Abstract
Background/Aims
The most commonly used immunosuppressant therapy after liver transplantation (LT) is a combination of tacrolimus and steroid. Basiliximab induction has recently been introduced; however, the most appropriate immunosuppression for hepatocellular carcinoma (HCC) patients after LT is still debated.
Methods
Ninety-three LT recipients with HCC who took tacrolimus and steroids as major immunosuppressants were included. Induction with basiliximab was implemented in 43 patients (46.2%). Mycophenolate mofetil (MMF) was added to reduce the tacrolimus dosage (n=28, 30.1%). The 1-year tacrolimus exposure level was 7.2 ± 1.3 ng/mL (mean ± SD).
Results
The 1- and 3-year recurrence rates of HCC were 12.9% and 19.4%, respectively. Tacrolimus exposure, cumulative steroid dosages, and MMF dosages had no impact on HCC recurrence. Induction therapy with basiliximab, high alpha fetoprotein (AFP; >400 ng/mL) and protein induced by vitamin K absence/antagonist-II (PIVKA-II; >100 mAU/mL) levels, and microvascular invasion were significant risk factors for 1-year recurrence (P<0.05). High AFP and PIVKA-II levels, and positive 18fluoro-2-deoxy-d-glucose positron-emission tomography findings were significantly associated with 3-year recurrence (P<0.05).
Conclusions
Induction therapy with basiliximab, a strong immunosuppressant, may have a negative impact with respect to early HCC recurrence (i.e., within 1 year) in high-risk patients.
Keywords: Immunosuppression, Basiliximab, Microvascular invasion, PIVKA-II, AFP, 18F-PET scan
INTRODUCTION
Hepatocellular carcinoma (HCC) is the seventh most common cancer and the third leading cause of cancer mortality worldwide.1 Liver transplantation (LT) has become the treatment of choice for the early stage of unresectable HCC patients because it offers complete tumor excision along with the removal of the carcinogenic liver. Unfortunately, tumor recurrence after LT still remains the main cause of death for HCC patients, and the incidence of recurrence is reported to be between 15% and 20%.2 Tumor progression is more rapid and aggressive in immunosuppressed patients following LT. The degree of the immunosuppression negatively affects the post-LT recurrence of HCC as well as the long-term survival of such patients.3
Attempts to identify clinical variables that influence tumor recurrence have resulted in improved selection criteria for patients with favorable HCC. Tumor size, number, differentiation, vascular invasion, and the serum alpha-fetoprotein (AFP) levels are potential markers for recurrence.4,5,6 Research into the relation between immunosuppressive regimens and tumor recurrence are ongoing in animal models and a few clinical studies. Calcineurin inhibitors and steroids dose-dependently increase the risk of HCC recurrence, although these are main immunosuppressants in LT recipients.7,8,9 Sirolimus has an anti-proliferative and anti-tumor effect,10 but is not approved for use in LT.11 The choice of immunosuppressive regimen for decreasing tumor recurrence risk is still a matter of debate.
Most research of immunosuppressants and HCC recurrence were analyzed in a deceased donor LT (DDLTs) setting. The immunosuppressant requirements are usually lower in recipients of living donor LTs (LDLTs) than in recipients of DDLTs. Recipients with hepatitis B related liver disease showed lower rejection rates compared to the other disease categories.12 Our center is a large volume LDLT center and has mostly adult recipients (around 80%) with hepatitis B related liver disease.13 In this patient population, our center usually follows our immunosuppressant guidelines for HCC recipients consisting of low levels of tacrolimus (5 to 8 ng/mL during the first year and 5 ng/mL thereafter) and steroids which are usually tapered down within 6 months. Basiliximab, a chimeric monoclonal antibody of the interleukin-2 receptor antagonist, has been shown to be useful as induction therapy in the setting of pre-transplant renal dysfunction because it allows minimization and delayed introduction of calcineurin inhibitors after LT.14 An induction therapy of basiliximab and addition of mycophenolate mofetil (MMF) has recently come into use in critically ill patients with encephalopathy or poor renal function, who were saved by delaying tacrolimus during the immediate post-LT period.
The objective of this study was to retrospectively investigate the effect(s) of different immunosuppressant exposures on HCC recurrence after LT-along with the many clinical, pathological, and histological factors-in a single large volume LDLT center.
METHODS
Patients
Between January 2005 and September 2009, 108 adult patients with HCC who received tacrolimus and steroids as the main immunosuppressant after LT at Seoul National University Hospital were evaluated. Of these patients, 15 patients (13.9%) were excluded: three patients with a history of other organ malignancy, four patients with metastasis in other organs at the time of the LT, and eight patients who changed main immunosuppressants within one year. Therefore, 93 patients were included as study subjects. Electronic medical records for these 93 patients were reviewed and the data collected.
Post-transplant surveillance for HCC recurrence included serum AFP, PIVKA-II level measurements during each outpatient clinic visit and abdominal CT scans for 1, 6, 12, 18, 24, and 36 months after transplantation. Chest CT scans and bone scans were conducted if HCC recurrence was suspected in any clinical or serological findings. The patients were evaluated via enhanced 3D-spiral CT scans, enhanced dynamic magnetic resonance imaging, and 18-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET) scans.
No neoadjuvant or adjuvant systemic chemotherapy was administered to the patients in this study.
All aspects of the retrospective study protocol were approved by our local Institutional Review Board (H-1110-100-382).
Clinical and pathological factors for HCC
In pre-transplant data, the recipient gender, age at the time of transplant, donor type, underlying liver disease, Child-Pugh class, medical Model for End-Stage Liver Disease (MELD) score without additional score for HCC, AFP level, protein induced by vitamin K absence-II (PIVKA-II) level, positive PET scans, and the pre-transplant treatment were reviewed. The pathological status of the explanted liver was reviewed; the number of tumors, tumor size, tumor differentiation, microvascular invasion (MVI), serosal invasion, intrahepatic metastasis, tumor stage, and Milan criteria fulfillment were documented. A positive PET scan finding was defined as an observed greater uptake of 18F-FDG in a primary HCC lesion than in a background liver.15 Tumor differentiation was classified in four grades according to Edmondson-Steiner (ES) criteria, and tumor stages were determined by pTNM classification (AJCC 7th edition). For post-transplant data, the presence of histologically proven acute rejection (Banff score) episodes and their treatment were analyzed.
Immunosuppressive therapy
Tacrolimus dosage was adjusted to the target trough blood level of 8 to 10 ng/mL for the first two weeks, followed by 5 to 8 ng/mL during the first year, and 5 ng/mL thereafter. Whole blood trough levels of tacrolimus were measured with a microparticle enzyme immunoassay (Tacrolimus, Abbott Laboratories, Abbott Park, IL). To assess the impact of tacrolimus exposure on tumor recurrence, average tacrolimus exposure was defined as the area under the curve (AUC) of tacrolimus levels plotted against time courses until the time to recurrence or one month, 3 month, 6 month, and one year after transplantation divided by the time of exposure to the tacrolimus.6 Tacrolimus blood levels at 3, 7, and 14 days, and 1, 3, 6, 9, and 12 months were used for calculating the AUC. The AUC was calculated by using the trapezoidal rule.
