Abstract
Organophosphate (OP)-based chemicals are used worldwide for many purposes and they have likely saved millions of people from starvation and disease. However, due to their toxicity they can also pose a significant environmental risk. While considerable research has focused on the acute symptoms and long-term consequences of overtly toxic exposures to OPs, less attention has been given to the subject of repeated exposures to levels that are not associated with acute symptoms (subthreshold exposures). There is clinical evidence indicating that this type of OP exposure can lead to prolonged deficits in cognition; however only a few studies have addressed this issue prospectively in animal models. In this study, repeated subthreshold exposures to the OP nerve agent diisopropylfluorophosphate (DFP) were evaluated in a 5-Choice Serial Reaction Time Task (5C-SRTT), an animal model of sustained attention. Adult rats were trained to stably perform the 5C-SRTT and then injected subcutaneously with vehicle or DFP 0.5 mg/kg every other day for 30 days. Behavioral testing occurred daily during the DFP-exposure period and throughout a 45 day (OP-free) washout period. Compared to vehicle-treated controls, DFP-treated rats exhibited deficits in accuracy, increases in omissions and timeout responses during the OP exposure period, while no significant effects on premature responses, perseverative responses, or response latencies were noted. While the increase in timeout responses remained detectible during washout, all other DFP-related alterations in 5C-SRTT performance abated. When the demands of the task were increased by the presentation of variable intertrial intervals, premature responses were also elevated in DFP-treated rats during the washout period. These results indicate that repeated exposures to subthreshold doses of DFP lead to reversible impairments in sustained attention as well as persistent impairments of inhibitory response control in rats.
Keywords: Organophosphate, Pesticide, Cholinesterase inhibitor, Cognition, Memory, Nerve Agent
1. Introduction
The class of chemicals known as the “organophosphates” (OPs) are found in hundreds of compounds including pesticides, herbicides, anthelmintics, chemical warfare (“nerve”) agents, ophthalmic agents, and industrial solvents, etc. (Katz and Brooks, 2010). In addition, the OP metrifonate has been documented to have moderate, but favorable effects on the cognitive symptoms in Alzheimer disease (Becker et al., 1998). The value of OPs as pesticides (their most common use) in optimizing agricultural productivity, the control of deadly vector-borne illnesses (e.g., malaria, yellow fever, typhus), and “nuisance” pests (e.g., flies, roaches, mosquitoes) is clear (reviewed, Cooper and Dobson, 2007) and they have likely saved millions of people from starvation and disease). Accordingly, OPs comprise the most widely used insecticides in the world and their use is increasing (see Ross et al., 2013). An unfortunate consequence of this widespread use, however, is the increasing number of OP poisonings worldwide, particularly in developing countries where adequate protective measures are often lacking (De Silva et al., 2006). While less likely for most people, the threat of OP exposure from intentional poisonings by rogue governments and terrorist organizations is also an ongoing concern. The Iraqi military (nerve agent) attacks on Kurdish civilians in the 1980s (Macilwain, 1993), the Tokyo Sarin attack in 1995 by domestic terrorists (Nagao et al., 1997), and the recent sarin attacks on civilians in Syria (United Nations Security Council Report, 2013) exemplify this concern.
The acute symptoms of OP toxicity have been studied extensively and are relatively-well characterized; however, the consequences of prolonged or repeated exposures to levels of OPs that produce no overt signs of acute toxicity are much less well understood. This type of exposure (referred to as “low level”, “subacute”, “subtoxic”, “subclinical”, and “subthreshold”) has been associated with prolonged neurological deficits including impairments of cognition. For example, a recently published meta-analysis of 14 studies (selected from more than 600) which fulfilled strict statistical criteria for inclusion and data from more than 1600 participants, found an association between subthreshold OP exposures and impaired neurobehavioral function. The domains of cognition most affected included, attention, working memory, executive function, visuospatial ability and visual memory (Ross et al., 2013). However, these authors commented in their discussion that it remains unclear whether the human health risks of exposure to some OPs have been underestimated or overestimated. Moreover, due to the retrospective nature of many of the relevant OP studies in humans, the variability of the testing methods used, the lack of knowledge on exposure levels, etc., the health outcomes in subjects chronically exposed to OPs, but never acutely poisoned, remain to be clearly elucidated (see reviews, Colosio et al., 2003; 2009). Prospective animal studies conducted in our laboratories and others appear to support the argument that chronic (or repeated) exposures to OPs at levels that are not associated with acute toxicity can indeed result in a variety of neurobehavioral effects, particularly cognitive deficits. For example, sustained deficits in delayed matching performance, sensorimotor gating, spatial learning and retention, recognition memory, and cognitive flexibility have been reported in association with subthreshold exposures to OPs (Bushnell et al., 1991, Terry et al., 2003; Terry et al., 2007; Terry et al., 2011; Yan et al., 2012; Terry et al., 2012). It is important to note, however, that in female rats gavaged 5 days a week for 4 weeks with the insecticide, chlorpyrifos (dose range 1–10 mg/kg/day) Maurissen and colleagues (Maurissen et al., 2000) found no deficits in a matching-to-position task.
We (Middlemore-Risher et al., 2010) have also observed protracted impairments of sustained attention and an increase in impulsive behaviors in rats exposed to subthreshold doses of the insecticide OP, chlorpyrifos (CPF) in a 5-Choice Serial Reaction Time Task (5C-SRTT), an animal model of sustained attention (see Robbins, 2002). In the study described here, we determined the effects of repeated subthreshold exposures to the prototypical nerve agent OP diisopropylfluorophosphate (DFP), on sustained attention in rats using the 5C-SRTT. The subthreshold dose of DFP (0.5 mg/kg) was determined in a previous study (Terry et al., 2011) and operationally defined as a dose that does not produce overt signs of cholinergic toxicity (e.g., fasciculations, seizures, diarrhea, excessive urination, salivation, etc., (see reviews, Rusyniak and Nanagas, 2004; Sungurtekin et al., 2006). DFP was originally developed by British researchers as a potential chemical warfare agent (see Saunders, 1957) and it possesses a great deal of structural homology with other highly toxic nerve agents such as sarin and soman, but is less potent (Hobbiger, 1972) and dangerous for laboratory personnel.
2. Materials and Methods
2.1. Compound Formulation
Rats (see section 2.2 below) received subcutaneous injections of vehicle (Kroger® Pure Peanut Oil) or DFP, CAS 55-91-4 (Sigma Aldrich D0879-1G Lot: 126K1306, St. Louis, MO) dissolved in vehicle over the course of a 30-day treatment period. All other chemicals were reagent grade or better and purchased from Fisher Scientific and Sigma Aldrich.
