Abstract
Switching from acetylation to methylation at histone H3 lysine 9 (K9) has recently been shown to contribute to euchromatin gene silencing. To identify genes silenced by K9 modifications, we probed a human CpG island microarray with DNA obtained by chromatin immunoprecipitation (ChIP) in a cancer cell line using an anti-H3-K9 methylated antibody or an anti-H3-K9 acetylated antibody. Of the 27 clones with the highest signal ratio of K9 methylation over acetylation (Me/Ac), 13 contained repetitive sequences. Among 14 nonrepetitive clones, we identified 11 genes (seven known and four previously undescribed), one EST, and two unknown fragments. Using ChIP-PCR, all 18 examined clones showed higher ratios of H3-K9 Me/Ac than the active gene control, P21, thus confirming the microarray data. In addition, we found a strong correlation between the K9 Me/Ac ratio and CpG island DNA methylation (R = 0.92, P < 0.01), and five of seven genes examined (megalin, thrombospondin-4, KR18, latrophilin-3, and phosphatidylinositol-3-OH kinase P101 subunit) showed lack of expression by RT-PCR and reactivation by DNA methylation and/or histone deacetylase inhibition, suggesting that these genes are true targets of silencing through histone modifications. All five genes also showed significant DNA methylation in a cell line panel and in primary colon cancers. Our data suggest that CpG island microarray coupled with ChIP can identify novel targets of gene silencing in cancer. This unbiased approach confirms the tight coupling between DNA methylation and histone modifications in cancer and could be used to probe gene silencing in nonneoplastic conditions as well.
Expression of genes in higher organisms critically depends on DNA accessibility. Any gene within condensed chromatin is silent. DNA methylation and histone modifications are two major epigenetic mechanisms that can affect gene expression through chromatin structure (1-3). Precise transcriptional regulation of a subset of genes is required to maintain normal cellular proliferation and differentiation. In fact, disruption of epigenetic mechanisms is closely linked to aberrant expression and leads to abnormal development and, potentially, malignant transformation (1, 4, 5).
Histone modifications have been reported to include acetylation, phosphorylation, methylation, ADP ribosylation, and ubiquitination (2). Multiple residues on each of the four core histones have been identified as potential modification sites, and some lysine (K) residues, such as histone 3-K9 (H3-K9), can be either methylated or acetylated. Different combinations of histone modifications at different residues may act synergistically or antagonistically to affect gene expression. Recent studies have shown that switching acetylation to methylation on H3-K9 contributes to euchromatin gene silencing in many organisms (6). Indeed, we recently described increased H3-K9 methylation and decreased H3-K9 acetylation in cells with promoter DNA methylation-associated silencing of the P16, MLH1, and MGMT genes (7). However, the global distribution of target genes silenced by methylation on H3-K9 has not been well characterized.
We have previously used an unbiased approach to clone targets of H3-K9 methylation using chromatin immunoprecipitation (ChIP) and found that most such clones contain Alu repetitive elements (8). An obvious functional significance of those findings was to provide a molecular mechanism for the known silencing of Alu elements. However, given that we did not find CpG island (CGI) promoter regions using that method, an alternate approach is needed to identify which nonrepeated genes are targets of silencing by H3-K9 modification.
Recently, CGI microarrays coupled with ChIP systems, in which direct targets of site-specific transcription factors can be identified under biologically relevant conditions, have been used to identify targets of the E2F transcription factor (9). Here we used this method for identifying genes controlled by H3-K9 modification. By applying the method to a cancer cell line, we found 12 genes [seven known genes; megalin, thrombospondin-4 (THBS4), KR18, latrophilin-3 (LPH3), phosphatidylinositol 3 OH kinase-P101 subunit (P101), and five previously undescribed genes] that were silenced by H3-K9 methylation, in association with DNA methylation. Our data show that CGI microarray, combined with ChIP using antimethylated H3-K9 and antiacetylated H3-K9 antibodies, is a powerful strategy for identifying targets of genes controlled by histone modifications.
Materials and Methods
Cell Lines and Culture Conditions. The colon cancer cell line, SW48, was grown in L-15 medium (GIBCO/BRL) plus 10% FBS in plastic tissue culture plates in a humidified atmosphere containing 5% CO2 at 37°C. The cells were grown to a density of 1.0 × 106 to 3.0 × 106 cells per dish before being harvested for crosslinking experiments. The cell line was obtained from the American Type Culture Collection. We also used DNA from the NCI 60 (Bethesda) collection of cancer cell line samples.
Trichostatin A (TSA) and 5-Aza-2′-deoxycytidine (DAC) Treatment of Cells. Cells were split 12-24 h before treatment. Cells were then treated with either DAC, 5 μM (Sigma), or PBS (control) daily for 3 days. TSA (300 nM, ICN) or an identical volume of ethanol (control) treatment was performed for 20 h.
Collection of Patient Samples. Forty-four primary colorectal cancer samples and 10 corresponding adjacent tissues were collected from consenting patients at the Johns Hopkins Hospital and M.D. Anderson Cancer Center in accordance with institutional policies. Tumors were selected solely based on tissue availability.
