Abstract
Toll-like receptors (TLRs) are known to be activated in Central Nervous System (CNS) viral infections and are recognized to be a critical component in innate immunity. Several reports state a role for particular TLRs in various CNS viral infections. However, excessive TLR activation was previously reported by us in correlation with a pathogenic, rather than a protective, outcome, in a model of SIV encephalitis. Here we aimed at understanding the impact of TLR-mediated pathways by evaluating the early course of pathogenesis in the total absence of TLR signaling during CNS viral infections. We utilized a mouse model of sublethal West Nile virus (WNV) infection. WNV is an emerging neurotropic flavivirus, and a significant global cause of viral encephalitis. The virus was peripherally injected into animals that simultaneously lacked two key adaptor molecules of TLR signaling, MyD88 and TRIF. On day 2 pi (post infection), MyD88/Trif−/− mice showed an increased susceptibility to WNV infection, and revealed an impairment in innate immune cytokines, when compared to wild type mice (WT). By day 6 pi, there was an increase in viral burden and robust expression of inflammatory cytokines as well as higher cell infiltration into the CNS in MyD88/Trif−/−, when compared to infected WT. A drastic increase in microglia activation, astrogliosis, and inflammatory trafficking were also observed on day 6 pi in MyD88/Trif−/−. Our observations show a protective role for TLR signaling pathways in preventing lethal encephalitis at early stages of WNV infection.
Keywords: West Nile Virus, Toll-like receptors (TLRs), MyD88, Trif
1. INTRODUCTION
Activation of Toll-like receptors (TLRs) on immune cells is known to be a critical innate immune component in viral infections, including within the CNS. Specifically, in the brain, TLR ligands are involved with activating microglia as well as astrocytes to control very early events in CNS pathogenesis (Hanke and Kielian, 2011). Furthermore, several reports show that particular Toll-like receptors are important in innate immunity (for a review see (O'Neill et al., 2013), especially against viral infections. For instance, triggering TLR3, which is expressed in astrocytes and microglia, effectively induces innate responses and improves adaptive antiviral immune responses (Akira et al., 2006; Kawai and Akira, 2006). Other experiments show that TLRs 3, 7/8 and 9 play a role in peripheral antiviral responses (Akira et al., 2006; Kawai and Akira, 2006; O'Neill et al., 2013). However, TLR signaling can be potentially antagonized to control CNS inflammation as a therapeuthic approach, for instance by blocking TLR4 signaling following systemic LPS exposure (Hines et al., 2013). Another example of the potential therapeutic character of TLR blocking comes from knock out studies, in which animals lacking particular TLR molecules, like TLR3, are protected against viral infections such as WNV in the CNS (Wang et al., 2004). This suggests that TLR signaling may participate in pathology. In fact, the excessive activation of the TLR pathways has been described in correlation with encephalitis in the SIV model of neuroAIDS, suggesting that aberrant TLR activation may result in a pathogenic rather than a protective outcome in infections of the CNS (Marcondes et al., 2013).
Here we investigated the impact of the complete absence of TLR pathways on the outcome of viral CNS pathogenesis. We utilized a mouse model of WNV infection, which has several commonalities with human WNV (low mortality and mild pathogenesis), to evaluate the characteristics of the CNS inflammatory pathology in animals that lack both MyD88 and TRIF adaptor molecules of TLR-mediated signaling.
West Nile Virus (WNV) is a neurotropic flavivirus transmitted by infected mosquitoes that has recently emerged as a primary source of viral encephalitis in the Western hemisphere (Campbell et al., 2002). Infection with WNV is characterized by an acute febrile episode that eventually progresses to encephalitis, meningitis, and flaccid paralysis. The mortality rate following neuroinvasive infection is approximately 10%, and 20-50% of WNV infected surviving patients have significant long-term morbidity (Campbell et al., 2002; Cook et al.; O'Leary et al., 2004; Samuel and Diamond, 2006).
Previous studies have established that both innate and adaptive immune response components play a role in protection against WNV infection. In humans and in mouse models, the essential role of TLRs has been highlighted (Daffis et al., 2008; Kong et al., 2008; Town et al., 2009; Wang et al., 2004; Welte et al., 2009; Xie et al., 2013). The TLR family is composed of at least 11-13 members, with each acting as a sensor of conserved microbial components, that drive the induction of immune responses (O'Neill et al., 2013; Qureshi and Medzhitov, 2003). For instance, peptidoglycan, lipopolysaccharide and flagellin are recognized by TLR2, TLR4 and TLR5, respectively (Yamamoto et al., 2004). Furthermore, TLR2, 3, 4, 7, and 9 are involved in viral recognition (Carty and Bowie, 2010; Carty and Bowie, 2011).
TLR signaling is mediated through adaptor molecules, including myeloid differentiation factor 88 (MyD88) and TIR domain-containing adapter-inducing IFN-α (TRIF). In the absence of these two adapter molecules, no discernable signaling pathway is known to arise from any of the TLRs [reviewed in (Beutler, 2009)]. MyD88 is believed to be capable of signaling through TLR5, TLR7, TLR8, and TLR9, while TRIF is the sole adapter molecule used by TLR3. TLR4 signaling is mediated through both MyD88 and TRIF. Currently, most studies that address the role of TLRs are focused on either individual TLRs or on one of the downstream adaptor signaling pathways. One study investigated the role of both MyD88 and TRIF in a model of lung damage by Klebsiella pneumoniae, where these molecules were shown to play a critical role in inflammatory cell migration and pathogenesis, and to exhibit a therapeutic potential in reducing a damaging inflammatory response (Cai et al., 2009). To the best of our knowledge, there has been no report to specifically study the neuroinflammatory response in the complete absence of TLR signaling in a model of CNS infection.
We have designed experiments to address whether TLR signaling pathways are essential innate immunity components in CNS pathogenesis, and whether their contribution occurs at an early time-point (2 days) or at day 6 pi. Using the minimal dose of WNV Eg101 isolate that is able to induce 50% mortality as innoculum (LD50), we observed detectable inflammatory pathology in the CNS, characterized by diffuse and focal inflammatory infiltrate, microglia activation and astrogliosis. In WT mice, both TLR3 and TLR7 were upregulated by WNV infection, suggesting that both MyD88 and TRIF can participate in the response that characterizes mortality and in the development of CNS pathology. Thus, for comparison, we infected animals that simultaneously lack MyD88 and TRIF adaptor molecules, where TLR signaling is completely absent. We evaluated survival and viral load in the CNS, and compared the expression of innate immune activation markers. Our findings establish a role for TLRs in the control of CNS viral replication, in the early control of brain cytokine and chemokine expression, and in the subsequent regulation of the pro-inflammatory environment in the CNS. Our results show that the absence of TLR-signaling impacts the host innate response at early time points, and that this absence plays a decisive role in determining the severity of brain inflammation and the survival to WNV infection.