2.2. Test Subjects and Drug Administration
A total of 40 male albino Wistar rats (approximately 3 months old) were purchased from Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA and housed individually in a temperature controlled room (25°C), maintained on a 12:12h (lights on at 7:00 am) with free access to water and food (Teklad Global Rodent Diet 2918, Harlan, Madison, WI, USA) during the first week. From week 2 until the end of the study animals were food restricted to approximately 85% of their age-dependent, free-feeding weights based upon Harlan Laboratories growth rate curves. This was accomplished by a daily ration of approximately 12 grams of food administered per rat on weekdays in the afternoon after behavioral testing, with supplemental food administered on weekends (up to a total of approximately 20 grams/day). When 24 subjects (N=12 per test group) achieved the performance criteria (see section 2.3.2 below) they received subcutaneous injections of vehicle or DFP, 0.5 mg/kg dissolved in vehicle in a volume of 0.7 ml/kg body weight every other day over a 30 day treatment period. The test subjects were weighed and monitored (in their home cages for a period of approximately 5 min each day) for visible cholinergic signs (diarrhea, excessive salivation or lacrimation, respiratory difficulties, muscle fasciculations) or other signs of distress throughout the study. This dosing procedure was selected based on previous studies by Terry et al., 2011, and was defined as subthreshold according to the definition provided above. Both the cholinergic signs assessments and the behavioral evaluations described below were conducted by technicians who were blinded to the treatment group.
All procedures employed during this study were reviewed and approved by the Georgia Regents University Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain and discomfort in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996.
2.3. Behavioral Experiments
The animals in each behavioral cohort were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments.
2.3.1. Test Apparatus
DFP and vehicle-treated rats were evaluated using an automated 5C-SRTT described previously (Middlemore-Risher et al., 2010; Terry et al., 2012). Training and testing in the 5C-SRTT was conducted using eight ventilated, sound attenuated operant chambers (Med Associates, St. Albans, VT, USA). Each operant chamber consisted of nine nose pokes/apertures (2.5 cm wide, 4 cm deep), four of which were closed off with metal inserts thus every other nose poke was available. The apertures, arranged on a curved panel 2 cm above the floor of the chamber, were equipped with a photocell beam to detect nose pokes. Each aperture was equipped with a lamp (2.8 W) on the rear wall that could be illuminated randomly and for varying durations. Food pellets (45 mg chow pellet, BioServ, Frenchtown, NJ, USA) were delivered automatically to a magazine, located on the opposite wall to the nose pokes, that was also equipped with a light that turned on to indicate that a pellet had been dispensed. The food magazine was equidistant from all nose poke apertures. The house light remained on for the entire session unless an error or omission occurred. The apparatus was controlled using MedPC software (Med Associates, St. Albans, VT, USA).
2.3.2. 5C-SRTT Training
Rats began 5C-SRTT training with the stimulus duration (SD) of 10 seconds, each session being 100 trials or 30 minutes in duration with intertrial intervals (ITI) of 5 seconds. An initial pellet was delivered to the magazine to facilitate the start of each session. One of the 5 nose poke apertures was illuminated randomly for 10 sec after which the light was extinguished. The animal was then required to respond correctly by nose poking the previously illuminated aperture within 5 sec of the light being extinguished. A correct response in the previously established time frame (5 sec) resulted in a pellet being dispensed into the magazine that was simultaneously illuminated for a maximum of 5 sec or until the animal retrieved the pellet. Collection of this pellet initiated the intertrial interval, a delay of 5 sec, before the next trial began. An incorrect response, premature response, or failure to respond within 5 sec of the light stimulus being extinguished (omission) resulted in a 5 sec timeout, marked by the extinction of the house light for 5 sec and no food reward, after which the animal initiated the next trial by a nose poke into the magazine. Subjects were trained 5 days per week until they reached stable performance levels (defined as 4 consecutive days at >80% accuracy, <20% omissions and completion of all 100 trials) at the 10 seconds stimulus duration Once criterion was achieved at a given stimulus duration, the animals were moved to the next more challenging stimulus duration (5, 2.5, 2.0, 1.5, 1.25 and 1.0 second).
5C-SRTT Performance Assessments
After the training criterion described above was reached at the 1.0 sec stimulus duration in 24 animals, subjects were assigned to one of two treatment groups (N=12 per test group, vehicle or DFP) and balanced for performance (% correct, omissions, perseverative, premature and timeout responses) as close as possible before OP exposure. This was based on the 5 day baseline results (see below) such that there were no statistically significant differences between the groups on any of the performance measures. The following mean values were obtained for the assembled vehicle and DFP groups, respectively, percent correct = 84.44, 84.44; omissions, 4.3, 4.8; premature responses, 6.5, 7.1; perseverative responses, 4.0, 5.6, timeout responses, 12.0, 15.2. Since the operant chambers were the same, subjects were initially assigned to a particular chamber during acquisition (prior to treatment group assignment). However, during the group selection, it was not possible to maintain this original chamber assignment. Since some animals, in the newly formed groups, were now to be tested be in a different chamber, the subjects were trained for an additional 5 day baseline period to ensure no “chamber artifact” on stable performance. Animals then remained in the newly assigned chamber (counterbalanced for treatment group) for the duration of the study. After the 5-day baseline period described above, OP treatment began. Rats (N=3) were also randomly selected from the test groups for blood collection as behavioral performance was consistent across animals. Rats were tested in the 5C-SRTT five days per week (on weekdays)
Subjects were tested at: 1) a standard 1.0 second stimulus duration with a 5 second intertrial interval, 2) a randomized, varying stimulus duration (VSD) of 0.25, 0.50 and 1 second with a 5 second intertrial interval, 3) and a randomized varying intertrial interval (VITI) of 1, 5, and 10 seconds with a 1 second stimulus duration. The VSD and VITI tasks were conducted (no more than one time in any given week) a total of six times during the study, one time during the baseline period prior to OP exposure, two times during the OP exposure period, and three times during the OP-washout period. For the To assess performance the following parameters were measured: % correct ((# correct/(# correct + # incorrect))x100), # of omissions (see description above in section 2.3.2), premature responses (total # of responses performed after the trial began but before onset of the light stimulus), timeout responses (total # of nose pokes made in any aperture during a timeout period, perseverative responses (total # of nose pokes performed after the correct response had been made but before collecting the reward), trials completed, latency to correct response (time taken from onset of nose poke light stimulus to making the correct nose poke response), latency to incorrect response (time taken from onset of nose poke light stimulus to making the incorrect nose poke response), and latency to reward (i.e., the magazine latency, time taken from making a correct nose poke response to retrieving the reward from the magazine).
2.4. Measurement of Cholinesterase Activity
From vehicle and DFP treated subjects (N=3 rats/treatment), blood samples (400 μl) were obtained at day 0, 14 and 30 of the OP-free washout period by tail nick and collected in lithium heparin coated vials. Samples were centrifuged for 15 min at 2500 × g at 4–5 °C and the resulting plasma was frozen and stored at −70 °C until analyzed. On the final day of washout (day 45) subjects (N=6 rats/treatment) were sacrificed and plasma and brain were collected. Subjects were anesthetized with isofluorane and 3.0 ml of blood was collected via cardiac puncture into Vacutainers containing potassium EDTA (BD, Franklin Lakes, NJ, USA). Following centrifuge, plasma was collected and stored at −70°C (see above). After the blood draw the animals were quickly decapitated, brains were removed within 3 minutes, washed 3 times in saline, weighted and quickly frozen for storage at −70°C until analysis.
