Abstract
Introduction
Although thorough pathologic nodal staging provides the greatest prognostic information in patients with potentially curable non-small cell lung cancer, N1 nodal metastasis is frequently missed. We tested the impact of corrective intervention with a novel pathology gross dissection protocol on intrapulmonary lymph node retrieval.
Methods
Retrospective review of consecutive lobectomy, or greater, lung resection specimens over a period of 15 months before and 15 months after training Pathologist's Assistants on the novel dissection protocol.
Results
141 specimens were examined before and 121 specimens after introduction of the novel dissection protocol. The median number of intrapulmonary lymph nodes retrieved increased from 2 to 5 (p<.0001), and the 75th – 100th percentile range of detected intrapulmonary lymph node metastasis increased from 0 – 5 to 0 – 17 (p=.0003). In multivariate analysis, the extent of resection, examination period (pre- or post-intervention), and pathologic N1 (vs. N0) status were most strongly associated with a higher number of intrapulmonary lymph nodes examined.
Conclusions
A novel pathology dissection protocol is a feasible and effective means of improving the retrieval of intrapulmonary lymph nodes for examination. Further studies to enhance dissemination and implementation of this novel pathology dissection protocol are warranted.
Keywords: non-small cell lung cancer, pathologic nodal staging, quality improvement
1. Introduction
Pathologic staging of resected lung cancer is the most accurate means of predicting future patient outcomes.1 However, there is increasing appreciation of limitations in the prognostic accuracy of the TNM staging system, which seems to be partly caused by variability in the thoroughness of examination.2-6 For example 46% of patients with pathologic node negative lung cancer die of recurrent disease within 5 years,1 the median number of examined lymph nodes in lung resection specimens is only approximately 3 – 5 in the US, 7,8 whereas it is repeatedly suggested that more than 10 lymph nodes need to be examined to minimize sampling error.7-10 At one extreme, 18% of ‘node negative’ resections in the US have no lymph nodes examined (pathologic NX),6 and 62% of ‘mediastinal node negative’ resections have no mediastinal nodes examined.4 The survival of patients with node-negative disease improves with the number of lymph nodes examined,7-10 while survival worsens in node-positive patients as the number (or proportion) of positive lymph nodes increases.11-16 Emphasizing the implication of sampling error, patients with pNX resections have a survival curve that closely approximates that of patients with pN1, not pN0.6
In a previous study, we demonstrated that about 60% of intrapulmonary lymph nodes are left unexamined in lung resection specimens, 11% of which have identifiable metastasis, representing 62% of all N1 lymph nodes with metastasis.17 In that study, 90% of patients who had curative-intent surgery for lung cancer had one or more unexamined lymph nodes and 12% of pathologic N0 resections had one or more unexamined lymph nodes with metastasis.17 However, our hypothesis-testing ‘special pathology examination protocol’, entailed making 0.3-0.5mm transverse sections of remnant lung resection specimens that had been fixed in formalin for several days. This thin-cut transverse dissection protocol is impractical for routine use in the clinical pathology laboratory because it is difficult to reproduce in fresh lung resection specimens, and the requirement for prolonged formalin fixation would unacceptably delay the processing of pathology specimens.
Therefore, we developed a novel modified gross dissection protocol to more readily fit into the routine flow of care. Our goal was to increase the number of intrapulmonary lymph nodes examined, leading up to larger future studies of the impact on nodal stage distribution. We now report how implementing this novel pathology dissection protocol affected lymph node retrieval from lung resection specimens.
2. Materials and Method
With Institutional Review Board approval, we retrospectively reviewed surgical pathology reports of all lobectomy, or greater, resections for lung cancer at 2 hospitals over the span of 10 quarters (30 months), from January 1, 2011 to June 30, 2013. The novel pathology gross dissection protocol was introduced as a quality improvement effort within a single surgical pathology group that provides histopathology services to the two hospitals. The protocol involved a series of blunt peri-bronchial dissections starting from the hilum to the periphery, with special attention to points of airway bifurcation where intrapulmonary lymph nodes aggregate (Fig 1).
Figure 1.
Bench protocol for novel pathology gross dissection of lung resection specimens.
Implementation
after pilot testing the protocol with one Pathologist's Assistant to demonstrate feasibility, all four other Pathologist's Assistants who routinely perform gross dissection of lung resection specimens in the department were trained on the same protocol by a thoracic pathologist (DS). The period of training was from May-June 2012 (quarter 6).
Approximately 6 months before starting this study, we introduced a surgical lymph node specimen collection kit to improve the intraoperative collection of mediastinal lymph nodes (stations 2 to 9). This kit has been described in detail elsewhere. 18 It contains 12 specimen collection cups, each pre-labeled with the International Association for the Study of Lung Cancer lymph node station name and number, thus: right and left upper paratracheal (2R, 2L), prevascular (3a), retrotracheal (3p), right and left lower paratracheal (4R, 4L), sub-aortic (5), para-aortic or phrenic (6), subcarinal (7), paraesophageal (8), pulmonary ligament (9), and hilar (10). One set of kits is labeled for right side resections, a different set for left side resections.