2. RESULTS
2.1. MyD88/Trif−/− mice are more susceptible to WNV infection
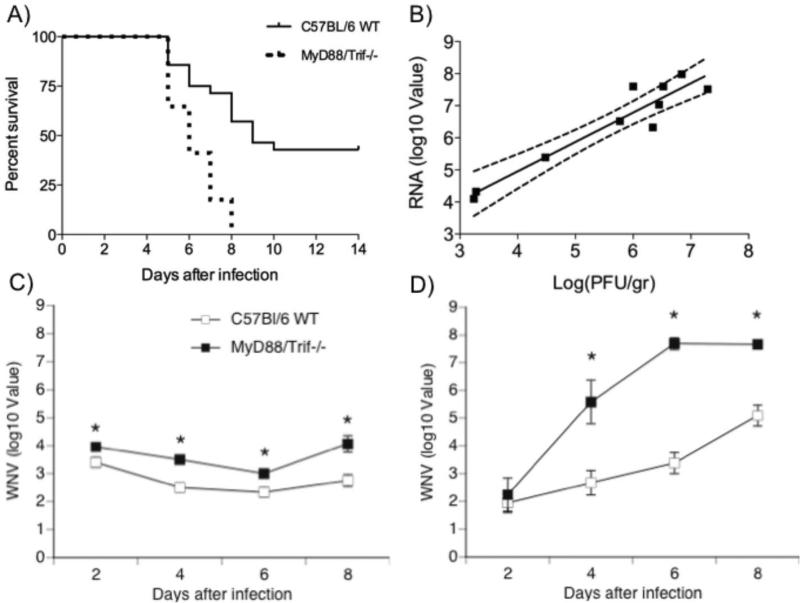
In WT mice, the intraperitoneal (ip) infection with WNV Eg101 strain at doses lower than 106 PFU did not produce any clinical sign of disease or mortality, and no TLR activation (data not shown). However, the ip injection of WNV Eg101 strain at a higher dose (107 PFU) induced significant mortality (58.1%) in C57Bl/6 WT mice (Fig 1A). MyD88/Trif−/− mice were significantly more vulnerable to WNV infection, since 100% of MyD88/Trif−/− mice succumbed to the infection by day 8 after infection (p<0.0001, Logrank test) (Fig 1A), and their viral load was significantly higher. The histopathological analysis of the brain, using this minimal dose of virus able to induce mortality in WT, revealed a mild and diffuse inflammatory infiltrate. Mice in both groups exhibited clinical signs of infection including weight loss, hunching back, fur ruffling and decreased activity. Viral load was measured in WNV-infected WT and MyD88/Trif−/− mice. We observed a strong correlation between WNV RNA levels by quantitative RT-PCR and viral particles by plaque assay (Fig 1B) (p<0.01, Two-tailed Pearson r correlation test r= 0.95). Thus, using qRT-PCR, we assessed WNV viral burden in the spleen (Fig 1C) and brain (Fig 1D) on days 2, 4, 6 and 8 after infection with 107 PFU. Splenic WNV levels from MyD88/Trif−/− mice were consistently higher than in WT mice during the course of infection (2, 4, 6 and 8 days after infection; Fig 1C). In spite of increased peripheral WNV replication in MyD88/Trif−/−, the penetration of WNV into the brain was similar in both groups at 2 dpi (Fig 1D). This suggests that the absence of TLR-mediated response does not affect viral entry across the Blood Brain Barrier (BBB), at least at early time-points. However, after day 4, a significantly higher viral load in the brain of MyD88/Trif−/− was observed compared to WT, which could be due to either higher viral replication, or late viral entry (Fig 1D). Although early viral entry into the brain was not affected, these results suggest that TLR signaling through MyD88 and TRIF adaptor molecules may be involved in mechanisms that control levels of WNV.
Figure 1.
Survival and viral burden analyses of wild-type C57BL/6 and MyD88/Trif−/− mice following WNV infection. (A) Survival curve of WT C57BL/6 (n=26) and MyD88/Trif−/− (n=17) mice infected with 1×107 PFU of WNV Eg101 strain. One hundred percent of MyD88/Trif−/− mice succumbed to the infection by 8 days after infection (p<0.0001, Logrank test). (B) There was a positive correlation between WNV PFU and WNV RNA levels in the brain of WT and MyD88/Trif −/− mice; p<0.01, r= 0.95 (Two-tailed Pearson r correlation test). WNV RNA levels from (C) spleen and (D) brain were determined by quantitative RT-PCR and expressed relative to 18 S at 2, 4, 6 and 8 days after infection. RT-PCR was performed in triplicate at each time point per group. Error bars represent +/− SD from the mean value. *: p<0.05.
We investigated whether the characteristics of the inflammatory CNS pathogenesis in animals lacking TLR adaptor molecules differed from WT mice during WNV infection in correlation with a higher mortality.
2.2. TLRs 3 and 7 are upregulated early after infection with a semi-lethal dose of WNV Eg101 strain
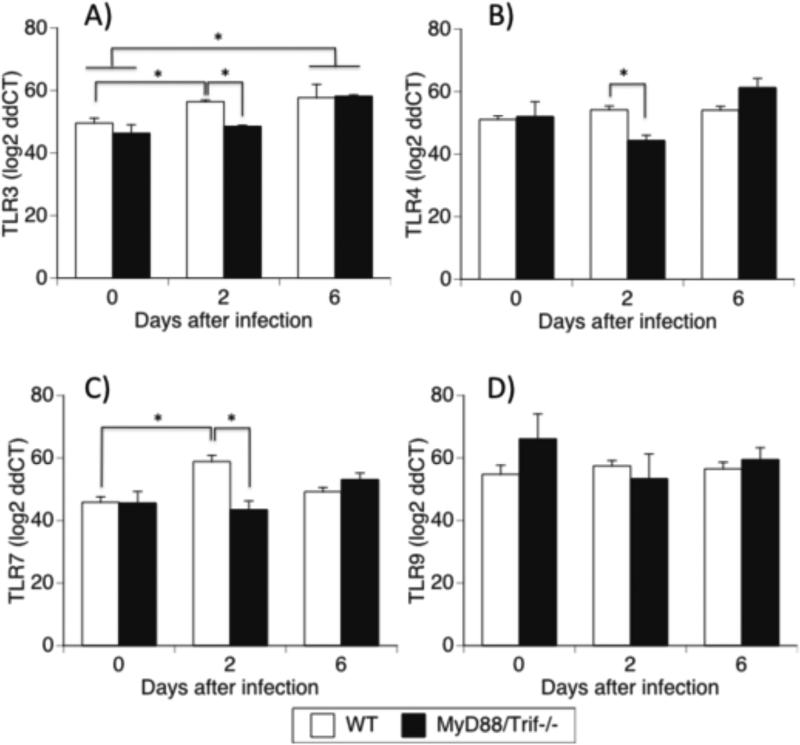
In WT animals, TLR3, which signals through MyD88 adaptor (Fig 2A), and TLR7, which signals through TRIF (Fig 2C), were both significantly upregulated 2 days following infection in comparison to the baseline. This suggests that not only do these two TLRs participate in response to viral infection, but that both TRIF and MyD88 downstream signaling pathways can be activated in this model. The expression of other TLRs known to be relevant in viral infections, such as TLR4 (Fig 2B) and TLR9 (Fig 2D), were not affected by the infection.