Cholinesterase activity was assessed in plasma and brain using a modification of the Ellman spectrophotometric method (Terry, et al., 2007) and similar that described previously by us Terry et al., 2011. Briefly, plasma or homogenate of whole brain (25% w/v in phosphate buffered saline) was added to 96 well plates with 300uL of reaction mixture (4.8uM acetylthiocholine iodide and 321uM dithiobisnitrobenzoate in 0.1 M Na2HPO4 buffer, pH 8.0; sigma) and shaken for 30 seconds on a Jitterbug, Boekel Scientific (Feasterville, PA, USA). Plates were then placed in Biotek uQuant plate reader (Philadelphia, PA, USA) and read at 412nm every 2 minutes for 16 minutes. The cholinesterase-mediated reaction rate (acetylthiocholine hydrolysis) was then calculated using the formula (moles/liter per min) = (Δ absorbance/min)/(1.36×104) then corrected for total protein (Coomassie Plus Assay; Pierce Biotechnology, Rockford, IL, USA). Cholinesterase activity was then expressed as nmoles acetylthiocholine hydrolyzed/min per mg protein.
2.5. Statistics
All statistical analyses were performed using SigmaPlot Version 11 (SPSS Inc., Chicago, IL) and statistical significance was assessed using an α level of 0.05.
Body Weights
To examine potential differences in body weights between the DFP and vehicle groups over the course of the study a two-factor repeated measures ANOVA was used. Animal nested within group was considered a random effect. Fixed effects in the model included treatment group (DFP or vehicle) and assessment day as well as the two-factor interaction between treatment group and assessment day. The statistical test of interest was the F-test for the two-factor interaction term and if statistically significant would indicate that the effect seen in the DFP group over the course of the study (the pattern of means across the study) was different than the effect seen in the vehicle group.
Cholinesterase Activity
To examine differences in plasma cholinesterase activity between DFP and vehicle over the washout period a two-factor repeated measures ANOVA was used. Animal nested within group was considered a random effect. Fixed effects in the model included treatment group (DFP or vehicle) and washout day (0, 14, 30 and 45 days) as well as the two-factor interaction between treatment group and washout day. The statistical test of interest was the F-test for the two-factor interaction term and when statistically significant would indicate that the effect seen in the DFP group over the washout period (the pattern of means across the washout period) was different than the effect seen in the vehicle group over the washout period. A Bonferroni (t-test) procedure was used to examine post-hoc pairwise differences on the adjusted least square means of the two-factor interaction term. A simple t-test was used to compare brain cholinesterase activity values at the end of the study.
Standard 5C-SRTT (Fixed SDs and ITIs)
The area under the curve (AUC) during two time periods (OP exposure period, OP washout period) was determined for each animal for: percent correct, omissions, premature response, perseverative response, and timeout responses. To examine differences in the AUC for each outcome between the DFP and vehicle group over the two time periods, a two-factor repeated measures ANOVA was used. Animal nested within group was considered a random effect. Fixed effects in the model included treatment group (DFP or vehicle) and time period as well as the two-factor interaction between treatment group and time period. The statistical test of interest was the F-test for the two-factor interaction term and when statistically significant would indicate that the effect seen in the DFP group over time (the pattern of means AUC across time) was different than the effect seen in the vehicle group over time. Regardless of whether the F-test for the two-factor interaction term was statistically insignificant (e.g. the mean response in both groups over time were parallel to each other), differences in AUCs at each time point between DFP and vehicle or differences between time points within treatment group could still be seen. A Bonferroni (t-test) procedure was used to examine post-hoc pairwise differences on the adjusted least square means of the two-factor interaction term.
5C-SRTT (Varying SDs and ITIs)
Of statistical interest here was not to compare session-to-session differences, rather to compare SD and ITI differences within session. To examine differences in the various outcome measures (percent correct, premature response, perseverative responses, etc.) between DFP and vehicle across SDs and ITIs a two-factor repeated measures ANOVA was used. Animal nested within group was considered a random effect. Fixed effects in the model included treatment group (DFP or vehicle) and SD (0.25, 0.50 and 1.00 sec) or ITI (1.0, 5.0 and 10.0 sec) as well as the two-factor interaction between treatment group and SD or ITI. The statistical test of interest was the F-test for the two-factor interaction term and when statistically significant would indicate that the effect seen in the DFP group over SD or ITI was different than the effect seen in the vehicle group over SD or ITI. Regardless of whether the F-test for the two-factor interaction term was statistically significant (e.g. the mean response in both groups over SD or ITI were parallel to each other), differences in means at each SD or ITI between DFP and vehicle or differences between SD and/or ITI within treatment group could still be seen. A Bonferroni (t-test) procedure was used to examine post-hoc pairwise differences on the adjusted least square means of the two-factor interaction term.
5C-SRTT-Response Latencies and Trials Completed
For both the standard and the VSD and VITI versions of the 5C-SRTT, latencies associated with correct and incorrect responses, magazine latencies, and the number of trials completed were averaged for each animal for sessions evaluated during the baseline period, the OP exposure period, and the washout period. To examine potential differences in the latencies and trials completed between DFP and vehicle groups a two-factor repeated measures ANOVA was used. Animal nested within group was considered a random effect. Fixed effects in the model included treatment group (DFP or vehicle) and assessment period as well as the two-factor interaction between treatment group and assessment period. The statistical test of interest was the F-test for the two-factor interaction term and if statistically significant would indicate that the effect seen in the DFP group over the three treatment periods (the pattern of means across the assessment periods) was different than the effect seen in the vehicle group.
3. Results
3.1 Subject Weights
Test subjects were weighed at the beginning of the study (day one of food restriction) and at multiple time points throughout the remainder of the study for accurate dosing purposes. For the 12 subjects (per treatment group) that met the training criteria (see section 2.3.2 above) the mean (± SEM) weights at the beginning of the study were, vehicle group = 327.33±12.13 grams; DFP group=365.25±14.03 grams, and the final day of the study, vehicle group = 409.00±3.95 grams; DFP group=412.00±4.32 grams. There were no statistically significant differences in weights between the test groups during any phase of the study and all 24 animals finished all components of the study.
3.2. Cholinesterase Activity
Fig 1A indicates the effects of DFP on plasma cholinesterase activity on the last day of drug administration (washout day zero) and at various time points during the OP-free washout period. Brain cholinesterase activity assessed at the end of the study (after the 45 day washout period is shown in Fig 1B. Statistical analysis indicated the following for the plasma cholinesterase activity comparison, main effect of treatment (F(1,4)= 0.81, p=0.42), day of washout (F(3,12)= 10.42, p<0.001), treatment x day of washout interaction (F(3,12)= 4.32, p=0.028). Post hoc analysis indicated that on the last day of drug administration (washout day zero), plasma cholinesterase activity was significantly (p=0.013) reduced in the DFP-treated subjects compared to vehicle-treated controls (i.e., by approximately 39%). At all other time points during the washout period, there were no group-related differences in plasma cholinesterase activity. This was also the case of for brain cholinesterase activity assessed at the end of the study (t-test p>0.05).
