Abstract
The binding of tumor necrosis factor α (TNFα) to cell surface receptors engages multiple signal transduction pathways, including three groups of mitogen-activated protein (MAP) kinases: extracellular-signal-regulated kinases (ERKs); the cJun NH2-terminal kinases (JNKs); and the p38 MAP kinases. These MAP kinase signalling pathways induce a secondary response by increasing the expression of several inflammatory cytokines (including TNFα) that contribute to the biological activity of TNFα. MAP kinases therefore function both upstream and down-stream of signalling by TNFα receptors. Here we review mechanisms that mediate these actions of MAP kinases during the response to TNFα.
1. Introduction
Tumor necrosis factor α (TNFα) is a master cytokine that mediates inflammatory responses and innate immunity. Moreover, TNFα is implicated in the pathogenesis of several diseases, including cancer, sepsis, rheumatoid arthritis, diabetes and inflammatory bowel disease [1]. Mechanisms that mediate the actions of TNFα have been intensively studied. Major pathways activated by TNFα include caspases, NF-κB, and mitogen-activated protein kinases (MAP kinases). Functional interaction between these signalling pathways can determine the physiological outcome of TNFα responses. Indeed, a systems biology approach is required to gain an understanding of the TNFα signalling network. This network response is further complicated by the finding that the early phase of TNFα signalling causes expression of inflammatory cytokines that initiate a secondary cytokine-mediated cellular response that contributes to the biological activity of TNFα [2]. This biphasic nature of TNFα signalling complicates biochemical analysis of TNFα signalling. For example, MAP kinases that are activated by TNFα cause increased expression of TNFα by target cells. Consequently, MAP kinases function both upstream and down-stream of TNFα signalling. Here we review mechanisms that mediate this dual role of MAP kinases in signal transduction mediated by TNFα.
2. Mechanisms of TNFα-stimulated MAP kinase activation
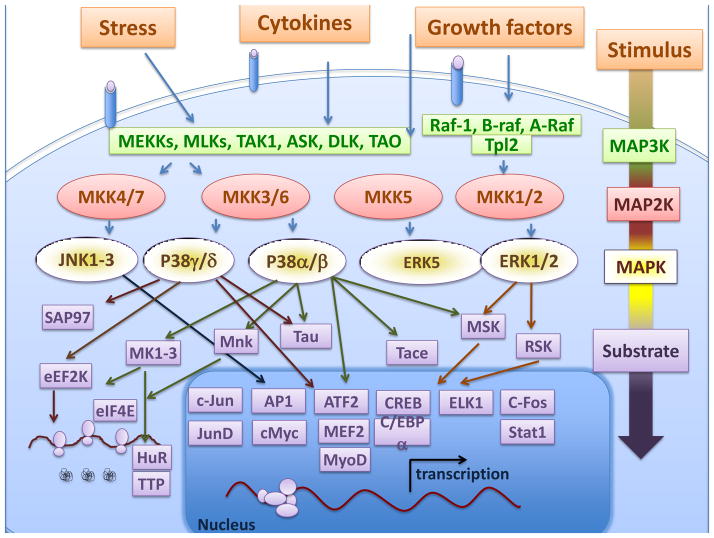
MAP kinase signalling cascades transduce a variety of extracellular signals that regulate cellular responses implicated in proliferation, differentiation and death [3–5]. Three groups of MAP kinases have been identified: the extracellular signal-regulated kinases (ERK); the p38 MAP kinases; and the cJun NH2-terminal kinases (JNK) (Figure 1). In general, ERKs are activated by mitogens and differentiation signals while the JNK and p38 MAP kinases are activated by stress stimuli. TNFα can activate all three groups of MAP kinases.
Figure 1. MAP kinase pathways.
A general feature of MAPK pathways is a canonical cascade consisting of a MAPK kinase kinases (MAP3K), a MAPK kinase (MAP2K) and a MAPK. Three groups of MAP kinases can be defined: ERK (extracellular signal-regulated kinase); JNK (c-Jun N-terminal kinase); and p38 MAP kinases. These pathways can be activated by many stimuli, including growth factors, inflammatory cytokines, and a wide spectrum of cellular stresses. The MAP kinases can phosphorylate downstream targets, including protein kinases, cytosolic substrates, and transcription factors.
MAP kinase pathways share a common structure formed by three sequentially acting protein kinases, including a MAP kinase kinase (MAP2K or MKK) and a MKK kinase (MAP3K or MKKK), although non-canonical exceptions (ERK3, ERK4, ERK7, and ERK8) have been described [6]. The canonical mechanism of MAP kinase activation is caused by MAP2K-mediated by phosphorylation of a pThr-Xaa-pTyr motif located in the MAP kinase T-loop [6]. The sequence of this T-loop motif is a defining feature of MAP kinases: Thr-Glu-Tyr (ERK); Thr-Gly-Tyr (p38); and Thr-Pro-Tyr (JNK). Each MAP2K, in turn, is activated by phosphorylation of Ser and/or Thr residues in the MAP2K T-loop by one or more members of the MAP3K protein family (Figure 1). The substrate specificity of MAP2Ks and MAP3Ks, docking interactions, and scaffold proteins define the different MAPK pathways [6–8].
Activated MAP kinases transform the external stimulus into the correct physiological responses by phosphorylation of downstream substrates, including transcription factors, cytoskeletal proteins, proteins involved in mRNA translation, and other protein kinases that contribute to the specificity, diversity, and amplification of the MAP kinase cascade (Figure 1). The protein kinases activated by MAP kinases include the p90 ribosomal S6 kinases (RSK), mitogen and stress activated kinases (MSK), the MAP kinase interacting kinases (MNK), and MAPK-activated protein kinases (MK) [6].