ChIP. The protocols for ChIP have been described (7). Briefly, SW48 cells are treated with 1% formaldehyde for 8 min to crosslink histones to DNA. After washing by cold PBS, the cell pellets are resuspended in lysis buffer (150 mM NaCl/25 mM Tris·Cl, pH 7.5/5 mM EDTA/1% Triton X-100/0.1% SDS/0.5% sodium deoxycholate) and sonicated for 8 sec × 7 times. The lysate is then divided into three fractions. The first lysate is incubated with 10 μl of anti-K9 methylated histone H3 antibody, anti-K9 acetylated histone H3 antibody, or anti-K4 methylated histone H3 antibody (all from Upstate Biotechnology, Lake Placid, NY) at 4°C overnight. The second lysate is incubated with TE buffer (10 mM Tris/1 mM EDTA, pH 8.0, 10 μl) at 4°C overnight as a negative control. The third lysate is used for input control. To collect the immunoprecipitated complexes, protein A-Sepharose beads (Pharmacia Biotech) are added and incubated for 1 h at 4°C. After washing, the beads are treated with RNase (50 μg/ml) for 30 min at 37°C and then proteinase K overnight. The crosslinks are then reversed by heating the sample at 65°C for 6 h. DNA is extracted by the phenol/chloroform method, ethanol-precipitated, and resuspended in water. To obtain DNA enriched with methylated or acetylated H3-K9 or no-antibody control for microarray, we performed ChIP from 60 plates of 10-cm dishes each with methylated or acetylated H3-K9 or without antibody.
CGI Microarray Analysis. The protocol for CGI microarray hybridization has been described (10). The resource material for preparing the microarray panel was derived from a CGI library. A total of 7,776 CGI clones were individually organized in 96-well culture chambers as master plates. A fraction (≈1 μl) of each clone was transferred to a well of separate 96-well PCR tubes by using the MULTI-PRINT replicator (V&P Science, San Diego). CGI inserts (0.2-2 kb) from these clones were amplified by PCR as described. We used the Affymetrix/GMS 417 microarrayer to dot unpurified PCR products (≈0.02 μl per dot, 0.1 μg/μl), in the presence of 20% DMSO, as microdots (150-μm diameter spaced at 300 μm) on poly-L-lysine-coated microscope slides. Spotted DNA was denatured before use. The combined methylated H3-K9- or acetylated H3-K9-specific samples and the combined no-antibody samples were then labeled and used to probe the microarray. Incorporation of amino-allyl dUTP (aa-dUTP, Sigma) into 2 μg each of methylated or acetylated K9-precipitated DNA and control no-antibody DNA was conducted by using the Bioprime DNA-labeling system protocol (Life Technologies, Grand Island, NY). Cy5 and Cy3 fluorescent dyes were coupled to aa-dUTP-labeled methylated K9 or acetylated K9-precipitated DNA and input DNA, respectively, and cohybridized to the microarray panel. Microarray protocols, including the hybridization and posthybridization washing procedures, can be found at www.microarrays.org. Hybridized slides were scanned with a GenePix 4000A scanner (Axon Instruments, Foster City, CA), and the acquired images were analyzed with the software gene pix pro 3.0. CGI tags having a Cy5/Cy3 ratio >2 were chosen as methylated or acetylated K9-specific signals.
Confirmation ChIP-PCR. ChIP product from SW48 was used for confirmation PCR with the oligonucleotide primers in Table 2, which is published as supporting information on the PNAS web site. The PCR products were visualized by 6% polyacrylamide gel electrophoresis and quantitated by densitometry or capillary electrophoresis by using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). To ensure that PCR amplification was in the linear range, each reaction was initially set up at different dilutions of DNA for varying amplification cycle number, and we selected the final PCR conditions accordingly. The assays were done in duplicate.
Bisulfite-PCR Methylation Analysis. We performed bisulfite treatment as reported previously (11). Briefly, 2 μg of genomic DNA were denatured with 2 M NaOH for 10 min, followed by incubation with 3 M sodium bisulfite (pH 5.0) for 16 h at 50°C. After treatment, DNA was purified by using a Wizard Miniprep Column (Promega), precipitated with ethanol, and resuspended in 30 μl of diluted water. We used in vitro spiroplasma sp-strain MQ1 (SssI)-treated DNA as a positive control for methylation studies. DNA extracted from the RKO cell line was treated by SssI methylase (New England BioLabs) according to the manufacturer's protocol. Two microliters of the aliquot was used as template for combined bisulfite restriction analysis (COBRA) with the oligonucleotide primers shown in Table 2. After amplification, 50-80% of the PCR products were digested with restriction enzymes as shown in Table 2. In this analysis, only methylated alleles change size after restriction enzyme digestion. PCR products were separated by 5% PAGE and stained with ethidium bromide.
RNA Extraction and RT-PCR. Total cellular RNA was extracted with TRIzol (GIBCO/BRL) according to the manufacturer's protocol. RNA was resuspended in diethyl pyrocarbonate-treated water and was quantitated by spectrophotometry. Reverse transcription reactions were done on 2 μg of total RNA and were performed by using Moloney murine leukemia virus-RT (Invitrogen) according to the manufacturer's protocol. cDNA was amplified by PCR by using oligonucleotides shown in Table 2. All reactions included negative controls where reverse transcriptase was omitted.