Methylprednisolone was initially given intravenously for the first 6 days after surgery and then changed to oral prednisolone. Steroids were progressively tapered to discontinuation by 3 to 6 months after LT. MMF was added in 28 patients (30.1%). The cumulative dosages of steroids and MMF during the first year were calculated. Basiliximab was introduced in 43 recipients (46.2%).
Statistical Analysis
Continuous variables are reported as the median (range) or mean ± the standard deviation, and differences in subgroups were compared using Student's t-test. Categorical variables were reported in a number of cases, and prevalence and differences in subgroups were compared using a chi-square test or Fisher's exact test with Yates correction. The optimal cut-off of tacrolimus exposure was obtained using receiver operating characteristic (ROC) curve. To analyze the impact of tumor recurrence by individual variables, a univariate Cox regression model was used. To identify independent variables that affect tumor recurrence, a stepwise multivariate Cox proportional hazard model was applied. Recurrence rates were calculated using survival analysis based on the Kaplan-Meier method, and a log-rank test was used to compare each subgroup. Null hypotheses of no difference were rejected if P-values were less than .05. Statistical analysis was performed using the SPSS Version 18.0 for Windows package (SPSS, Chicago, IL).
RESULTS
The demographic data and tumor characteristics
The demographic data and tumor characteristics of the study populations are described in Table 1. The most common original liver disease was related to hepatitis B virus (83.9%). LDLT was performed in 90.3% (n=84) of the patients. The mean medical MELD score without additional point for HCC was 15.0±7.23 at the time of transplantation. The patients with serum AFP level of more than 400 ng/mL were 16.1% (n=15) and the patients with PIVKA-II level of more than 100 mAU/mL were 22.6% (n=21). Thirty-three percent (n=31) had undergone some form of pretransplant treatment against HCC, i.e., trans-arterial chemoembolization, percutaneous ethanol injection therapy, or radiofrequency ablation including multiplex treatment. Based on the results of imaging studies, 75.3% (n=70) of the patients met radiologic Milan criteria. The PET scans were positive in 18.1% (n=17).
Table 1.
Characteristics of the patients and their tumors
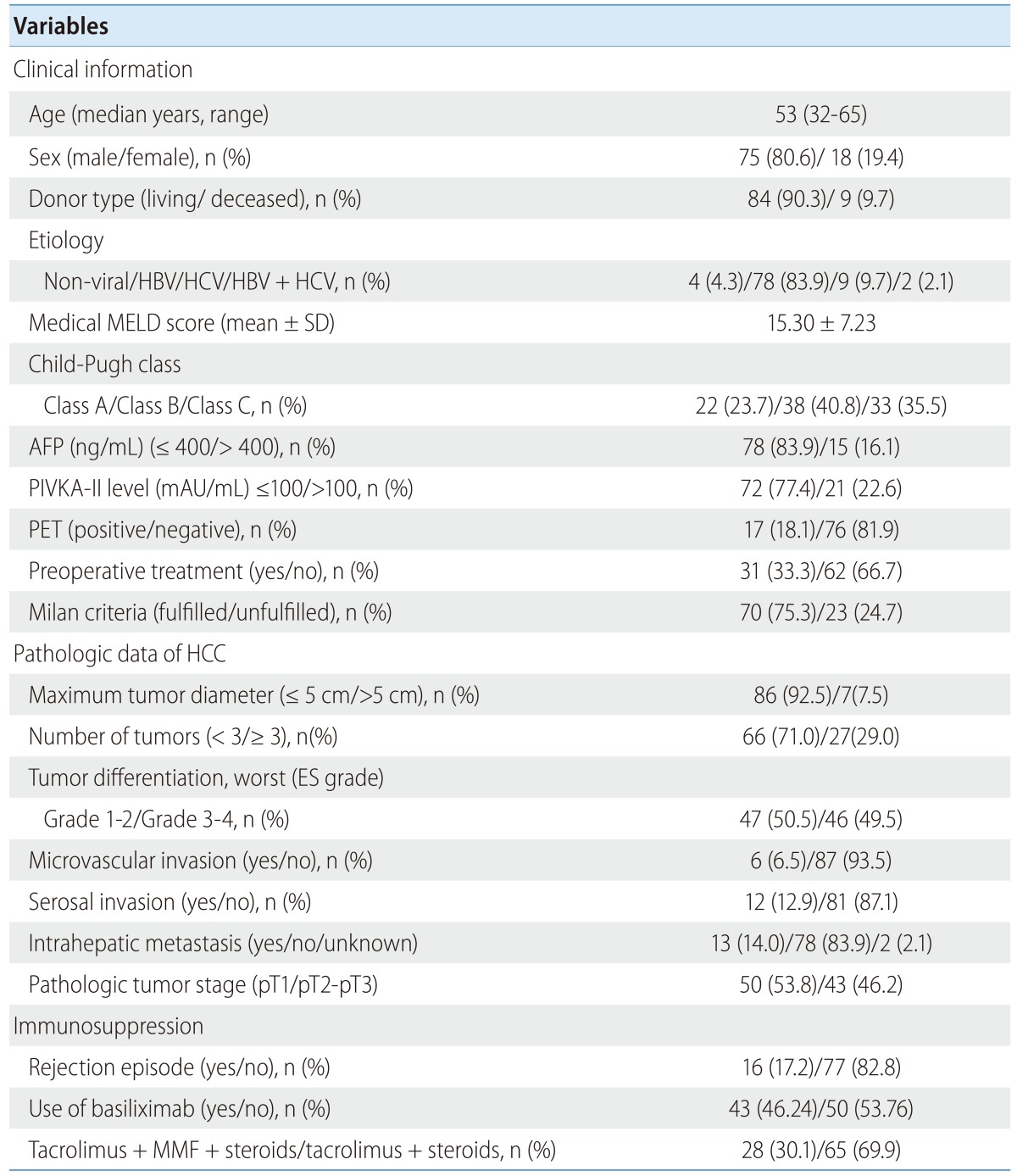
HBV, Hepatitis B virus; HCV, Hepatitis C virus; MELD, Model for End-Stage Liver Disease; SD, standard deviation; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence II; ES, Edmondson-Steiner; MMF, Mycophenolate mofetil; PD, Prednisolone; PET, positron emission tomography.
Pathologic data showed that maximum tumor diameter of more than 5 cm was present in 7.5% (n=7) of the patients, and more than three tumors were present in 29.0% (n=27). A total of 49.5% (n=46) patients showed grades 3 and 4 differentiation based on a worst of ES score system. MVI was noted in 6.5% (n=6) of the patients, serosal invasion in 12.9% (n=12), and intrahepatic metastasis in 14.0% (n=13) of the patients. The pathologic tumor stage was pT1 in 53.8% (n=50), and more than pT2 in 46.2% (n=43).