2.1. ERK MAP kinase signaling pathways
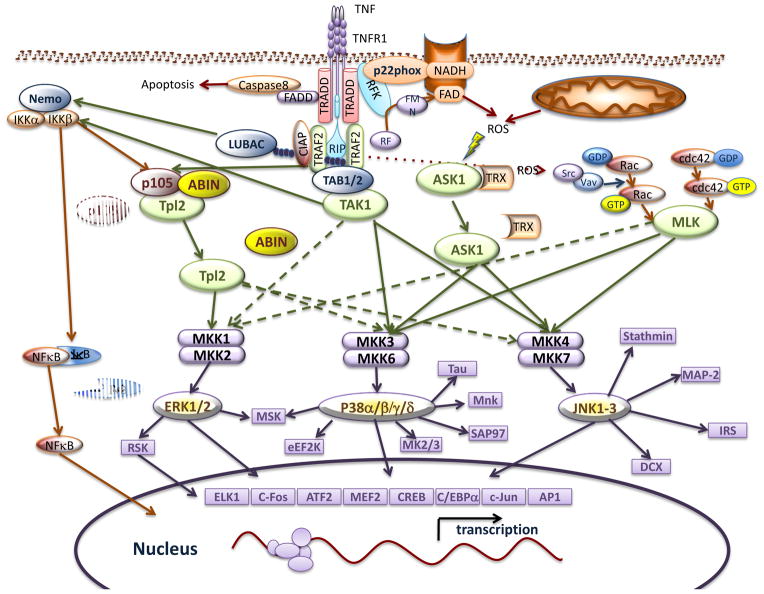
The ERK1 and ERK2 MAP kinases are activated by the MAP2K isoforms MKK1 and MKK2 [6]. The activation of MKK1/2 by TNFα is mediated largely by the MAP3K isoform Tumor Progression Locus 2 (TPL2) [9]. The mechanisms that account for TNFα regulation of the TPL2-MKK1/2-ERK1/2 [10] pathway remains unclear, but detailed studies of this pathway in the response to the endotoxin lipopolysaccharide (LPS) have been reported [10, 11]. TPL2 forms a complex with ABIN and p105 NF-kappaB1 in resting cells and is inactive (Figure 2). TPL2 activation requires Ubch5-promoted (K11, K63 or linear) polyubiquitin chain-dependent activation of the MAP3K isoform TGFβ-activated kinase 1 (TAK1) and phosphorylation/activation of IκB kinase 2 (IKK2) that is recruited to the receptor signalling complex primarily by polyubiquitin chains synthesized by the linear ubiquitin assembly complex (LUBAC) [12]. The activated IKK2 phosphorylates p105, which induces K48 polyubiquitin chain-dependent proteasomal degradation of p105 NF-kappaB1 and release of active TPL2 from the p105/ABIN ternary complex [13, 14]. IKK2 also phosphorylates TPL2 on the activating site Ser-400 [15, 16]. Further studies are required to determine whether this endotoxin pathway (TAK1-IKK2-TPL2-MKK1/2-ERK1/2) differs from the signalling pathway that is regulated by TNFα to activate ERK. In particular, the required role for TAK1 in this pathway is unclear because TAK1 function appears to be cell type-specific [17] and deficiency of the ubiquitin-binding subunits of TAK1 (Tab2−/− Tab3−/−) does not block TNFα signaling in fibroblasts [18].
Figure 2. Activation of MAP kinases by TNFα.
TNFα causes the activation of ERK, JNK, and p38 MAP kinases. TNFα-stimulated ERK activation is primarily mediated by the TAK1-IKK2-TPL2 pathway. In contrast, TNFα-stimulated activation of stress-activated MAP kinases (p38 and JNK) is mediated by multiple pathways, including those engaged by ASK1, MLK, TAK1, and TPL2.
2.2. Stress-activated MAP kinase signalling pathways
The JNK and p38 MAP kinase signalling pathways are collectively named stress-activated MAP kinases and are potently activated in cells treated with TNFα [19, 20]. These pathways are engaged by similar MAP3K isoforms, but diverge during the activation of MAP2K isoforms that selectively activate JNK and p38 MAP kinases (Figure 2). The JNK family includes three members (JNK1, JNK2, and JNK3) and four members of the p38 MAP kinase family have been identified (p38α, p38β, p38γ, and p38δ).
JNK is activated by the MAP2K isoforms MKK4 and MKK7 [19]. Indeed, compound MKK4/7-deficiency (Mkk4−/− Mkk7−/− mice) prevents TNFα-stimulated JNK activation [21]. MKK4 and MKK7 preferentially phosphorylate JNK on Tyrosine and Threonine, respectively [22]. Efficient dual phosphorylation and activation of JNK therefore requires collaborative actions of both MKK4 and MKK7 [21]. Studies of primary fibroblasts demonstrate that TNFα is a potent activator of MKK7, but is a poor activator of MKK4 [21]. Consequently, MKK7 is essential for TNF-stimulated JNK activation, while MKK4 contributes to the maximum extent of JNK activation [21]. The mechanism that accounts for the selective activation of MKK7 by TNFα has not been defined. Interestingly, this pathway appears to be re-wired in immortalized cells in which MKK4 and MKK7 are co-regulated [23].
The p38 MAP kinases can be activated by MKK3, MKK4, and MKK6 in vitro, but p38 MAP kinase activation in vivo is primarily mediated by MKK3 and MKK6 [24]. Indeed p38β, p38γ, and p38δ MAP kinases are not activated in Mkk3−/− Mkk6−/− fibroblasts [25]. In contrast, p38α MAP kinase can be activated by MKK4 in Mkk3−/− Mkk6−/− fibroblasts in response to stress exposure, but this action of MKK4 is largely redundant with MKK3 and MKK6 [24]. Studies of fibroblasts exposed to TNFα indicate that MKK3 and MKK6 contribute equally to the activation of p38α and p38β MAP kinase, MKK3 is the major activator of p38δ MAP kinase, and MKK6 is the major activator of p38γ MAP kinase [24, 25].
The activation of p38 MAP kinases (by MKK3 and MKK6) and JNK (by MKK4 and MKK7) is induced by members of the MAP3K protein kinase family. Roles for ASK1, MEKK, MLK, TAK1, and TPL2 isoforms of MAP3K in the TNFα response have been reported. The relative importance of these pathways appears to be cell type-dependent and context-specific. Mechanisms that account for the selective involvement of these MAP3K isoforms in TNFα signalling have not been defined.
2.2.1. Apoptosis-sensing kinase 1 (ASK1)
The MAP3K isoform ASK1 has been implicated in the activation of JNK and p38 MAP kinases caused by TNFα [26]. The mechanism is mediated by TNFα-stimulated production of reactive oxygen species (ROS) by Riboflavin kinase-mediated activation of NADPH oxidase [27] and ROS derived from endoplasmic reticulum stress or mitochondria. In the basal state, ASK1 forms an inactive complex with thioredoxin that is dissociated by exposure to ROS [28]. The released ASK1 forms complexes with TRAF2 and TRAF6 that promote the oligomerization, phosphorylation, and activation of ASK1 [29–32]. This mechanism of JNK and p38 MAP kinase pathway activation has been associated with sustained TNFα signalling during the cell death response.
While ASK1 is broadly expressed in different tissues, the related protein kinase ASK2 is expressed in a limited number of tissues, including keratinocytes and the intestinal epithelium [33, 34]. ASK2 function is dependent on ASK1 and both proteins form complexes in the active state [34]. It is established that ASK2 contributes to JNK and p38 MAP kinase activation and the regulation of inflammatory cytokine expression [33], but the role of ASK2 in the TNFα signalling response has not been defined.
2.2.2. MAP and ERK kinase kinase (MEKK)
There are four members of the MEKK sub-group of MAP3K [35]. It has been reported that the MAP3K isoform MEKK1 is essential for TNFα-stimulated JNK activation [36], but this conclusion has not been confirmed [37]. Nevertheless, it remains possible that MEKKs contribute to MAP kinase regulation by TNFα-related signalling mechanisms, including TRAIL and CD40L [38]. Moreover, MEKK isoforms may contribute to the function of TNFα-activated TAK1 by the formation of TAK1 complexes with MEKK3 [39] and possibly with other MEKK isoforms. Questions relating to the role of MEKK isoforms remain unresolved and further studies are warranted.