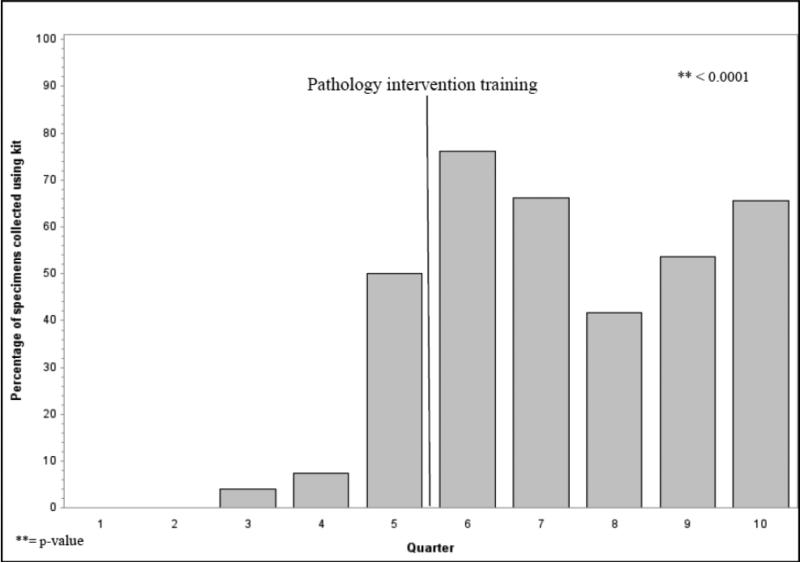
In the right side kits, the collection cups for stations 2R, 4R, 7, 8, 9 and 10R are conspicously marked as mandatory for sampling. Collection cups for stations 4L, 5, 6, 7, 8, 9 and 10L are similarly marked in the left side kits. The kit includes station 10, an N1 nodal station that is anatomically located such that it has to be collected by the surgeon during the operation, without which pathologists would have no access. The penetration of use of this kit evolved over the time course of this study (Figure 2). We abstracted patient demographic and clinical information from hospital records, and lymph node examination results from the final pathology reports.
Figure 2.
Evolution of surgical lymph node specimen collection kit use over timespan of study.
Outcomes
the primary outcome was the number of intrapulmonary lymph nodes retrieved by the Pathologist's Assistants before and after implementation of the novel pathology dissection protocol. We also examined the rate of detection of lymph node metastasis. Because station 10 was likely to be impacted by use of the kit, which specifically mandates surgeons to collect lymph nodes from this station during the operation, we also analyzed the influence of kit use.
Statistical analysis
we used the Pearson Chi-squared test to examine the relationship between categorical variables, and the t-test for continuous variables. For the number of lymph nodes variable, we used the Mann-Whitney test to examine differences in medians due to the right skewed distribution. We also performed a linear trend test to assess the number of lymph nodes retrieved over time.
For the multivariate analysis of the number of lymph nodes retrieved, we used Poisson regression based on the Generalized Estimating Equations (GEE) to account for cluster within Pathologist's Assistants. We also controlled for the following potential confounders: age, race, sex, histology, T-category, node metastasis status, extent of resection, intervention period, surgical kit use, and Pathologist's Assistant. All potential confounders were simultaneously entered into the model. In addition, for the number of lymph nodes with metastasis, we used zero inflated Poisson regression to compare the distributions because of the excessive zeros in the distributions of lymph nodes with metastasis. The possibility of having zero lymph node metastasis was modeled with intervention period, Pathologist's Assistant, extent of surgical resection and T-category. No weighting was used in any of the analyses. The 95% confidence interval (95% CI) was estimated and two-sided p-values were set at the <0.05 significance level. All statistical analyses were performed using SAS version 9.4 PROC GENMOD, PROC COUNTREG (SAS Institute Inc., Cary, NC, USA, 2013).
3. Results
Demographic, clinical and treatment characteristics
Over the 30 month timespan covered by this project, 262 patients had lobectomy, or greater, resection for lung cancer at the two hospitals served by the pathology group: 141 patients before (‘pre-‘ era) and 121 after (‘post-‘ era) the new pathology dissection protocol was introduced. The clinical and demographic characteristics of the two cohorts of patients were very similar (Table 1). However, despite the similarity in median tumor size between both groups of patients, those in the ‘pre’ era had a higher frequency of T2, T3 and T4 tumors, and the ‘post’ era had more patients with T1 tumors (p<.01). The extent of resection was similar between both groups.
Table 1.