Statistics. Correlation analyses were done by using excel software (Microsoft). Two-sided P values are shown, and P < 0.05 was considered statistically significant.
Results
We performed ChIP by using antimethylated and antiacetylated H3-K9 antibodies. The immunoprecipitated DNA was used to probe a microarray containing 7,776 CGIs, as described (10). We selected the 27 clones that showed the highest ratio of H3-K9 methylation over acetylation (K9 Me/Ac) for further analysis (Table 1). All clones were resequenced for verification. The length of positive CGI clones on the microarray ranged from 163 to 807 bp. To characterize the positive clones, we searched a 2-kb genomic sequence surrounding the CGI clones by blast (www.ncbi.nlm.nih.gov) and blat (genome.ucsc.edu) searches. The CGI clones corresponded to 11 genes, including seven known and four previously undescribed genes (spliced EST), and one was near an EST. Two clones had no homology to any gene or ESTs. Thirteen clones contained repeat elements, either short interspersed transposable element, long terminal repeat, long interspersed transposable element, or ribosomal DNA. All 14 clones that did not contain repeat elements had CGIs within 1 kb. The CGIs corresponded to the 5′ promoter area of all 11 genes identified.
Table 1. Targets of H3-K9 modification identified by ChIP-CpG microarray.
| Signal on microarray
|
Density of ChIP-PCR
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | K9 Me | K9 Ac | K9 Me/Ac | K9 Me | K9 Ac | K4 Me | K9 Me/Ac | Length | Locus | CpG* | Accession no. | Location | Gene |
| 1 | 29 | 2 | 14.50 | 0.6 | 1.0 | 1.1 | 0.6 | 786 | 1q32.1 | Y | AL445483 | 124331-125116 | Undescribed gene (spliced EST) |
| 2 | 21 | 8 | 2.63 | 1.2 | 0.6 | 0.6 | 2.0 | 357 | 18q21.1 | N | AC027216 | 121993-122349 | HERVH48 (LTR) |
| 3 | 8 | 6 | 1.33 | 163 | 1q25.1 | N | AL031599 | 52868-53030 | MSTA (LTR) | ||||
| 4 | 12 | 11 | 1.09 | 1.0 | 0.9 | 1.4 | 1.1 | 221 | 20q11.21 | Y | AL359765 | 6215-6435 | L2 (LINE) |
| 5 | 8 | 8 | 1.00 | 483 | 1p31.2 | N | AL391820 | 26845-26363 | LTR5B (LTR) | ||||
| 6 | 12 | 12 | 1.00 | 0.9 | 0.4 | 0.6 | 2.3 | 386 | 2q31.1 | Y | AC009336 | 132133-132518 | Unknown |
| 7 | 11 | 12 | 0.92 | 0.6 | 1.1 | 1.3 | 0.5 | 694 | 6P21.33 | Y | AL929592 | 18847-18154 | G7b |
| 8 | 13 | 18 | 0.72 | 1.1 | 0.9 | 1.3 | 1.2 | 522 | 19p13.11 | Y | AC005793 | 21068-21589 | AluSq (within 146 bp) |
| 9 | 18 | 25 | 0.72 | 1.1 | 0.4 | 0.3 | 2.8 | 762 | 20q11.21 | N | AL121762 | 48594-49356 | MIRb (SINE) |
| 10 | 21 | 35 | 0.60 | 0.6 | 1.0 | 1.1 | 0.6 | 420 | 1q42.12 | Y | AL365444 | 84014-84433 | EST (nonspliced) |
| 11 | 16 | 27 | 0.59 | 452 | 15 random | N | Z61873 | 1-179 | AluSq | ||||
| 12 | 44 | 76 | 0.58 | 0.8 | 0.5 | 0.5 | 1.6 | 718 | 2q31-q32.1 | Y | AC008178 | 105732-105003 | Megalin, gp330 |
| 13 | 20 | 36 | 0.56 | 0.6 | 0.2 | 0.3 | 3.0 | 308 | 5q13 | Y | AC112184 | 156594-156901 | Thrombospondin 4 |
| 14 | 9 | 17 | 0.53 | 452 | rDNA | N | U13369 | 28256-28707 | Ribosomal DNA | ||||
| 15 | 13 | 25 | 0.52 | 649 | 14q32.33 | N | AL590362 | 12867-13515 | AluY (within 30 bp) | ||||
| 16 | 11 | 22 | 0.50 | 556 | 2p11.2 | N | AC006453 | 22070-22625 | AluSq (within 165 bp) | ||||
| 17 | 14 | 30 | 0.47 | 1.3 | 0.5 | 0.6 | 2.6 | 724 | 12q14.1 | Y | AC078789 | 49373-50096 | New gene (spliced EST) |
| 18 | 14 | 31 | 0.45 | 544 | 8q24.3 | Y | AC087815 | 38519-37975 | AluJb (134bp up), EST | ||||
| 19 | 20 | 47 | 0.43 | 1.0 | 0.4 | 0.6 | 2.5 | 630 | 19q13.42 | Y | AC010328 | 43303-43932 | KR18 |
| 20 | 8 | 19 | 0.42 | 1.5 | 0.8 | 0.8 | 1.9 | 696 | 20q11.21 | Y | AL121762 | 33498-32857 | Unknown |
