of HLH
The exact incidence or prevalence of HLH is not known. This orphan syndrome was not listed in the SEER database until 2010 and it is believed that a significant number of cases remain undiagnosed and underreported. Based on the available data, the incidence of HLH varies by geographic region. It has been reported to occur in anywhere from 1/50,000 live births in Sweden (5), to 7.5/10,000 live births in Turkey (6), an unusually high reported prevalence attributed to increased consanguinity. The prevalence in the United States is estimated to be approximately 1/100,000 live births based on a study recently performed at three large academic centers in Texas (7), with 1 case of HLH expected per 3000 inpatient admissions in tertiary care pediatric hospitals (8, 9). There is no predilection for gender or race.
HLH has been historically categorized as “primary” (i.e. caused by an underlying genetic defect in infants) and “secondary” (i.e. triggered by another process, such as an infection or neoplasm, most commonly in older children and adults). This distinction is falling out of favor, however, given discovery of shared genetic defects between patients with primary and secondary HLH, as well as our improved understanding that a trigger event is often necessary for full manifestation of disease in both types. As a result, “familial” and “acquired” HLH are currently the preferred nomenclature -- with familial cases sharing known underlying genetic defects. However, it is more appropriate to think of HLH syndrome as a continuum of hyperinflammatory diseases rather than two distinct subsets, with disease penetrance and latency being likely driven by additional genetic drivers and modifiers yet to be identified.
Familial HLH
Familial HLH comprises about 25% of all HLH; a number that is more likely going to increase in the coming years with the recent boom in sequencing and genetic testing. There are 5 subtypes of familial HLH, referred to as FHL1-5, based on underlying genetic defects, which are inherited in an autosomal recessive fashion (Table 1) (10-14). Linkage analysis has identified genes on chromosomes 6, 9, 10, 17 and 19 to be associated with familial HLH; all of these with the exception of a yet to be identified gene on chromosome 9q encode proteins involved in intracellular vesicle trafficking, and their mutations lead to defective cytotoxicity. In addition, a number of congenital immune deficiency syndromes, such as Chédiak-Higashi syndrome, Hermansky-Pudlak syndrome type 2, Griscelli syndrome type 2, X-linked lymphoproliferative (XLP) disease types 1 and 2, X-linked severe combined immunodeficiency (X-SCID) and lymphoproliferative syndrome 1 (LPSA1) can be associated with HLH, and present either at the time of initial diagnosis or during the course of the disease (Table 2) (15-21).
Table 1.
Familial HLH subtypes and associated gene defects
| HLH Subtype | Genetic defect | Protein function | References |
|---|---|---|---|
| FHL1 | 9q21.3-locus 6 | unknown | (11) |
| FHL2 | PFR1 | pore forming cytolytic protein | (12) |
| FHL3 | UNC13D | secretion of cytolytic granules | (10) |
| FHL4 | STX11 | intracellular vesicle transport | (13) |
| FHL5 | (UNC18B) | membrane fusion | (14) |
FHL = familial HLH
HLH = hemophagocytic lymphohistiocytosis
Table 2.
Congenital immune deficiencies associated with HLH
| Immune deficiency | Genetic defect |
Protein function | Ref |
|---|---|---|---|
| Chédiak-Higashi syndrome | LYST | microtubule-assisted endosomal protein sorting | (16) |
| Hermansky-Pudlak S. type 2 | AP3B1 | protein trafficking, AP3 complex subunit | (18) |
| Griscelli syndrome type 2 | RAB27A | vesicle fusion and trafficking | (17) |
| XLP type 1 | SHD21A | involved in regulating SLAM signaling | (15) |
| XLP type 2 | BIRC4/XIAP | inhibits caspase 3 and 7 proteases | (19) |
| X-SCID | IL2RG | subunit of IL2,4,7,9,15,21 receptors | (20) |
| LPSA1 | ITK | mediates TCR activation of B-1-integrins | (21) |
X- SCID = X-linked severe combined immunodeficiency
XLP = X-linked lymphoproliferative disease
LPSA1 = lymphoproliferative syndrome type 1
Acquired HLH
In acquired HLH, which makes up majority of HLH in both children and adults and is by definition not associated with a known genetic defect, the hyperinflammatory state is triggered by infectious, autoimmune or neoplastic conditions. The most common infectious etiologies associated with HLH include viral infections, such as EBV, CMV, parvovirus, HSV, VZV, HHV8, HIV, influenza, and hepatitis A, B and C viruses, among others (22-26). EBV-associated HLH is the best characterized entity among all and gives us some insight into the mechanisms of acquired HLH, as described below (27). A number of bacterial infections (gram negative rods, Mycoplasma species and Mycobacterium tuberculosis) (28, 29), parasitic infections (Plasmodium, Leishmania, and Toxoplasma species), and fungal infections (Cryptococcal, Candidal and Pneumocystis species) (30), have also been implicated (reviewed in (31)).
Malignancy, especially hematologic malignancy, has been a long appreciated trigger of acquired HLH. Similarly to autoimmune diseases, HLH may be the part of the initial clinical presentation, or may arise from cancer progression or treatment. Malignancies most commonly associated with HLH include peripheral T-cell and NK-cell lymphomas, anaplastic large cell lymphoma, acute lymphoblastic leukemia, acute erythroid leukemia, and Hodgkin disease (32-38). The link of between T-cell and NK-cell diseases and HLH is not incidental, as proper T and NK-cell function is required for clearance of antigenic stimuli and termination of the inflammatory response. Aberrant T-cell and NK-cell activation in these disorders results in excessive cytokine production and sustained macrophage activation. Associations with solid malignancies, such as lung, prostate and hepatocellular carcinoma, have also been made (39-41), although these are much more rare. In addition, there are multiple case reports of HLH following umbilical cord blood transplantation or organ transplantation (42, 43). We currently have no understanding why some solid malignancies but not others are more likely to be associated with HLH.
A number of autoimmune diseases, especially rheumatologic illnesses, have been linked to an HLH-like syndrome, and grouped together as macrophage activation syndromes (MAS). Among these, systemic juvenile inflammatory arthritis (sJIA) leads the way, followed by systemic lupus erythematosus, Still’s disease, rheumatoid arthritis, Kawasaki disease, dermatomyositis, polyarteritis nodosa, sarcoidosis, and Sjögren’s syndrome (44). In some cases, MAS can be the initial presentation of the disease, whereas in other cases, it may be triggered by an infection or a flare of the underlying systemic autoimmune disease (45, 46). Drug induced hypersensitivity syndromes, such as DRESS (drug reaction with eosinophilia and systemic symptoms) have been linked to HLH as well (47).
PATHOPHYSIOLOGY
Familial HLH
Insight into the pathophysiology of HLH has been facilitated by seminal discoveries related to famililial HLH syndromes. First, Stepp and colleagues identified loss of function mutations in the PRF1 gene in patients with familial HLH linked to 10q21-22 in 1999. This uncovered the critical role of perforin-dependent cytotoxicity for normal T-cell and NK-cell function (12). Perforin is a cytolytic protein that creates pores in the cell membranes of target cells, leading to their osmotic lysis. Perforin and granzyme proteins are normally stored in specialized secretory vacuoles of T and NK cells. Upon encountering infected cells, or tumor and antigen presenting cells, the cells release the granules, mediating cell lysis. Effective cell lysis leads to decreased antigen stimulation and facilitates a feedback loop termed “activation induced cell death,” which limits the immune response. Perforin is critical for maintenance of this negative feedback loop, and absent or decreased levels of perforin in HLH lead to sustained overactivation of antigen presenting cells and an uncontrolled hyperinflammatory state. This hyperinflammation is accompanied by a cytokine storm – i.e. high levels of a number of cytokines, including IFNγ, IL-6, IL-10, IL-12, IL-16, IL-18, and soluble IL-2R (CD25), among others. The high cytokine milieu in return leads to sustained macrophage activation and uncontrolled cellular ingestion independent of proper cell-cell interactions and recognition. This includes ingestion of blood cells in the marrow, or hemophagocytosis, which is one of the hallmarks of HLH. Macrophage and histiocyte infiltration leads to hepatosplenomegaly, often accompanied by transaminitis and hyperbilirubinemia. In addition, activated macrophages release plasminogen activator, promoting fibrinolysis and resulting in hypofibrinogenemia. Macrophages also upregulate heme-oxygenase, driving up serum ferritin levels (48). Many additional features of HLH are the consequence of elevated cytokine levels. For example, pancytopenia and hypertriglyceridemia are thought to be a result of excessive levels of TNFα and IFNγ, which suppress hematopoiesis and inhibit lipoprotein lipase, respectively (49). On the other hand, prolonged fevers are most likely driven by high levels of IL-1 and IL-6 levels (reviewed in (3)).