Fig 1.
The effects of DFP (0.5 mg/kg) on plasma (A) and brain (B) cholinesterase (ChE) activity (nmoles acetylthiocholine hydrolyzed/min per mg protein). From the test subjects (N=3/treatment), plasma was obtained at various points during the OP-free washout period up until day 30. On the final day of washout (day 45) subjects were sacrificed and plasma and brain were obtained (N=6/treatment). * = Statistically significant difference (p<0.05) between DFP-treated and vehicle-treated controls. Each bar represents the mean ± S.E.M
3.3. Standard 5C-SRTT (Fixed SDs and ITIs)
In the standard version of the 5C-SRTT there was a decrease in percent correct during exposure to DFP when compared to vehicle matched controls (Fig 2A) and this deficit eventually abated during the 45 day washout period. Statistical analysis of the area under the curves (AUCs, see Fig insets) for percent correct indicated the following, main effect of treatment, (F(1,22)= 3.72, p=0.067), time point (F(1,22)= 6043.86, p<0.001), treatment x time point interaction (F(1,22)= 4.47, p=0.046). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p=0.020) decrease in the AUC for percent correct compared to vehicle treated animals during the OP-exposure period, but not during the washout period. DFP exposure also resulted in an increase in the number of omissions during the OP exposure period (Fig 2B), an effect that also abated during the washout period. Statistical analysis of the AUCs (see Fig insets) for omissions indicated the following, main effect of treatment, (F(1,22)= 7.21, p=0.013), time point (F(1,22)= 0.05, p=0.82), treatment x time point interaction (F(1,22)= 2.01, p=0.17). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p=0.005) increase in the AUC for omissions compared to vehicle treated animals during the OP-exposure period, and a nearly significant (p=0.079) increase during the washout period.
Fig 2.
The effects of DFP (0.5 mg/kg) on % correct (A) and the number of omissions (B) in the standard version of the 5C-SRTT (fixed SDs and ITIs) during the 30 day every other day exposure period and during the 45 day (OP-free) washout period. The scatter plot insets illustrate the area under the curve (AUC) for the % correct and the number of omissions by test session (OP exposure period and washout period, respectively). * = p<0.05; ** = p<0.01, indicates a statistically significant difference between DFP-treated (N=12) and vehicle-treated (N=12) controls. Each symbol in the line plot represents the mean ± S.E.M
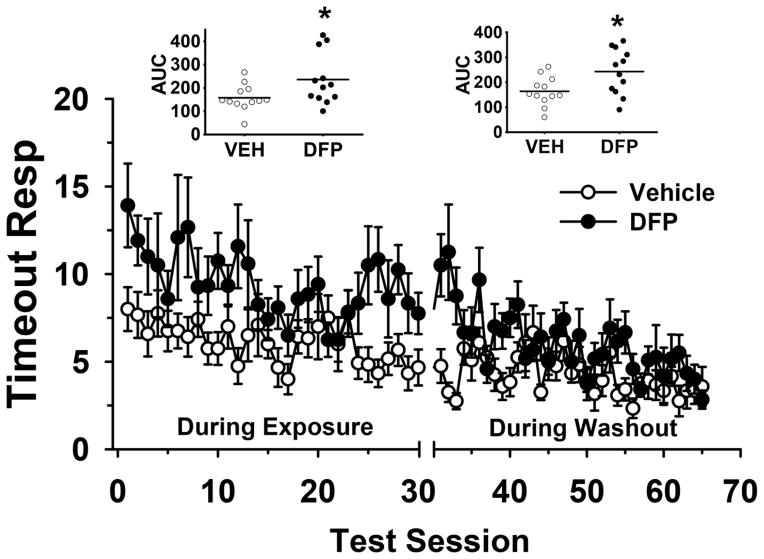
The number of premature and perseverative responses is shown in Fig 3A and 3B, respectively. AUCs are illustrated in the Fig insets. While premature and perseverative responses were generally higher in the DFP-treated subjects compared to vehicle-treated subjects throughout the study, none of the specific group (DFP versus VEH) comparisons reached the required level of statistical significance (i.e., all p values were >0.05). The number of timeout responses is shown in Fig 4. AUCs are illustrated in the Fig insets. Statistical analysis of the AUCs (see Fig insets) for timeout responses indicated the following, main effect of treatment, (F(1,22)= 7.95, p=0.010), time point (F(1,22)= 0.12, p=0.73), treatment x time point interaction (F(1,22)= 0.01, p=0.98). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p=0.025) increase in the AUC for timeout responses compared to vehicle treated animals during the OP-exposure period and this effect persisted during the washout period (p=0.023).
Fig 3.
The effects of DFP (0.5 mg/kg) on the number of premature responses (A) and perseverative responses (B) in the standard version of the 5C-SRTT (fixed SDs and ITIs) during the 30 day every other day exposure period and during the 45 day (OP-free) washout period. The scatter plot insets illustrate area under the curve (AUC) for the number of premature responses and perseverative responses by test session (OP exposure period and washout period, respectively). DFP-treated (N=12) and vehicle-treated controls (N=12). Each symbol in the line plot represents the mean ± S.E.M.
Fig 4.
The effects of DFP (0.5 mg/kg) on the number of timeout responses in the standard version of the 5C-SRTT (fixed SDs and ITIs) during the 30 day every other day exposure period and during the 45 day (OP-free) washout period. The scatter plot insets illustrate the area under the curve (AUC) for the number of timeout responses by test session (OP exposure period and washout periods, respectively). * = p<0.05, indicates a statistically significant difference between DFP-treated (N=12) and vehicle-treated (N=12) controls. Each symbol in the line plot represents the mean ± S.E.M.
The mean latencies associated with correct responses, incorrect responses, and reward collection (magazine latency) at baseline (i.e., before drug exposure), during the drug exposure period, and during the washout period are provided in Table 1. There were no statistically significant (DFP-exposure-related) differences in any if these measures.
Table 1.