Survival Outcomes
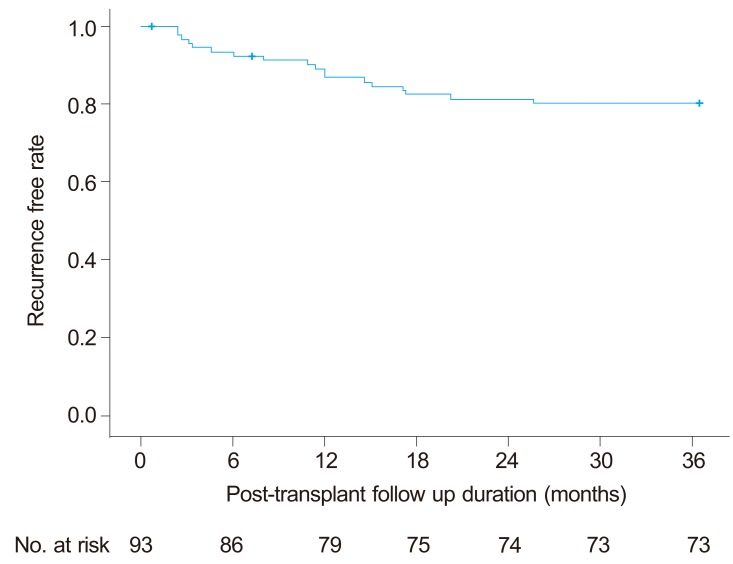
The mean follow-up period for the entire study population was 923±344 (21-1095) days. Recurrence of HCC was observed in 12 patients (12.9%) within one year after LT and in 18 patients (19.4%) within 3 year of LT. In other words, the overall one- and three-year recurrence free rate were 87.1% and 80.6%, respectively (Fig.1). Recurrence was diagnosed between 71 and 771 days after LT (median 333 days). The sites of recurrence were the graft liver (n=4), lungs (n=3), bones (n=3), peritoneal seeding (n=3), celiac lymph node (n=1), adrenal gland (n=1), and multiple organs (n=4). Two patients died without HCC recurrence and are regarded as censored cases.
Figure 1.
Recurrence-free interval curve after liver transplantation in patients with HCC. The 1- and 3-year recurrence-free rates were 87.1% and 80.6%, respectively.
Analysis of Immunosuppressant
The average one month tacrolimus exposure was 9.32±2.04 ng/mL, 3 month was 8.80±1.74 ng/mL, 6 month was 8.10±1.47 ng/mL, and one year was 7.23±1.31 ng/mL. There were no significant differences between the patients with and without 1-year recurrence in tacrolimus average exposure during the entire course of this study (Table 2). However, one year tacrolimus average exposure in patients with 3-year recurrence (7.97±1.35 ng/mL) was higher than patients without recurrence (7.05±1.24 ng/mL) (P=0.015). A cut-off point of one year average exposure to tacrolimus ≥ 7.5 ng/mL was identified by ROC analysis, with an AUC of 0.701 (95% CI 0.562-0.840), sensitivity of 72.2% and specificity of 64.9%. There was a high proportion of patients with high exposure to tacrolimus (≥ 7.5 ng/mL) in 3-year recurrence group (72.2%) than non-recurrence group (35.2%) (P=0.007) (Table 3).
Table 2.
Comparison of the characteristics of patients with/without 1-year recurrence
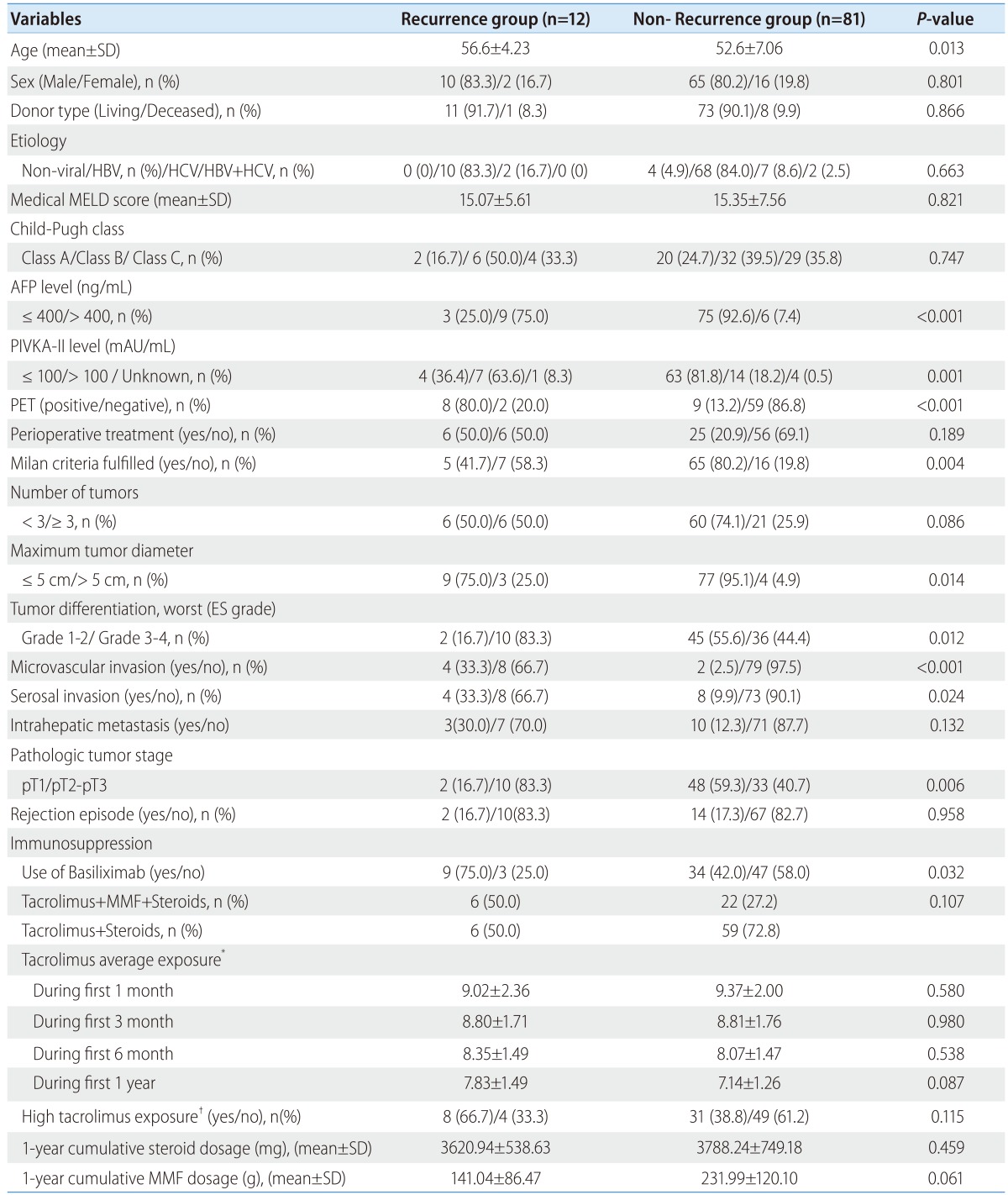
HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, model for End-Stage Liver Disease; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence II; MMF, mycophenolate mofetil.