2.2.3. Transforming growth factor β-activated protein kinase 1 (TAK1)
TAK1 plays a key role in ubiquitin-mediated signal transduction by the TNF receptor. The functional TAK1 complex in vivo includes the ubiquitin binding proteins TAB2 or TAB3 that interact with Ubch5-dependent (K11, K63, and/or linear) polyubiquitin chains formed by TNF receptor signalling complexes [40]. This ubiquitin-mediated activation of TAK1 triggers the JNK pathway (mediated by MKK4 and MKK7) and the p38 MAP kinase pathway (mediated by MKK3 and MKK6). The TAK1 pathway also activates ERK MAP kinases by an IKK2/TPL2-dependent pathway (Figure 2).
TAK1 was thought to be an essential mediator of TNFα-stimulated JNK and p38 MAP kinase activation [41]. However, more recent studies have led to a re-evaluation of the role of TAK1. For example, TAK1-deficiency in embryonic fibroblasts was reported to reduce, but not eliminate, TNFα-stimulated stress-activated MAP kinase activation [42]. This observation indicates that TAK1 may play a partially redundant role of TNF-stimulated activation of stress-activated MAP kinases. Moreover, myeloid TAK1-deficiency was found to cause no change in MAP kinase activation in peritoneal macrophages and caused an unexpected (and ROS-dependent) increase in MAP kinase activation in neutrophils [43]. Similarly, compound deficiency of TAB2 and TAB3 did not prevent TNFα signaling [18]. Nevertheless, other studies do support a role for TAK1 in the activation of stress-activated MAP kinases in myeloid cells [44]. On balance, it is likely that TAK1 plays a contributing role in the activation of stress-activated MAP kinases that is partially redundant with other MAP3K pathways. Further studies to firmly establish this conclusion are warranted.
2.2.4. Mixed-lineage protein kinases (MLK)
It is established that TNFα can activate Rho family GTPases, including Rac/Cdc42 [45–48] and RhoA [49]. These Rho family proteins mediate actions of TNFα on cytoskeletal re-organization [45, 49] and the production of ROS [27, 50]. The mechanisms that account for the activation of these small GTPases are unclear, but the exchange factors GEF-H1 [49] and p115RhoGEF [51] are implicated in TNF-stimulated RhoA activation. More recently, it has been demonstrated that the exchange factor Vav, activated by Src family tyrosine kinases, contributes to TNFα-stimulated Rac/Cdc42 activation [42].
Rac/Cdc42 can activate the JNK signalling pathway [52, 53] mediated by the mixed-lineage protein kinase (MLK) pathway [54]. Four members of the MLK family have been identified [55]. Two of these isoforms are widely expressed in many tissues (MLK2 & MLK3) while the other MLK family members (MLK1 & MLK4) are expressed in a limited number of tissues, including the brain. Activated Rac/Cdc42 bind to a conserved CRIB motif that causes MLK activation by disruption of an intramolecular SH3-domain mediated autoinhibitory interaction [55]. The role of this pathway in vivo has been tested using mouse models. Thus, compound disruption of the Mlk2 and Mlk3 genes causes markedly reduced activation of JNK and p38 MAP kinases, but does not alter TNFα-stimulated ERK activation [42]. A similar phenotype was caused by germ-line knock-in mutation of the MLK CRIB motif [42]. More recently, the interaction of MLK3 with TRAF2/5 and K63 polyubiquitination of MLK3 have also been proposed to contribute to MLK3 regulation [56–58]. These data demonstrate that the MLK pathway contributes to TNFα-stimulated activation of stress-activated MAP kinases.
2.2.5. Tumor Progression Locus 2 (TPL2)
The TPL2 pathway has primarily been implicated in ERK activation [10]. Nevertheless, cell type-specific roles for TPL2 in TNFα-stimulated activation of stress-activated MAP kinases have been reported [9]. The mechanism that accounts for the cell type specificity of this response is unclear. Nevertheless, TPL2 may partially contribute to stress-activated MAP kinase activation in TNF-treated cells.
3. Mechanism of MAP kinase regulation of TNFα expression
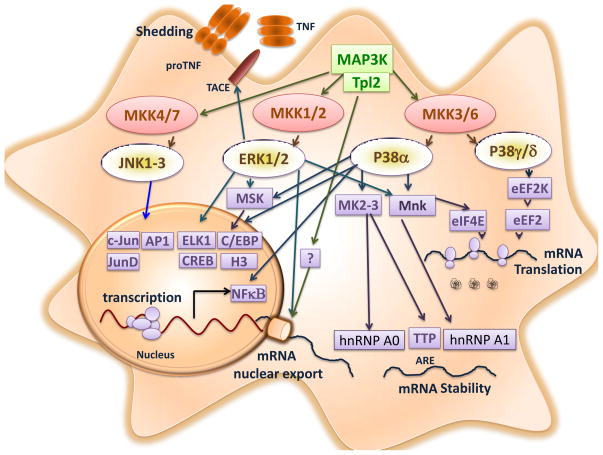
TNFα plays a central role in innate immunity and inflammation. Consequently, the production of TNFα is tightly regulated to prevent exaggerated or persistent inflammation. The regulation of TNFα expression is mediated by both transcriptional and post-transcriptional mechanisms (Figure 3), including Tnfα mRNA transcription, Tnfα mRNA nuclear export, Tnfα mRNA stability, translation of pro-TNFα, and shedding of mature TNFα from the cell membrane [59]. The precursor form pro-TNFα is a membrane protein that contains the mature cytokine in its extracellular domain [60, 61]; soluble TNFα is released upon cleavage of pro-TNFα by the metalloproteinase TNFα converting enzyme (TACE) [62]. Key regulators of Tnfα mRNA expression include NF-κB and members of the mitogen-activated protein kinase (MAPK) family. Moreover, MAP kinases play central roles in TNFα post-transcriptional regulation, including nuclear export of Tnfα mRNA, Tnfα mRNA stabilization through cis-elements in the 3′ untranslated-region (3′-UTR), Tnfα mRNA translation, and shedding of soluble TNFα [63, 64].
Figure 3. MAP kinases control of TNFα biosynthesis.
MAPK regulates TNFα expression by several mechanisms. Tnfα gene transcription can be regulated by ERK, JNK and p38α MAP kinases (and by down-stream protein kinases, including MSK1/2). In addition, the ERK pathway regulates Tnfα mRNA export to the cytoplasm, Tnfα mRNA stability, Tnfα mRNA translation initiation (mediated by MNK), and TNFα shedding by TACE phosphorylation. The p38α MAP kinase pathway regulates Tnfα mRNA translation initiation and Tnfα mRNA stability (mediated by the MNK and MK2/3 pathways). Moreover, the p38γ/δ MAP kinase pathway controls TNFα translation elongation by phosphorylation and inactivation of eukaryotic elongation factor 2 Kinase (eEF2K).
3.1. Control of TNFα expression by the ERK pathway
Pharmacological inhibition of the ERK pathway was shown to reduce TNFα production by leukocytes [65]. Subsequent studies indicated that the ERK1 and ERK2 pathways play an important role in regulating cytokine production by both transcriptional [66, 67] and post-transcriptional mechanisms [68].