Response Latencies and Trials Completed in the 5C-SRTT
| Standard Task | ||||||
|---|---|---|---|---|---|---|
| Treatment
|
Latency Correct (s)
|
Latency Incorrect (s)
|
||||
| Baseline | OP Exposure | OP Washout | Baseline | OP Exposure | OP Washout | |
| Vehicle | 0.82±0.03 | 0.84±0.02 | 0.83±0.02 | 1.87±0.08 | 1.91±0.07 | 2.01±0.07 |
| DFP | 0.78±0.03 | 0.91±0.04 | 0.82±0.03 | 1.96±0.13 | 2.29±0.10 | 1.99±0.08 |
|
Magazine Latencies (s)
|
Trials Completed
|
|||||
| Baseline | OP Exposure | OP Washout | Baseline | OP Exposure | OP Washout | |
| Vehicle | 1.51±0.11 | 1.49±0.10 | 1.40±0.08 | 100.00±0.00 | 99.94±0.06 | 100.00±0.00 |
| DFP | 1.45±0.10 | 1.59±0.10 | 1.43±0.07 | 100.00±0.00 | 97.18±0.96 | 100.00±0.00 |
| Variable Stimulus Duration Task | ||||||
|---|---|---|---|---|---|---|
| Treatment
|
Latency Correct (s)
|
Latency Incorrect (s)
|
||||
| Baseline | OP Exposure | OP Washout | Baseline | OP Exposure | OP Washout | |
| Vehicle | 0.84±0.03 | 0.80±0.03 | 0.82±0.03 | 1.89±0.11 | 1.75±0.11 | 2.03±0.03 |
| DFP | 0.76±0.04 | 0.89±0.03 | 0.82±0.03 | 1.90±0.10 | 2.00±0.09 | 1.91±0.13 |
|
Magazine Latencies (s)
|
Trials Completed
|
|||||
| Baseline | OP Exposure | OP Washout | Baseline | OP Exposure | OP Washout | |
| Vehicle | 1.46±0.11 | 1.44±0.13 | 1.54±0.13 | 99.67±0.33 | 100.00±0.00 | 100.00±0.00 |
| DFP | 1.40±0.09 | 1.44±0.10 | 1.41±0.09 | 100.00±0.00 | 98.00±2.00 | 99.72±0.28 |
| Variable Intertrial Interval Task | ||||||
|---|---|---|---|---|---|---|
| Treatment
|
Latency Correct (s)
|
Latency Incorrect (s)
|
||||
| Baseline | OP Exposure | OP Washout | Baseline | OP Exposure | OP Washout | |
| Vehicle | 0.95±0.04 | 0.95±0.04 | 0.88±0.03 | 2.48±0.16 | 2.29±0.17 | 2.10±0.16 |
| DFP | 1.00±0.06 | 1.11±0.06 | 0.87±0.03 | 2.22±0.25 | 2.16±0.16 | 2.27±0.16 |
|
Magazine Latencies (s)
|
Trials Completed
|
|||||
| Baseline | OP Exposure | OP Washout | Baseline | OP Exposure | OP Washout | |
| Vehicle | 1.63±0.17 | 1.37±0.10 | 1.29±0.08 | 67.67±5.15 | 70.58±6.15 | 81.14±5.21 |
| DFP | 1.31±0.09 | 1.50±0.10 | 1.41±0.12 | 60.50±5.94 | 62.75±4.76 | 69.17±5.33 |
All data are presented as the mean ± SEM. N=12 per treatment group. Standard Task = fixed stimulus duration and fixed intertrial interval.
3.4. Varying Stimulus Duration (VSD)
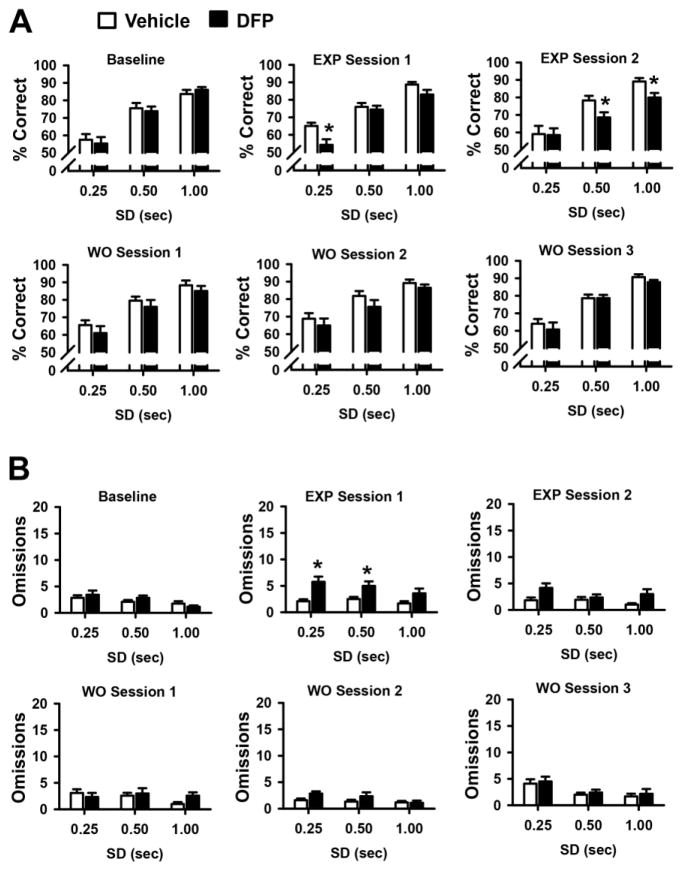
The effects of a pseudorandom presentation of three different stimulus durations (SDs) on percent correct administered during baseline sessions and sessions conducted during the OP exposure period and OP- free washout periods are shown in Fig 5A. As expected, during all test sessions, there was a significant decrease in accuracy as the SD was decreased (p<0.001 for the effect of SD). This effect was exacerbated during the DFP exposure periods, but abated during the OP-free washout period. Specifically, during OP exposure session 1 statistical analysis revealed the following, main effect of treatment, (F(1,11)= 6.76, p=0.025), SD (F(2,22)= 81.64, p<0.001), treatment x SD (F(2,22)= 3.04, p=0.068). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p=0.002) decrease in percent correct at the 0.25 sec SD compared to vehicle-treated controls. During exposure session 2 statistical analysis revealed the following, main effect of treatment, (F(1,11)= 5.03, p=0.046), SD (F(2,22)= 27.77, p<0.001), treatment x SD (F(2,22)= 2.63, p=0.098). Post hoc analysis indicated that DFP-treated rats exhibited a significant decrease in percent correct at the 0.5 sec (p=0.023) and 1.0 sec SD (p=0.044) compared to vehicle-treated controls. The effects of VSDs on omissions are illustrated in Fig 5B. During OP exposure period 1 statistical analysis revealed the following, main effect of treatment, (F(1,11)= 6.54, p=0.027), SD (F(2,22)= 6.07, p=0.008), treatment x SD (F(2,22)= 2.72, p=0.088). Post hoc analysis indicated that DFP-treated rats exhibited a significant increase in omissions at the 0.25 (p=0.006) and 0.5 sec SD (p=0.045) compared to vehicle-treated controls. There were no other statistically significant effects on omissions during VSD test sessions. The effects of VSDs on the number of premature and perseverative responses are shown in Fig 6A and 6B, respectively. In this portion of the analysis there were no significant treatment-related (DFP versus VEH) differences observed. The effects of VSDs on the number of timeout responses are illustrated in Fig 7. During OP exposure session 1 statistical analysis revealed the following, main effect of treatment, (F(1,11)= 1.03, p=0.33), SD (F(2,22)= 12.72, p<0.001), treatment x SD (F(2,22)= 5.56, p=0.011). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p=0.009) increase in timeout responses at the 0.25 sec SD compared to vehicle-treated controls. During OP exposure session 2 statistical analysis revealed the following, main effect of treatment, (F(1,11)= 6.15, p=0.031), SD (F(2,22)= 3.48, p=0.049), treatment x SD (F(2,22)= 1.62, p=0.22). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p=0.006) increase in timeout responses at the 0.25 sec SD compared to vehicle-treated controls. There were no other statistically significant effects on timeout responses during VSD test sessions. There were also no effects of DFP on response or reward latencies or trials completed during these sessions (see Table 1).