A mouse deficient in Prf1 recapitulates all features of the HLH phenotype upon infection with lymphocytic choriomeningitic virus, including a rapidly fatal course with death only 15 days after infection (50). Further characterization of this model suggested that CD8+ cytotoxic T-cells, but not NK cells, are necessary for the development of the full HLH phenotype, at least in mice. Furthermore, Prf1-deficient mice demonstrated increased levels of a number of cytokines similar to humans, including TNFα, IFNγ, IL6, IL10, and IL18, but neutralization of only IFNγ in vivo resulted in “a cure” of 90% animals. This has led to a number of preclinical studies and an ongoing clinical trial of a monoclonal antibody targeting IFNγ in patients with reactivated HLH (NCT01818492; Table 6).
Table 6.
Summary of the current clinical trials targeting HLH syndrome
| ACTIVE, RECRUITING PATIENTS | |||||
| Clinical trial |
Type of
study |
Targeted disease(s) | Drug regimen |
Patient
population |
Sponsor |
| NCT01104025 | Phase II | HLH | ATG, dexamethasone, etoposide | < 18 years | Children’s Hospital Medical Center, Cincinnati, OH, USA |
| NCT01547143 | Phase II | HLH, excluding malignancy and MAS |
cyclosporine, dexamethasone, etoposide, allo-SCT |
18-80 years | Asan Medical Center, Seoul, Korea |
| NCT01818908 | Phase II | NHL with HLH | DA-EPOCH | 15-80 years | Jiangsu Province Hospital, Nanjing, Jiangsu, China |
| NCT01818492 | Phase II | reactivated HLH, excluding MAS and malignancy |
NI-0501, cyclosporine, dexamethasone |
< 18 years | NovImmune SA, Geneva, Switzerland |
| NCT00368355 | Phase II | HLH, XLP, (AML, ALL, MDS, NHL, CML) |
T-cell depleted haploidentical- HSCT |
< 55 years | Baylor College of Medicine, Houston, TX, USA |
| NCT01494103 | Phase I | HLH, XLP, (AML, ALL, MDS, NHL, CML) |
haplo-identical T cells, caspase 9 suicide gene, AP1903 |
Undefined | Baylor College of Medicine, Houston, TX, USA |
| NCT01125319 | N/A | acquired HLH | Blood sampling to investigate NK and T cell function |
> 18 years | Assistance Publique Hopitaux De Marseille, Marseille, France |
| NCT01095146 | N/A | MAS, excluding HLH | retrospective study to define MAS diagnostic criteria |
Undefined | Amrita Institute of Medical Sciences & Research Center, Kerala, India |
| NCT01652092 | Phase II | HLH, SCID, XLP, Griscelli, Chediak-Higashi, etc |
myeloablative and RIC allo- HSCT |
< 50 years | Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA |
| NCT01821781 | Phase II | HLH, SCID, XLP, Griscelli, Chediak-Higashi, etc |
RIC-HSCT | < 21 years | Washington University School of Medicine, St. Louis, MO, USA |
| NCT00919503 | Phase II | nonmalignant diseases | allo-HSCT, treosulfan | < 54 years | Fred Hutchinson Cancer Research Center, Seattle, WA, USA |
| NCT01050855 | Phase II | nonmalignant diseases, immune deficiencies |
RIC-HSCT | 6 months - 25 years |
Children’s Hospital of Philadelphia, Philadelphia, PA, USA |
| NCT00553098 | Phase II | nonmalignant diseases, immune deficiencies |
RIC-HSCT | < 54 years | Fred Hutchinson Cancer Research Center, Seattle, WA, USA |
| ACTIVE, NOT RECRUITING PATIENTS | |||||
| Clinical trial |
Type of
study |
Targeted disease(s) | Drug regimen |
Patient
population |
Sponsor |
| NCT00426101 | Phase III | HLH | HLH-2004, allo-HSCT | < 18 years | Karolinska University Hospital, Stockholm, Sweden |
| NCT00334672 | Phase III | HLH | etoposide, dexamethasone, cyclosporine, allo HSCT |
< 17 years | Children’s Cancer and Leukaemia Group, UK |
| NCT00006056 | Phase II | HLH; XLP type 1, 2; Chediak- Higashi |
allo-HSCT | < 55 years | Fairview University Medical Center, Minneapolis, MN, USA |
| NCT00710892 | Phase I | HLH, AML, MDS, CML, ALL | T cell depletion, inducible caspase 9 suicide gene |
< 65 years | Baylor College of Medicine, Houston, TX, USA |
| NCT00176826 | Phase II/ III |
HLH, XLP, Chediak-Higashi, Griscelli, |
in vivo T cell depletion, allo- HSCT |
< 55 years | Masonic Cancer Center, University of Minnesota, Minneapolis, MN |
| NCT00176865 | Phase II | HLH, XLP, Chediak-Higashi, Griscelli, |
non-myeloablative HSCT | < 35 years | Masonic Cancer Center, University of Minnesota, Minneapolis, MN |
Abbreviations used: AML = acute myeloid leukemia
ALL = acute lymphoblastic leukemia
allo = allogeneic
ATG = anti-thymocyte globulin
AP-1903 = lipid-permeable tacrolimus analogue with homodimerizing activity, used to “activate” caspase 9 suicide gene
CML = chronic myeloid leukemia
DA EPOCH = dose adjusted EPOCH (etoposide, doxorubicin, vincristine, rituximab, cyclophosphamide, prednisone)
HLH = hemophagocytic lymphohistiocytosis
HLH-2004 = HLH-2004 treatment protocol (dexamethasone, etoposide, cyclosporine)
HSCT = hematopoietic stem cell transplant
MAS = macrophage activation syndrome
MDS= myelodysplastic syndrome
NHL = non-Hodgkin lymphoma
NI-0501 = anti-interferon γ monoclonal antibody
RIC = reduced intensity conditioning
SCID = severe combined immunodeficiency
XLP = X-linked lymphoproliferative disease
Following the discovery of PRF1 mutations, 3 additional groups identified mutations in UNC13D, STX11, and STXB2, as genetic defects associated with familial HLH linked to 17q25, 6q24, and 19p13, respectively. Perhaps not surprisingly, all of these genetic defects converge on the same pathway regulating trafficking and exocytosis of cytotoxic granules of T and NK cells. Therefore, a defective cytolytic pathway and a concomitant inability to terminate an inflammatory response to antigen stimulation seem to be the main drivers of familial HLH.
It remains unclear why some patients with familial HLH present early in life while others don’t develop the disease until late adulthood. Most cases of familial HLH occur within the first 6 months of life, although there are many outliers. The oldest case reported to our knowledge is that of a 62-year old man with a compound heterozygous mutation of PRF1 (51). A plausible explanation for this heterogeneity involves hypomorphic alleles. Indeed, in a study of 175 adults with sporadic HLH, 14% were found to have hypomorphic alleles (missense and splice-site mutations) in PRF1, MUNC13-4, and STXBP2 (52). Additional studies have found that patients carrying PRF1 null mutations, which correlate with absent levels of perforin expression, develop HLH within the first three months of life. Conversely, patients with missense mutations in PRF1 gene, who express residual perforin protein, don’t develop disease until later in life (53, 54). Age of onset varies among different genetic defects as well – for example, loss of function PRF1 mutations, as compared to UNC13D and STX11 mutations, are associated with early (i.e. <6 months) onset of disease (55). Still, there are multiple case reports of children or adults carrying the same PRF1 gene mutations presenting with distinct disease patterns; the basis for this phenotypic heterogeneity is unknown.
Acquired HLH
Mechanisms driving acquired HLH are not clear. One plausible hypothesis at this time is that T- and NK-cell dysfunction driving the HLH phenotype is somehow caused by chronic antigen stimulation in a setting of a viral infection or malignancy. The best studied example is that of EBV-associated HLH. In this case it is believed that the EBV latent membrane protein (LMP1) interferes with the T cell adapter protein, signaling lymphocyte activation molecule-associated protein (SAP), which in return results in excessive T cell activation and Th1 cytokine secretion (56). With the advent of whole exome and whole genome sequencing and its application to this rare disease subset, it is quite likely that we will uncover new genetic drivers and modifiers and be able to link many of these “acquired HLH” cases to genes found in familial HLH, or identify a new subset of those patients with genetic predisposition to HLH.