3.1.1. Transcriptional regulation of Tnfα gene expression by the ERK pathway
NF-κB is a primary transcription factor that controls Tnfα gene expression. Cis-acting regulatory elements that control maximal Tnfα gene expression include region I (with an overlapping Sp1/Erg-1 site) and region II (with CRE and NF-κB sites) [69]. Tnfα gene expression therefore requires a group of transcription factors (NFκB, EGR-1, ELK-1, ATF-2 and AP-1) that bind these two regions of the Tnfαpromoter [70, 71]. Indeed, full Tnfα promoter induction may require interaction between several of these transcription factors, including NF-κB, EGR-1 and cJun/AP-1 [72, 73]. However, there is evidence that the transcription factor requirement for Tnfα gene expression is cell type dependent [74]. ERK-stimulated Tnfα gene expression may be mediated by activation of one or more of these transcription factors, including members of the AP-1 group. ERKs may also control Tnfα gene expression by phosphorylation and activation of the protein kinases MSK1/2 that phosphorylate histone H3 and the transcription factor cyclic-AMP responsive-element-binding protein (CREB) and activating transcription factor-1 (ATF-1) [75, 76]. Moreover, MSK1/2 can promote the expression of IL10, which acts as in an inhibitory feed-back loop that blocks TNFα expression by macrophages [11].
3.1.2. ERK and nuclear export of Tnfα mRNA
ERK signalling can control post-transcriptional regulation of TNFα production by promoting nuclear export of Tnfα mRNA to the cytoplasm. Mice lacking TPL2 exhibit defects in ERK pathway activation and produce low levels of TNFα after endotoxin exposure [77]. It was postulated that ERKs control nucleocytoplasmatic mRNA transport in these mice by targeting the AU-rich elements (AREs) in the 3′ untranslated region (UTR) of TNFα mRNA [77]. It was later demonstrated that Tnfα RNA nuclear export requires not only the ARE elements but also interaction with two proteins involved in nucleocytoplasm transport, TAP and NxT1 [78]. The importance of ERK in the control of Tnfα mRNA nuclear export is supported by a study of dominant negative ERK mutants [78]. However, a recent report showed that TPL2 catalytic activity can regulate TNFα production independently of ERK activation [13]. Further studies to unambiguously identify TPL2 substrates that regulate TNFα production are required [79]. This is an important area for future research because TPL2 represents a possible anti-inflammatory drug target [79].
3.1.3. ERK and TNFα mRNA translation
ERK and p38 MAP kinase cooperate to phosphorylate and activate the MNK1 and MNK2 protein kinases [80, 81]. MNK1 is recruited to the translation initiation complex by eIF4G, where it promotes cap-dependent translation by phosphorylating eIF4E [82]. MNK1 also phosphorylates the translational silencer hnRNP A1, allowing its release from AU-rich elements (AREs) in the 3′ untranslated region (UTR) of Tnfα mRNA [83].
3.1.4. ERK and TNFα shedding
ERK1/2 have been proposed to promote the processing of pro-TNFα by phosphorylation of TNFα converting enzyme (TACE) [84], the protease that cleaves pro-TNFα to release secreted TNFα [85]. This mechanism may control TNFα secretion [84]. Confirmation that this phosphorylation event is essential for TNFα secretion would require the generation of mice expressing a mutated form of TACE that is refractory to ERK-mediated phosphorylation.
3.2. Control of TNFα expression by the JNK pathway
Studies of embryonic fibroblasts demonstrate that the JNK signalling pathway is not essential for Tnfα gene expression [86]. However, JNK is required for Tnfα gene expression by hematopoietic cells [87]. Mechanistic studies of macrophages demonstrate that the requirement of JNK for Tnfα expression reflects a defect in macrophage polarization [88]. Specifically, JNK was required for the differentiation of pro-inflammatory M1 macrophages that secrete TNFα. Further studies are required to define the mechanism that accounts for the role of JNK in M1 macrophage polarization.
3.3. Control of TNFα expression by p38 MAP kinase pathway
p38 MAPK was first identified as the primary target of anti-inflammatory pyridinyl imidazole drugs that inhibit endotoxin-stimulated production of TNFα [89]. Four p38 MAPK isoforms have been identified. These isoforms are widely expressed, although expression of p38α MAP kinase is low in brain where p38β MAP kinase is the major isoform [90]. The p38γ MAP kinase is expressed in all tissues and is expressed at high levels in muscle [90]. The p38δ MAP kinase is highly expressed in a limited number of tissues, including neutrophils and endocrine glands [20, 64, 91, 92]. The p38 MAPK family can be sub-divided into two subsets on the basis of sequence homology, substrate specificity, and sensitivity to chemical inhibitors [94]: 1) p38α and p38β MAP kinases; and 2) p38γ and p38δ MAP kinases. These p38 MAPKs are strongly activated by exposure to stress and by inflammatory cytokines, including TNFα [93].
The identification of physiological substrates for p38α and p38β MAP kinases was facilitated by the availability of specific inhibitors of these enzymes, such as the cell-permeant pyridinyl imidazole SB203580 and related compounds [95]. In contrast, the lack of specific inhibitors for p38γ and p38δ MAP kinases has slowed the elucidation of their biological roles. However, the generation of a potent inhibitor (BIRB796) that can inhibit all p38 MAP kinase isoforms [95] and the use of p38γ/p38δ MAP kinase knock-out mouse models [96] are now helping to identify the physiological roles of these p38 MAPK isoforms.
The early availability of inhibitors of p38α/β MAP kinase resulted in the discovery that these isoforms regulated TNFα expression. However, the dual specificity of these inhibitors for both p38α and p38β MAP kinases prevented the analysis of the relative importance of these p38 MAP kinase isoforms. Subsequent studies have relied on the use knockout mice for this analysis. These studies demonstrated that p38β-deficiency did not affect TNFα production [90]. In contrast, p38α was found to be a key player in the control of TNFα production [97]. The central role of p38α MAP kinase in TNFα expression was confirmed by a chemical genetic approach [98]. This role of p38α MAP kinase is mediated by regulation of Tnfα mRNA stability and translation initiation. More recently, the p38γ and p38δ MAP kinase isoforms were found to play an important role in TNFα production by regulating Tnfα mRNA translation elongation [64].
3.2.1. The p38 MAPK pathway and transcriptional regulation of TNFα
Inhibition of p38 MAP kinase reduces Tnfα mRNA expression by a mechanism that is mediated, in part, by actions on the Tnfα gene promoter [99]. Indeed, studies of p38α-deficient macrophages demonstrates reduced recruitment of RNA Pol II to the Tnfα promoter [97]. This effect of p38α MAP kinase-deficiency may reflect defects in p38 MAP kinase-dependent activation of CREB [97], CCAAT/enhancer-binding protein β (C/EBPβ) [97], C/EBP homologous protein-1 (CHOP) [100], myocyte enhancer factor 2C (MEF2C) [101], and activating transcription factor-2 (ATF2) [102]. Moreover, p38α MAP kinase increases NF-κB expression [103] and may mark promoters for increased NF-κB recruitment by p38α MAP kinase-dependent histone H3 phosphorylation of selected chromatin targets [104]. p38α MAP kinase (in cooperation with ERK) was also shown to regulate NF-κB by activating MSK1 [105]. These data indicate that p38α MAP kinase regulates Tnfα gene transcription by multiple mechanisms.