*Tacrolimus average exposure was defined as the area under the curve (AUC) of tacrolimus levels plotted against time courses until the time to recurrence or 1, 3, 6 month and one year after transplantation.
†High tacrolimus exposure was defined as one year average tacrolimus exposure ≥7.5 ng/mL.
Table 3.
Comparison of the characteristics of patients with/without 3-year recurrence
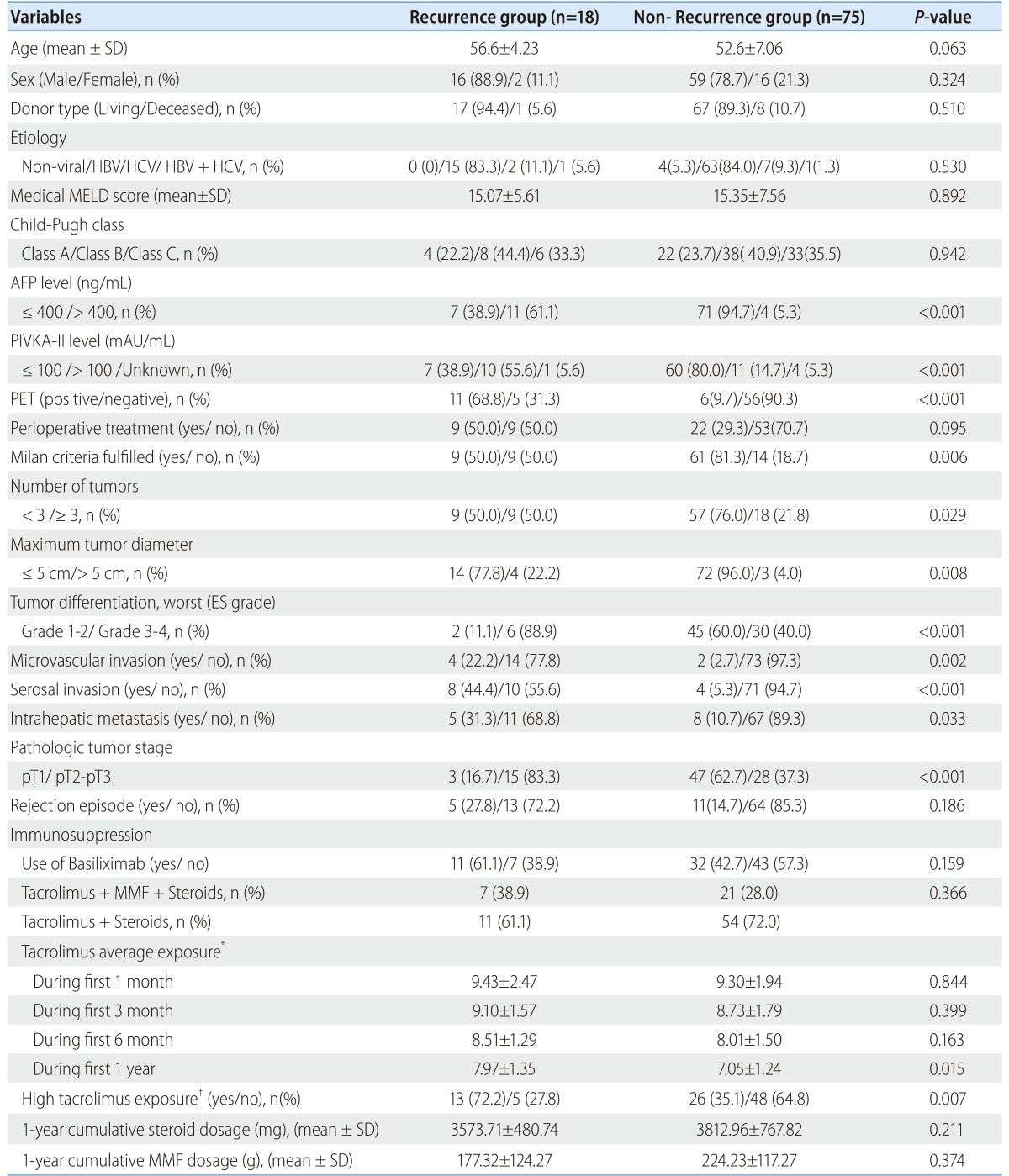
HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, model for End-Stage Liver Disease; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence II; MMF, mycophenolate mofetil
*Tacrolimus average exposure was defined as the area under the curve (AUC) of tacrolimus levels plotted against time courses until the time to recurrence or 1, 3, 6 month and one year after transplantation.
†High tacrolimus exposure was defined as one year average tacrolimus exposure ≥ 7.5 ng/mL.
The one year cumulative steroid dosage was 3,940.10±1417.16 (2,060-14,296) mg. There were no differences in cumulative steroid dosage between the patients with one-year HCC recurrence (3,620.9±538.6 mg) and the patients without recurrence (3,788.2±749.2 mg) (P=0.459). The cumulative steroid dosage was not different between the patients with 3-year HCC recurrence (3,575.7±480.7 mg) and the patients without recurrence (3,813.0±767.8 mg) (P=0.211).
The one year cumulative MMF dosage was 201.6±11.9 (120.0-412.0) g. The cumulative MMF dosage was not different between the patients with one-year HCC recurrence (141.0±86.5 g) and the patients without recurrence (232.0±120.1 g) (P=0.061). The cumulative MMF dosage was also not different between patients with 3-year HCC recurrence (177.3±124.3 g) and patients without recurrence (224.2±117.3 g) (P=0.374).
The basiliximab induction therapy was more frequently used in one-year recurrence group than non-recurrence group (75.0% vs. 42.0%, P=0.032); however the frequency of basiliximab induction therapy was not significantly different between patients with and without 3-year recurrence (61.1% vs. 42.7%, P=0.159)
Factors affecting the one-year recurrence outcomes
With regard to non-pharmacological factors, pre-transplant AFP levels over 400 ng/mL (P<0.001), PIVKA-II levels over 100 mAU/mL (P=0.002), tumor sizes over 5 cm (P=0.018), tumor differentiation of G3-G4 (P=0.025), PET positive (P<0.001), MVI (P<0.001), serosal invasion (P=0.026), pathologic tumor stage pT2-pT3 (P=0.016), and unfulfilled radiologic Milan criteria (P=0.005) were variables that significantly affected one-year HCC recurrence by univariate analysis. With regard to pharmacological factors, and use of induction therapy with basiliximab (P=0.043) affected one year HCC recurrence by univariate analysis (Table 4).