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
Clinical and Laboratory Findings
The diagnostic criteria for HLH have been developed and updated by the FHL Study Group of the Histiocyte Society and are summarized in Table 3. Based on the most recently published guidelines, diagnosis of HLH is established either by documenting presence of one of the mutations linked to familial HLH or by fulfillment of 5 out of 8 relatively non-specific criteria: fever, splenomegaly, cytopenias involving two or more lines, hypertriglyceridemia and/or hypofibrinogenemia, hemophagocytosis in bone marrow, spleen or lymph nodes, low or absent NK-cell activity, ferritin >500 and soluble CD25 (IL-2 receptor) > 2400 U/mL (57, 58). Whereas most patients with HLH develop most if not all of these features at the peak of their disease, it may often be at a time when it is too late to initiate treatment. Hence, some of the features may be absent at a time when rapid recognition of the disease process and initiation of treatment are essential. The presence of some but not other features may be partly driven by the underlying genetics of the disease. For example, patients with null PRF1 mutations have been found to have the highest levels of ferritin and soluble IL-2 receptor as opposed to patients with other underlying genetic defects, and may therefore be picked up more easily at the outset (59). Conversely, many of the cardinal features of HLH are relatively common in critically ill patients, and therefore a high degree of suspicion is necessary to trigger initiation of appropriate work-up and timely treatment.
Table 3.
Diagnostic Criteria for HLH
|
A, Molecular diagnosis consistent with HLH
|
| OR |
| B, Fulfillment of 5/8 criteria: |
| 1. Fever |
| Temperature > 38.5 C for > 7 days |
| 2. Splenomegaly |
| Spleen tip palpated > 3cm below left costal margin |
| 3. Cytopenias involving 2 or more lines |
| Hb < 9 g/dL, or Plt < 100,000/uL, or ANC <1000/uL |
| 4. Hypertriglyceridemia and/or hypofibrinogenemia |
|
fasting triglycerides > 2mmol/L, or >3SD above age-adjusted normal
fibrinogen < 1.5 g/L, or < 3SD below age-adjusted normal |
| 5. Hemophagocytosis in bone marrow, spleen or lymph nodes |
| 6. Low or absent NK-cell activity |
| determined by 51-Cr release assay |
| 7. Ferritin > 500 ug/L (84% sensitivity) |
| > 10,000 (90% sensitvity, 96% specificity) |
| 8. soluble CD25 (IL-2 receptor) > 2400 U/mL |
| > 2 SD above the age-adjusted mean |
Adapted from: Henter JI, et al. Semin Oncol 1991;18:29-33
Henter JI, et al. Pediatr Blood Cancer 2007; 48:124-13
The majority of the laboratory work-up necessary to establish the diagnosis of HLH includes testing done routinely with a fast turn-around time (e.g. CBC, triglycerides, fibrinogen, and ferritin). These findings, combined with a good clinical exam and synthesis of the entire clinical picture should be enough to raise suspicion of HLH in an appropriate clinical setting. For example, a 3-month old infant with fevers, pancytopenia, hepatosplenomegaly associated with abnormal liver function tests and coagulopathy, high ferritin level, and abnormal CNS findings, should be strongly considered for initiation of empiric treatment of HLH before the results of any of the more time intensive diagnostic assays, such as genetic testing, bone marrow biopsy or NK cell assays become available. Additional assays, which may be helpful to confirm the diagnosis, include cytotoxicity assays and flow-cytometry quantification of perforin (60), SAP and XIAP protein levels. The use of perforin staining may be particularly useful as a screening test in families with known history of HLH. The presence of a perforin mutation strongly correlates with absence of intracellular perforin staining in CD8+ and CD56+ T cells, as well as NK cells (60).
The appropriate cut-off level for ferritin in the diagnosis of patients with HLH has been a topic of debate. The cut-off of ferritin >500 was included in the original 1991 HLH diagnostic criteria and carried over in the updated 2004 guidelines, with the caveat that the sensitivity of this test is only 84% based on the results of the HLH-94 study (58). However, many patients, including those with rheumatologic disease and hemochromatosis, or patients receiving chronic red blood cell transfusions, can demonstrate ferritin levels >500 in the absence of HLH (Table 4A). Retrospective analysis of 330 inpatients with ferritin level >500 identified a value of 10,000 as having 90% sensitivity and 96% specificity for HLH (8). Furthermore, the same group established that the rate of ferritin decline, as well as the absolute ferritin value at the time of diagnosis have significant prognostic values (61). Of note, the level of soluble IL-2 receptor being greater than 2 age-adjusted standard deviations above the mean (roughly 2400 U/mL) has been found to be a more sensitive test for HLH (sensitivity of 93%) than ferritin level (62). As with ferritin, it has been found to carry prognostic implications as well (63). However, soluble IL-2 receptor level continues to require referral to a reference laboratory in most medical centers without the benefit of a rapid turnaround time (summarized in Table 4B).
Table 4A.
Differential diagnosis of isolated elevation of ferritin and sIL-2R
| ELEVATED FERRITIN | ELEVATED SOLUBLE IL-2 RECEPTOR |
|---|---|
|
| |
| Still’s disease | Autoimmune diseases |
| Acute and chronic inflammation | Malignancy |
| Autoimmune diseases | Gestational trophoblastic disease |
| Chronic renal failure | Infection (HIV, TB) |
| Malignancy, esp. hematologic | Cardiomyopathy and MI |
| Hemochromatosis | Allograft rejection in transplantation |
| Chronic RBC transfusion Infection |
|
| Acute or chronic liver disease Metabolic syndrome |
|
Table 4B.
Comparison of predictive values of ferritin and sIL-2 R testing in HLH
| FERRITIN | |
| >500 ug/L | 100% sensitivity, 80 specificity for diagnosis of HLH(a) |
| >10,000 ug/L | 90% sensitivity, 96% specificity for diagnosis of HLH(b) |
| ferritin decrease <50% | odds ratio of death 17.42 compared to ferritin decrease >96%(c) |
| >11,000 in the first 3 weeks | odds ratio of death 5.6 compared to ferritin <11,000 in the first 3 weeks(c) |
| SOLUBLE IL-2 RECEPTOR | |
| > 2400 (2SD above mean) | 93% sensitivity for diagnosis of HLH(d) |
| <10,000 | 78% survival(e) |
| >10,000 | 35% survival(e) |
Based on Henter JI et al. Pediatr Blood Cancer. 2007;48(2):124-31
Allen CE et al. Pediatr Blood Cancer. 2008;50(6):1227-35
Lin TF et al. Pediatr Blood Cancer. 2011;56(1):154-5
Komp Dm et al. Blood. 1989;73(8):2128-32
Imashuku S et al. Blood. 1995;86(12):4706-7.
A new promising laboratory marker, soluble CD163 (sCD163), which is a receptor for hemoglobin-haptoglobin complexes and a marker of macrophage activation, has potential value as a diagnostic test for HLH. Unlike ferritin and IL-2, which are produced by a number of tissue and cell types, including liver, spleen, heart, kidney, or T-cells under a variety of nonspecific inflammatory conditions, CD163 expression is restricted to the macrophage/monocyte lineage only. sCD163 has been mainly investigated in patients with MAS, where combination of sIL-2 receptor and sCD163 testing can lead to identification of patients with subclinical MAS (64). Its value in the diagnosis of HLH in the absence of autoimmune disease has not been established.
Pathologic Findings
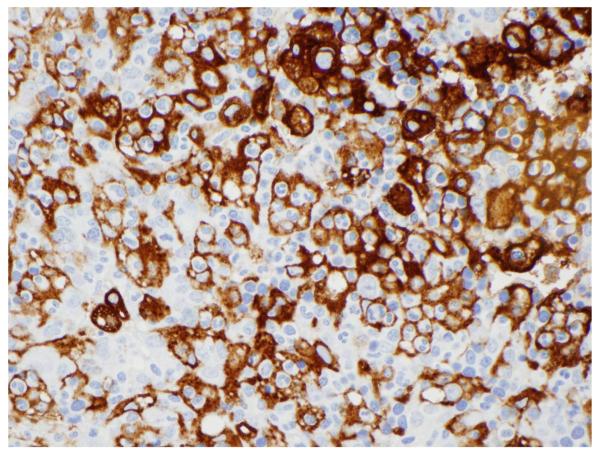
The key pathologic finding of HLH is the presence of hemophagocytosis in bone marrow, spleen or lymph nodes. Hemophagocytosis refers to the ingestion of both mature and immature red blood cells, white blood cells, or platelets by activated macrophages. Immunohistochemical staining of tissue macrophages with CD163 or CD68 can be quite helpful in highlighting this process (Figure 1). However, documentation of hemophagocytosis is not necessary to establish the diagnosis of HLH, nor is it consistently picked up even in patients with documented familial HLH. Conversely, hemophagocytosis is not solely restricted to HLH and can be present in other inflammatory conditions. A recently published small case control study of bone marrow biopsies performed in 6 patients with HLH along with 20 random controls showed that the sensitivity of hemophagocytosis was 83% with a specificity of only 60% (65).