Patient characteristics pre- and post-introduction of a novel pathology gross dissection protocol.
| Characteristics | Before N=141 | After N=121 | p-value |
|---|---|---|---|
| Age, years, median (range) | 69 (36 - 87) | 68 (44 - 89) | 0.799 |
| Age category, n (%) | 0.601 | ||
| < 65 | 49 (34.8) | 35 (28.9) | |
| 65-74 | 66 (46.8) | 62 (51.2) | |
| >74 | 26 (18.4) | 24 (19.8) | |
| Sex, n (%) | 0.533 | ||
| Female | 61 (43.3) | 57 (47.1) | |
| Male | 80 (56.7) | 64 (52.9) | |
| Race | 0.258 | ||
| Black | 35 (24.8) | 23 (19.0) | |
| White | 106 (75.2) | 98 (81.0) | |
| Clinical characteristics | |||
| Tumor size cm, median (range) | 2.5 (0.8 – 18.0) | 2.5 (0.5 – 12.0) | 0.715 |
| T-category, n (%) | 0.001 | ||
| 1 | 57 (40.4) | 59 (48.8) | |
| 2 | 54 (38.3) | 44 (36.4) | |
| 3 | 26 (18.4) | 16 (13.2) | |
| 4 | 4 (2.8) | 2 (1.6) | |
| N-category, n (%) | 0.448 | ||
| 0 | 104 (73.8) | 90 (74.4) | |
| 1 | 17 (12.1) | 19 (15.7) | |
| 2 | 20 (14.2) | 12 (9.9) | |
| Histology n (%) | 0.136 | ||
| Adenocarcinoma | 78 (55.3) | 79 (65.3) | |
| Squamous cell carcinoma | 20 (14.2) | 9 (7.4) | |
| Others | 43 (30.5) | 33 (27.3) | |
| Treatment characteristics | |||
| 1. Extent of resection, n (%) | 0.578 | ||
| Bilobectomy | 7 (5.0) | 3 (2.7) | |
| Lobectomy | 125 (88.7) | 110 (90.9) | |
| Pneumonectomy | 9 (6.4) | 8 (6.4) | |
| 2. Number of nodes examined, median (IQR) | |||
| N1 stations | 3 (1 - 7) | 8 (4 - 16) | < 0.0001 |
| N1 minus station 10 | 2 (1 - 4) | 5 (3 - 11) | < 0.0001 |
| Stations 1-9 | 3 (0 - 6) | 6 (4 – 10) | < 0.0001 |
| All stations | 8 (4 - 11) | 15 (10 - 24) | < 0.0001 |
| 3. Cases with >0 nodes from specific stations, n (%) | |||
| Station 10 | 96 (68.1) | 103 (85.1) | 0.001 |
| N1 minus station 10 | 108 (76.6) | 108 (89.3) | 0.007 |
| Stations 1-9 | 102 (72.3) | 109 (90.1) | 0.0003 |
| 4. Number of nodes with metastasis, median (range) | |||
| N1 stations with metastasis | 0 (0 – 5) | 0 (0 – 17) | <0.0001 |
| Intrapulmonary stations with metastasis | 0 (0 – 5) | 0 (0 – 17) | 0.0003 |
| Mediastinal stations with metastasis | 0 (0 - 8) | 0 (0 – 3) | 0.278 |
| All stations with metastasis | 0 (0 – 12) | 0 (0 – 19) | 0.019 |