Figure 2.
Expression of relevant TLRs in the brain of WNV infected WT and MyD88/Trif−/− mice. Quantitative RT-PCR analyses of TLRs mRNA levels in the brain of C57BL/6 WT and MyD88/Trif−/− were measured in uninfected as well as 2 and 6 days after infection with 1×107 PFU of WNV. Total RNA was analyzed for the expression of (A) TLR3, (B) TLR4, (C) TLR7 and (D) TLR9. The data are expressed as log 2 of ddCT ± SEM of mRNA copies, normalized to the expression of GAPDH. Experiments were performed in 5 animals per group, and in triplicates at each time point. * p<0.05; (ANOVA, Bonferroni test).
In MyD88/Trif−/−, the upregulation of TLR3 and TLR7 on day 2 pi were suppressed (Fig 2A and 2C). Nevertheless, the expression of TLR3 was still increased on day 6, regardless of the presence of adaptor molecules (Fig 2A). In MyD88/Trif−/− animals, the expression of TLRs 4 and 9 (Fig 2B and 2D, respectively) were not affected by infection at any of the analyzed time points.
2.3. Expression of pro-inflammatory cytokines and chemokines in the brain of WNV infected mice
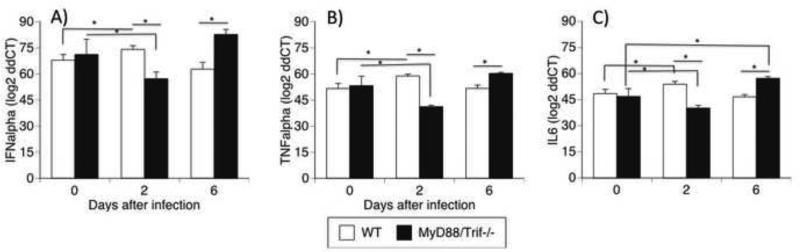
We measured the expression of innate immune cytokines IFNα, TNFα, and IL-6 in the brains of uninfected and WNV-infected WT and MyD88/Trif−/− mice on days 2 and 6 after infection.
There was no difference between uninfected WT and MyD88/Trif−/− at baseline regarding levels of either IFNα, TNFα, or IL-6. In WT animals on day 2 pi, WNV infection significantly upregulated these cytokines[g69]and was followed by a return to baseline on day 6 (Fig 3). In contrat, on day 2 pi with WNV infection in MyD88/Trif−/− brains, there was a significant decrease in IFNα (Fig 3A), TNFα (Fig 3B) and IL6 (Fig 3C). This initial decrease was followed by a return to baseline at day 6 for IFNα (Fig 3A) and TNFα (Fig 3B), and an upregulation of IL6 (Fig 3C). At day 6 pi, the levels of these cytokines were significantly higher in MyD88/Trif−/− when compared to WT animals (Fig 3). These data suggest that MyD88/Trif−/− mice may have a deficit in the early production of antiviral IFNα and innate immunity cytokines, followed by a robust recovery in correlation with the increase in viral load.
Figure 3.
Transcriptional levels of cytokines in the brains of WNV infected WT and MyD88/Trif−/− mice in uninfected, or 2 and 6 days after infection. Quantitative RT-PCR analyses of cytokine mRNA levels in the brain of C57BL/6 WT and MyD88/Trif−/− were measured in uninfected as well as 2 and 6 days after infection with 1×107 PFU of WNV. Total RNA was analyzed for the expression of (A) IFNα, (B) TNFα and (C) IL-6. The data are expressed as log 2 of ddCT ± SEM of mRNA copies, normalized to the expression of GAPDH. Experiments were performed in 5 animals per group, and in triplicates at each time point. * p<0.05; (ANOVA, Bonferroni test).
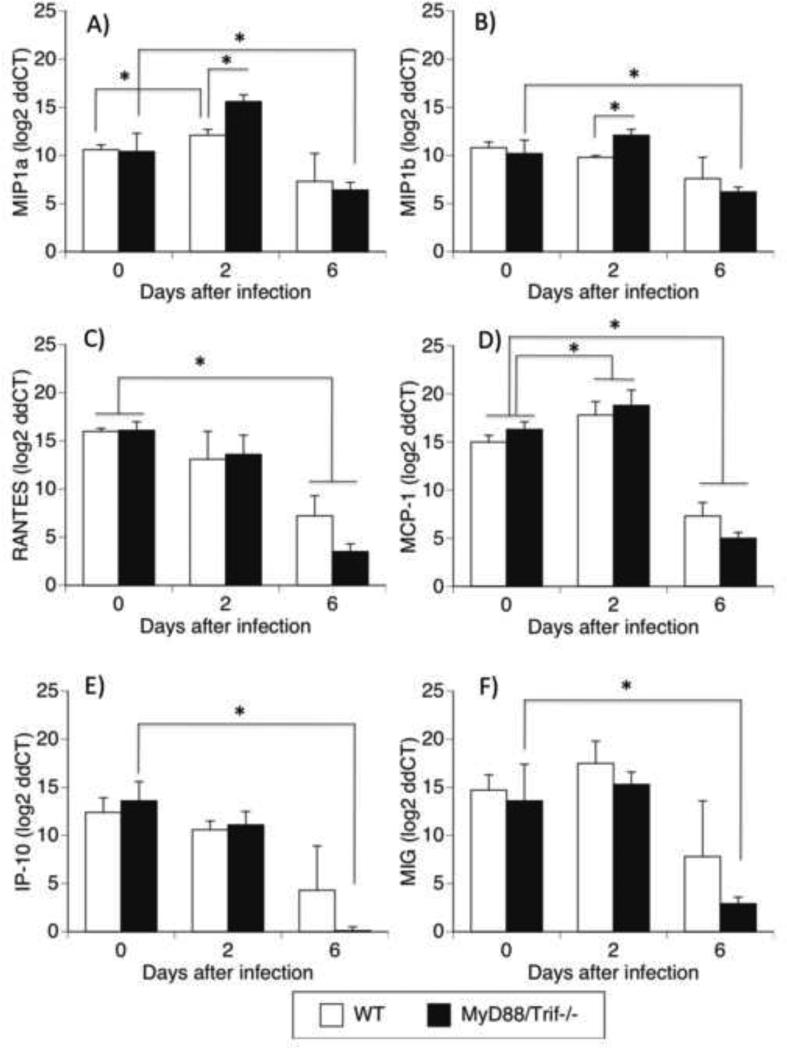
We also investigated the levels of chemokines previously reported to play a role in CNS pathogenesis (Cartier et al., 2005; Garcia-Tapia et al., 2007; Sellner et al., 2005). MIP1α (Fig 4a), MIP1β (Fig 4B), RANTES (Fig 4C), MCP1 (Fig 4D), IP-10 (Fig 4E), and MIG (Fig 4F) were measured in WNV-infected brain tissues in WT and MyD88/Trif−/− mice by qRT-PCR. The baseline levels of these chemokines in the brain of uninfected WT and MyD88/TRIF−/− mice were similar. WNV infection in the brain of WT mice significantly upregulated MIP1α (Fig 4A) and MCP1 (Fig 4D) on day 2 pi, while MIP1b (Fig 4B), RANTES (Fig 4C), IP-10 (Fig 4E) and MIG (Fig 4F) did not change compared to pre-infection baseline. On day 6 pi, WT animnals had a significant decrease in RANTES (Fig 4C) and MCP-1 (Fig 4D). MyD88/Trif−/− mice on day 2 pi had a significant increase of MCP1 (Fig 4D) compared to baseline. In addition, levels of MIP1a and MIP1b in MyD88/Trif−/− on day 2 pi were significantly higher than WT animals. On day 6 pi, MyD88/Trif−/− mice experienced a significant decrease in all of the chemokines analyzed, in comparison to baseline (Fig 3). In summary, while WT have an modest increase of MIP1α and MCP1, MyD88/Trif−/− mice have a more robust upregulation of MIP1α, MIP1β and MIG at early time-points. In addition, on day 6 pi, while WT animals showed a decrease in RANTES and MCP1, MyD88/Trif−/− mice had a significant drop in all the chemokines.