| 21 | 12 | 29 | 0.41 | 1.2 | 0.5 | 0.5 | 2.4 | 458 | 4q12-q13.3 | Y | AC092643 | 143188-143645 | Latrophilin-3 (LPH3) |
| 22 | 124 | 329 | 0.38 | 398 | 6q11.2 | N | AL596065 | 9605-10002 | LTR1 (LTR), EST | ||||
| 23 | 13 | 35 | 0.37 | 552 | 14q32.33 | N | AL512356 | 18118-18669 | MER4-int (LTR) | ||||
| 24 | 15 | 42 | 0.36 | 1.1 | 0.5 | 0.5 | 2.2 | 488 | 17p13.1 | Y | AC090610 | 7301-7788 | PI3-Kinase P101 Subunit |
| 25 | 19 | 57 | 0.33 | 0.6 | 0.8 | 0.8 | 0.8 | 807 | 1q24-25.3 | Y | AL032864 | 2555-1824 | Undescribed gene (spliced EST) |
| 26 | 10 | 35 | 0.29 | 0.7 | 1.1 | 1.6 | 0.6 | 323 | Xq22-q24 | Y | AC004835 | 34531-34853 | PRMC1 |
| 27 | 16 | 56 | 0.29 | 1.3 | 1.2 | 1.2 | 1.1 | 636 | 19q13.41 | Y | AC011468 | 110279-110914 | Undescribed gene (spliced EST) |
LTR, long terminal repeat; SINE, short interspersed transposable element.
CpG island within 1 kb of clone.
To confirm that the identified clones were truly bound by methylated K9 but not acetylated K9 in vivo, we performed standard ChIP for all 14 nonrepetitive clones and for four randomly selected repeat elements (Fig. 1). For amplification of the 14 nonrepetitive clones, primer sets were designed within the CGIs and also close to the clones themselves. For amplification of the four repeat element clones, at least one primer was located in the flanking (nonrepetitive) region to ensure that we were studying that specific repeat element. For controls we used P21, P16, and MLH1. In SW48, P21 is expressed and shows a high degree of K9 acetylation and K4 methylation and a low degree of K9 methylation, whereas P16 and MLH1 show a low degree of K9 acetylation and K4 methylation and a high degree of K9 methylation, as we reported previously (7) (Fig. 1 A). All selected clones examined by ChIP-PCR showed higher levels of K9 methylation than P21, thus validating the microarray data. In addition, 13 of 18 clones examined showed higher levels of K9 methylation than acetylation, which was similar to P16 and MLH1. Interestingly, in all four repeat element clones (C2, 4, 8, and 9), K9 methylation was more than two times higher than that in P21. K9 acetylation density and K4 methylation density were well correlated, except for C4 (L2) and C8 (AluSq). In these two clones, K9 methylation was higher than K9 acetylation, whereas K4 methylation was higher than K9 methylation. We calculated the ratios of K9 methylation over acetylation (K9 Me/Ac) for all clones (Fig. 1B). The ratio in 13 clones was more than two times higher than that in P21. The other five clones showed only moderately to slightly higher ratios than P21. These data generally confirm the validity of the ChIP microarray hybridizations
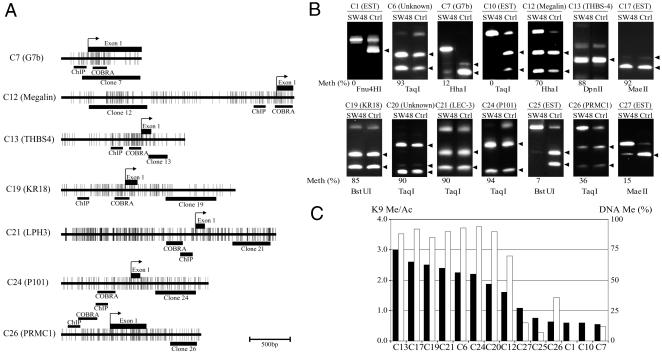
Fig. 1.
Confirmation of histone H3-K9 modification targets. (A) A ChIP experiment using SW48 cells is shown. Antibodies against histone H3-methylated K9 (K9 Me), histone H3-acetylated K9 (K9 Ac), histone H3-methylated K4 (K4 Me), or no antibody (-) were used. A fixed portion of the total input (0.4%) was also examined by PCR. P21, P16, and MLH1 promoter regions were used for control. P21 showed a low degree of K9 methylation and a high degree of K9 acetylation and K4 methylation with gene activation. By contrast, P16 and MLH1 showed a high degree of K9 methylation and a low degree of K9 acetylation and K4 methylation. The intensity of the PCR bands was quantitated by an Agilent 2100 bioanalyzer. Ratios of precipitated DNA over input DNA were calculated as a relative precipitated fold shown below each sample. (B) Ratios of K9 methylation density over K9 acetylation density (K9 Me/Ac) were calculated for each clone (open bars) and controls (closed bars) and are shown on the y axis.