Figure 1.
Extensive hemophagocytosis highighted by macrophage specific CD163 staining of the spleen of a patient with HLH. (Photo courtesy of Dr. Russell Ryan)
Differential Diagnosis
Making a diagnosis of HLH can be challenging since most of the clinical and laboratory features of HLH are quite non-specific. This is particularly the case in the setting of the intensive care unit (ICU) where distinguishing critically ill patients with sepsis or multiorgan failure from HLH may be quite problematic. In fact, the majority of HLH patients in the ICU have symptoms identical to those with septic shock. Additional diagnoses on the differential include Langerhans cell histiocytosis, certain infections, neonatal hemochromatosis and metabolic diseases.
The hyperinflammatory state of HLH, similar to sepsis, is characterized by a cytokine storm, with exceedingly high levels of proinflammatory cytokines, such as IFNγ, TNFα, IL-2, IL-4, IL-6 and IL-10. T-helper 1 (Th1) and Th2 cytokine profiling using cytometric bead array performed in children 10 years or younger with a new diagnosis of HLH versus age-matched healthy controls, patients with infectious mononucleosis, and patients with gram negative rod sepsis, revealed distinct patterns of cytokine levels among the four different conditions (66).
HLH, unlike sepsis or acute EBV infection, could be distinguished by high levels of IFNγ and IL-10, and slightly increased levels of IL-6. Furthermore, a level of IL-10 >2000 pg/mL was found to be an unfavorable predictor of treatment response and outcome. The same group then went on to investigate the diagnostic accuracy of this cytokine pattern in a prospective study of 756 patients who were admitted with fever to a hematology/oncology unit at Children’s Hospital of Zhejiang University in Hangzhou, China. When using the diagnostic criteria of IFNγ > 75 pg/mL and IL-10 > 60 pg/mL for a diagnosis of HLH, they achieved a sensitivity of 98.9% and specificity of 93% (67) (Table 5). Measurement of cytokine levels is currently not routinely performed in most hospital settings; however, this study suggests that incorporation of rapid cytokine profiling, especially in the ICU setting, may be extremely helpful in facilitating diagnosis of bona fide HLH.
Table 5.
Cytokine level patterns in children with HLH, sepsis, infectious mononucleosis, and healthy controls
| IFNγ | IL-10 | IL-6 | |
|---|---|---|---|
| HLH |

|

|

|
| Sepsis |

|

|

|
| Infectious mononucleosis |

|

|

|
| Healthy controls | ↔ | ↔ | ↔ |
Adapted from Tang Y et al, Br J Haematol 2008; 143: 84-91
IFNγ >75 pg/mL and IL-10 > 60 pg/mL have 98.9% sensitivity and 93% specificity for HLH
TREATMENT
HLH-94 and HLH-2004 treatment protocols
The mortality of HLH used to be about 95%, with a median survival of 1-2 months, prior to initiation of HLH-directed therapy (57, 68). Early recognition and initiation of therapy are therefore of utmost importance, and urgent referral to a hematologist-oncologist is critical. In spite of progress in the understanding of the mechanisms underlying familial HLH, it has not led to improved targeted treatment of HLH patients. The basic principle of current therapy is to combine immunosuppressive and cytotoxic therapy to target the hyperinflammatory state, regardless of the type of HLH (familial or acquired), followed by transplantation in cases of familial HLH, relapsing or persistent disease.
Two major prospective international therapeutic studies sponsored by the Histiocyte Society have been aimed at improving the dismal prognosis of patients with this orphan syndrome. Of note, both of these studies were performed in children and adolescents only, with very limited data currently available for treatment of adults with HLH. The HLH-94 study enrolled 249 patients between 1994-2008 who fulfilled strictly defined inclusion criteria, including age <16 years, no history of previous cytotoxic therapy or cyclosporin use, no known malignancy, and fulfillment of all 5 diagnostic criteria of HLH defined at the time (fever, splenomegaly, cytopenias, hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis in the bone marrow, spleen or lymph nodes). All patients were treated with the same 8-week long induction regimen, which included dexamethasone (10mg/m2 for 2 weeks, 5mg/m2 for 2 weeks, 2.5mg/m2 for 2 weeks, 1.25mg/m2 for 1 week, followed by 1 week taper) and etoposide (150 mg/m2 twice weekly for 2 weeks, 150 mg/m2 once weekly for 6 weeks). Patients with neurologic symptoms also received intrathecal methotrexate (once weekly during weeks 3-6 at age-appropriate dosing) (68, 69).
Patients with acquired HLH whose disease resolved at the completion of induction were allowed to stop treatment (and restart with relapse). Those patients with familial disease or persistent or relapsing disease continued treatment with maintenance therapy and allogeneic stem cell transplantation (SCT). Of note, since the study was initiated years before the first identification of a disease-causing mutation in HLH, presence of an affected sibling was used as a proxy for familial disease. Maintenance therapy in HLH-94 included dexamethasone pulses (10mg/m2 every 2 weeks, Days 1-3), cyclosporine daily (goal trough 200ug/L) and etoposide (150mg/m2, administered once every two weeks, on Day 8). Patients continued maintenance therapy until they were able to proceed to allogeneic SCT (68). At a median follow-up of 6.2 years, the estimated 5-year probability of survival was 54% ± 6%. 124 patients underwent SCT, with a 5-year survival of 66 ± 8% and a non-significant trend toward improved survival for those with no active disease entering SCT. No patients with familial HLH survived without a SCT, and 97% of patients who died within the first year had active disease. Forty nine patients (20%) survived without a transplant, the majority of whom had infection-related disease, predominantly EBV. All patients who underwent successful SCT remained without disease recurrence. Remaining patients who did not undergo SCT and were more than a year out from completing treatment did not show any recurrence. Jaundice, edema and elevated creatinine at the time of presentation were correlated with poor outcome, whereas older age of presentation, female gender and lack of neurologic symptoms and hepatomegaly were good prognostic markers (69). Since the majority of deaths were observed during the first year, and more than 70% were due to sepsis during the first 8 weeks, PCP and antifungal prophylaxis during treatment is critical. Overall, treatment of patients with the HLH-94 protocol led to about 50% improvement in survival in this patient population and was a major step forward in the management of this disease.
Although HLH-94 treatment significantly improved the outcomes of patients with HLH, early relapses were a major problem, with 24% patients dying prior to SCT. The HLH-2004 protocol was developed to intensify the induction regimen of HLH-94 and decrease the number of relapsed cases. Furthermore, enrollment (which has been now completed as of 2011) was based on the more updated diagnostic criteria (outlined in Table 3), with the target population <18 years old. The major addition to the protocol is initiation of cyclosporine during induction (target trough of 200μg/L). Estimated study completion is in December 2016. HLH-94 protocol therefore continues to be the standard of care of first line treatment for HLH in 2013 outside of clinical trials.
There are very few data to guide the management of adults with HLH since both the HLH-94 and HLH-2004 protocols included children and adolescents only. A number of retrospective case series have been published and highlight exceedingly high mortality when limited or delayed treatment is offered (70-72). For example, a retrospective study of 18 adult patients with HLH diagnosed at a single institution between 2004 and 2009 and treated predominantly with non-etoposide regimens describes a mortality rate of 72% (71). This highlights the need for additional clinical studies including this patient population. At our institution, we follow the HLH-94 guidelines in the management of adult patients with HLH with the exception of those with MAS.
Salvage Treatment Options
Treatment of patients with refractory disease has been difficult, with limited data published on the success of different salvage regimens. Alemtuzumab, a humanized monoclonal antibody targeting CD52, has been successfully used as a salvage therapy and a bridge to transplantation in a small number of patients (73, 74). A recent study demonstrated that 77% of patients with refractory HLH were able to proceed to SCT after alemtuzumab administration. At a median follow-up of 870 days after the first alemtuzumab administration, the long-term probability of survival using this salvage regimen was estimated at 64% (74). A novel salvage therapy with a monoclonal antibody neutralizing IFN-γ is currently being investigated in a clinical trial (NCT01818492; Table 6).