Table 4.
Univariate and multivariate Cox regression analyses of risk factors for 1-year recurrence
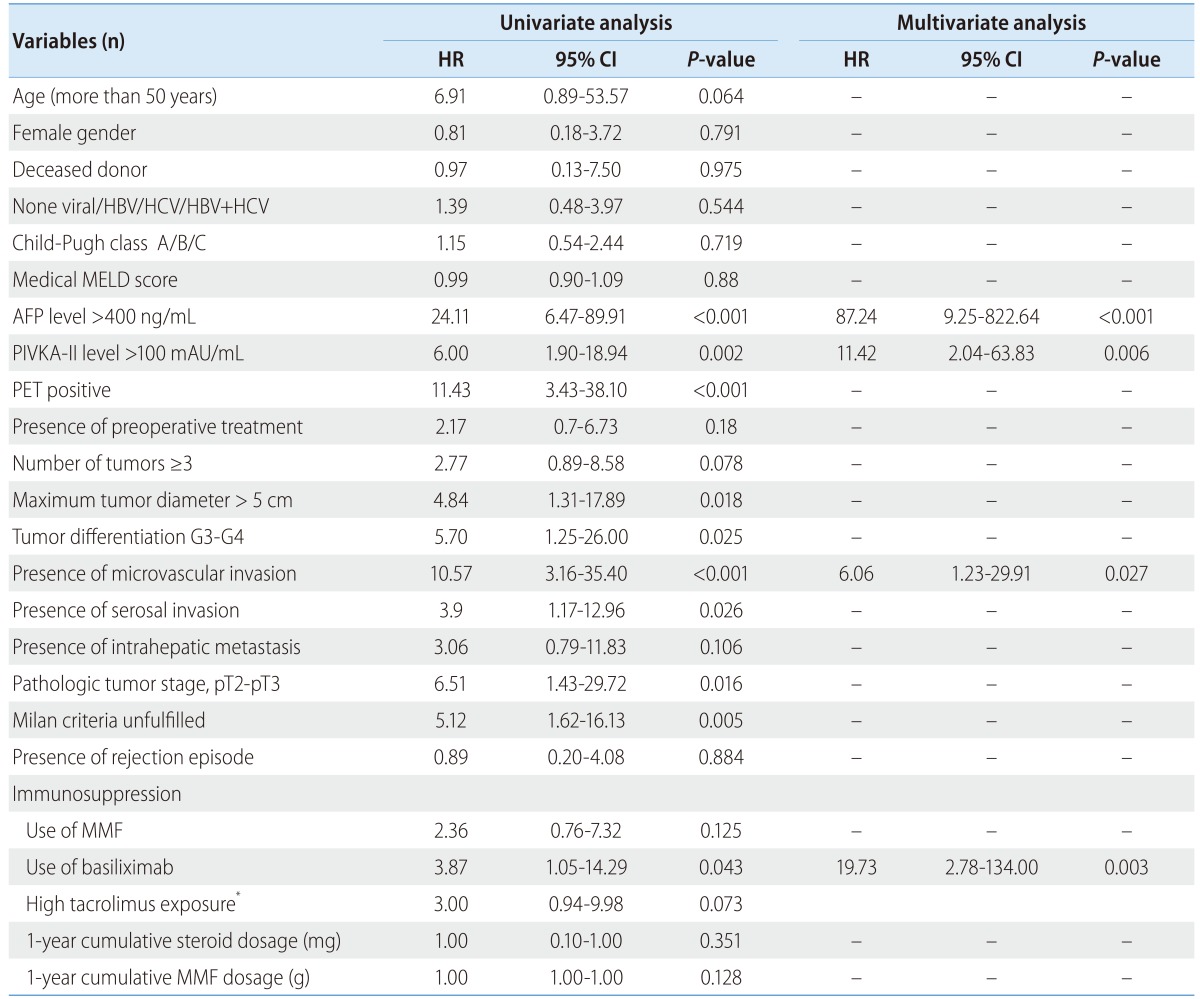
HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, model for End-Stage Liver Disease; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence II; MMF, mycophenolate mofetil
*High tacrolimus exposure was defined as one year average tacrolimus exposure ≥ 7.5 ng/mL.
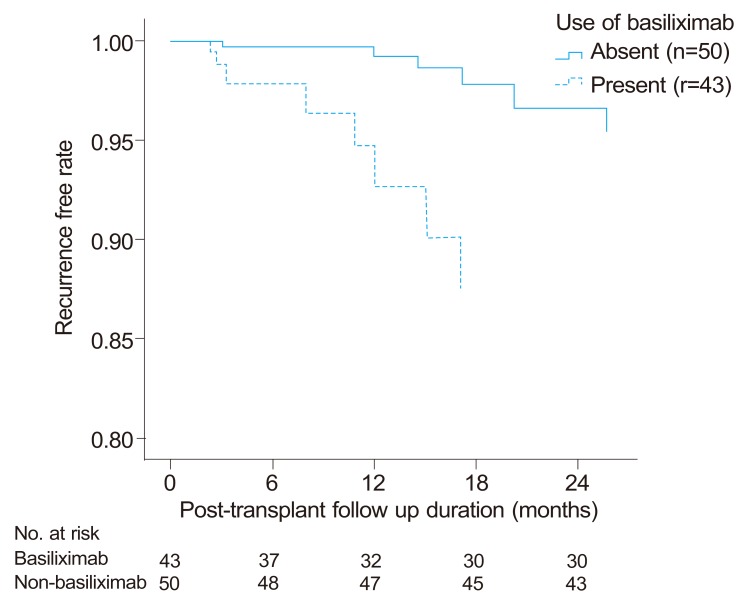
In multivariate analysis, the variates found significant (p-value<0.05) by univariate analysis except tumor size which is associated with both Milan criteria and tumor stage were included. In multivariate Cox regression analysis adjusted for age, gender, and medical MELD score, AFP levels (HR: 87.24, 95% CI: 9.25-822.64, P<0.001), induction therapy with basiliximab (HR: 19.73, CI: 2.78-134.00, P=0.003), PIVKA-II levels (HR: 11.42, CI: 2.04-63.83, P=0.006), and MVI (HR: 6.06, CI: 1.23-29.91, P=0.0271) (Fig.2) were independently and significantly associated with one-year recurrence outcome (Table 4).
Figure 2.
Recurrence-free interval curve according to induction therapy with basiliximab (Cox-adjusted model for age, gender, AFP and PIVKA-II levels, microvascular invasion, and Model for End-Stage Liver Disease score). Induction therapy with basiliximab, high AFP (>400 ng/mL) and PIVKA-II (>100 mAU/mL) levels, and microvascular invasion were significant risk factors for 1-year recurrence (P<0.05).