To study the mechanisms of the observed alterations in histone code, we next examined DNA methylation status by COBRA in the 14 nonrepetitive clones (Fig. 2). The CGIs of seven representative genes are illustrated in Fig. 2 A. We designed COBRA primers close to the transcription start site of all genes. Clones with a high ratio of K9 Me/Ac (C6, 12, 13, 17, 19, 20, 21, and 24) all showed a high level of DNA methylation (Fig. 2B). Of the clones with slightly or moderately increased levels of K9 Me/Ac, three (C7, C25, and C26) showed partial DNA methylation, whereas four showed no DNA methylation. Because COBRA can analyze the methylation status of only one or two CpG sites at a time, we used two different restriction enzymes for digestion and found that both gave identical results (data not shown). The DNA methylation status and ratio of K9 Me/Ac by ChIP-PCR are summarized in Fig. 2C. Every clone whose K9 Me/Ac ratio was >1.5 also showed high levels of DNA methylation. However, clones whose K9 Me/Ac ratio was <1.1 showed a low level of DNA methylation. Overall, we found a strong concordance between DNA methylation and the ratio of K9 Me/Ac in these 14 clones (R = 0.92, P < 0.01).
Fig. 2.
DNA methylation status of unique identified clones. (A) CpG maps of the five genes analyzed in this study. For each gene, CpG density is indicated by short vertical bars. Exon 1 is indicated by black rectangles on the top, whereas location of the clones is indicated by black rectangles on the bottom. Arrows point to known or presumed transcription start sites. Areas analyzed by ChIP and COBRA are indicated by black bars on the bottom. (B) COBRA analysis of each gene. We used spiroplasma sp-strain MQ1 methylase-treated DNA as a positive control for methylation studies (Ctrl). Arrows indicate the methylated alleles. In each case, methylation density is indicated below each sample as a percentage. (C) Correlation between the ratio of K9 methylation over acetylation (K9 Me/Ac) in ChIP-PCR and the level of DNA methylation. Ratio of K9 Me/Ac in ChIP-PCR and the percentage of DNA methylation are shown on the left and right y axes, respectively. Black and white bars indicate the ratio of K9 Me/Ac and the level of DNA methylation, respectively. Bisulfite-treated DNA was amplified with gene-specific primers. PCR products were digested with enzymes specific to methylated alleles (indicated below each bar). There is a strong correlation between the ratio of K9 Me/Ac and the level of DNA methylation (R = 0.92, P < 0.01).
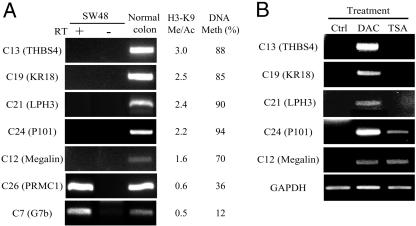
To confirm silencing, we next performed expression analysis by RT-PCR for the seven known genes identified [C7, G7b; C12, megalin; C13, thrombospondin-4 (THBS4); C19, KR18; C21, latrophilin-3 (LPH3); C24, phosphatidylinositol-3-OH kinase P101 subunit (P101); and C26, progesterone receptor membrane component 1 (PRMC1)]. Five genes (megalin, THBS4, KR18, LPH3, and P101) were silenced in SW48, whereas two genes (G7b and PRMC1) were expressed (Fig. 3). This expression status was very consistent with both K9 Me/Ac and DNA methylation status. We next examined the effects of treatment with the DNA-methyltransferase inhibitor DAC or the histone deacetylase inhibitor TSA on the silenced genes. DAC could reactivate all five silenced genes (Fig. 3). Interestingly, TSA could also reactivate megalin and P101, although those CpG promoters were highly methylated. To confirm that our method was detecting H3-K9 target promoters selectively, we preliminarily examined several clones showing high signals on a CGI microarray probed with ChIP DNA products from SW48 using an H3-K9 antibody that does not recognize the methylated moiety specifically. We randomly selected 10 positive genes from this microarray and found that only one gene was silenced (data not shown).
Fig. 3.
RNA expression of each gene as determined by RT-PCR. (A) All seven identified known genes are expressed in normal colon. For each gene, the ratio of histone H3-K9 methylation over acetylation (H3-K9 Me/Ac) using ChIP-PCR and the percentage of DNA methylation level (DNA Meth) are shown (Right). Gene expression, the ratio of K9 Me/Ac, and DNA methylation are all correlated in these genes. (B) SW48 cells were treated with DAC and TSA. DAC reactivates all five silenced genes. TSA also reactivates megalin and P101, even though the CGI promoters are highly methylated.