Stem Cell Transplantation in HLH
Hematopoietic stem cell transplantation offers the best overall cure rates for HLH, and is currently indicated for patients with familial HLH, relapsed refractory HLH, those with persistent disease and those with CNS disease (75). For pediatric patients undergoing meyloablative allogeneic SCT following HLH-94 protocol, overall survival rates at 4-5 years have been found to be in the range of 50-66% in different studies (76-78), with improved survival rates for those patients having matched related and matched unrelated, versus haploidentical and mismatched unrelated donors. There have been a number of recent reports indicating that non-myeloablative (i.e. reduced-intensity conditioning) SCT with fludarabine based regimens (e.g. fludarabine, melphalan and alemtuzumab) leads to significantly improved survival rates of 84-92% (75, 79, 80). This is particularly the case in patients with XIAP (81). There are a number of ongoing clinical trials investigating the role of reduced intensity conditioning SCT in the treatment of HLH (Table 6), although there is currently no randomized trial comparing myeloablative versus non-myeloablative SCT in this context. There are very limited data available for post-transplant outcomes of adult patients with HLH. In a small single institution case series of 23 adults with acquired HLH not associated with lymphoma, 5 out of 7 patients who were able to achieve a clinical remission or remained stable on maintenance therapy underwent related donor allogeneic SCT with fludarabine and melphalan conditioning. 4 out of 5 transplanted patients survived, all of whom were treated on HLH-94 protocol. The single transplanted patient who did not survive received treatment with immunosuppressive agents only and was transplanted at a time of “treatment-dependent relapse.” Two additional patients in clinical remission after immunosuppressive treatment survived without SCT (72). At our institution, we recommend consideration of SCT for all adult patients with HLH in remission or those stable on maintenance therapy.
Care of HLH Patients in the ICU Setting
Patients diagnosed with HLH often require care in the intensive care unit due to their rapidly deteriorating clinical condition. Their clinical presentation is often identical to those patients with septic shock and multi-organ failure. In fact, we suspect that a number of patients admitted to the ICU with presumed septic shock, who have a negative infectious work-up and proceed to be unresponsive to sepsis-directed therapy may have undiagnosed and untreated HLH syndrome. Therefore, it is critical for both pediatric and adult intensivists to entertain the diagnosis of HLH in any septic patient who is not responsive to treatment and does not have an identifiable source of infection.
Conversely, the majority of deaths of HLH patients undergoing HLH-directed therapy occur from sepsis and multiorgan failure (69). In addition, these patients can experience significant and often fatal hemorrhagic episodes due to severe coagulopathy from their liver disease. Therefore, early communication with the blood bank team is critical, aggressive blood product support may be necessary, and only life-saving surgical interventions should be pursued. Given the strong immunosuppressive nature of HLH treatment, all patients should receive PCP and anti-fungal prophylaxis, with a low threshold to initiate broad spectrum antimicrobial and antifungal coverage. Based on a recent retrospective study of 56 HLH patients in a medical ICU, increased hospital death was associated with presence of shock at the time of ICU admission as well as thrombocytopenia, with platelet count <30 g/l. Conversely, co-occurrence of Castleman disease and B-cell lymphoma predicted improved survival (82).
Treatment of Acquired HLH
There has been a long standing debate about how to best treat patients with acquired HLH with an identifiable trigger. Although there is ongoing controversy over how to approach patients with malignancy-related HLH, there is general agreement that patients with infection associated HLH should be treated on an HLH protocol. This is especially evident in the studies of EBV associated HLH, where initiation of etoposide-containing HLH regimens within 4 weeks of diagnosis significantly increased probability of long-term survival (x90%, vs 57% long-term survival in patients receiving etoposide early, vs late or not at all) (83, 84). There is recent evidence that addition of rituximab to the HLH treatment protocol in children and adults with EBV-associated familial and acquired HLH may reduce EBV viral load and serum ferritin, which correlates with improved survival (72). Additional prospective studies will be required to study such regimens in a more rigorous way.
The majority of acquired HLH cases not associated with malignancy or rheumatologic disease are triggered by an infection, which is frequently not identified or identified postmortem. In addition, the diagnosis and treatment of HLH is often delayed in patients who are treated for a presumed infection and subsequently miss the window of opportunity for a timely and effective HLH-directed treatment. Therefore, based on the experience in our medical center, we favor timely initiation of HLH-94 treatment protocol in all patients with HLH except those with underlying rheumatologic disease (MAS subset) who usually respond to steroids and immunosuppressants alone.
As noted above, recent studies have implicated hypomorphic alleles for fHLH genes in about 15% of acquired HLH; however, there are no data to indicate whether their prognosis differs from those patients in whom such polymorphisms are not identified. Consequently, although identification of these alleles may further confirm the diagnosis and be important for family counseling, testing for mutations should not delay therapy, nor should it influence the clinical decisions regarding transplantation.
Clinical Trials
If possible, all patients with HLH should be treated on a clinical protocol. There are currently 13 active clinical trials recruiting patients with HLH, MAS, and immune deficiencies that can be associated with HLH (summarized in Table 6). These trials include studies investigating new induction, as well as maintenance regimens, using both established as well as novel agents. For example, among studies targeting induction, NCT01104025 trial is a Phase II trial in children younger than 18 years, in with induction combines ATG (previously used successfully at a single European center), with dexamethasone and etoposide (the backbone of HLH-94). Among some of the more novel agents, anti-interferon γ neutralizing antibodies used as a salvage regimen in combination with cyclosporine and dexamethasone, and T-cell depleted allogeneic SCT, followed by infusion of donor T-cells transduced with a caspase 9 suicide gene, are currently being investigated in this patient population as well. There are almost no data available on treatment of adults with HLH, and only a single Phase II study (NCT01547143), which is currently recruiting patients, has been designed to study treatment of HLH in the adult population. Additional studies that target this patient population are therefore sorely needed.
CONCLUSIONS AND FUTURE DIRECTIONS
Much progress has been made in our understanding of the genetic basis and pathogenesis of familial HLH, although we have yet to translate this knowledge into more effective targeted therapies for our patients. The advent of inexpensive high-throughput sequencing and other novel techniques of mutation discovery promises to dramatically improve our understanding of the pathophysiology of HLH, especially among the group of “acquired” cases. Currently, our uncertainty about HLH diagnosis significantly impairs prognostic assessment and therapeutic decision making. Whole exome sequencing of patients with HLH is currently being carried out at the Cincinnati Children’s Hospital Medical Center. It is hoped that this analysis will not only lead to identification of new disease-causing alleles, but will also lead to discovery of previously unappreciated disease modifiers, which likely contribute to the heterogeneity of the phenotype. Improved understanding of the pathogenesis of HLH will in return significantly accelerate both diagnostic marker as well as drug development, with the common goals of rapid diagnosis and more targeted treatment of this highly heterogeneous group of critically ill patients.