Interestingly, p38 MAP kinases can also inhibit NF-κB activity following exposure to TNFα [106, 107]. This inhibitory pathway may be mediated by p38 MAP kinase-mediated negative regulation of TAK1 [108]. The p38 MAP kinases may therefore function both as an activator of Tnfα gene transcription and as part of a negative autoregulatory mechanism that limits TNFα expression.
3.2.2. The p38 MAPK pathway and post-transcriptional regulation of TNFα
Early studies using p38 MAP kinase inhibitors revealed significant reductions in TNFα production by macrophages independently of major changes in Tnfα mRNA abundance [89]. Subsequent studies identified MK2, a protein kinase that is phosphorylated and activated by p38α MAP kinase, as a critical mediator of post-transcriptional regulation of TNFα expression [109]. The protein kinase MNK1 (activated by p38α MAP kinase) also contributes to post-transcriptional regulation of TNFα expression by phosphorylation of eIF4E and promotion of cap-dependent translation [82]. It is now established that p38α MAP kinase controls post-transcriptional TNFα production by two primary mechanisms: 1) Tnfα mRNA stabilization; and 2) Tnfα mRNA translation initiation. These forms of regulation are mediated, in part, by regulatory cis-elements (e.g. AU-rich elements (AREs)) in the Tnfα mRNA 3′untranslated region (UTR) [110] that serve as binding sites for proteins that stabilize Tnfα mRNA [111] and can regulate Tnfα mRNA translation [11, 112].
It has been demonstrated that Tnfα mRNA that lacks 3′ UTR AREs is unresponsive to post-transcriptional regulation [112, 113]. The loss of AREs increases Tnfα mRNA stability and prevents translational repression [47]. The molecular mechanisms regulating ARE-dependent Tnfα mRNA regulation are not completely understood. However, various ARE-binding proteins have been identified as substrates of the p38 MAPK pathway, including hnRNP A0 [114], tristetraprolin (TTP) [115–117] and hnRNP A1 [83].
TNFα production is down-regulated by the zinc finger protein TTP, an mRNA-binding protein that interacts with the AREs in the Tnfα mRNA. The inhibitory effect of TTP on TNFα production was identified by the presence of cachexia in TTP knockout mice [118] caused by increased TNFα expression due to loss of the feedback negative regulation of TNFα production caused by ARE binding by TTP and stabilization of Tnfα mRNA [119]. Further studies using mice with myeloid-specific TTP-deficiency indicated that TTP regulates both Tnfα mRNA stability and translation [120]. Importantly, TTP is required for the defect in TNFα production observed in MK2 knockout mice [121]. MK2 regulates the expression, stability, and binding of TTP to the ARE elements controlling TNFα expression [121]. Similarly, MNK1 (activated by p38α MAP kinase) phosphorylates the translational silencer hnRNP A1, allowing its release from AREs in Tnfα mRNA [83]. The MK2-mediated phosphorylation of TTP [122] causes sequestration of TTP/mRNA complexes by 14-3-3 proteins [117] that prevent the recruitment of deadenylases [123, 124] and result in target mRNA stabilization. Moreover, MK2-mediated phosphorylation of TTP facilitates an exchange on Tnfα mRNA AREs with human antigen R (HUR), which initiates the translation of Tnfα mRNA [125]. Since translation of Ttp mRNA is also regulated by MK2 [119, 122], this mechanism ensures tight control of the inflammatory response. The relative importance of these p38α MAP kinase-dependent pathways that control Tnfα mRNA stabilization and translation remains to be established.
Recent studies have demonstrated that the p38γ and p38δ MAP kinase isoforms also play key roles in post-transcriptional regulation of TNFα biosynthesis [64, 126]. Studies using mice with myeloid-specific p38γ/δ gene ablation identified defects in translational elongation of nascent pro-TNFα protein [64]. This defect in translation elongation was mediated by eukaryotic elongation factor 2 (eEF2) kinase [64], a protein kinase that promotes translational elongation and is regulation by inhibitory phosphorylation by the p38γ/δ MAP kinases [24]. Studies of mice with whole body p38γ/δ MAP kinase deficiency identified a defect in ERK activation (caused by TPL2 instability) [126], although decreased ERK activation was not detected in mice with myeloid-specific p38γ/δ gene ablation [24]. This difference in ERK pathway regulation in whole body p38γ/δ knockout mice versus myeloid-specific p38γ/δ gene ablation mice may reflect differences in mutational compensation in these mice. The possible role of ERK pathway defects in the phenotype caused by p38γ/δ MAP kinase-deficiency warrants further study.
4. Conclusions
Significant progress towards understanding the interplay between MAP kinases and TNFα has been achieved. However, significant questions remain to be resolved. In particular, the diversity and partial redundancy of the MAP3K pathways that are engaged by TNFα signalling reveals significant complexity. What mechanisms account for the partial redundancy of MAP3K isoforms? What is the cause of cell-type specific differences in MAP3K function? In addition, significant questions remain concerning the relative roles of MAP kinase-mediated Tnfα mRNA stabilization, translational initiation, and translational elongation. Are these regulatory mechanisms co-equal or do specific mechanisms predominate under specific physiological circumstances? These important questions need to be addressed by future research.
Highlights.