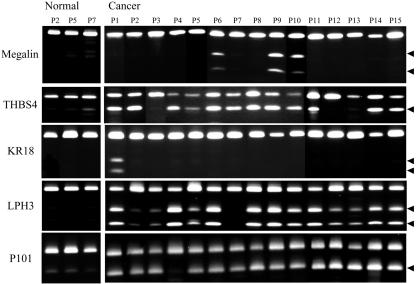
We next examined the methylation status of the five genes, megalin, THBS4, KR18, LPH3, and P101, in primary colorectal cancer patients and cell lines from multiple tissues. Representation of COBRA analysis of normal and cancerous tissues in colorectal cancer patients for megalin, THBS4, KR18, LPH3, and P101 is shown in Fig. 4. We summarized the methylation status of the five genes in Fig. 5, which is published as supporting information on the PNAS web site. We randomly examined 10 noncancerous tissues from corresponding colon cancer patients. Megalin and KR18 had no detectable methylation in normal samples studied using the COBRA assay. By contrast, there was methylation of THBS4, LPH3, and P101 in a subset of cases (median methylation density 7.9%, 2.6%, and 18.7%, respectively). Because low levels of methylation have an uncertain biological significance and can occasionally be found in normal tissues, we used a cutoff of 15% methylation to call a cancer methylation positive.
Fig. 4.
Representative examples of methylation analysis in colon cancer patients by COBRA. Bisulfite-treated DNA from cancer and paired normal tissues was amplified and digested with restriction enzymes that cleave only the methylated CpG sites. The arrows indicate the methylated alleles. Patient numbers are indicated above each lane.
In cancerous tissues, 10 (23%), 33 (75%), 7 (16%), 35 (80%), and 41 (93%) of 44 patients showed aberrant methylation of megalin, THBS4, KR18, LPH3, and P101, respectively (median methylation density 26.9%, 18.2%, 44.9%, 41.9%, and 41.4%, respectively). Methylation status in multiple tissue cell lines showed that 18 (31%), 45 (78%), 6 (10%), 20 (34%), and 46 (79%) cell lines showed methylation of megalin, THBS4, KR18, LPH3, and P101, respectively. It is noteworthy that aberrant promoter methylation of these genes did not equally arise in all tissues. For instance, megalin was not methylated in nonsmall cell lung cancer (NSCLC) and brain cancer (CNS). KR18 was not methylated in breast cancer, NSCLC and CNS. LPH3 was not methylated in melanoma and CNS. Interestingly, methylation of all five genes was found most frequently in colon cancer cell lines.
Discussion
Modifications of histone tails are thought to specify a code that regulates the expression of genes (2). Given the number of sites and the variety of possible modifications, the combinatorial possibilities are extremely large. However, among the many possible modifications of histone tails, histone H3-K9 and K27 methylation are the only modifications that have been well documented to be linked to euchromatic gene silencing so far (6). Switching from acetylation to methylation at histone H3-K9 contributes to gene silencing in cells with promoter DNA methylation-associated silencing (7, 12, 13). Thus, here we focused on identification of genes silenced through histone H3-K9 modification, because tumor suppressor gene inactivation by H3-K9 modification has been reported to be involved in carcinogenesis. We demonstrated that chromatin immunoprecipitated DNA with antibodies to histone H3-methylated K9 or acetylated K9 can be used as hybridization probes on a CGI microarray to allow the unbiased discovery of target promoters. Using ChIP-PCR, we were able to confirm the validity of the microarray data, and the identification of silenced genes ultimately proves the value of this approach. Overall, only 2/27 clones were likely false positives.
The value of the approach described here lies in the fact that it is relatively unbiased and depends solely on histone modifications. Thus, in addition to studying disease states such as cancer, one could use this technique to identify genes silenced during development and/or differentiation. By contrast, gene expression microarrays do not distinguish silenced states (accompanied by histone modifications) from physiologic (or transient) decreased expression. DNA methylation-based gene identification is also powerful (14-16) but is limited to the subset of genes for which silencing is accompanied by promoter methylation. Indeed, in the absence of unbiased approaches such as the one described here, it has been difficult to ascertain whether histone modifications are always linked to promoter DNA methylation. Finally, a similar approach has been recently described by using methyl-binding protein antibodies (17), but this approach is also tightly linked to promoter DNA methylation.
As performed here, this approach to studying chromatin modifications will mostly be useful for identifying target genes. The overall survey likely underestimates the total number of hypermethylated/hypoacetylated loci in this cell line, because the CGI fragments on the chip may not be situated in the ideal location (i.e., promoter and first exon regions) for probing, the whole genome is not represented, and we used rigid criteria for positive hybridization signals. With additional loci on the microarrays and careful testing and validation of the hybridization procedures, it may eventually be possible to use this approach to obtain a snapshot of gene-specific modifications in a given tissue or cell line. This would be very useful for studying gene silencing that is potentially unlinked to DNA methylation (for example, in normal tissues or in disease situations accompanied by deregulation of chromatin modifiers such as MLL). It would also be of interest to apply this technique to cells exposed to DNA methylation inhibitors in vitro (16) or in vivo (18).