Acknowledgments
Funding: The authors received no financial support for the research and/or authorship of this article.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article
Contributing authors: ZT – Zuzana_Tothova@dfci.harvard.edu; (617) 355-9060 NB – Nancy_Berliner@dfci.harvard.edu; (617) 732-5840
REFERENCES
- 1.Farquhar JW, Claireaux AE. Familial haemophagocytic reticulosis. Arch Dis Child. 1952 Dec;27(136):519–25. doi: 10.1136/adc.27.136.519. PubMed PMID: 13008468. Epub 1952/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med. 2012;63:233–46. doi: 10.1146/annurev-med-041610-134208. PubMed PMID: 22248322. Epub 2012/01/18. eng. [DOI] [PubMed] [Google Scholar]
- 3.Rosado FG, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013 Jun;139(6):713–27. doi: 10.1309/AJCP4ZDKJ4ICOUAT. PubMed PMID: 23690113. Epub 2013/05/22. eng. [DOI] [PubMed] [Google Scholar]
- 4.Weitzman S. Approach to hemophagocytic syndromes. Hematology Am Soc Hematol Educ Program. 2011;2011:178–83. doi: 10.1182/asheducation-2011.1.178. PubMed PMID: 22160031. Epub 2011/12/14. eng. [DOI] [PubMed] [Google Scholar]
- 5.Henter JI, Elinder G, Soder O, Ost A. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand. 1991 Apr;80(4):428–35. doi: 10.1111/j.1651-2227.1991.tb11878.x. PubMed PMID: 2058392. Epub 1991/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 6.Gurgey A, Gogus S, Ozyurek E, Aslan D, Gumruk F, Cetin M, et al. Primary hemophagocytic lymphohistiocytosis in Turkish children. Pediatr Hematol Oncol. 2003 Jul-Aug;20(5):367–71. PubMed PMID: 12775534. Epub 2003/05/31. eng. [PubMed] [Google Scholar]
- 7.Niece JA, Rogers ZR, Ahmad N, Langevin AM, McClain KL. Hemophagocytic lymphohistiocytosis in Texas: observations on ethnicity and race. Pediatr Blood Cancer. 2010 Mar;54(3):424–8. doi: 10.1002/pbc.22359. PubMed PMID: 19953651. Epub 2009/12/03. eng. [DOI] [PubMed] [Google Scholar]
- 8.Allen CE, Yu X, Kozinetz CA, McClain KL. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008 Jun;50(6):1227–35. doi: 10.1002/pbc.21423. PubMed PMID: 18085676. Epub 2007/12/19. eng. [DOI] [PubMed] [Google Scholar]
- 9.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011 Oct 13;118(15):4041–52. doi: 10.1182/blood-2011-03-278127. PubMed PMID: 21828139. Epub 2011/08/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003 Nov 14;115(4):461–73. doi: 10.1016/s0092-8674(03)00855-9. PubMed PMID: 14622600. Epub 2003/11/19. eng. [DOI] [PubMed] [Google Scholar]
- 11.Ohadi M, Lalloz MR, Sham P, Zhao J, Dearlove AM, Shiach C, et al. Localization of a gene for familial hemophagocytic lymphohistiocytosis at chromosome 9q21.3-22 by homozygosity mapping. Am J Hum Genet. 1999 Jan;64(1):165–71. doi: 10.1086/302187. PubMed PMID: 9915955. Epub 1999/01/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999 Dec 3;286(5446):1957–9. doi: 10.1126/science.286.5446.1957. PubMed PMID: 10583959. Epub 1999/12/03. eng. [DOI] [PubMed] [Google Scholar]
- 13.zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005 Mar 15;14(6):827–34. doi: 10.1093/hmg/ddi076. PubMed PMID: 15703195. Epub 2005/02/11. eng. [DOI] [PubMed] [Google Scholar]
- 14.Cote M, Menager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009 Dec;119(12):3765–73. doi: 10.1172/JCI40732. PubMed PMID: 19884660. Epub 2009/11/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arico M, Imashuku S, Clementi R, Hibi S, Teramura T, Danesino C, et al. Hemophagocytic lymphohistiocytosis due to germline mutations in SH2D1A, the X-linked lymphoproliferative disease gene. Blood. 2001 Feb 15;97(4):1131–3. doi: 10.1182/blood.v97.4.1131. PubMed PMID: 11159547. Epub 2001/02/13. eng. [DOI] [PubMed] [Google Scholar]
- 16.Rubin CM, Burke BA, McKenna RW, McClain KL, White JG, Nesbit ME, Jr., et al. The accelerated phase of Chediak-Higashi syndrome. An expression of the virus-associated hemophagocytic syndrome? Cancer. 1985 Aug 1;56(3):524–30. doi: 10.1002/1097-0142(19850801)56:3<524::aid-cncr2820560320>3.0.co;2-z. PubMed PMID: 2988747. Epub 1985/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 17.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000 Jun;25(2):173–6. doi: 10.1038/76024. PubMed PMID: 10835631. Epub 2000/06/03. eng. [DOI] [PubMed] [Google Scholar]
- 18.Enders A, Zieger B, Schwarz K, Yoshimi A, Speckmann C, Knoepfle EM, et al. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood. 2006 Jul 1;108(1):81–7. doi: 10.1182/blood-2005-11-4413. PubMed PMID: 16551969. Epub 2006/03/23. eng. [DOI] [PubMed] [Google Scholar]
- 19.Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006 Nov 2;444(7115):110–4. doi: 10.1038/nature05257. PubMed PMID: 17080092. Epub 2006/11/03. eng. [DOI] [PubMed] [Google Scholar]
- 20.Grunebaum E, Zhang J, Dadi H, Roifman CM. Haemophagocytic lymphohistiocytosis in X-linked severe combined immunodeficiency. Br J Haematol. 2000 Mar;108(4):834–7. doi: 10.1046/j.1365-2141.2000.01923.x. PubMed PMID: 10792291. Epub 2000/05/03. eng. [DOI] [PubMed] [Google Scholar]
- 21.Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009 May;119(5):1350–8. doi: 10.1172/JCI37901. PubMed PMID: 19425169. Epub 2009/05/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClain K, Gehrz R, Grierson H, Purtilo D, Filipovich A. Virus-associated histiocytic proliferations in children. Frequent association with Epstein-Barr virus and congenital or acquired immunodeficiencies. Am J Pediatr Hematol Oncol. 1988;10(3):196–205. Fall. PubMed PMID: 2845831. Epub 1988/01/01. eng. [PubMed] [Google Scholar]
- 23.Chen TL, Wong WW, Chiou TJ. Hemophagocytic syndrome: an unusual manifestation of acute human immunodeficiency virus infection. Int J Hematol. 2003 Dec;78(5):450–2. doi: 10.1007/BF02983819. PubMed PMID: 14704039. Epub 2004/01/06. eng. [DOI] [PubMed] [Google Scholar]
- 24.Fardet L, Blum L, Kerob D, Agbalika F, Galicier L, Dupuy A, et al. Human herpesvirus 8-associated hemophagocytic lymphohistiocytosis in human immunodeficiency virus-infected patients. Clin Infect Dis. 2003 Jul 15;37(2):285–91. doi: 10.1086/375224. PubMed PMID: 12856221. Epub 2003/07/12. eng. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki N, Morimoto A, Ohga S, Kudo K, Ishida Y, Ishii E. Characteristics of hemophagocytic lymphohistiocytosis in neonates: a nationwide survey in Japan. J Pediatr. 2009 Aug;155(2):235–8. e1. doi: 10.1016/j.jpeds.2009.02.050. PubMed PMID: 19446847. Epub 2009/05/19. eng. [DOI] [PubMed] [Google Scholar]
- 26.Dutta U, Mittal S, Ratho RK, Das A. Acute liver failure and severe hemophagocytosis secondary to parvovirus B19 infection. Indian J Gastroenterol. 2005 May-Jun;24(3):118–9. PubMed PMID: 16041107. Epub 2005/07/26. eng. [PubMed] [Google Scholar]
- 27.Imashuku S. Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol. 2002 Dec;44(3):259–72. doi: 10.1016/s1040-8428(02)00117-8. PubMed PMID: 12467966. Epub 2002/12/07. eng. [DOI] [PubMed] [Google Scholar]
- 28.Risdall RJ, Brunning RD, Hernandez JI, Gordon DH. Bacteria-associated hemophagocytic syndrome. Cancer. 1984 Dec 15;54(12):2968–72. doi: 10.1002/1097-0142(19841215)54:12<2968::aid-cncr2820541226>3.0.co;2-4. PubMed PMID: 6498770. Epub 1984/12/15. eng. [DOI] [PubMed] [Google Scholar]
- 29.Brastianos PK, Swanson JW, Torbenson M, Sperati J, Karakousis PC. Tuberculosis-associated haemophagocytic syndrome. Lancet Infect Dis. 2006 Jul;6(7):447–54. doi: 10.1016/S1473-3099(06)70524-2. PubMed PMID: 16790385. Epub 2006/06/23. eng. [DOI] [PubMed] [Google Scholar]
- 30.Numata K, Tsutsumi H, Wakai S, Tachi N, Chiba S. A child case of haemophagocytic syndrome associated with cryptococcal meningoencephalitis. J Infect. 1998 Jan;36(1):118–9. doi: 10.1016/s0163-4453(98)93594-0. PubMed PMID: 9515682. Epub 1998/11/20. eng. [DOI] [PubMed] [Google Scholar]