| 5. Patients with lymph node metastasis | |||
| Station 10 with metastasis | 11 (7.8) | 10 (8.3) | 0.707 |
| N1 minus station 10 with metastasis | 24 (17.0) | 22 (18.2) | 0.806 |
| Stations 1-9 with metastasis | 20 (14.2) | 11 (9.1) | 0.203 |
| N1 stations with metastasis | 31 (22.0) | 26 (21.5) | 0.922 |
| All stations with metastasis | 37 (26.2) | 31 (25.6) | 0.909 |
| 6. Kit use, n (%) | |||
| Yes | 25 (17.7) | 72 (59.5) | < 0.0001 |
| No | 116 (82.3) | 49 (40.5) | |
Evolution of number of lymph nodes examined and proportion of patients with lymph nodes examined from specific locations
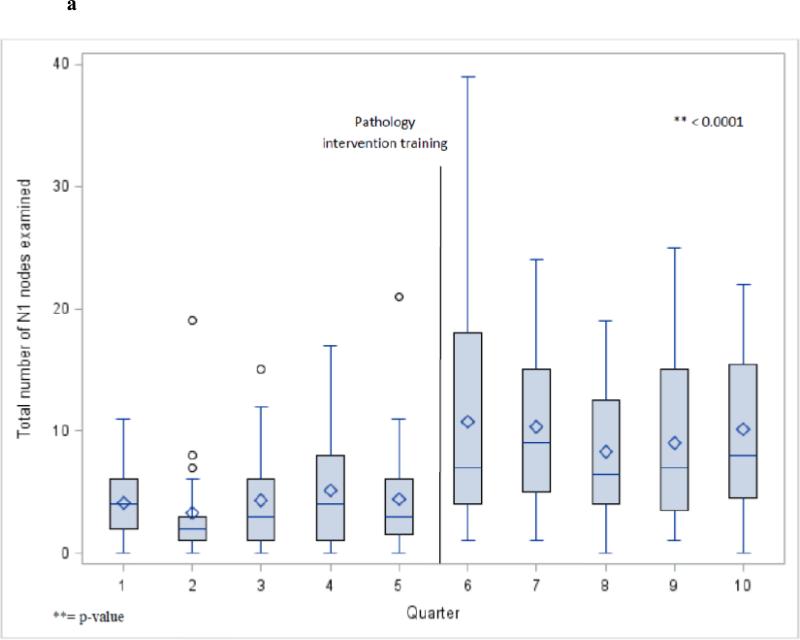
The number of lymph nodes examined approximately doubled in the era after introduction of the novel dissection protocol. For example the median number of intrapulmonary nodes increased from 2 to 5 (p<.0001), and the total N1 nodes (including station 10 [hilar]) increased from 3 to 8 (Figure 3). The median number of mediastinal lymph nodes increased from 3 to 6, and the median number of lymph nodes examined from all stations increased from 8 in the pre-intervention era to 15 in the post-intervention era (p<.0001 for both comparisons). The proportion of patients who had one or more lymph nodes examined from mediastinal stations (2-9), hilar station (10) and also from intrapulmonary lymph nodes (stations 11-14) increased significantly in the post-intervention period (Table 1).
Figure 3.
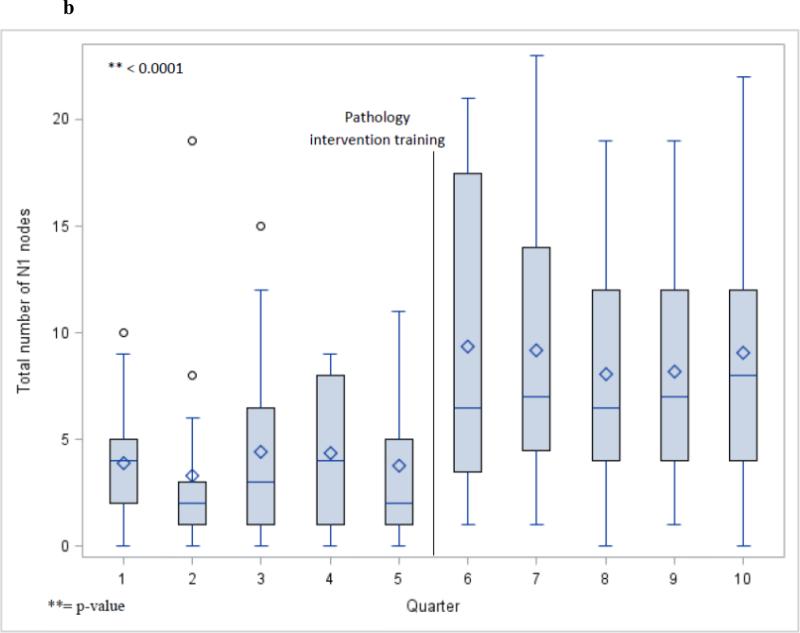
Total N1 station nodes examined per quarter for all cases (a) and lobectomy cases (b).
Evolution of number of lymph node metastasis
There was no difference in nodal stage distribution between patients before and after the novel pathology intervention protocol was introduced. However, more lymph nodes with metastasis were detected after introduction of the novel protocol. For example, the 75th to 100th percentile range of detected intrapulmonary lymph node metastasis was 0 to 5 in the pre era, 0 to 17 in the post era (p= 0.0003). For total lymph node metastasis, the 75th to 100th percentile range was 1 to 12 in the pre- era, and 1 to 19 in the post era (p<0.002).