Half of the clones we identified were related to repetitive elements. This is an underestimation, because the CGI microarray was depleted in repetitive sequences. These data confirm our prior study showing enrichment for methylated H3-K9 at Alu repeat elements (8) and suggest that a major function of histone code modifications is suppression of repeat elements. It is interesting that all 14 nonrepetitive clones studied showed an inverse correlation between K9 methylation and each of K9 acetylation and K4 methylation, whereas two repetitive elements (L2 and AluSq) showed high levels of both K9 and K4 methylation, suggesting that histone H3 modification patterns in some repetitive elements might be different from those of euchromatin genes. Indeed, K4 methylation has been reported to be required for rDNA silencing in Saccharomyces cerevisiae (19).
In Neurospora crassa, genomic DNA methylation patterns are partially controlled by the methylation status of histones (20). In mammals, it is still unclear which comes on first, DNA methylation or histone modifications, with data supporting both possibilities (7, 21). In this study, we found a strong correlation between the level of DNA methylation and the ratio of K9 Me/Ac. According to these unbiased results, histone K9 modification and DNA methylation frequently (if not always) coexist at silenced loci in this cell line, supporting the notion that the interactions between DNA methylation and histone H3 lys9 methylation form a reinforcing silencing loop. Our data do not address which modification comes first, but it will be interesting to use this microarray approach in other cells to see whether genes with such histone modifications are always accompanied by DNA methylation.
In the seven identified known genes, we found a strong correlation between the ratio of K9 Me/Ac, DNA methylation status, and expression. Five genes, megalin, thrombospondin-4 (THBS4), KR18, latrophilin-3 (LPH3), and phosphatidylinositol 3 OH kinase-P101 subunit (P101), were truly silenced in SW48, with a substantially high ratio of K9 Me/Ac and a high level of DNA methylation. Two additional genes, G7b and PRMC1, were expressed in SW48 but showed partial methylation on the promoter CGI and a moderately elevated ratio K9 Me/Ac, suggesting possibly monoallelic or heterogeneous methylation. These results suggest that the histone dynamics at silenced loci in cancer, described so far for a handful of genes only, are fairly universal.
It is unknown whether silencing and inactivation of the five genes identified here are related to carcinogenesis. Megalin is a member of the low-density lipoprotein (LDL) receptor gene family, which can bind to multiple ligands and possibly activate intracellular signaling cascades. It is located in the apical surfaces of epithelial tissues and mediates the endocytic uptake of diverse macromolecules (lipoprotein, protease, and antiproteinase). Interestingly, inhibitors of LDL production have been reported to have antineoplastic effects, suggesting a potential link to neoplasia. THBS4 is a member of the extracellular calcium-binding protein family, which is involved in cell proliferation, adhesion, and migration. Remarkably, THBS1 (22) and THBS2 (23) have also been shown to be silenced in some cancers. KR18 is a member of the Krüppel-related zinc finger protein family. One-third of the proteins in this family contain a Krüppel associated box domain, which has been shown to be a potent repressor of transcription by interacting with the TIF I/KAP-1/KRIP-1 family (24). LPH3 is a cell-surface G protein-linked receptor with very large extra and intracellular domains. Based on its structure, it seems likely that LPH3 has a physiological function as a cell-adhesion molecule coupled to signal transduction. P101 is a noncatalytic domain of phosphatidylinositol 3-kinase γ (PI3Kγ), which is related to cellular proliferation, growth, apoptosis, and cytoskeletal rearrangements (25). Interestingly, one strain of mice lacking P110γ, a catalytic domain of PI3Kγ, developed colon carcinomas (26), raising the possibility that p110γ might function as a tumor-suppressor gene, although other strains did not show this effect (27), and this issue is not resolved. Interestingly, P110γ itself has been shown to have hypermethylation in colon cancer (28). Further studies will be needed to determine the functional significance of inactivation of these genes in cancers.
Conclusion
Our study suggests that a CGI microarray coupled with ChIP using anti-H3-K9 methylated and acetylated antibodies is a powerful technique to identify target genes silenced by the epigenetic machinery. This technique will be useful in identifying genes silenced in normal and pathologic conditions, potentially independent of DNA methylation states.
Supplementary Material
Acknowledgments
This work was supported by research grants from the National Institutes of Health [R33 CA-89837 (to J.-P.J.I.) and RO1 CA-69065 (to T.H.M.H.)] and by the George and Barbara Bush Fund for Innovative Cancer Research. Y.K. is supported by the Uehara Memorial Foundation of Japan.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ChIP, chromatin immunoprecipitation; TSA, trichostatin A; DAC, 5-aza-2′-deoxycytidine; CpG island, CGI; COBRA, combined bisulfite restriction analysis.