MAP kinases play a dual role in TNFα signaling
ERK, JNK, and p38 MAP kinases promote TNFα expression
TNFα is a potent activator of ERK, JNK, and p38 MAP kinases
Acknowledgments
G.S. is supported by the Ramón y Cajal Program and grants ERC 260464, EFSD 2030, MICINN SAF2010-19347 and Comunidad de Madrid S2010/BMD-2326; CNIC is supported by the Ministry of Economy and Competitiviness and the Pro-CNIC Foundation. R.J.D. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, Gjertson D, Braun J, Reed E, Singh RR. TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol. 2008;126:121–36. doi: 10.1016/j.clim.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK. The response of human epithelial cells to TNF involves an inducible autocrine cascade. Cell. 2006;124:1225–39. doi: 10.1016/j.cell.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Nebreda AR, Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem Sci. 2000;25:257–60. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 4.Schieven GL. The biology of p38 kinase: a central role in inflammation. Curr Top Med Chem. 2005;5:921–8. doi: 10.2174/1568026054985902. [DOI] [PubMed] [Google Scholar]
- 5.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–65. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 6.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 7.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 8.Enslen H, Davis RJ. Regulation of MAP kinases by docking domains. Biol Cell. 2001;93:5–14. doi: 10.1016/s0248-4900(01)01156-x. [DOI] [PubMed] [Google Scholar]
- 9.Das S, Cho J, Lambertz I, Kelliher MA, Eliopoulos AG, Du K, Tsichlis PN. Tpl2/cot signals activate ERK, JNK, and NF-kappaB in a cell-type and stimulus-specific manner. J Biol Chem. 2005;280:23748–57. doi: 10.1074/jbc.M412837200. [DOI] [PubMed] [Google Scholar]
- 10.Gantke T, Sriskantharajah S, Sadowski M, Ley SC. IkappaB kinase regulation of the TPL-2/ERK MAPK pathway. Immunol Rev. 2012;246:168–82. doi: 10.1111/j.1600-065X.2012.01104.x. [DOI] [PubMed] [Google Scholar]
- 11.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–92. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZJ. Ubiquitination in signaling to and activation of IKK. Immunol Rev. 2012;246:95–106. doi: 10.1111/j.1600-065X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang HT, Papoutsopoulou S, Belich M, Brender C, Janzen J, Gantke T, Handley M, Ley SC. Coordinate regulation of TPL-2 and NF-kappaB signaling in macrophages by NF-kappaB1 p105. Mol Cell Biol. 2012;32:3438–51. doi: 10.1128/MCB.00564-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beinke S, Robinson MJ, Hugunin M, Ley SC. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol Cell Biol. 2004;24:9658–67. doi: 10.1128/MCB.24.21.9658-9667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roget K, Ben-Addi A, Mambole-Dema A, Gantke T, Yang HT, Janzen J, Morrice N, Abbott D, Ley SC. IkappaB kinase 2 regulates TPL-2 activation of extracellular signal-regulated kinases 1 and 2 by direct phosphorylation of TPL-2 serine 400. Mol Cell Biol. 2012;32:4684–90. doi: 10.1128/MCB.01065-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson MJ, Beinke S, Kouroumalis A, Tsichlis PN, Ley SC. Phosphorylation of TPL-2 on serine 400 is essential for lipopolysaccharide activation of extracellular signal-regulated kinase in macrophages. Mol Cell Biol. 2007;27:7355–64. doi: 10.1128/MCB.00301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajibade AA, Wang HY, Wang RF. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 2013;34:307–16. doi: 10.1016/j.it.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Ori D, Kato H, Sanjo H, Tartey S, Mino T, Akira S, Takeuchi O. Essential roles of K63-linked polyubiquitin-binding proteins TAB2 and TAB3 in B cell activation via MAPKs. J Immunol. 2013;190:4037–45. doi: 10.4049/jimmunol.1300173. [DOI] [PubMed] [Google Scholar]
- 19.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 20.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–17. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 21.Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–26. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleming Y, Armstrong CG, Morrice N, Paterson A, Goedert M, Cohen P. Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem J. 2000;352(Pt 1):145–54. [PMC free article] [PubMed] [Google Scholar]
- 23.Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–15. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–78. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remy G, Risco AM, Inesta-Vaquera FA, Gonzalez-Teran B, Sabio G, Davis RJ, Cuenda A. Differential activation of p38MAPK isoforms by MKK6 and MKK3. Cell Signal. 2010;22:660–7. doi: 10.1016/j.cellsig.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–8. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M, Kleinridders A, Wunderlich T, Kashkar H, Utermohlen O, et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460:1159–63. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- 28.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2:389–95. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue J, Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem. 2005;280:37033–40. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- 31.Fujino G, Noguchi T, Matsuzawa A, Yamauchi S, Saitoh M, Takeda K, Ichijo H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27:8152–63. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iriyama T, Takeda K, Nakamura H, Morimoto Y, Kuroiwa T, Mizukami J, Umeda T, Noguchi T, Naguro I, Nishitoh H, et al. ASK1 and ASK2 differentially regulate the counteracting roles of apoptosis and inflammation in tumorigenesis. EMBO J. 2009;28:843–53. doi: 10.1038/emboj.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K, Shimozono R, Noguchi T, Umeda T, Morimoto Y, Naguro I, Tobiume K, Saitoh M, Matsuzawa A, Ichijo H. Apoptosis signal-regulating kinase (ASK) 2 functions as a mitogen-activated protein kinase kinase kinase in a heteromeric complex with ASK1. J Biol Chem. 2007;282:7522–31. doi: 10.1074/jbc.M607177200. [DOI] [PubMed] [Google Scholar]
- 35.Schlesinger TK, Fanger GR, Yujiri T, Johnson GL. The TAO of MEKK. Front Biosci. 1998;3:D1181–6. doi: 10.2741/a354. [DOI] [PubMed] [Google Scholar]
- 36.Xia Y, Makris C, Su B, Li E, Yang J, Nemerow GR, Karin M. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc Natl Acad Sci U S A. 2000;97:5243–8. doi: 10.1073/pnas.97.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K, Oka Y, et al. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc Natl Acad Sci U S A. 2000;97:7272–7. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallagher E, Enzler T, Matsuzawa A, Anzelon-Mills A, Otero D, Holzer R, Janssen E, Gao M, Karin M. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat Immunol. 2007;8:57–63. doi: 10.1038/ni1421. [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Yamamoto M, Akira S, Hayashi H, Su B, Inoue J. Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci Signal. 2009;2:ra66. doi: 10.1126/scisignal.2000387. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Chen ZJ. Regulation of NF-kappaB by ubiquitination. Curr Opin Immunol. 2013;25:4–12. doi: 10.1016/j.coi.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33:522–30. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Kant S, Swat W, Zhang S, Zhang ZY, Neel BG, Flavell RA, Davis RJ. TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Genes Dev. 2011;25:2069–78. doi: 10.1101/gad.17224711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ajibade AA, Wang Q, Cui J, Zou J, Xia X, Wang M, Tong Y, Hui W, Liu D, Su B, et al. TAK1 negatively regulates NF-kappaB and p38 MAP kinase activation in Gr-1+CD11b+ neutrophils. Immunity. 2012;36:43–54. doi: 10.1016/j.immuni.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eftychi C, Karagianni N, Alexiou M, Apostolaki M, Kollias G. Myeloid TAK1 acts as a negative regulator of the LPS response and mediates resistance to endotoxemia. PLoS One. 2012;7:e31550. doi: 10.1371/journal.pone.0031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–65. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 46.Kim BC, Lee MN, Kim JY, Lee SS, Chang JD, Kim SS, Lee SY, Kim JH. Roles of phosphatidylinositol 3-kinase and Rac in the nuclear signaling by tumor necrosis factor-alpha in rat-2 fibroblasts. J Biol Chem. 1999;274:24372–7. doi: 10.1074/jbc.274.34.24372. [DOI] [PubMed] [Google Scholar]