Fig 5.
The effects of DFP (0.5 mg/kg) on (A) % correct and (B) the number of omissions in the variable stimulus duration (VSD) version of the 5C-SRTT at baseline (before OP exposure), two sessions during the 30 day every other day OP exposure (EXP) period and three sessions during the 45 day (OP-free) washout (WO) period. * = p<0.05, indicates a statistically significant difference between DFP-treated (N=12) and vehicle-treated (N=12) controls. Each bar represents the mean ± S.E.M
Fig 6.
The effects of DFP (0.5 mg/kg) on (A) the number of premature responses and (B) the number of perseverative responses in the variable stimulus duration (VSD) version of the 5C-SRTT at baseline (before OP exposure), two sessions during the 30 day every other day OP exposure (EXP) period and three sessions during the 45 day (OP-free) washout (WO) period. DFP-treated (N=12) and vehicle-treated (N=12) controls. Each bar represents the mean ± S.E.M
Fig 7.
The effects of DFP (0.5 mg/kg) on the number of timeout responses in the variable stimulus duration (VSD) version of the 5C-SRTT at baseline (before OP exposure), two sessions during the 30 day every other day OP exposure (EXP) period and three sessions during the 45 day (OP-free) washout (WO) period. * = p<0.05, indicates a statistically significant difference between DFP-treated (N=12) and vehicle-treated (N=12) controls. Each bar represents the mean ± S.E.M
3.5. Varying Intertrial Intervals (VITIs)
The effects of a pseudorandom presentation of three different ITIs on percent correct during baseline sessions and sessions conducted during the OP exposure period and OP- free washout periods are shown in Fig 8A. The effects of VITIs on the number of omissions are illustrated in Fig 8B. Statistical analysis indicated that the only significant (treatment-related) differences in these analyses were in the number of omissions during exposure session 2, main effect of treatment, (F(1,11)= 6.51, p=0.027), ITI (F(2,22)= 22.84, p<0.001), treatment x ITI (F(2,22)= 1.67, p=0.21). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p=0.005) increase in omissions at the 1.0 sec ITI compared to vehicle-treated controls. Fig 9 illustrates the effects of VITIs on premature and perseverative responding. As expected, during all test sessions, there was a significant (p<0.001 for the effect of ITI) increase in premature responding at the longer (10 sec) ITI. Statistically significant treatment-related effects were found in 3 of the test sessions, OP exposure session 1 and washout sessions 2 and 3. During OP exposure session 1 the following statistical results were obtained, main effect of treatment, (F(1,11)= 5.22, p=0.042), ITI (F(2,22)= 204.62, p<0.001), treatment x ITI (F(2,22)= 4.34, p=0.027). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p=0.001) decrease in premature responses at the 10.0 sec ITI compared to vehicle-treated controls. During OP washout session 2 the following statistical results were obtained, main effect of treatment, (F(1,11)= 10.19, p=0.009), ITI (F(2,22)= 184.44, p<0.001), treatment x ITI (F(2,22)= 10.38, p<0.001). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p<0.001) increase in premature responses at the 10.0 sec ITI compared to vehicle-treated controls. During OP washout session 3 the following statistical results were obtained, main effect of treatment, (F(1,11)= 7.52, p=0.019), ITI (F(2,22)= 129.93, p<0.001), treatment x ITI (F(2,22)= 6.27, p=0.007). Post hoc analysis indicated that DFP-treated rats exhibited a significant (p<0.001) increase in premature responses at the 10.0 sec ITI compared to vehicle-treated controls. There were no statistically significant treatment-related effects on the number of perseverative responses. The effects of VITIs on the number of timeout responses are illustrated in Fig 10. There were no statistically significant treatment-related effects on the number of timeout responses. Likewise, there were no significant effects of DFP on response or reward latencies during the VITI sessions (see Table 1). While the number of trials completed was reduced in all animals exposed to varying ITIs, there were no statistically significant differences between the DFP- and vehicle-treated subjects (Table 1).
Fig 8.
The effects of DFP (0.5 mg/kg) on (A) % correct and (B) the number of omissions in the variable intertrial interval (VITI) version of the 5C-SRTT at baseline (before OP exposure), two sessions during the 30 day every other day OP exposure (EXP) period and three sessions during the 45 day (OP-free) washout (WO) period. ** = p<0.01, indicates a statistically significant difference between DFP-treated (N=12) and vehicle-treated (N=12) controls. Each bar represents the mean ± S.E.M
Fig 9.
The effects of DFP (0.5 mg/kg) on (A) the number of premature responses and (B) the number of perseverative responses variable intertrial interval (VITI) version of the 5C-SRTT at baseline (before OP exposure), two sessions during the 30 day every other day OP exposure (EXP) period and three sessions during the 45 day (OP-free) washout (WO) period. * = p<0.05; *** = p<0.001, indicates a statistically significant difference between DFP-treated (N=12) and vehicle-treated (N=12) controls. Each bar represents the mean ± S.E.M
Fig 10.
The effects of DFP (0.5 mg/kg) on the number of timeout responses in the variable intertrial interval (VITI) version of the 5C-SRTT at baseline (before OP exposure), two sessions during the 30 day every other day OP exposure (EXP) period and three sessions during the 45 day (OP-free) washout (WO) period. DFP-treated (N=12) and vehicle-treated (N=12) controls. Each bar represents the mean ± S.E.M
4. Discussion
The results of this study can be summarized as follows: 1) repeated exposure to a relatively low (subthreshold) dose of DFP (0.5 mg/kg) was associated with modest (but statistically significant) impairments in accuracy of a standard 5C-SRTT (i.e., with fixed ITIs and SDs, see Fig 1A), as well as an increase in the number of omissions (Fig 1B), and timeout responses (Fig 4), 2) with the exception of the elevation in timeout responses, these alterations eventually abated during the OP-free washout period, 3) when the demands of the task were increased by varying the stimulus durations, DFP-related impairments in accuracy (Fig 5A), increases in omissions (Fig 5B) and increases in timeout responses (Fig 7) were observed during the OP exposure period, but they also abated during the OP-free washout period, 4) conversely, when the demands of the task were increased by varying the intertrial intervals, premature responses (Fig 9A) actually decreased during the DFP exposure period, but increased during the washout period (increases that persisted until the end of the study and long after cholinesterase activity levels had returned to control levels in plasma).