- 31.Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007 Dec;7(12):814–22. doi: 10.1016/S1473-3099(07)70290-6. PubMed PMID: 18045564. Epub 2007/11/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falini B, Pileri S, De Solas I, Martelli MF, Mason DY, Delsol G, et al. Peripheral T-cell lymphoma associated with hemophagocytic syndrome. Blood. 1990 Jan 15;75(2):434–44. PubMed PMID: 2153036. Epub 1990/01/15. eng. [PubMed] [Google Scholar]
- 33.Menard F, Besson C, Rince P, Lambotte O, Lazure T, Canioni D, et al. Hodgkin lymphoma-associated hemophagocytic syndrome: a disorder strongly correlated with Epstein-Barr virus. Clin Infect Dis. 2008 Aug 15;47(4):531–4. doi: 10.1086/590152. PubMed PMID: 18611160. Epub 2008/07/10. eng. [DOI] [PubMed] [Google Scholar]
- 34.Miyahara M, Sano M, Shibata K, Matsuzaki M, Ibaraki K, Shimamoto Y, et al. B-cell lymphoma-associated hemophagocytic syndrome: clinicopathological characteristics. Ann Hematol. 2000 Jul;79(7):378–88. doi: 10.1007/s002770000155. PubMed PMID: 10965786. Epub 2000/08/31. eng. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien MM, Lee-Kim Y, George TI, McClain KL, Twist CJ, Jeng M. Precursor B-cell acute lymphoblastic leukemia presenting with hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008 Feb;50(2):381–3. doi: 10.1002/pbc.20950. PubMed PMID: 16856156. Epub 2006/07/21. eng. [DOI] [PubMed] [Google Scholar]
- 36.Okuda T, Sakamoto S, Deguchi T, Misawa S, Kashima K, Yoshihara T, et al. Hemophagocytic syndrome associated with aggressive natural killer cell leukemia. Am J Hematol. 1991 Dec;38(4):321–3. doi: 10.1002/ajh.2830380412. PubMed PMID: 1746541. Epub 1991/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki S, Nakamura F, Nasu R, Nannya Y, Ichikawa M, Kurokawa M. Haemophagocytic lymphohistiocytosis is a recurrent and specific complication of acute erythroid leukaemia. Br J Haematol. 2011 Jun;153(5):669–72. doi: 10.1111/j.1365-2141.2010.08544.x. PubMed PMID: 21275966. Epub 2011/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu Y, Tanae K, Takahashi N, Kohri M, Arai E, Bessho M, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010 Feb;34(2):263–6. doi: 10.1016/j.leukres.2009.07.001. PubMed PMID: 19640585. Epub 2009/07/31. eng. [DOI] [PubMed] [Google Scholar]
- 39.Tatsuta H, Hoso T, Takagi T, Kakimoto T, Shimada T, Sakamoto H, et al. Small cell lung cancer presenting as hemophagocytic syndrome. Nihon Naika Gakkai Zasshi. 2005 Apr 10;94(4):756–8. doi: 10.2169/naika.94.756. PubMed PMID: 15865006. Epub 2005/05/04. jpn. [DOI] [PubMed] [Google Scholar]
- 40.Koizumi K, Haseyama Y, Machino R, Sato Y, Sawada K, Koike T. The hemophagocytic syndrome in prostate cancer revealed by disseminated carcinomatosis of the bone marrow. J Urol. 2002 Sep;168(3):1101–2. doi: 10.1016/S0022-5347(05)64588-0. PubMed PMID: 12187236. Epub 2002/08/21. eng. [DOI] [PubMed] [Google Scholar]
- 41.Sakai T, Shiraki K, Deguchi M, Itoh N, Konishi T, Takase K, et al. Hepatocellular carcinoma associated with hemophagocytic syndrome. Hepatogastroenterology. 2001 Sep-Oct;48(41):1464–6. PubMed PMID: 11677988. Epub 2001/10/27. eng. [PubMed] [Google Scholar]
- 42.Takagi S, Masuoka K, Uchida N, Ishiwata K, Araoka H, Tsuji M, et al. High incidence of haemophagocytic syndrome following umbilical cord blood transplantation for adults. Br J Haematol. 2009 Nov;147(4):543–53. doi: 10.1111/j.1365-2141.2009.07863.x. PubMed PMID: 19709082. Epub 2009/08/28. eng. [DOI] [PubMed] [Google Scholar]
- 43.Karras A, Thervet E, Legendre C. Hemophagocytic syndrome in renal transplant recipients: report of 17 cases and review of literature. Transplantation. 2004 Jan 27;77(2):238–43. doi: 10.1097/01.TP.0000107285.86939.37. PubMed PMID: 14742988. Epub 2004/01/27. eng. [DOI] [PubMed] [Google Scholar]
- 44.Atteritano M, David A, Bagnato G, Beninati C, Frisina A, Iaria C, et al. Haemophagocytic syndrome in rheumatic patients. A systematic review. Eur Rev Med Pharmacol Sci. 2012 Oct;16(10):1414–24. PubMed PMID: 23104659. Epub 2012/10/30. eng. [PubMed] [Google Scholar]
- 45.Wong KF, Hui PK, Chan JK, Chan YW, Ha SY. The acute lupus hemophagocytic syndrome. Ann Intern Med. 1991 Mar 1;114(5):387–90. doi: 10.7326/0003-4819-114-5-387. PubMed PMID: 1992881. Epub 1991/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 46.Morris JA, Adamson AR, Holt PJ, Davson J. Still’s disease and the virus-associated haemophagocytic syndrome. Ann Rheum Dis. 1985 May;44(5):349–53. doi: 10.1136/ard.44.5.349. PubMed PMID: 4004365. Epub 1985/05/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben m’rad M, Leclerc-Mercier S, Blanche P, Franck N, Rozenberg F, Fulla Y, et al. Drug-induced hypersensitivity syndrome: clinical and biologic disease patterns in 24 patients. Medicine (Baltimore) 2009 May;88(3):131–40. doi: 10.1097/MD.0b013e3181a4d1a1. PubMed PMID: 19440116. Epub 2009/05/15. eng. [DOI] [PubMed] [Google Scholar]
- 48.Knutson MD, Vafa MR, Haile DJ, Wessling-Resnick M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood. 2003 Dec 1;102(12):4191–7. doi: 10.1182/blood-2003-04-1250. PubMed PMID: 12907459. Epub 2003/08/09. eng. [DOI] [PubMed] [Google Scholar]
- 49.Selleri C, Sato T, Anderson S, Young NS, Maciejewski JP. Interferon-gamma and tumor necrosis factor-alpha suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol. 1995 Dec;165(3):538–46. doi: 10.1002/jcp.1041650312. PubMed PMID: 7593233. Epub 1995/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 50.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004 Aug 1;104(3):735–43. doi: 10.1182/blood-2003-10-3413. PubMed PMID: 15069016. Epub 2004/04/08. eng. [DOI] [PubMed] [Google Scholar]
- 51.Nagafuji K, Nonami A, Kumano T, Kikushige Y, Yoshimoto G, Takenaka K, et al. Perforin gene mutations in adult-onset hemophagocytic lymphohistiocytosis. Haematologica. 2007 Jul;92(7):978–81. doi: 10.3324/haematol.11233. PubMed PMID: 17606450. Epub 2007/07/04. eng. [DOI] [PubMed] [Google Scholar]
- 52.Zhang K, Jordan MB, Marsh RA, Johnson JA, Kissell D, Meller J, et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood. 2011 Nov 24;118(22):5794–8. doi: 10.1182/blood-2011-07-370148. PubMed PMID: 21881043. Epub 2011/09/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldmann J, Le Deist F, Ouachee-Chardin M, Certain S, Alexander S, Quartier P, et al. Functional consequences of perforin gene mutations in 22 patients with familial haemophagocytic lymphohistiocytosis. Br J Haematol. 2002 Jun;117(4):965–72. doi: 10.1046/j.1365-2141.2002.03534.x. PubMed PMID: 12060139. Epub 2002/06/13. eng. [DOI] [PubMed] [Google Scholar]
- 54.Molleran Lee S, Villanueva J, Sumegi J, Zhang K, Kogawa K, Davis J, et al. Characterisation of diverse PRF1 mutations leading to decreased natural killer cell activity in North American families with haemophagocytic lymphohistiocytosis. J Med Genet. 2004 Feb;41(2):137–44. doi: 10.1136/jmg.2003.011528. PubMed PMID: 14757862. Epub 2004/02/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horne A, Ramme KG, Rudd E, Zheng C, Wali Y, al-Lamki Z, et al. Characterization of PRF1, STX11 and UNC13D genotype-phenotype correlations in familial hemophagocytic lymphohistiocytosis. Br J Haematol. 2008 Oct;143(1):75–83. doi: 10.1111/j.1365-2141.2008.07315.x. PubMed PMID: 18710388. Epub 2008/08/20. eng. [DOI] [PubMed] [Google Scholar]
- 56.Chuang HC, Lay JD, Hsieh WC, Wang HC, Chang Y, Chuang SE, et al. Epstein-Barr virus LMP1 inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome. Blood. 2005 Nov 1;106(9):3090–6. doi: 10.1182/blood-2005-04-1406. PubMed PMID: 16002423. Epub 2005/07/09. eng. [DOI] [PubMed] [Google Scholar]
- 57.Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL Study Group of the Histiocyte Society. Semin Oncol. 1991 Feb;18(1):29–33. PubMed PMID: 1992521. Epub 1991/02/01. eng. [PubMed] [Google Scholar]