- 47.Puls A, Eliopoulos AG, Nobes CD, Bridges T, Young LS, Hall A. Activation of the small GTPase Cdc42 by the inflammatory cytokines TNF(alpha) and IL-1, and by the Epstein-Barr virus transforming protein LMP1. J Cell Sci. 1999;112 (Pt 17):2983–92. doi: 10.1242/jcs.112.17.2983. [DOI] [PubMed] [Google Scholar]
- 48.Hanna AN, Berthiaume LG, Kikuchi Y, Begg D, Bourgoin S, Brindley DN. Tumor necrosis factor-alpha induces stress fiber formation through ceramide production: role of sphingosine kinase. Mol Biol Cell. 2001;12:3618–30. doi: 10.1091/mbc.12.11.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kakiashvili E, Speight P, Waheed F, Seth R, Lodyga M, Tanimura S, Kohno M, Rotstein OD, Kapus A, Szaszi K. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem. 2009;284:11454–66. doi: 10.1074/jbc.M805933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–87. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 51.Peng J, He F, Zhang C, Deng X, Yin F. Protein kinase C-alpha signals P115RhoGEF phosphorylation and RhoA activation in TNF-alpha-induced mouse brain microvascular endothelial cell barrier dysfunction. J Neuroinflammation. 2011;8:28. doi: 10.1186/1742-2094-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–46. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 53.Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–57. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 54.Teramoto H, Coso OA, Miyata H, Igishi T, Miki T, Gutkind JS. Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem. 1996;271:27225–8. doi: 10.1074/jbc.271.44.27225. [DOI] [PubMed] [Google Scholar]
- 55.Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663–72. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- 56.Humphrey RK, Yu SM, Bellary A, Gonuguntla S, Yebra M, Jhala US. Lysine 63-linked ubiquitination modulates mixed lineage kinase-3 interaction with JIP1 scaffold protein in cytokine-induced pancreatic beta cell death. J Biol Chem. 2013;288:2428–40. doi: 10.1074/jbc.M112.425884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korchnak AC, Zhan Y, Aguilar MT, Chadee DN. Cytokine-induced activation of mixed lineage kinase 3 requires TRAF2 and TRAF6. Cell Signal. 2009;21:1620–5. doi: 10.1016/j.cellsig.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sondarva G, Kundu CN, Mehrotra S, Mishra R, Rangasamy V, Sathyanarayana P, Ray RS, Rana B, Rana A. TRAF2-MLK3 interaction is essential for TNF-alpha-induced MLK3 activation. Cell Res. 2010;20:89–98. doi: 10.1038/cr.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov. 2009;8:480–99. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 60.Utsumi T, Akimaru K, Kawabata Z, Levitan A, Tokunaga T, Tang P, Ide A, Hung MC, Klostergaard J. Human pro-tumor necrosis factor: molecular determinants of membrane translocation, sorting, and maturation. Mol Cell Biol. 1995;15:6398–405. doi: 10.1128/mcb.15.11.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 62.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 63.O’Malley WE, Achinstein B, Shear MJ. Journal of the National Cancer Institute, Vol. 29, 1962: Action of bacterial polysaccharide on tumors. II. Damage of sarcoma 37 by serum of mice treated with Serratia marcescens polysaccharide, and induced tolerance. Nutr Rev. 1988;46:389–91. doi: 10.1111/j.1753-4887.1988.tb05376.x. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Teran B, Cortes JR, Manieri E, Matesanz N, Verdugo A, Rodriguez ME, Gonzalez-Rodriguez A, Valverde A, Martin P, Davis RJ, et al. Eukaryotic elongation factor 2 controls TNF-alpha translation in LPS-induced hepatitis. J Clin Invest. 2013;123:164–78. doi: 10.1172/JCI65124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trotta R, Kanakaraj P, Perussia B. Fc gamma R-dependent mitogen-activated protein kinase activation in leukocytes: a common signal transduction event necessary for expression of TNF-alpha and early activation genes. J Exp Med. 1996;184:1027–35. doi: 10.1084/jem.184.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutault K, Hazzalin CA, Mahadevan LC. Combinations of ERK and p38 MAPK inhibitors ablate tumor necrosis factor-alpha (TNF-alpha) mRNA induction. Evidence for selective destabilization of TNF-alpha transcripts. J Biol Chem. 2001;276:6666–74. doi: 10.1074/jbc.M005486200. [DOI] [PubMed] [Google Scholar]
- 67.Hoffmeyer A, Grosse-Wilde A, Flory E, Neufeld B, Kunz M, Rapp UR, Ludwig S. Different mitogen-activated protein kinase signaling pathways cooperate to regulate tumor necrosis factor alpha gene expression in T lymphocytes. J Biol Chem. 1999;274:4319–27. doi: 10.1074/jbc.274.7.4319. [DOI] [PubMed] [Google Scholar]
- 68.Deleault KM, Skinner SJ, Brooks SA. Tristetraprolin regulates TNF TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways. Mol Immunol. 2008;45:13–24. doi: 10.1016/j.molimm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 69.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem. 1997;272:17795–801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 70.Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH, Fenton MJ, Gordon DC, Dunn IF, Goldfeld AE. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol Cell Biol. 2000;20:6084–94. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kishore R, McMullen MR, Cocuzzi E, Nagy LE. Lipopolysaccharide-mediated signal transduction: Stabilization of TNF-alpha mRNA contributes to increased lipopolysaccharide-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding. Comp Hepatol. 2004;3 (Suppl 1):S31. doi: 10.1186/1476-5926-2-S1-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–62. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 73.Zhu W, Downey JS, Gu J, Di Padova F, Gram H, Han J. Regulation of TNF expression by multiple mitogen-activated protein kinase pathways. J Immunol. 2000;164:6349–58. doi: 10.4049/jimmunol.164.12.6349. [DOI] [PubMed] [Google Scholar]
- 74.Means TK, Pavlovich RP, Roca D, Vermeulen MW, Fenton MJ. Activation of TNF-alpha transcription utilizes distinct MAP kinase pathways in different macrophage populations. J Leukoc Biol. 2000;67:885–93. doi: 10.1002/jlb.67.6.885. [DOI] [PubMed] [Google Scholar]
- 75.Dyson MH, Thomson S, Inagaki M, Goto H, Arthur SJ, Nightingale K, Iborra FJ, Mahadevan LC. MAP kinase-mediated phosphorylation of distinct pools of histone H3 at S10 or S28 via mitogen- and stress-activated kinase 1/2. J Cell Sci. 2005;118:2247–59. doi: 10.1242/jcs.02373. [DOI] [PubMed] [Google Scholar]
- 76.Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22:2871–81. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–83. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 78.Skinner SJ, Deleault KM, Fecteau R, Brooks SA. Extracellular signal-regulated kinase regulation of tumor necrosis factor-alpha mRNA nucleocytoplasmic transport requires TAP-NxT1 binding and the AU-rich element. J Biol Chem. 2008;283:3191–9. doi: 10.1074/jbc.M705575200. [DOI] [PubMed] [Google Scholar]
- 79.George D, Salmeron A. Cot/Tpl-2 protein kinase as a target for the treatment of inflammatory disease. Curr Top Med Chem. 2009;9:611–22. doi: 10.2174/156802609789007345. [DOI] [PubMed] [Google Scholar]
- 80.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–33. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–20. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahalingam M, Cooper JA. Phosphorylation of mammalian eIF4E by Mnk1 and Mnk2: tantalizing prospects for a role in translation. Prog Mol Subcell Biol. 2001;27:132–42. [PubMed] [Google Scholar]
- 83.Buxade M, Parra JL, Rousseau S, Shpiro N, Marquez R, Morrice N, Bain J, Espel E, Proud CG. The Mnks are novel components in the control of TNF alpha biosynthesis and phosphorylate and regulate hnRNP A1. Immunity. 2005;23:177–89. doi: 10.1016/j.immuni.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 84.Rousseau S, Papoutsopoulou M, Symons A, Cook D, Lucocq JM, Prescott AR, O’Garra A, Ley SC, Cohen P. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. J Cell Sci. 2008;121:149–54. doi: 10.1242/jcs.018671. [DOI] [PubMed] [Google Scholar]