The DFP-related impairments in accuracy and the increases in omissions in the absence of changes in magazine latencies (see below) observed in this study suggest that repeated exposures to DPF results in impairments of sustained attention. Further, the increases in premature responses (i.e., inappropriate nose pokes during the intertrial interval before the target stimulus has been presented) in the VITI task as well as timeout responses (i.e., nose pokes made during the timeout interval which occurred after an incorrect response or premature response) during the OP washout period are indicative of protracted (DFP-related) impulsive-like behavior and/or cognitive inflexibility (i.e., the inability to alter behavior in reaction to changing situational demands, in this case, disorganized responses that are not tied to the stimulus presentation, see Amitai and Markou, 2010). There are several observations that lead us to conclude that the effects of DFP were related to deficits of attention/executive function as opposed to general deficits in motivation, locomotor activity, malaise etc. As noted above, we did not observe any alterations in the magazine latency (i.e., the latency to collect food rewards) which has been described as an index of motivation in the 5C-SRTT (see review, Robbins, 2002). In addition, we did not observe any significant alterations in the total number of trials completed or in the response latencies (i.e., in this portion of the study in the standard version of the 5C-SRTT with fixed SDs and it is, see Table 1). In the 5C-SRTT, subjects must nose poke the food magazine after an incorrect response (i.e., selection of incorrect stimulus aperture), premature response, or an omission (i.e., when 5 seconds expired after the stimulus presentation without a nose poke) in order to initiate the next trial. Therefore, significant delays in initiation would be exemplified by a decrease in the total number of trials completed (which, as noted above, did not occur).
An additional objective of the experiments described in this manuscript was determine if repeated exposures to DFP might result in an increase in vulnerability to alterations of attention and inhibitory response control when the demands of the task were increased. This was accomplished by varying the stimulus durations and intertrial intervals. The VSD version of the 5C-SRTT is used to increase attentional load, thus it often results in decreased accuracy (% correct), the main measure of attentional performance of the task (Higgins and Breysse, 2008; Bari et al., 2008). As expected, when tested using VSDs, all experimental subjects had a significant (stimulus-dependent) decrease in accuracy. However, the magnitude of the decrease in accuracy was greater in rats exposed to DFP during the OP exposure period indicating a vulnerability to deficits of sustained attention when attentional load is increased. This effect was not evident during the OP-free washout period.
The VITI version of the 5C-SRTT is often used to increase the demand on the inhibition of inappropriate responding by making the appearance of the stimuli unpredictable. This version of the task often results in increased premature responses which are generally interpreted as a form of impulsive behavior (see review, Robbins 2002; Bari et al., 2008; Amitai and Markou, 2011). Predictably, when the test subjects were exposed to a pseudorandom presentation of different ITIs, there was a significant increase in premature responses (compared to when fixed ITIs were presented), particularly at the longer (10 sec) ITIs. Interestingly (as noted above), subjects exposed to DFP had significantly fewer premature responses compared to controls during the OP exposure period; however, during the washout period; they increased in DFP-treated animals. This increase in premature responding persisted throughout the washout period and is suggestive of a protracted (DFP-related) loss of impulse control. The basis for the decrease in premature responses observed in one session during the DFP exposure period is unclear, but could be (in some manner) related to the increase in omissions. We have observed a similar decrease in perseverative responding (another inhibitory response control measure) during OP exposure and in increase during washout period in a previous 5C-SRTT study where the OP chlorpyrifos was evaluated (Middlemore-Risher et al., 2010).
As noted in the Introduction, we have also observed protracted impairments of sustained attention and an increase in impulsive behaviors in rats repeatedly exposed to subthreshold doses of the insecticide OP, chlorpyrifos (Middlemore-Risher et al., 2010). Collectively, these results combined with other previous work in our laboratory suggest that both DFP and CPF can lead to deficits in sustained attention, executive function/cognitive flexibility (Terry et al., 2012) as well as impairments of spatial learning and recall (i.e., DFP, Terry et al., 2011; CPF- Terry et al., 2003; Terry et al., 2007). Importantly, these domains of cognition appear among those commonly affected in humans who have been exposed to subthreshold doses of OPs (Ross et al., 2013). The prospective animal studies described here thus support the argument that chronic (or repeated) exposures to OPs at levels that are not associated with acute toxicity can indeed result in cognitive deficits.
The neurobiological substrates of these persistent behavioral effects of OPs have not been fully elucidated, but substantial evidence now suggests that cholinesterase inhibition alone is insufficient as an explanation. It has been suggested that interactions of OPs with non-cholinesterase targets may contribute to the more delayed and persistent effects observed following chronic exposure to OPs (see reviews, Lotti and Moretto, 2005; Costa, 2006). The list of non-cholinesterase targets for OPs (in environmentally relevant doses) is growing and now includes a variety of proteins, receptors, and enzymes (and neurobiological processes that rely on these macromolecules) (see reviews, Casida and Quistad, 2005; Lopachin and Decaprio, 2005; Terry, 2012). Studies in our laboratory have indicated that OPs can have protracted (deleterious) effects on cytoskeletal and motor proteins involved in axonal transport, neurotrophins and their receptors, mitochondria, especially their morphology and movement in axons, and cholinergic marker proteins (e.g., choline acetyltransferase and the α7-nicotinic acetylcholine receptor) (see review Terry, 2012). It is important that these effects and others potential mechanisms of OP toxicity be further investigated so that effective therapeutic strategies can be developed.
Finally, one potential limitation to this study relates to the well-documented miotic effect of OPs (primarily associated with acutely toxic doses), and, the fact that DFP has been used as a miotic agent (administered topically) for the treatment of glaucoma in humans. While our study evaluated a subthreshold dose of DFP (0.5 mg/kg administered subcutaneously), even subtle drug effects on the pupil diameter could (at least theoretically) contribute to alterations in performance of a visual-based task such as the 5C-SRTT (especially accuracy of the task). It is unclear if miotic effects of DFP would alter the inhibitory response measures that were assessed, however. It is also important to note, that to our knowledge, miosis has only been documented in rats after topical application to the eye (Mattio et al., 1984) or after peripherally administered doses (4.0–14.0 mg/kg) that were much higher than the one evaluated in the current study (see Moser, 1995).
5. Conclusion
In conclusion, the results of this rodent study support the premise that repeated, subthreshold exposures to nerve agents OPs like DFP may lead to deficits in sustained attention and to impairments of inhibitory response control in the absence of acute (cholinergic) side effects or motivational deficits. While some of these effects were persistent (e.g., timeout responses, premature responses specifically in the VITI task), most of the effects appeared to be reversible.
Research Highlights.
Repeated exposures to the organophosphate DFP lead to reversible impairments of sustained attention.
Repeated exposures to DFP lead to persistent impairments of inhibitory response control.
DFP-related behavioral effects occurred the absence of acute side effects or motivational deficits.
Acknowledgments
The authors thank Ms. Ashley Davis for administrative assistance in preparing this article. This work was supported by The National Institute of Environmental Health (NIEHS-RO1-ES012241 (AVT).