References
- 1.Jones, P. A. & Takai, D. (2001) Science 293, 1068-1070. [DOI] [PubMed] [Google Scholar]
- 2.Jenuwein, T. & Allis, C. D. (2001) Science 293, 1074-1080. [DOI] [PubMed] [Google Scholar]
- 3.Jaenisch, R. & Bird, A. (2003) Nat. Genet. 33 Suppl., 245-254. [DOI] [PubMed] [Google Scholar]
- 4.Jones, P. A. & Laird, P. W. (1999) Nat. Genet. 21, 163-167. [DOI] [PubMed] [Google Scholar]
- 5.Baylin, S. B., Herman, J. G., Graff, J. R., Vertino, P. M. & Issa, J. P. J. (1998) Adv. Cancer Res. 72, 141-196. [PubMed] [Google Scholar]
- 6.Lachner, M. & Jenuwein, T. (2002) Curr. Opin. Cell Biol. 14, 286-298. [DOI] [PubMed] [Google Scholar]
- 7.Kondo, Y., Shen, L. & Issa, J. P. (2003) Mol. Cell. Biol. 23, 206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo, Y. & Issa, J. P. (2003) J. Biol. Chem. 278, 27658-27662. [DOI] [PubMed] [Google Scholar]
- 9.Weinmann, A. S., Yan, P. S., Oberley, M. J., Huang, T. H. & Farnham, P. J. (2002) Genes Dev. 16, 235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan, P. S., Chen, C. M., Shi, H., Rahmatpanah, F., Wei, S. H. & Huang, T. H. (2002) J. Nutr. 132, 2430S-2434S. [DOI] [PubMed] [Google Scholar]
- 11.Clark, S. J., Harrison, J., Paul, C. L. & Frommer, M. (1994) Nucleic Acids Res. 22, 2990-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen, C. T., Weisenberger, D. J., Velicescu, M., Gonzales, F. A., Lin, J. C., Liang, G. & Jones, P. A. (2002) Cancer Res. 62, 6456-6461. [PubMed] [Google Scholar]
- 13.Fahrner, J. A., Eguchi, S., Herman, J. G. & Baylin, S. B. (2002) Cancer Res. 62, 7213-7218. [PubMed] [Google Scholar]
- 14.Shi, H., Wei, S. H., Leu, Y. W., Rahmatpanah, F., Liu, J. C., Yan, P. S., Nephew, K. P. & Huang, T. H. (2003) Cancer Res. 63, 2164-2171. [PubMed] [Google Scholar]
- 15.Toyota, M., Ho, C., Ahuja, N., Jair, K.-W., Ohe-Toyota, M., Baylin, S. B. & Issa, J. P. J. (1999) Cancer Res. 59, 2307-2312. [PubMed] [Google Scholar]
- 16.Suzuki, H., Gabrielson, E., Chen, W., Anbazhagan, R., van Engeland, M., Weijenberg, M. P., Herman, J. G. & Baylin, S. B. (2002) Nat. Genet. 31, 141-149. [DOI] [PubMed] [Google Scholar]
- 17.Ballestar, E., Paz, M. F., Valle, L., Wei, S., Fraga, M. F., Espada, J., Cigudosa, J. C., Huang, T. H. & Esteller, M. (2003) EMBO J. 22, 6335-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Issa, J. P., Garcia-Manero, G., Giles, F. J., Mannari, R., Thomas, D., Faderl, S., Bayar, E., Lyons, J., Rosenfeld, C. S., Cortes, J., et al. (2004) Blood 103, 1635-1640. [DOI] [PubMed] [Google Scholar]
- 19.Briggs, S. D., Bryk, M., Strahl, B. D., Cheung, W. L., Davie, J. K., Dent, S. Y., Winston, F. & Allis, C. D. (2001) Genes Dev. 15, 3286-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamaru, H. & Selker, E. U. (2001) Nature 414, 277-283. [DOI] [PubMed] [Google Scholar]
- 21.Bachman, K. E., Park, B. H., Rhee, I., Rajagopalan, H., Herman, J. G., Baylin, S. B., Kinzler, K. W. & Vogelstein, B. (2003) Cancer Cell 3, 89-95. [DOI] [PubMed] [Google Scholar]
- 22.Li, Q., Ahuja, N., Burger, P. C. & Issa, J. P. J. (1999) Oncogene 18, 3284-3289. [DOI] [PubMed] [Google Scholar]
- 23.Toyota, M., Kopecky, K. J., Toyota, M. O., Jair, K. W., Willman, C. L. & Issa, J. P. (2001) Blood 97, 2823-2829. [DOI] [PubMed] [Google Scholar]
- 24.Collins, T., Stone, J. R. & Williams, A. J. (2001) Mol. Cell. Biol. 21, 3609-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foukas, L. C. & Okkenhaug, K. (2003) Arch. Biochem. Biophys. 414, 13-18. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki, T., Irie-Sasaki, J., Horie, Y., Bachmaier, K., Fata, J. E., Li, M., Suzuki, A., Bouchard, D., Ho, A., Redston, M., et al. (2000) Nature 406, 897-902. [DOI] [PubMed] [Google Scholar]
- 27.Barbier, M., Attoub, S., Calvez, R., Laffargue, M., Jarry, A., Mareel, M., Altruda, F., Gespach, C., Wu, D., Lu, B., et al. (2001) Nature 413, 796. [DOI] [PubMed] [Google Scholar]
- 28.Semba, S., Itoh, N., Ito, M., Youssef, E. M., Harada, M., Moriya, T., Kimura, W. & Yamakawa, M. (2002) Clin. Cancer Res. 8, 3824-3831. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.