- 58.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007 Feb;48(2):124–31. doi: 10.1002/pbc.21039. PubMed PMID: 16937360. Epub 2006/08/29. eng. [DOI] [PubMed] [Google Scholar]
- 59.Ueda I, Ishii E, Morimoto A, Ohga S, Sako M, Imashuku S. Correlation between phenotypic heterogeneity and gene mutational characteristics in familial hemophagocytic lymphohistiocytosis (FHL) Pediatr Blood Cancer. 2006 Apr;46(4):482–8. doi: 10.1002/pbc.20511. PubMed PMID: 16365863. Epub 2005/12/21. eng. [DOI] [PubMed] [Google Scholar]
- 60.Kogawa K, Lee SM, Villanueva J, Marmer D, Sumegi J, Filipovich AH. Perforin expression in cytotoxic lymphocytes from patients with hemophagocytic lymphohistiocytosis and their family members. Blood. 2002 Jan 1;99(1):61–6. doi: 10.1182/blood.v99.1.61. PubMed PMID: 11756153. [DOI] [PubMed] [Google Scholar]
- 61.Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011 Jan;56(1):154–5. doi: 10.1002/pbc.22774. PubMed PMID: 20842751. Epub 2010/09/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Komp DM, McNamara J, Buckley P. Elevated soluble interleukin-2 receptor in childhood hemophagocytic histiocytic syndromes. Blood. 1989 Jun;73(8):2128–32. PubMed PMID: 2786434. Epub 1989/06/01. eng. [PubMed] [Google Scholar]
- 63.Imashuku S, Hibi S, Sako M, Ishida Y, Mugishima H, Chen J, et al. Soluble interleukin-2 receptor: a useful prognostic factor for patients with hemophagocytic lymphohistiocytosis. Blood. 1995 Dec 15;86(12):4706–7. PubMed PMID: 8541568. Epub 1995/12/15. eng. [PubMed] [Google Scholar]
- 64.Bleesing J, Prada A, Siegel DM, Villanueva J, Olson J, Ilowite NT, et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007 Mar;56(3):965–71. doi: 10.1002/art.22416. PubMed PMID: 17328073. Epub 2007/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 65.Goel S, Polski JM, Imran H. Sensitivity and specificity of bone marrow hemophagocytosis in hemophagocytic lymphohistiocytosis. Ann Clin Lab Sci. 2012;42(1):21–5. Winter. PubMed PMID: 22371906. Epub 2012/03/01. eng. [PubMed] [Google Scholar]
- 66.Tang Y, Xu X, Song H, Yang S, Shi S, Wei J, et al. Early diagnostic and prognostic significance of a specific Th1/Th2 cytokine pattern in children with haemophagocytic syndrome. Br J Haematol. 2008 Oct;143(1):84–91. doi: 10.1111/j.1365-2141.2008.07298.x. PubMed PMID: 18673367. Epub 2008/08/05. eng. [DOI] [PubMed] [Google Scholar]
- 67.Xu XJ, Tang YM, Song H, Yang SL, Xu WQ, Zhao N, et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012 Jun;160(6):984–90. e1. doi: 10.1016/j.jpeds.2011.11.046. PubMed PMID: 22226576. Epub 2012/01/10. eng. [DOI] [PubMed] [Google Scholar]
- 68.Henter JI, Samuelsson-Horne A, Arico M, Egeler RM, Elinder G, Filipovich AH, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002 Oct 1;100(7):2367–73. doi: 10.1182/blood-2002-01-0172. PubMed PMID: 12239144. Epub 2002/09/20. eng. [DOI] [PubMed] [Google Scholar]
- 69.Trottestam H, Horne A, Arico M, Egeler RM, Filipovich AH, Gadner H, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011 Oct 27;118(17):4577–84. doi: 10.1182/blood-2011-06-356261. PubMed PMID: 21900192. Epub 2011/09/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajagopala S, Singh N, Agarwal R, Gupta D, Das R. Severe hemophagocytic lymphohistiocytosis in adults-experience from an intensive care unit from North India. Indian J Crit Care Med. 2012 Oct;16(4):198–203. doi: 10.4103/0972-5229.106501. PubMed PMID: 23559726. Epub 2013/04/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shabbir M, Lucas J, Lazarchick J, Shirai K. Secondary hemophagocytic syndrome in adults: a case series of 18 patients in a single institution and a review of literature. Hematol Oncol. 2011 Jun;29(2):100–6. doi: 10.1002/hon.960. PubMed PMID: 20809477. Epub 2010/09/03. eng. [DOI] [PubMed] [Google Scholar]
- 72.Park HS, Kim DY, Lee JH, Kim SD, Park YH, Lee JS, et al. Clinical features of adult patients with secondary hemophagocytic lymphohistiocytosis from causes other than lymphoma: an analysis of treatment outcome and prognostic factors. Ann Hematol. 2012 Jun;91(6):897–904. doi: 10.1007/s00277-011-1380-3. PubMed PMID: 22147006. Epub 2011/12/08. eng. [DOI] [PubMed] [Google Scholar]
- 73.Strout MP, Seropian S, Berliner N. Alemtuzumab as a bridge to allogeneic SCT in atypical hemophagocytic lymphohistiocytosis. Nat Rev Clin Oncol. 2010 Jul;7(7):415–20. doi: 10.1038/nrclinonc.2010.40. PubMed PMID: 20404855. Epub 2010/04/21. eng. [DOI] [PubMed] [Google Scholar]
- 74.Marsh RA, Allen CE, McClain KL, Weinstein JL, Kanter J, Skiles J, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013 Jan;60(1):101–9. doi: 10.1002/pbc.24188. PubMed PMID: 22522603. Epub 2012/04/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper N, Rao K, Gilmour K, Hadad L, Adams S, Cale C, et al. Stem cell transplantation with reduced-intensity conditioning for hemophagocytic lymphohistiocytosis. Blood. 2006 Feb 1;107(3):1233–6. doi: 10.1182/blood-2005-05-1819. PubMed PMID: 16219800. Epub 2005/10/13. eng. [DOI] [PubMed] [Google Scholar]
- 76.Horne A, Janka G, Maarten Egeler R, Gadner H, Imashuku S, Ladisch S, et al. Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis. Br J Haematol. 2005 Jun;129(5):622–30. doi: 10.1111/j.1365-2141.2005.05501.x. PubMed PMID: 15916685. Epub 2005/05/27. eng. [DOI] [PubMed] [Google Scholar]
- 77.Ouachee-Chardin M, Elie C, de Saint Basile G, Le Deist F, Mahlaoui N, Picard C, et al. Hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis: a single-center report of 48 patients. Pediatrics. 2006 Apr;117(4):e743–50. doi: 10.1542/peds.2005-1789. PubMed PMID: 16549504. Epub 2006/03/22. eng. [DOI] [PubMed] [Google Scholar]
- 78.Baker KS, Filipovich AH, Gross TG, Grossman WJ, Hale GA, Hayashi RJ, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2008 Aug;42(3):175–80. doi: 10.1038/bmt.2008.133. PubMed PMID: 18454181. Epub 2008/05/06. eng. [DOI] [PubMed] [Google Scholar]
- 79.Cooper N, Rao K, Goulden N, Webb D, Amrolia P, Veys P. The use of reduced-intensity stem cell transplantation in haemophagocytic lymphohistiocytosis and Langerhans cell histiocytosis. Bone Marrow Transplant. 2008 Oct;42(Suppl 2):S47–50. doi: 10.1038/bmt.2008.283. PubMed PMID: 18978744. Epub 2008/11/26. eng. [DOI] [PubMed] [Google Scholar]
- 80.Marsh RA, Vaughn G, Kim MO, Li D, Jodele S, Joshi S, et al. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010 Dec 23;116(26):5824–31. doi: 10.1182/blood-2010-04-282392. PubMed PMID: 20855862. Epub 2010/09/22. eng. [DOI] [PubMed] [Google Scholar]
- 81.Marsh RA, Rao K, Satwani P, Lehmberg K, Muller I, Li D, et al. Allogeneic hematopoietic cell transplantation for XIAP deficiency: an international survey reveals poor outcomes. Blood. 2013 Feb 7;121(6):877–83. doi: 10.1182/blood-2012-06-432500. PubMed PMID: 23131490. Epub 2012/11/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buyse S, Teixeira L, Galicier L, Mariotte E, Lemiale V, Seguin A, et al. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 2010 Oct;36(10):1695–702. doi: 10.1007/s00134-010-1936-z. PubMed PMID: 20532477. Epub 2010/06/10. eng. [DOI] [PubMed] [Google Scholar]
- 83.Imashuku S. Treatment of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis (EBV-HLH); update 2010. J Pediatr Hematol Oncol. 2011 Jan;33(1):35–9. doi: 10.1097/MPH.0b013e3181f84a52. PubMed PMID: 21088619. Epub 2010/11/23. eng. [DOI] [PubMed] [Google Scholar]
- 84.Imashuku S, Kuriyama K, Teramura T, Ishii E, Kinugawa N, Kato M, et al. Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J Clin Oncol. 2001 May 15;19(10):2665–73. doi: 10.1200/JCO.2001.19.10.2665. PubMed PMID: 11352958. Epub 2001/05/16. eng. [DOI] [PubMed] [Google Scholar]