Impact of use of the surgical specimen collection kit
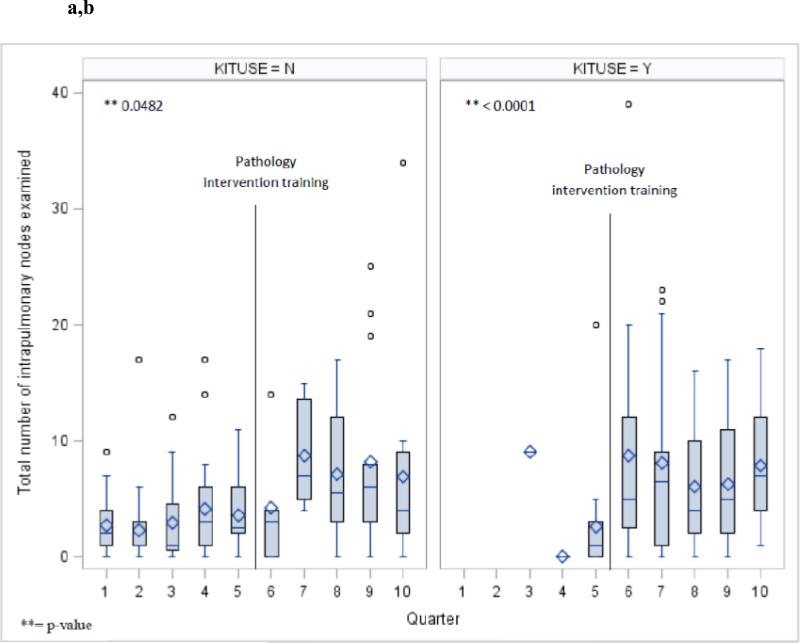
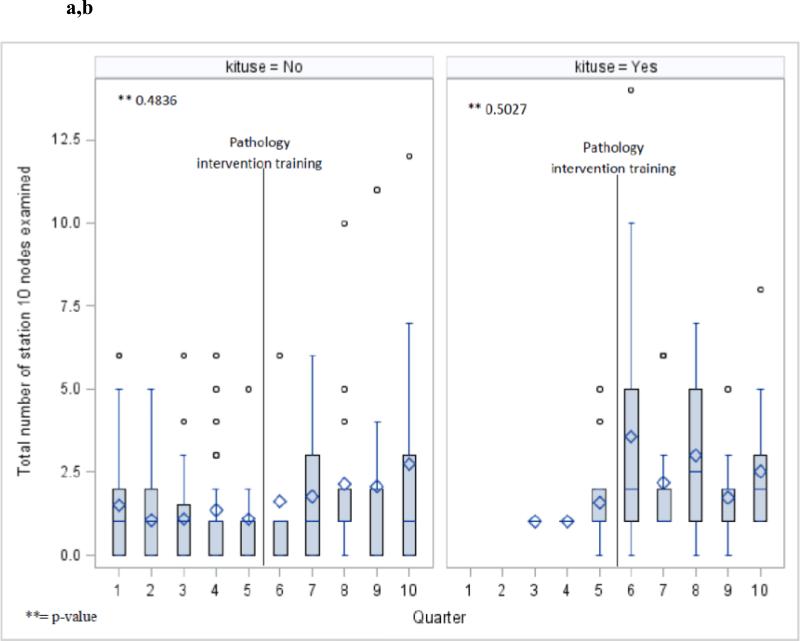
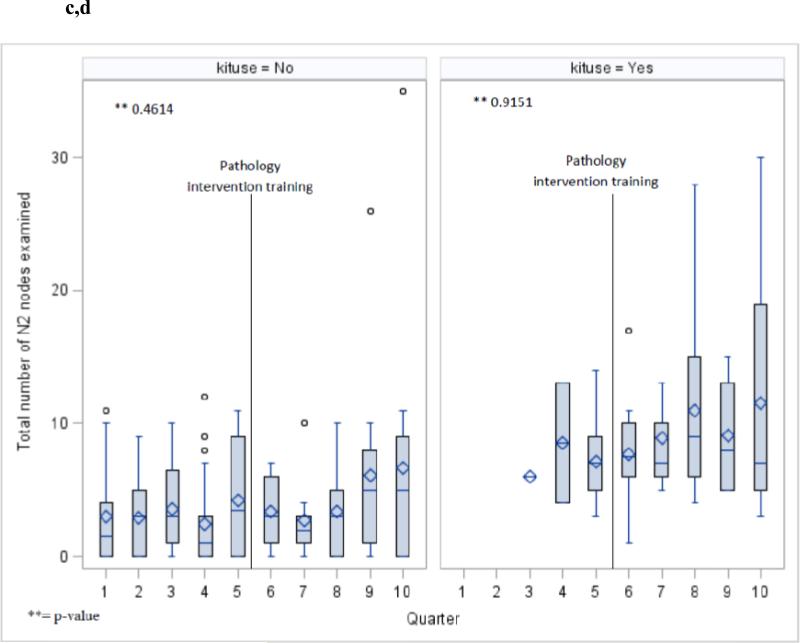
The aggregate penetration of use of the surgical specimen collection kit increased from 18% of all cases in the pre-era, to 60% in the post-era (p<.0001). Although there was fluctuation from quarter to quarter in the penetration of kit use, the overall trend was toward increased penetration over time (Fig 2). The increase in the intrapulmonary lymph node count was dependent on the era, and independent of use of the kit (Fig 4 a,b), in contradistinction to the station 10 and N2 lymph node counts which were mainly influenced by whether or not the kit was used, and independent of the introduction of the novel intrapulmonary lymph node dissection protocol (Fig 5 a-d).