Figure 4.
Transcriptional levels of chemokines in the brains of WNV infected WT and MyD88/Trif−/− mice in uninfected, or 2 days and 6 days after infection. Quantitative RT-PCR analyses of chemokine mRNA levels in the brain of WT and MyD88/Trif−/− were measured in uninfected as well as 2 and 6 days after infection with 1×107 PFU of WNV, Eg101 strain. Total RNA was analyzed for the expression of (A) MIP1α, (B) MIP1β, (C) RANTES, (D) CCL2 (MCP1), (E) IP-10, and (F) MIG. The data are expressed as Median ± SEM of chemokine mRNA copies per copy of 18S. Experiments were performed in triplicates at each time point per group. * p<0.05 (ANOVA, Bonferroni test).
2.4. Infiltration of inflammatory cells is increased in the brain of MyD88/Trif −/− mice
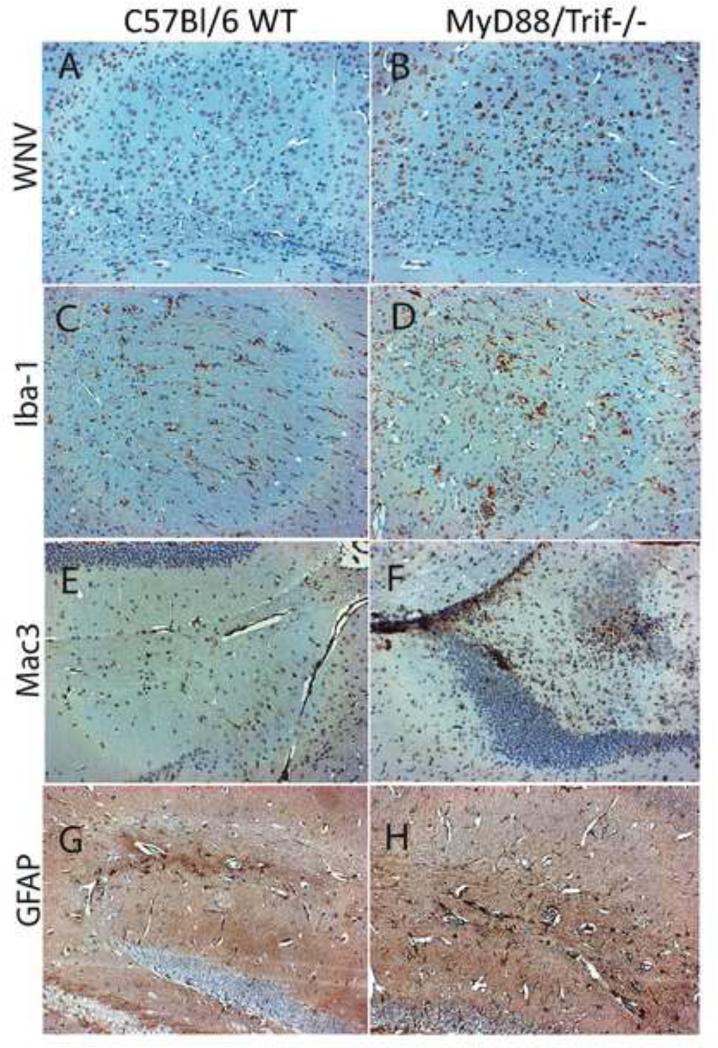
To further define the role of MyD88 and Trif-mediated TLR signaling in the inflammatory pathogenesis of WNV in the CNS, we performed immunohistochemistry to examine the distribution of virus and infiltrating inflammatory cells in the brain. We evaluated brain tissue sections in non-moribund WT and MyD88/Trif−/− mice at day 6 after infection. Significantly higher numbers of WNV-infected neurons were observed in the brain of MyD88/Trif−/− mice compared to WT (Fig 5A and 5B). Virus-infected cells were distributed throughout the brain and did not seem to follow a preferential pattern. The increased viral expression correlated with a higher level of microglia activation in MyD88/Trif−/− mice, as characterized by the enhanced expression of Iba-1 (shown in Fig 5C and 5D). In addition, MyD88/Trif−/− mice had a higher number of cells that stained positively for Mac-3, a macrophage marker (Fig 5F), than WT mice (Fig 5E). The macrophages in MyD88/Trif−/− were predominantly localized in the perivascular area, but they also appeared as foci in the parenchyma (Fig 5F), while in WT animals, they showed a diffuse distribution pattern. The number of activated astrocytes were also increased in WNV infected MyD88/Trif−/− (Fig 5 H) in comparison to WT mice (Fig 5G).
Figure 5. Histopathology of the brains of C57BL/6 WT and MyD88/Trif−/− animals at day 6 after WNV infection.
Brain sections were immunohistochemically stained for detection of specific cell types and infected cells. (A), (C), (E) and (G) represent brain sections from infected C57BL/6 WT mice and (B), (D), (F) and (H) represent brain sections from infected MyD88/Trif−/− mice. (A) and (B) show WNV-positively stained neurons; (C) and (D) show Iba-1+ activated microglia, (E) and (F) show Mac3+ infiltrating macrophages; and (G) and (H) show GFAP+ astroglia. MyD88/Trif−/−animals show a more robust inflammatory response in correlation with brain viral load at day 6 after infection.