- 85.Moss ML, Jin SL, Becherer JD, Bickett DM, Burkhart W, Chen WJ, Hassler D, Leesnitzer MT, McGeehan G, Milla M, et al. Structural features and biochemical properties of TNF-alpha converting enzyme (TACE) J Neuroimmunol. 1997;72:127–9. doi: 10.1016/s0165-5728(96)00180-4. [DOI] [PubMed] [Google Scholar]
- 86.Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol. 2003;23:2871–82. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das M, Sabio G, Jiang F, Rincon M, Flavell RA, Davis RJ. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–60. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–22. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–46. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 90.Beardmore VA, Hinton HJ, Eftychi C, Apostolaki M, Armaka M, Darragh J, McIlrath J, Carr JM, Armit LJ, Clacher C, et al. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol Cell Biol. 2005;25:10454–64. doi: 10.1128/MCB.25.23.10454-10464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ittner A, Block H, Reichel CA, Varjosalo M, Gehart H, Sumara G, Gstaiger M, Krombach F, Zarbock A, Ricci R. Regulation of PTEN activity by p38delta-PKD1 signaling in neutrophils confers inflammatory responses in the lung. J Exp Med. 2012;209:2229–46. doi: 10.1084/jem.20120677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sumara G, Formentini I, Collins S, Sumara I, Windak R, Bodenmiller B, Ramracheya R, Caille D, Jiang H, Platt KA, et al. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136:235–48. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–75. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 94.Risco A, Cuenda A. New Insights into the p38gamma and p38delta MAPK Pathways. J Signal Transduct. 2012;2012:520289. doi: 10.1155/2012/520289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuma Y, Sabio G, Bain J, Shpiro N, Marquez R, Cuenda A. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem. 2005;280:19472–9. doi: 10.1074/jbc.M414221200. [DOI] [PubMed] [Google Scholar]
- 96.Sabio G, Arthur JS, Kuma Y, Peggie M, Carr J, Murray-Tait V, Centeno F, Goedert M, Morrice NA, Cuenda A. p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. Embo J. 2005;24:1134–45. doi: 10.1038/sj.emboj.7600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang YJ, Chen J, Otsuka M, Mols J, Ren S, Wang Y, Han J. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180:5075–82. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- 98.O’Keefe SJ, Mudgett JS, Cupo S, Parsons JN, Chartrain NA, Fitzgerald C, Chen SL, Lowitz K, Rasa C, Visco D, et al. Chemical genetics define the roles of p38alpha and p38beta in acute and chronic inflammation. J Biol Chem. 2007;282:34663–71. doi: 10.1074/jbc.M704236200. [DOI] [PubMed] [Google Scholar]
- 99.Campbell J, Ciesielski CJ, Hunt AE, Horwood NJ, Beech JT, Hayes LA, Denys A, Feldmann M, Brennan FM, Foxwell BM. A novel mechanism for TNF-alpha regulation by p38 MAPK: involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J Immunol. 2004;173:6928–37. doi: 10.4049/jimmunol.173.11.6928. [DOI] [PubMed] [Google Scholar]
- 100.Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science. 1996;272:1347–9. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 101.Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–9. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- 102.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–55. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–63. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 104.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 105.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–24. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bowie AG, O’Neill LA. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol. 2000;165:7180–8. doi: 10.4049/jimmunol.165.12.7180. [DOI] [PubMed] [Google Scholar]
- 107.Alpert D, Schwenger P, Han J, Vilcek J. Cell stress and MKK6b-mediated p38 MAP kinase activation inhibit tumor necrosis factor-induced IkappaB phosphorylation and NF-kappaB activation. J Biol Chem. 1999;274:22176–83. doi: 10.1074/jbc.274.32.22176. [DOI] [PubMed] [Google Scholar]
- 108.Cheung PC, Campbell DG, Nebreda AR, Cohen P. Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha. EMBO J. 2003;22:5793–805. doi: 10.1093/emboj/cdg552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–7. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 110.Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986;83:1670–4. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grzybowska EA, Wilczynska A, Siedlecki JA. Regulatory functions of 3′UTRs. Biochem Biophys Res Commun. 2001;288:291–5. doi: 10.1006/bbrc.2001.5738. [DOI] [PubMed] [Google Scholar]
- 112.Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk HD, Holtmann H, Kollias G, Gaestel M. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem. 2002;277:3065–8. doi: 10.1074/jbc.C100685200. [DOI] [PubMed] [Google Scholar]
- 113.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 114.Zaru R, Ronkina N, Gaestel M, Arthur JS, Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat Immunol. 2007;8:1227–35. doi: 10.1038/ni1517. [DOI] [PubMed] [Google Scholar]
- 115.Chrestensen CA, Schroeder MJ, Shabanowitz J, Hunt DF, Pelo JW, Worthington MT, Sturgill TW. MAPKAP kinase 2 phosphorylates tristetraprolin on in vivo sites including Ser178, a site required for 14-3-3 binding. J Biol Chem. 2004;279:10176–84. doi: 10.1074/jbc.M310486200. [DOI] [PubMed] [Google Scholar]
- 116.Bollig F, Winzen R, Gaestel M, Kostka S, Resch K, Holtmann H. Affinity purification of ARE-binding proteins identifies polyA-binding protein 1 as a potential substrate in MK2-induced mRNA stabilization. Biochem Biophys Res Commun. 2003;301:665–70. doi: 10.1016/s0006-291x(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 117.Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P. MK2-induced tristetraprolin: 14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313–24. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, et al. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–54. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 119.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–5. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 120.Qiu LQ, Stumpo DJ, Blackshear PJ. Myeloid-specific tristetraprolin deficiency in mice results in extreme lipopolysaccharide sensitivity in an otherwise minimal phenotype. J Immunol. 2012;188:5150–9. doi: 10.4049/jimmunol.1103700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol. 2006;26:2399–407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol. 2001;21:6461–9. doi: 10.1128/MCB.21.9.6461-6469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clement SL, Scheckel C, Stoecklin G, Lykke-Andersen J. Phosphorylation of tristetraprolin by MK2 impairs AU-rich element mRNA decay by preventing deadenylase recruitment. Mol Cell Biol. 2011;31:256–66. doi: 10.1128/MCB.00717-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marchese FP, Aubareda A, Tudor C, Saklatvala J, Clark AR, Dean JL. MAPKAP kinase 2 blocks tristetraprolin-directed mRNA decay by inhibiting CAF1 deadenylase recruitment. J Biol Chem. 2010;285:27590–600. doi: 10.1074/jbc.M110.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tiedje C, Ronkina N, Tehrani M, Dhamija S, Laass K, Holtmann H, Kotlyarov A, Gaestel M. The p38/MK2-driven exchange between tristetraprolin and HuR regulates AU-rich element-dependent translation. PLoS Genet. 2012;8:e1002977. doi: 10.1371/journal.pgen.1002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Risco A, del Fresno C, Mambol A, Alsina-Beauchamp D, MacKenzie KF, Yang HT, Barber DF, Morcelle C, Arthur JS, Ley SC, et al. p38gamma and p38delta kinases regulate the Toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Proc Natl Acad Sci U S A. 2012;109:11200–5. doi: 10.1073/pnas.1207290109. [DOI] [PMC free article] [PubMed] [Google Scholar]