Figure 4.
Total number of intrapulmonary nodes examined per quarter in cases without (a) and with (b) use of the surgical specimen collection kit during the two pathology intervention eras.
Figure 5.
Number of lymph nodes examined from station 10 without (a) and with (b) use of the surgical specimen collection kit during the two pathology intervention eras. Number of mediastinal lymph nodes examined without (c) and with (d) use of the specimen collection kit during the two pathology intervention eras.
Multivariate analysis of factors associated with retrieval of total N1 and intrapulmonary N1 lymph nodes
In the model which included age, race, sex, histology, T-category, N-category, tumor size, extent of resection and intervention period (pre or post), the extent of resection and intervention period had the strongest association with higher intrapulmonary lymph node counts (p<.0001). Pathologic N1 category was also significantly associated with higher number of lymph nodes examined, in comparison to pN0 (p<.01 for total N1 nodes [ie. including station 10] and <.0001 for intrapulmonary lymph node count [ie. excluding station 10]). Male sex and nonadenocarcinoma histology were less strongly associated with higher lymph node retrieval. All other variables were not independently associated with N1 nodal retrieval (Table 2).
Table 2.
Multivariate analysis of factors influencing the total N1 and intrapulmonary lymph node retrieval rate.
| Variables | Nodes examined |
|||
|---|---|---|---|---|
| All N1 nodes | Intrapulmonary | |||
| RR (95% C.I) | p-value | RR (95% C.I) | p-value | |
| Intervention period | ||||
| Before | 1.00 | - | 1.00 | - |
| After | 2.27 (2.09 – 2.46) | < 0.0001 | 2.52 (2.32 – 2.75) | < 0.0001 |
| Age Category | ||||
| < 65 | 1.00 | - | 1.00 | - |
| 65-74 | 1.00 (0.89 – 1.11) | 0.922 | 0.91 (0.84 – 0.99) | 0.030 |
| >74 | 1.06 (0.87 – 1.29) | 0.558 | 0.93 (0.80 – 1.09) | 0.373 |
| Race | ||||
| White | 1.00 | - | 1.00 | - |
| African American | 0.84 (0.60 – 1.17) | 0.307 | 0.72 (0.50 – 1.03) | 0.072 |
| Sex | ||||
| Female | 1.00 | - | 1.00 | - |
| Male | 1.22 (1.09 – 1.36) | 0.001 | 1.34 (1.14 – 1.59) | 0.001 |
| Histology | ||||
| Non-adenocarcinoma | 1.00 | - | 1.00 | - |
| Adenocarcinoma | 0.92 (0.81 – 1.04) | 0.177 | 0.87 (0.79 – 0.95) | 0.002 |
| N-category | ||||
| N0 | 1.00 | - | 1.00 | - |
| N1 | 1.80 (1.40 – 2.32) | < 0.0001 | 2.15 (1.57 – 2.93) | < 0.0001 |
| N2 | 1.10 (0.88 – 1.38) | 0.419 | 0.97 (0.72 – 1.30) | 0.838 |
| T-category | ||||
| 1 (Ref) | 1.00 | - | 1.00 | - |
| 2 | 0.90 (0.80 – 1.01) | 0.084 | 0.92 (0.74 – 1.14) | 0.418 |
| 3 | 1.34 (1.12 – 1.58) | 0.001 | 1.25 (1.01 – 1.55) | 0.041 |
| Extent of Resection | ||||
| Greater than lobectomy | 1.00 | - | 1.00 | - |
| Lobectomy | 0.74 (0.66 – 0.83) | < 0.0001 | 0.58 (0.51 – 0.65) | < 0.0001 |
4. Discussion
Patients who undergo surgical resection constitute the overwhelming majority of long term survivors of lung cancer. However, 56% of recipients of resection die within 5 years, most with regional or distant recurrence.19 With the advent of beneficial adjuvant systemic therapy, there has been increased interest in identifying high risk ‘early stage’ patients who might benefit from proven adjuvant therapies,20-22 or who might be candidates for clinical trials of novel adjuvant treatments. The primary predictor of benefit from adjuvant therapy is the presence of lymph node metastasis. Accurate nodal staging enhances post-operative prognostication, management, and survival.