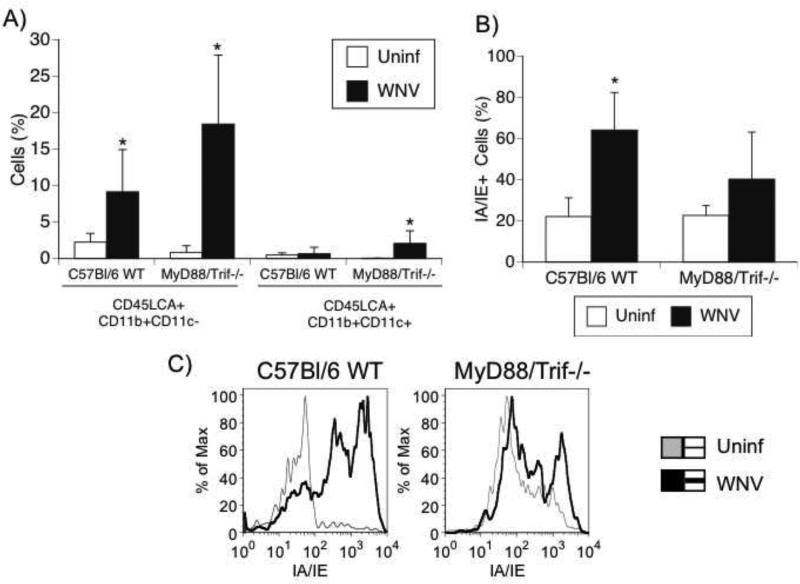
Using flow cytometry in brain-derived cell suspensions, we identified the subpopulations of the infiltrating cells observed upon histological analysis. CD45LCA is a cell-surface antigen expressed by all circulating leukocytes and lymphocytes (Beverley et al., 1988; Beverley, 1991; Janossy et al., 1989). MyD88/Trif−/− animals presented a significantly higher number of CD45LCA+ infiltrating cells than did WT mice at 6 days after infection in the brain. WT mice presented a significant 4-fold increase in the CD45LCA+CD11b+CD11c- macrophage population in the brain after infection (p<0.05), while MyD88/Trif−/− mice presented a 21.8-fold enrichment of these same cells (p<0.01). Also, WT mice did not enrich CD45LCA+ CD11b+CD11c+ myeloid dendritic cells in the brain after infection, but MyD88/Trif−/− mice had a significant increase (p<0.05) in this population (Fig 6A). However, brain-infiltrating macrophages failed to efficiently up-regulate class II expression in MyD88/Trif−/− mice compared to WT, as assessed by FACS (Fig 6B and 6C). This observation suggests that although abundant, macrophages in the brain of WNV-infected MyD88/Trif−/− may have functional impairments. These findings suggest that MyD88/Trif−/− mice have a very robust inflammatory infiltrate in the brain 6 days after infection, when compared to WT animals, and IA/IE expression deficits.
Figure 6.
Brain-infiltrating macrophages and myeloid dendritic cells in WNV-infected C57BL/6 WT and MyD88/Trif−/− mice. Uninfected controls and WNV-infected C57BL/6 WT and MyD88/Trif−/−mice at day 6 after infection were perfused and brain-infiltrating innate immune cells were evaluated using flow cytometry. Values represent the average ± SD of 4 uninfected and 7 infected animals in each group. (A) Percentage of CD45LCA+ CD11b+ CD11c- cells represent infiltrating macrophages and CD45LCA+ CD11b+ CD11c+ cells represent myeloid dendritic cells in the brain of controls and WNV-infected C57BL/6 and MyD88/Trif−/− mice. *p<0.05. (B) Class II expression on the surface of infiltrating innate immune cells. Brain-infiltrating macrophages failed to efficiently up-regulate class II expression (IA/IE) in MyD88/Trif−/− mice compared to C57Bl/6 WT mice. (C) Representative histograms showing the expression of class II molecules on uninfected (gray line) and infected (black line) C57BL/6 WT and MyD88/Trif−/− mice.
2.5. TLR7−/− and TLR3−/− mice are susceptible to WNV infection
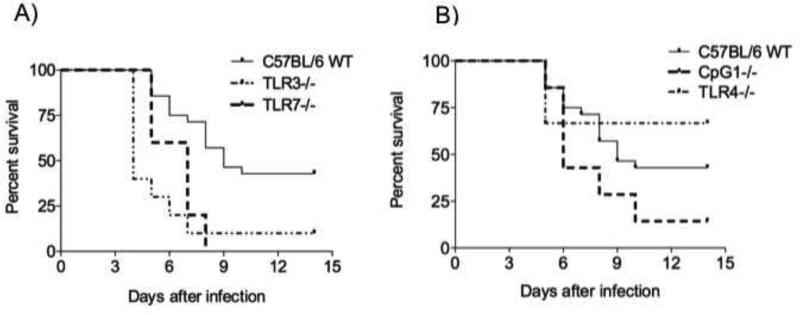
TLRs are located on cell membrane or intracellular membranes. Unlike other TLRs, TLR3, 7, and 9 are exclusively localized in intracellular compartments, suggesting that these intracellular TLRs recognize nucleic acids following the internalization and lysing of viruses. TLR3 and TLR7 can detect virus-derived double-stranded RNAs (dsRNAs) and single-stranded RNAs (ssRNAs), respectively. TLR9 ligands are known to be ssDNAs carrying unmethylated CpG motif (Hemmi et al., 2000). To determine whether individual TLRs that are involved in the immune response against viruses are involved in WNV infection, we analyzed the susceptibility of TLR3−/− and TLR7−/− after WNV infection. We also studied the susceptibility of an intracellular localized TLR (TLR9) and a cell membrane localized TLR (TLR4) as controls. These analysis demonstrated that TLR7−/− and TLR3−/− mice are significantly more susceptible to WNV infection (Fig 7A and 7B). However, there was no significant difference in the susceptibility of TLR9 deficient and TLR4−/− mice to WNV infection (Fig 7C).
Figure 7.
Survival curve of TLR3−/−, TLR7−/−, TLR4−/− and CpG1 mice infected with WNV. TLR3−/− and TLR7−/− mice are highly susceptible to WNV infection. C57BL/6 WT (n=26), TLR3−/− (n=13), TLR4−/− (n=6), TLR7−/− (n=5), and CpG1 (n=7) mice were infected with 1×107 PFU of WNV Eg101 strain. (A) TLR3−/− (p<0.05) and TLR7−/− (p <0.001) mice are more susceptible to WNV infection compared to C57BL/6 WT mice (Logrank test). (B) The susceptibility of TLR4−/− and CpG1 mice to WNV infection was equivalent compared to what was observed in C57BL/6 WT animals (p>0.05).