Factors affecting the 3-year recurrence outcome
With regard to non-pharmacological factors, pre-transplant AFP levels over 400 ng/mL (P<0.001), PIVKA-II levels over 100 mAU/mL (P<0.001), tumor sizes over 5 cm (P=0.002), tumor differentiation of G3-G4 (P=0.002), PET positive (P<0.001), MVI (P=0.001), serosal invasion (P<0.001), TNM stage pT2-pT3 (P=0.002), and unfulfilled radiologic Milan criteria (P=0.004), number of tumors more than 3 (P=0.027), and intrahepatic metastasis (P=0.022) were variables that significantly affected HCC recurrence by univariate analysis. With regard to pharmacological factors, one year average tacrolimus exposure more than 7.5 ng/mL (P=0.008) affected 3-year HCC recurrence by univariate analysis (Table 5).
Table 5.
Univariate and multivariate Cox regression analyses of risk factors for 3-year recurrence
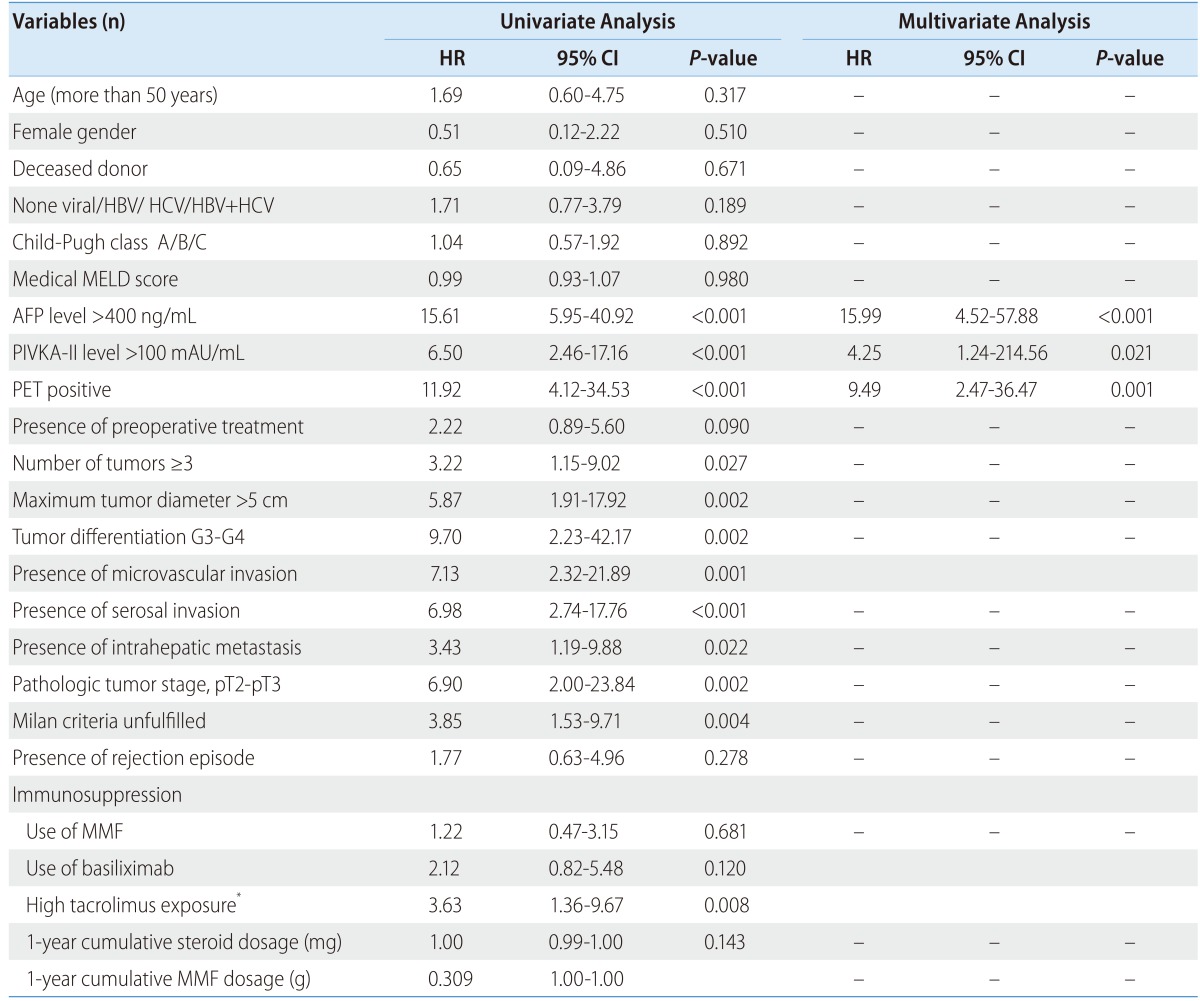
HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, model for End-Stage Liver Disease; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence II; MMF, Mycophenolate mofetil
*High tacrolimus exposure was defined as one year average tacrolimus exposure ≥ 7.5 ng/mL.
In multivariate analysis, the variates found significant (P-value<0.05) except tumor size and tumor number which are associated with both Milan criteria and tumor stage were included. By a multivariate Cox regression analysis adjusted for age, gender, and medical MELD score, AFP levels (HR: 15.99, 95% CI: 4.42-57.88, P<0.001), PIVKA-II levels (HR: 4.25, CI: 1.24-214.56, P=0.021), and PET positive (HR: 9.49, CI: 2.47-36.47, P=0.001) were independently and significantly associated with three-year recurrence outcome (Table 5).
DISCUSSION
Immunosuppressive therapy after LT has changed over the last 10 years. Minimization of calcineurin inhibitor exposure and steroid therapy combination with MMF has become widely adopted. Although postoperative immunosuppression can accelerate tumor growth, there are only a few studies that investigate the possible influence of different immunosuppressive agents on HCC recurrence after liver transplantation.4,6 In contrast, it is widely accepted that the pathologic status of HCC is closely related to the risk of HCC recurrence, and patient survival has improved by establishing Milan criteria for selecting transplant candidates.5,16,17,18,19,20 In this regard, we focused on immunosuppressive agents and the early recurrence (within one year) of HCC because exposure to immunosuppressants was maximal during this period, the HCC which were recurred within one year might have aggressive nature, and the late recurrence of HCC might be related to many complex factors. In our study, basiliximab induction therapy was the independent risk factor of early recurrence within one-year. This could be explained by the effect of strong immunosuppression on the early period of transplant on the recurrence of aggressive nature of HCC. The impact of the immunosuppression disappeared during follow up analysis for 3-year recurrence.