This understanding has led to renewed focus on opportunities to improve the accuracy of routine pathologic nodal staging.23 The strong association between survival and the number of lymph nodes examined 7-10,24 or found to harbor metastasis, 11-16 coupled with recognition that most lung resection cases in large US databases cluster around the lowest end of the lymph node number spectrum,6-8,10 has raised interest in strategies designed to improve lymph node yield. Some strategies target the intraoperative lymph node harvest,18 others may be designed to encourage more thorough retrieval of lymph nodes within the resected lung specimen.17
Having previously established a high rate of inadvertent loss of lymph nodes in discarded lung tissue,17 we developed and field-tested a practical, but more thorough tissue dissection protocol which demonstrably increased the intrapulmonary lymph node yield. Although we have not shown a striking impact on the nodal stage distribution (the ultimate point of all such interventions), this study was not designed to achieve this. Rather, our primary intentions were to demonstrate the practicality and ready adoptability of this novel dissection protocol within an active surgical pathology laboratory in a community level hospital, and to evaluate the impact on the intrapulmonary lymph node retrieval rate. We hypothesize that once routinely achieved, more thorough intrapulmonary lymph node examination will lead to an increased rate of detection of N1 nodal metastasis (and upward stage redistribution) in appropriately designed future studies. The validity of this hypothesis is supported by the significant increase at the 75th – 100th percentile end of the spectrum of number of lymph nodes with metastasis during the post-intervention era, and by the multivariate analysis showing pN1 cases had significantly more lymph nodes examined than pN0 cases, an association that has previously been reported in a community-wide dataset.25
The potential survival impact of any such redistribution of nodal stage is suggested by studies that show the inferior survival rate of patients with intrapulmonary nodal metastasis, in comparison to those with pN0.26-29 Other studies show a difference in survival between subsets of patients with pN1 who have varying numbers of lymph node metastasis.13,16 Because the opportunity for quality improvement in nodal staging accuracy exists in surgical practice (the hilar and mediastinal nodal harvest,18 clarity of specimen labeling,18,30 security of specimen transfer from the operating room to the pathology laboratory),31 as well as pathology practice, it is desirable to implement interventions that target practice in both areas, as we have done. However, it is also important to clarify the contribution of each intervention. Therefore, we staggered the intervention implementation process, introducing the surgical lymph node kit 6 months before we introduced the novel pathology dissection protocol. Our report reveals the beneficial interaction of these two interventions in a pragmatic implementation design. However, we are able to separate the individual contribution of each intervention (Figs 2-5).
The combination of interventions is ultimately what will provide the greatest increase in the thoroughness of nodal staging evaluation, and therefore the most accurate pathologic stage information for use in post-operative management decision-making.32 We are actively engaged in a tri-state regional dissemination and implementation study, in which the combination of interventions will be rigorously studied to evaluate the impact on the workload of operating room and pathology laboratory staff, overall stage distribution, and patient survival. These studies will provide a robust cost-effectiveness and overall economic impact analysis of the proposed changes in practice.
Acknowledgments
Funding support: NIH R01CA172253 (Osarogiagbon).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rusch VW, Crowley J, Giroux DJ, et al. The IASLC lung cancer staging project: Proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:603–612. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- 2.Gephardt GN, Baker PB. Lung carcinoma surgical pathology report adequacy. Arch Pathol Lab Med. 1996;120:922–927. [PubMed] [Google Scholar]
- 3.Farooq A, Osarogiagbon RU, Allen JW, et al. Accuracy and comprehensiveness of pathology reportage after lung cancer resection. J Clin Oncol. 2009;27(suppl):15s. abstr 6523. [Google Scholar]
- 4.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early stage non-small cell lung cancer in the surveillance, epidemiology and end results database. J Thorac Oncol. 2012;7:1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 5.Osarogiagbon RU. Predicting survival of patients with resectable non-small cell lung cancer: Beyond TNM. J Thorac Dis. 2012;4:214–216. doi: 10.3978/j.issn.2072-1439.2012.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osarogiagbon RU, Yu X. Non-examination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013;96:1178–89. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Varlotto JM, Recht A, Nikolov M, et al. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer. 2009;115:851–8. doi: 10.1002/cncr.23985. [DOI] [PubMed] [Google Scholar]