3. DISCUSSION
The Eg101 WNV mouse model replicates the human disease that is endemic in the Nile Delta region, where sero-prevalence among adults was shown to be 61%, but with little or no evidence of disease (Hurlbut et al., 1956). In order to produce detectable CNS pathology and mortality, we have used high-doses (107 PFU) of the WNV Eg101 strain inoculated intraperitoneally. Such a high dose was able to induce 50% mortality in WT mice. At lower doses, the Eg101 strain caused a very mild CNS pathology in mice, with no mortality, when compared to studies with its’ more virulent lineage 1 relative, the northeastern USA isolate NY99 that can induce a mortality above 60% with an innoculum of 100 pfu or less (Shrestha and Diamond, 2004).
The ip injection of WNV Eg101 was chosen as a model for several reasons. It shares a few unique characteristics with the human WNV infection and to the pathogenesis of other CNS viral infections. For instance, given that close to 100% of the animals died in the absence of TLR signaling, our model reproduces the fact that in humans, lethality is mainly associated to immune-compromised individuals (Murray et al., 2006). In addition, studies have shown that mosquitoes deliver up to 106 PFUs of infectious virus per bite into the host (Styer et al., 2007). Here we used the lowest dose of WNV Eg101 able to induce mortality in WT mice, to examine whether the absence of TLR signaling impacts survival and pathology in a positive or negative way. Other strains of WNV, such as the NY99 strain, which is most commonly used in experimental models, induces a highly severe pathology that could potentially mask a potential positive impact for the absence of TLR adaptor molecules. Thus, our model is milder than the NY99 model, and yet pathogenic enough to allow the observation of both, positive or negative outcomes, upon manipulation in the system. The MyD88/Trif−/− mice were highly susceptible to WNV Eg101, mainly due to a decrease in the expression of innate immune cytokines with antiviral properties, such as IFNα, TNFα and IL6, and an early production of chemokines, such as MIP1α, MIP1β, and MCP-1. These characteristics could be sufficient to favor early viral spread and to support a late inflammatory infiltrate, with poor capacity to contain the virus.
Given that both groups of animals had similar levels of virus in the brain on day 2 after infection, the absence of signaling through MyD88 and TRIF did may not have affect the ability of the WNV to cross the BBB. However, at later time points, the viral load in the CNS reached markedly increased in MyD88/Trif−/− mice in comparison to WT animals. The differences observed between WT and MyD88/Trif−/− mice cannot be attributed to a differential baseline expression of individual TLRs (TLR3, 4, 7, 9), as brain RNA expression levels were similar in both strains. The baseline levels of chemokines and inflammatory cytokines were also similar in both groups.
Interestingly, the characterization of the cytokine environment in MyD88/Trif−/− compared to WT showed that the absence of TLR signaling impacted WNV-infection in the CNS, both at day 2 and at day 6 post infection (pi). In the total absence of TLR signaling, animals developed a suppression of inflammatory cytokines IFNα, TNFα and IL6 compared to infected WT animals, and an increase in MIP1α, MIP1β, and MCP-1, as early as 2 days pi. This was followed by a drastic increase in viral replication accompanied by an enhanced expression of IL6, microglia and astrocyte activation, as well as increased CNS inflammatory cell infiltrate on day 6 pi. Thus, TLR signaling may modulate the course of infection by promoting innate antiviral cytokines at early time points. It is paradoxical that on day 6, a robust inflammatory response develops following an early (day 2 pi) impairment in the innate immune functions, which happens in the context of an absence in TLR-signaling, and is likely a result of increased viral burden. Microglia activation was detected by an increase in Iba-1 expression. Mac3, used to visualize macrophages, can also detect dendritic cells (DC). We have morphologically identified both microglia enhancement and macrophage infiltration in correlation with the mentioned charateristics of the cytokine environment, which may support the presence of inflammatory cells on day 6. Regarding the DC subpopulation, based on the expression of other surface markers such as CD11b and CD11c, we have detected a modest but significant contribution of these cell types to the inflammatory burden in MyD88/Trif−/− mice.
The detection of a strong inflammatory infiltrate is in contrast to what was observed in animals that lacked MyD88 but had TRIF-mediated signaling, in a model using the NY99 strain (Szretter et al.). MyD88−/− animals were also susceptible to WNV in correlation with a decrease in chemokine and inflammatory cytokine production and drastically reduced inflammatory infiltrate in the CNS. Here, both TRIF and MyD88-dependent pathways are impaired, and the impact of these defects is detectable at very early time points. Tissue tropism and restriction of virus entry into the CNS in WNV infection is largely controlled by the innate immune response (Cho et al.; Suthar et al.; Suthar et al.). It is important to acknowledge that in our WNV Eg101 model, susceptibility was accompanied by a high inflammatory cell infiltration on day 6 pi, while in other NY99 models there was an increase in viral load that occurred in association with a lack of inflammatory response (Shirato et al., 2006; Szretter et al.), and which was detectable at later time points. The two strains differ in the envelope sequence, which may cause WNV Eg101 to induce a milder pathology (Beasley et al., 2002) compared to NY99, what may relate with differences in the levels of chemokines in the brain between the two strains (Shirato et al., 2004). In addition to viral strain, routes of infection and doses could be responsible for differences between each reported models.
The expression of IA/IE (MHC class II) molecules was also impaired in the absence of MyD88 and TRIF. Further studies are necessary to rule out a role for antigen-presenting function, which might cause an impaired adaptative immune response that is crucial for dealing with the virus (Shrestha and Diamond, 2004).
Our results indicate that both TLR3 (TRIF-dependent signaling) and TLR7 (MyD88-dependent signaling) are required for controlling WNV infection. However, we did not find a protective role for TLR4 (MyD88 and TRIF-dependent) or TLR9 (MyD88-dependent signaling) in our WNV infection model. This indicates that characteristics of the TLR ligands may cooperate for the role of particular TLRs to infection. However, it is clear that both MyD88 and TRIF dependent signaling are critical for protection against lethal WNV infection by confering different beneficial characteristics to ameliorate pathogenesis.
Regarding the role of TLR3, conflicting results have been reported. Using a lethal dose of the CT-2741 isolate, Wang et al reported that TLR3−/− mice were more resistant in correlation with a reduced inflammatory pathology, which potentially mediates viral penetration and neuronal injury (Wang et al., 2004). On the other hand, Daffis et al showed that animals lacking TLR3 or the downstream signaling component IRF-3, were more vulnerable to WNV infection (Daffis et al., 2008). Interestingly, deficiency of TLR3 was associated with enhanced viral replication in primary cortical neuron cultures and in vivo after intracranial inoculation (Daffis et al., 2008). TLR7−/− mice were also more susceptible to semi-lethal doses of WNV (Welte et al., 2009). This was attributed to a decrease in brain immune cell infiltration mostly mediated by the disruption of IL-23 production (Town et al., 2009). However, no difference in susceptibility was found between WT and TLR7−/− mice when low doses of WNV NY99-6480 isolate were administered intradermally (Welte et al., 2009). Thus, the relative role of different TLRs in WNV infection is highly dependent on particularities of the model, not only affecting the occupancy of molecules on the cell surface and binding strength, but their interactions with different cell subpopulations. Importantly, in our model, and contrary to our initial hypothesis, the absence of TLR signaling caused a severe inflammatory pathology in the CNS.