In this study, exposure to immunosuppressant was analyzed using only within first postoperative year data to avoid the possible bias of a longer follow-up period of non-recurrent patients, as 66.7% of the recurrent patients were detected within one year after transplantation. The average tacrolimus exposure during first 1, 3, and 6 month was not significantly different between recurrence group and non-recurrence group. However, one year average tacrolimus exposure was higher in patients with recurrence. Because 38.9% of recurrence patients are detected within 6 months and the tacrolimus levels usually go to the lower end of the target range as time goes on after transplantation, the shorter follow up time of the patients who recurred early might lead to the higher calculated average tacrolimus exposure. On the other hand, despite the difference of average one year tacrolimus exposure, average tacrolimus exposure is not an independent risk factor of HCC recurrence, which is inconsistent with previous results demonstrating the effects of calcineurin inhibitors on HCC recurrence.7,8,21 Vivarelli et al. reported that the cumulative dose of cyclosporine during the first year was an independent risk factor for five-year recurrence free survival.4 The same group reported that overexposure to tacrolimus (cut-off level of 10 ng/mL) during the first year after transplantation increased the risk of HCC recurrence.6 The suggested mechanism was that calcineurin inhibitors are involved in increased tumor growth factor-β, vascular endothelial growth factor, and the inhibition of DNA repair.21 One of the reasons we could not observe a negative effect of high tacrolimus exposure on HCC recurrence may be due to the relatively low one-year average tacrolimus exposure in both the recurrence (7.97±1.35) and non-recurrence groups (7.05±1.24) in this study compared with that reported in the non-recurrence group in the previous study.4 This kind of low-level tacrolimus maintenance therapy and the early tapering of steroids was possible due to most of our cases being living donor recipients with underlying hepatitis B related liver disease.12,14 Many investigators reported that early steroid withdrawal regimens reduce tumor recurrence and do not increase rejection rates.9 In our study, correlation between one-year cumulative steroid dosage and tumor recurrence was not observed because most of the patients withdrew steroids after approximately six months, and because the difference between cumulative dosages was not significant.
The Child-Pugh classes and medical MELD scores of the patients treated with basiliximab were different from the patients in the non-basiliximab group (data not shown) as expected. This selection bias could be due to the limitations of the retrospective design of this study. However, Child-Pugh classes and MELD scores were not shown as significant risk factors for HCC recurrence in the Cox-adjusted regression analysis. Induction therapy with anti-lymphocyte antibodies such as OKT-3 (anti-CD3 antibody) or anti-thymocyte globulin has been reported to increase the risk of HCC recurrence.22 Although in previous reports, basiliximab induction in combination with tacrolimus-based immunosuppressive regimens did not increase the risk of post-transplant lymphoproliferative disease, the direct impact of basiliximab on HCC recurrence has not been investigated.23,24,25 In a study conducted by Ramirez et al., three (18%) recipients with HCC developed and died of metastatic HCC in the basiliximab group compared with none in the placebo group, not statistically significant.22 In a recent study based on large registry data, the use of CD25 antibody induction improved the survival rate after transplantation for HCC. However, they were not able to access the data on HCC recurrence.10 Although some research using anti-CD25 antibodies in cancer immunotherapy has been conducted, the basiliximab doses used for those purposes is different from that used in transplantation. Okita et al have demonstrated that, at low concentrations of basiliximab (under 0.06 µg/mL), CD4+CD25high regulatory T (T-reg) cells were selectively inhibited, allowing the tumor cells to escape from the host immune attack.26 Also, at concentrations under 0.01 µg/mL, basiliximab enhanced interferon-gamma production of activated peripheral blood mononuclear cells. However, at a concentration of 1µg/mL, an almost complete reduction of CD4+CD25low cells to an undesirable degree, along with CD4+CD25high cells, and the inhibition of interferon-gamma production occurs when basiliximab concentration is over 0.1 µg/mL.26 After a single 20-mg injection of basiliximab, the immediate serum concentrations of basiliximab generally ranged from 5-10 mg/L and declined over time, with a terminal half-life 13.4 days.27 Thus a relatively high dose of basiliximab (20 mg) is currently used in transplantation and results in a long-lasting, complete disappearance of all CD25+ cells, including the tumor-specific effector cytotoxic T-cells involved in tumor eradication. Therefore, more research is needed to confirm the correlation between induction therapy with basiliximab and HCC recurrence.
In this study, we analyzed other factors, including pathologic findings, as well as the exposure of immunosuppressive agents affecting the early recurrence of HCC. Consistent with other studies, pre-transplant MVI, AFP and PIVKA-II levels, were significantly and independently correlated with HCC recurrence.28,29,30,31,32,33,34,35,36 Pre-operative needle core biopsy provides exact histological information on tumors, but this procedure is not recommended due to the risk of tumor seeding. Therefore, correlation between tumor markers, i.e., serum AFP, PIVKA-II, and post-transplant histological findings is important to predict HCC recurrence after LT. Among several pathologic factors, MVI is one of the strongest prognostic risk factors for tumor recurrence.33,34,35
According to this study results, basiliximab induction therapy might have a negative impact as a strong immunosuppression on early HCC recurrence within one year. Therefore, basiliximab induction therapy should be cautious for the high risk patients of early HCC recurrence according to the guidance of pre-transplant risk factors. If the patient's pre-transplant medical condition is poor requiring basiliximab induction therapy, but the patient has HCC with poor biology, i.e., high AFP/PIVKA-II levels, a baseline immunosuppressant with a calcineurin inhibitor and a steroid must be lowered or delayed in order to avoid excessive immunosuppression. However, due to the limitations of retrospective design and relatively small sample size of our study, prospective randomized studies with large sample size are needed to confirm the impact of basiliximab induction therapy on early HCC recurrence after LT.