Footnotes
Conflict of Interest Statement
None of the authors of this manuscript have any financial, personal, or other conflicts of interest that could have inappropriately influenced the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amitai N, Markou A. Disruption of performance in the five choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol Psychiatry. 2010;68(1):5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Comparative effects of different test day challenges on performance in the 5-choice serial reaction time task. Behav Neurosci. 2011;125(5):764–74. doi: 10.1037/a0024722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3(5):759–67. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Padilla SS, Ward T, Pope CN, Olszyk VB. Behavioral and neurochemical changes in rats dosed repeatedly with diisopropylfluorophosphate. Journal of Pharmacology and Experimental Therapeutics. 1991;256:741–50. [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Serine hydrolase targets of organophosphorus toxicants. Chemico-Biological Interactions. 2005;157–158:277–83. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Colosio C, Tiramani M, Maroni M. Neurobehavioral effects of pesticides: state of the art. Neurotoxicology. 2003;24:577–591. doi: 10.1016/S0161-813X(03)00055-X. [DOI] [PubMed] [Google Scholar]
- Colosio C, Tiramani M, Brambilla G, Colombi A, Moretto A. Neurobehavioural effects of pesticides with special focus on organophosphorus compounds: which is the real size of the problem? Neurotoxicology. 2009;30:1155–1161. doi: 10.1016/j.neuro.2009.09.001. 2009. [DOI] [PubMed] [Google Scholar]
- Cooper J, Hans Dobson. The benefits of pesticides to mankind and the environment. Crop Protection. 2007;26:1337–48. [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clinica Chimica Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- De Silva HJ, Samarawickrema NA, Wickremasinghe AR. Toxicity due to organophosphorus compounds: what about chronic exposure? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100:803–6. doi: 10.1016/j.trstmh.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Breysse N. Rodent model of attention: the 5-Choice serial reaction time task. Curr Protoc Pharmacol. 2008:5.49.1–5.49.20. doi: 10.1002/0471141755.ph0549s41. [DOI] [PubMed] [Google Scholar]
- Hobbiger F. Pharmacology of anticholinesterase drugs. In: Zaimis E, editor. Neuromuscular junction. Handbook of experimental pharmacology. Berlin: Springer Verlag; 1972. pp. 487–581. [Google Scholar]
- Katz KD, Brooks DE. Medscape from WebMD. Medscape, LLC; New Yorl, New York: Mar 16, 2010. [accessed 2011 Sept 1]. Organophosphate Toxicity Emergency Medicine. Available: http://emedicine.medscape.com/article/167726. [Google Scholar]
- Lopachin RM, Decaprio AP. Protein adduct formation as a molecular mechanism in neurotoxicity. Toxicological Sciences. 2005;86(2):214–25. doi: 10.1093/toxsci/kfi197. [DOI] [PubMed] [Google Scholar]
- Lotti M, Moretto A. Organophosphate-induced delayed polyneuropathy. Toxicological Reviews. 2005;24:37–49. doi: 10.2165/00139709-200524010-00003. [DOI] [PubMed] [Google Scholar]
- Macilwain C. Study proves Iraq used nerve gas. Nature. 1993 May 6;363(6424):3. doi: 10.1038/363003b0. [DOI] [PubMed] [Google Scholar]
- Mattio TG, Richardson JS, Giacobini E. Effects of DFP on iridic metabolism and release of acetylcholine and on pupillary function in the rat. Neuropharmacology. 1984 Oct;23(10):1207–14. doi: 10.1016/0028-3908(84)90241-7. [DOI] [PubMed] [Google Scholar]
- Maurissen Jacques-Pj, Shankar Mamtha R, Mattsson Joel L. Chlorpyrifos: Lack of cognitive effects in adult Long-Evans rats. Neurotoxicology and Teratology. 2000;22(2):237–246. doi: 10.1016/s0892-0362(99)00062-8. [DOI] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Buccafusco JJ, Terry AV., Jr Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicology and Teratology. 2010;32:415–24. doi: 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VC. Comparisons of the Acute Effects of Cholinesterase Inhibitors Using a Neurobehavioral Screening Battery in Rats. Neurotoxicology and Teratology. 1995;17(6):617–625. doi: 10.1016/0892-0362(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Becker Robert E, Colliver Jerry A, Markwell Stephen J, Moriearty Pamela L, Unni Latha K, Vicari Sandra. Effects of metrifonate on cognitive decline in Alzheimer disease: A double-blind, placebo-controlled, 6-month study. Alzheimer Disease and Associated Disorders. 1998;12(1):54–57. doi: 10.1097/00002093-199803000-00009. [DOI] [PubMed] [Google Scholar]
- Nagao M, Takatori T, Matsuda Y, Nakajima M, Iwase H, Iwadate K. Definitive evidence for the acute sarin poisoning diagnosis in the Toyko subway. Toxicol Appl Pharmacol. 1997;144 (1):198–203. doi: 10.1006/taap.1997.8110. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002 Oct;163(3–4):362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Ross SM, McManus IC, Harrison V, Mason O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol. 2013 Jan;43(1):21–44. doi: 10.3109/10408444.2012.738645. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Nañagas KA. Organophosphate poisoning. Semin Neurol. 2004 Jun;24(2):197–204. doi: 10.1055/s-2004-830907. [DOI] [PubMed] [Google Scholar]
- Saunders BC. Some aspects of the chemistry and toxic action of organic compounds containing phosphorus and fluorine. Cambridge: University Press; 1957. p. 42. [Google Scholar]
- Sungurtekin H, Gürses E, Balci C. Evaluation of several clinical scoring tools in organophosphate poisoned patients. Clin Toxicol (Phila) 2006;44(2):121–6. doi: 10.1080/15563650500514350. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Stone JD, Buccafusco JJ, Sickles DW, Prendergast MA. Repeated, Subthreshold Exposures to Chlorpyrifos in Rats: Hippocampal Damage, Impaired Axonal Transport and Deficits in Spatial Learning. Journal of Pharmacology and Experimental Therapeutics. 2003;305:375–84. doi: 10.1124/jpet.102.041897. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Beck WD, Truan JN, Middlemore ML, Williamson LN, et al. Chronic, Intermittent Exposure to Chlorpyrifos in Rats: Protracted Effects on Axonal Transport, Neurotrophin Receptors, Cholinergic Markers, and Information Processing. Journal of Pharmacology and Experimental Therapeutics. 2007;322:1117–28. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Gearhart DA, Beck WD, Middlemore-Risher ML, Truan JN, et al. Repeated, intermittent exposures to diisopropylfluorophosphate in rats: protracted effects on cholinergic markers, nerve growth factor-related proteins, and cognitive function. Neuroscience. 2011;10;176:237–53. doi: 10.1016/j.neuroscience.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV., Jr Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther. 2012 Jun;134(3):355–65. doi: 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Beck WD, Warner S, Vandenhuerk L, Callahan PM. Chronic impairments in spatial learning and memory in rats previously exposed to chlorpyrfos or diisopropylfluorophosphate. Neurotoxicology and Teratology. 2012;34:1–8. doi: 10.1016/j.ntt.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Mission to Investigate Allegations of the Use of Chemical Weapons in the Syrian Arab Republic. Report on the Alleged Use of Chemical Weapons in the Ghouta Area of Damascus on 21 August 2013.
- Yan C, Jiao L, Zhao J, Yang H, Peng S. Repeated exposures to chlorpyrifos lead to spatial memory retrieval impairment and motor activity alteration. Neurotoxicol Teratol. 2012 Jul;34(4):442–9. doi: 10.1016/j.ntt.2012.05.053. [DOI] [PubMed] [Google Scholar]