It is important to acknowledge that the dysruption of MyD88 may affect signaling through the IL1R (Wesche et al., 1997; Yamamoto et al., 2002), which may contribute to the dysfunctional acute inflammatory response at early time points in WNV infection, in a TLR-independent fashion (Durrant et al., 2013). Further studies are necessary to address the combined effects of TLRs and IL1R signaling in the pathogenesis of viral infections of the CNS, such as WNV.
In summary, signaling through TLR adaptors MyD88 and TRIF in WNV Eg101 infection modulated the acute inflammatory response to prevent exacerbated viral replication, and to subsequently prevent a chemokine-associated CNS inflammatory pathology, linked to the development of lethal encephalitis.
4. Experimental Procedures
Animals
Eight to twelve week-old male C57BL/6 mice, MyD88/Trif double knockout (MyD88/Trif−/−), TLR3−/−, TLR4−/− and TLR7−/− and Tlr9CpG1 / CpG1 mice were housed in the vivarium of The Scripps Research Institute (TSRI). All knockout mice were transferred to a C57BL/6 background by repeated backcrossing (Hill, 1998). TLR9CpG1/CpG1 or CpG1 mice has specific missense mutation in Tlr9 gene, which specifically affects TLR9 signaling (Tabeta et al., 2004). All knockout animals were kind gifts from Dr. Bruce Beutler while at TSRI, La Jolla, CA. Mice were bred and maintained in pathogen-free conditions at TSRI. All experiments were carried out in compliance with the rules of the Animal Use Committee of TSRI.
Cells and viruses
BHK-21 cells were cultured as previously described (Diamond et al., 2000). The WNV strain Eg101 (obtained from MA Brinton, Georgia State University, GA) was amplified by infecting SCID mice, and brains were harvested and kept frozen at –80°C until processing with the aid of a Dounce homogenizer. Processed brains were centrifuged at 1200 g for 10 min and supernatants were quantified for viral load and used as stocks.
Plaque Assay
Virus production was titrated by plaque assays using BHK21 cells. BHK21 cells were seeded in 6-well (2.5×105 cells/well) plates in DMEM with 10% FBS overnight. Medium was then removed, serial dilutions of virus supernatants in DMEM were added (0.20 ml/well and the cells were incubated for 1 h at 37°C with shaking every 15 minutes. Subsequently, MEM containing 5% FBS and 1% low-melting-point agarose (SeaPlaque; FMC Bioproducts, Rockland, Maine; 3 ml/well for 6-well plates) was added, and the plates were incubated at 37°C for 72 hours. The plaques were visualized after 10% formaldehyde fixation (>1 h at room temperature) and removal of the agarose plug by staining briefly (15 to 30 s) with a 1% crystal violet solution. Virus concentrations were determined as PFU per milliliter.
CNS Cell Isolation
Brains were harvested following cardiac perfusion with PBS containing 5mM EDTA. A sterile plunger was used to flush the brains through a sterile 70 μm nylon cell-strainer (BD Falcon 352350, BD Biosciences, San Jose, CA). DNAse I (28U/ml) and Collagenase I (50U/ml) were added to the brain cells solution. Digestion was performed at 37 °C for 1 hour with frequent shaking. Afterwards, cells were pelleted and washed with HBSS containing 3% FCS. The cell pellets were subjected to a 1.033/1.088 g/ml Percoll (Pharmacia) gradient, washed, and quantified with a hemocytometer.
Flow Cytometry
Brain cells were placed in 96-well round bottom plates (Corning) at 106 cells/well. Cells were stained with anti-CD45LCA, anti-CD11b, anti-CD11c, anti CD86, anti-IA/IE, anti-CD4, and anti-CD8 (all antibodies from BD Bioscience) resuspended in Staining Buffer (HBSS containing 2% FBS and 0.2% Sodiem Azide) at 4°C for 40 min. Samples were washed twice with Staining Buffer and fixed in 4% PFA. Acquisition was performed in BD Digital LSR II -1, -2, and -3 (BD Biosciences) using DiVa 6.0 Software (BD Biosciences), followed by analysis in FlowJo Software (Tree Star, Inc., Ashland, OR).
Immunohistochemistry
Tissues were fixed in 10% buffered formalin, embedded in paraffin, and cut into 5-μm sections. Following H&E staining and microscopic examination, representative sections of brain were stained through a basic indirect protocol, using an antigen retrieval method (heated to 95°C in 0.01M Citrate buffer, pH6.39 for 45 min and then left for 20 min to steep). Antigens identified were West Nile Virus glicoproteins E and M (Thermo Scientific Pierce Antibodies, Rockford, IL), Mac-3 (BD Pharmingen), Iba-1 (Wako Pure Chemical Industries, Osaka, Japan) and GFAP (BioLegend, San Diego, CA).
RNA extraction and Real-time RT-PCR
Total RNA was isolated from WNV-infected tissues using Trizol reagent (Invitrogen) and cDNA was synthesized using SuperScript™ First-Strand Synthesis Systems (Invitrogen). Specific RNA transcripts were quantified through the use of real-time PCR using primers designed using Primer3 Primer Designer Tool (SourceForge.Net, Mountain View, CA). Calculated copies were normalized against copies of the housekeeping gene 18S and GAPDH. Quantitative real-time PCR reactions were performed using a Stratagene Mx3000P Q-PCR machine, and an ABI 7900HT machine. All RT-PCR experiments were performed in triplicate at each time point per animal, in at least 5 animals per group, as designated in the figures’ legends, and normalized by the expression of GAPDH.
Statistics
Statistical analyses were performed using GraphPad InStat version 3.0 and GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Survival results between groups were compared using Logrank test. Correlation between WNV RNA levels by quantitative RT-PCR and viral particles by plaque assay was assayed using Two-tailed Pearson r correlation test. Two-way ANOVA, together with post hoc Bonferroni multiple comparison test was used for evaluating the impact of the absence of TLRs along the infection, between groups and in comparison to baseline. P values <0.05 were considered significant.
Highlights.
MyD88 and Trif regulate CNS expression of anti-viral cytokines and affect survival in WNV infection.
WNV-infected MyD88/Trif−/− had early viremia peaks and encephalitis compared to WT mice.
WNV in MyD88/Trif −/− increased gliosis and exacerbated inflammatory influx.
TLR signaling pathways prevent WNV-induced lethal encephalitis at early stages of infection.