Abbreviations
- 18-FDG PET
18-fluoro-2-deoxy-D-glucose positron emission tomography
- AFP
alpha-fetoprotein
- AUC
area under the curve
- CI
confidence interval
- CT
computed tomography
- DDLT
deceased donor liver transplantation
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- LDLT
living donor liver transplantation
- LT
liver transplantation
- MELD
model for end-stage liver disease
- MMF
Mycophenolate mofetil
- MVI
microvascular invasion
- PIVKA-II
protein induced by vitamin K absence-II
Footnotes
The authors have no conflicts to disclose.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26:109–118. doi: 10.1111/j.1432-2277.2012.01562.x. [DOI] [PubMed] [Google Scholar]
- 3.Molmenti EP, Klintmalm GB. Liver transplantation in association with hepatocellular carcinoma: an update of the International Tumor Registry. Liver Transpl. 2002;8:736–748. doi: 10.1053/jlts.2002.34879. [DOI] [PubMed] [Google Scholar]
- 4.Vivarelli M, Bellusci R, Cucchetti A, Cavrini G, De Ruvo N, Aden AA, et al. Low recurrence rate of hepatocellular carcinoma after liver transplantation: Better patient selection or lower immunosuppression? Transplantation. 2002;74:1746–1751. doi: 10.1097/00007890-200212270-00017. [DOI] [PubMed] [Google Scholar]
- 5.Fiorentino M, Altimari A, Ravaioli M, Gruppioni E, Gabusi E, Corti B, et al. Predictive value of biological markers for hepatocellular carcinoma patients treated with orthotopic liver transplantation. Clin Cancer Res. 2004;10:1789–1795. doi: 10.1158/1078-0432.ccr-1149-3. [DOI] [PubMed] [Google Scholar]
- 6.Parfitt JR, Marotta P, Alghamdi M, Wall W, Khakhar A, Suskin NG, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007;13:543–551. doi: 10.1002/lt.21078. [DOI] [PubMed] [Google Scholar]
- 7.Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, et al. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008;248:857–862. doi: 10.1097/SLA.0b013e3181896278. [DOI] [PubMed] [Google Scholar]
- 8.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZS, He F, Zeng FJ, Jiang JP, Du DF, Liu B. Early steroid withdrawal after liver transplantation for hepatocellular carcinoma. World J Gastroenterol. 2007;13:5273–5276. doi: 10.3748/wjg.v13.i39.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration (FDA) Rapamune highlights of prescribing information. [Accessed 2013]. FDA web site, <http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021083s045,021110s052lbl.pdf>.
- 12.Kwekkeboom J, Tha-In T, Tra WM, Hop W, Boor PP, Mancham S, et al. Hepatitis B immunoglobulins inhibit dendritic cells and T cells and protect against acute rejection after liver transplantation. Am J Transplant. 2005;5:2393–2402. doi: 10.1111/j.1600-6143.2005.01029.x. [DOI] [PubMed] [Google Scholar]
- 13.Yi NJ, Lee KW, Kong SY, Park KU, Lee KB, Hong G, et al. Outcome of various treatments for posttransplant hepatitis B virus recurrence. World J Surg. 2013;37:812–819. doi: 10.1007/s00268-013-1914-z. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez CB, Bozdin A, Frank A, Maley W, Doria C. Optimizing use of basiliximab in liver transplantation. Transpl Res Risk Manag. 2010;2:1–10. [Google Scholar]
- 15.Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, et al. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl. 2006;12:1655–1660. doi: 10.1002/lt.20861. [DOI] [PubMed] [Google Scholar]
- 16.Ringe B, Wittekind C, Bechstein WO, Bunzendahl H, Pichlmayr R. The role of liver transplantation in hepatobiliary malignancy: a retrospective analysis of 95 patients with particular regard to tumor stage and recurrence. Ann Surg. 1989;209:88–98. doi: 10.1097/00000658-198901000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva MF, Wigg AJ. Current controversies surrounding liver transplantation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1217–1226. doi: 10.1111/j.1440-1746.2010.06335.x. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M Practice guidelines committee, American association for the study of liver diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 19.Cescon M, Ravaioli M, Grazi GL, Ercolani G, Cucchetti A, Bertuzzo V, et al. Prognostic factors for tumor recurrence after a 12-year, single-center experience of liver transplantations in patients with hepatocellular carcinoma. J Transplant. 2010;2010:pii:904152. doi: 10.1155/2010/904152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 21.Majno P, Giostra E, Mentha G Geneva liver cancer study. Is there a customised immunosuppressive regimen for patients transplanted with hepatocellular carcinoma? J Hepatol. 2005;43:577–584. doi: 10.1016/j.jhep.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, et al. Role of immunosuppression and tumor differentiation in predicting recurrence after liver transplantation for hepatocellular carcinoma: a multicenter study of 412 patients. World J Gastroenterol. 2006;12:7319–7325. doi: 10.3748/wjg.v12.i45.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez CB, Doria C, di Francesco F, Iaria M, Kang Y, Marino IR. Basiliximab induction in adult liver transplant recipients with 93% rejection-free patient and graft survival at 24 months. Transplant Proc. 2006;38:3633–3635. doi: 10.1016/j.transproceed.2006.10.110. [DOI] [PubMed] [Google Scholar]
- 24.Gruttadauria S, Vasta F, Mandala L, Cintorino D, Piazza T, Spada M, et al. Basiliximab in a triple-drug regimen with tacrolimus and steroids in liver transplantation. Transplant Proc. 2005;37:2611–2613. doi: 10.1016/j.transproceed.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 25.Chapman TM, Keating GM. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs. 2003;63:2803–2835. doi: 10.2165/00003495-200363240-00009. [DOI] [PubMed] [Google Scholar]
- 26.Okita R, Yamaguchi Y, Ohara M, Hironaka K, Okawaki M, Nagamine I, et al. Targeting of CD4+CD25high cells while preserving CD4+CD25low cells with low-dose chimeric anti-CD25 antibody in adoptive immunotherapy of cancer. Int J Oncol. 2009;34:563–572. [PubMed] [Google Scholar]
- 27.Onrust SV, Wiseman LR. Basiliximab. Drugs. 1999;57:207–213. doi: 10.2165/00003495-199957020-00006. [DOI] [PubMed] [Google Scholar]
- 28.Vivarelli M, Dazzi A, Zanello M, Cucchetti A, Cescon M, Ravaioli M, et al. Effect of different immunosuppressive schedules on recurrence free survival after liver transplantation for hepatocellular carcinoma. Transplantation. 2010;89:227–231. doi: 10.1097/TP.0b013e3181c3c540. [DOI] [PubMed] [Google Scholar]
- 29.Montaser LM, Abbas OM, Saltah AM, Waked IA. Circulating AFP mRNA as a possible indicator of hematogenous spread of HCC cells: a possible association with HBV infection. J Egypt Natl Canc Inst. 2007;19:48–60. [PubMed] [Google Scholar]
- 30.Lee KW, Suh KS. Liver transplantation for advanced hepatocellular carcinoma. J Korean Soc Transplant. 2010;24:4–12. [Google Scholar]
- 31.Fujiki M, Takada Y, Ogura Y, Oike F, Kaido T, Teramukai S, et al. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant. 2009;9:2362–2371. doi: 10.1111/j.1600-6143.2009.02783.x. [DOI] [PubMed] [Google Scholar]
- 32.Shirabe K, Itoh S, Yoshizumi T, Soejima Y, Taketomi A, Aishima S, et al. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol. 2007;95:235–240. doi: 10.1002/jso.20655. [DOI] [PubMed] [Google Scholar]
- 33.Salizzoni M, Romagnoli R, Lupo F, David E, Mirabella S, Cercutti E, et al. Microscopic vascular invasion detected by anti-CD34 immunohistochemistry as a predictor of recurrence of hepatocellular carcinoma after liver transplantation. Transplantation. 2003;76:844–848. doi: 10.1097/01.TP.0000083555.06337.8E. [DOI] [PubMed] [Google Scholar]
- 34.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 35.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 36.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]