- 8.Ou SI, Zell JA. Prognostic Significance of the Number of Lymph Nodes Removed at Lobectomy in Stage IA Non-small Cell Lung Cancer. J Thorac Oncol. 2008;3:880–886. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig MS, Goodman M, Miller DL, et al. Postoperative Survival and the Number of Lymph Nodes Sampled During Resection of Node-Negative Non-Small Cell Lung Cancer. CHEST. 2005;128:1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 10.Osarogiagbon RU, Ogbata O, Yu X. Number of Lymph Nodes Associated With Maximal Reduction of Long-Term Mortality Risk in Pathologic Node-Negative Non-Small Cell Lung Cancer. Ann Thorac Surg. 2013 Nov 20; doi: 10.1016/j.athoracsur.2013.09.058. pii: S0003-4975(13)02145-0. doi: 10.1016/j.athoracsur.2013.09.058. [Epub ahead of print] PMID: 24266949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1:120–125. [PubMed] [Google Scholar]
- 12.Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg. 2008;85:211–215. doi: 10.1016/j.athoracsur.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Jonnalagadda S, Smith C, Mhango G, et al. The Number of Lymph Node Metastases as a Prognostic Factor in Patients With N1 Non-small Cell Lung Cancer. CHEST. 2011;140:433–440. doi: 10.1378/chest.10-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei S, Asamura H, Kawachi R, et al. Which is the Better Prognostic Factor for Resected Non-Small Cell Lung Cancer The Number of Metastatic Lymph Nodes or the Currently Used Nodal Stage Classification? J Thorac Oncol. 2011;6:310–318. doi: 10.1097/JTO.0b013e3181ff9b45. [DOI] [PubMed] [Google Scholar]
- 15.Nwogu CE, Groman A, Hahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg. 2012;93:1614–20. doi: 10.1016/j.athoracsur.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonnalagadda S, Arcinega J, Smith C, Wisnivesky JP. Validation of the lymph node ratio as a prognostic factor in patients with N1 non-small cell lung cancer. Cancer. 2011;117:4724–4731. doi: 10.1002/cncr.26093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol. 2012;30:2823–2828. doi: 10.1200/JCO.2011.39.2589. [DOI] [PubMed] [Google Scholar]
- 18.Osarogiagbon RU, Miller LE, Ramirez RA, et al. Use of a surgical specimen-collection kit to improve mediastinal lymph-node examination of resectable lung cancer. J Thorac Oncol. 2012;7:1276–1282. doi: 10.1097/JTO.0b013e318257fbe5. [DOI] [PubMed] [Google Scholar]
- 19.Pfannschmidt J, Muley T, Bulzebruck H, Hoffmann H, Dienemann H. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer. 2007;55:371–377. doi: 10.1016/j.lungcan.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 20.The International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 21.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 22.Pignon J, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 23.Osarogiagbon RU, Darling GE. Towards optimal pathologic staging of resectable non-small cell lung cancer. Transl Lung Cancer Res. 2013;2:364–371. doi: 10.3978/j.issn.2218-6751.2013.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Lin CJ, Hsu W, et al. Long-term results of pathological stage I non-small cell lung cancer: validation of using the number of totally removed lymph nodes as a staging control. Eur J Cardiothorac Surg. 2003;24:994–1001. doi: 10.1016/s1010-7940(03)00567-0. [DOI] [PubMed] [Google Scholar]
- 25.Osarogiagbon RU, Allen JW, Farooq A, et al. Pathologic lymph node staging practice and stage-predicted survival after resection of lung cancer. Ann Thorac Surg. 2011;91:1486–93. doi: 10.1016/j.athoracsur.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 26.Riquet M, Manac’h D, Le Pimpec-Barthes F, et al. Prognostic significance of surgical-pathologic N1 disease in non-small cell carcinoma of the lung. Ann Thorac Surg. 1999;67:1572–6. [PubMed] [Google Scholar]
- 27.Osaki T, Nagashima A, Yoshimatsu T, et al. Survival and characteristics of lymph node involvement in patients with N1 non-small cell lung cancer. Lung Cancer. 2004;43:151–157. doi: 10.1016/j.lungcan.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Maeshima AM, Tsuta K, Asamura H, Tsuda H. Prognostic implication of metastasis limited to segmental (level 13) and/or subsegmental (level 14) lymph nodes in patients with surgically resected nonsmall cell lung carcinoma and pathologic N1 lymph node status. Cancer. 2012;118:4512–8. doi: 10.1002/cncr.27424. [DOI] [PubMed] [Google Scholar]
- 29.Rena O, Boldorini R, Papalia E, et al. Metastasis to subsegmental and segmental lymph nodes in patients resected for non-small cell lung cancer: prognostic impact. Ann Thorac Surg. 2014 Jan 27; doi: 10.1016/j.athoracsur.2013.11.051. [Epub ahead of print]. PMID: 24480258. [DOI] [PubMed] [Google Scholar]
- 30.Makary MA, Epstein J, Pronovost PJ, Millman EA, Hartmann EC, Freischlag J. Surgical specimen identification errors: A new measure of quality in surgical care. Surgery. 2007;141:450–5. doi: 10.1016/j.surg.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Molnar TF. A new device for the identification of lymph nodes at lung cancer surgery. Eur J Cardiothorac Surg. 2007;31:311–312. doi: 10.1016/j.ejcts.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 32.Osarogiagbon RU, Ramirez RA, Wang CG, Miller LE, Smeltzer MM, Sareen S, Javed AY, Robbins SG, Khandekar A, Wolf BA, Gibson J, Spencer D, Robbins ET. Dual intervention to improve pathologic staging of resectable lung cancer. Ann Thorac Surg. 2013;96:1975–81. doi: 10.1016/j.athoracsur.2013.07.009. [DOI] [PubMed] [Google Scholar]