Acknowledgements
The authors thank Dr. Bruce Beutler, Dr. Xin Du, Kasper Hoebe, Marcie Kritzik, Mary Cleary, Patrick Secrest and Cody Fine for their help and constructive comments. The authors also thank Nikki Bortell (TSRI) for critically reading the manuscript. This is the manuscript number #22038 of the Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have no financial conflict of interests.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Beasley DW, Li L, Suderman MT, Barrett AD. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology. 2002;296:17–23. doi: 10.1006/viro.2002.1372. [DOI] [PubMed] [Google Scholar]
- Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227:248–63. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley PC, Merkenschlager M, Terry L. Phenotypic diversity of the CD45 antigen and its relationship to function. Immunol Suppl. 1988;1:3–5. [PubMed] [Google Scholar]
- Beverley PC. CD45 isoform expression: implications for recirculation of naive and memory cells. Immunol Res. 1991;10:196–8. doi: 10.1007/BF02919692. [DOI] [PubMed] [Google Scholar]
- Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. 2009;183:6629–38. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–29. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Carty M, Bowie AG. Recent insights into the role of Toll-like receptors in viral infection. Clin Exp Immunol. 2010;161:397–406. doi: 10.1111/j.1365-2249.2010.04196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M, Bowie AG. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem Pharmacol. 2011;81:825–37. doi: 10.1016/j.bcp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Cho H, Proll SC, Szretter KJ, Katze MG, Gale M, Jr., Diamond MS. Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat Med. 19:458–64. doi: 10.1038/nm.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Xu X, Yablonsky EJ, Sakata N, Tripp JH, Hess R, Piazza P, Rinaldo CR. Demographic and clinical factors associated with persistent symptoms after West Nile virus infection. Am J Trop Med Hyg. 83:1133–6. doi: 10.4269/ajtmh.2010.09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Suthar MS, Gale M, Jr., Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82:10349–58. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, Harris E. Modulation of Dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol. 2000;74:4957–66. doi: 10.1128/jvi.74.11.4957-4966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant DM, Robinette ML, Klein RS. IL-1R1 is required for dendritic cell-mediated T cell reactivation within the CNS during West Nile virus encephalitis. J Exp Med. 2013;210:503–16. doi: 10.1084/jem.20121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tapia D, Hassett DE, Mitchell WJ, Jr., Johnson GC, Kleiboeker SB. West Nile virus encephalitis: sequential histopathological and immunological events in a murine model of infection. J Neurovirol. 2007;13:130–8. doi: 10.1080/13550280601187185. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond) 2011;121:367–87. doi: 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hill WG. Selection with recurrent backcrossing to develop congenic lines for quantitative trait loci analysis. Genetics. 1998;148:1341–52. doi: 10.1093/genetics/148.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines DJ, Choi HB, Hines RM, Phillips AG, MacVicar BA. Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PLoS One. 2013;8:e60388. doi: 10.1371/journal.pone.0060388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut HS, Rizk F, Taylor RM, Work TH. A study of the ecology of West Nile virus in Egypt. Am J Trop Med Hyg. 1956;5:579–620. doi: 10.4269/ajtmh.1956.5.579. [DOI] [PubMed] [Google Scholar]
- Janossy G, Bofill M, Rowe D, Muir J, Beverley PC. The tissue distribution of T lymphocytes expressing different CD45 polypeptides. Immunology. 1989;66:517–25. [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–7. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Kong KF, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, Fikrig E, Montgomery RR. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82:7613–23. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Spina C, Bustamante E, Fox H. Increased toll-like receptor signaling pathways characterize CD8+ cells in rapidly progressive SIV infection. Biomed Res Int. 2013;2013:796014. doi: 10.1155/2013/796014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, Baraniuk S, Resnick M, Arafat R, Kilborn C, Cain K, Shallenberger R, York TL, Martinez D, Hellums JS, Hellums D, Malkoff M, Elgawley N, McNeely W, Khuwaja SA, Tesh RB. Risk factors for encephalitis and death from West Nile virus infection. Epidemiol Infect. 2006;134:1325–32. doi: 10.1017/S0950268806006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DR, Marfin AA, Montgomery SP, Kipp AM, Lehman JA, Biggerstaff BJ, Elko VL, Collins PD, Jones JE, Campbell GL. The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis. 2004;4:61–70. doi: 10.1089/153036604773083004. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors -redefining innate immunity. Nat Rev Immunol. 2013;13:453–60. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- Qureshi ST, Medzhitov R. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 2003;4:87–94. doi: 10.1038/sj.gene.6363937. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80:9349–60. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellner J, Dvorak F, Zhou Y, Haas J, Kehm R, Wildemann B, Meyding-Lamade U. Acute and long-term alteration of chemokine mRNA expression after anti-viral and anti-inflammatory treatment in herpes simplex virus encephalitis. Neurosci Lett. 2005;374:197–202. doi: 10.1016/j.neulet.2004.10.054. [DOI] [PubMed] [Google Scholar]
- Shirato K, Kimura T, Mizutani T, Kariwa H, Takashima I. Different chemokine expression in lethal and non-lethal murine West Nile virus infection. J Med Virol. 2004;74:507–13. doi: 10.1002/jmv.20205. [DOI] [PubMed] [Google Scholar]
- Shirato K, Miyoshi H, Kariwa H, Takashima I. The kinetics of proinflammatory cytokines in murine peritoneal macrophages infected with envelope protein-glycosylated or non-glycosylated West Nile virus. Virus Res. 2006;121:11–6. doi: 10.1016/j.virusres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78:8312–21. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 2007;3:1262–70. doi: 10.1371/journal.ppat.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Brassil MM, Blahnik G, McMillan A, Ramos HJ, Proll SC, Belisle SE, Katze MG, Gale M., Jr. A systems biology approach reveals that tissue tropism to West Nile virus is regulated by antiviral genes and innate immune cellular processes. PLoS Pathog. 9:e1003168. doi: 10.1371/journal.ppat.1003168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Diamond MS, Gale M., Jr. West Nile virus infection and immunity. Nat Rev Microbiol. 11:115–28. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- Szretter KJ, Daffis S, Patel J, Suthar MS, Klein RS, Gale M, Jr., Diamond MS. The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system. J Virol. 84:12125–38. doi: 10.1128/JVI.01026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–21. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, Anderson JF, Flavell RA, Fikrig E. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–53. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Welte T, Reagan K, Fang H, Machain-Williams C, Zheng X, Mendell N, Chang GJ, Wu P, Blair CD, Wang T. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J Gen Virol. 2009;90:2660–8. doi: 10.1099/vir.0.011783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–47. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- Xie G, Welte T, Wang J, Whiteman MC, Wicker JA, Saxena V, Cong Y, Barrett AD, Wang T. A West Nile virus NS4B-P38G mutant strain induces adaptive immunity via TLR7-MyD88-dependent and independent signaling pathways. Vaccine. 2013;31:4143–51. doi: 10.1016/j.vaccine.2013.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–72. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol. 2004;40:861–8. doi: 10.1016/j.molimm.2003.10.006. [DOI] [PubMed] [Google Scholar]