Abstract
The mechanism by which neutrophils [polymorphonuclear leukocyte (PMNs)] are stimulated to move across epithelial barriers at mucosal surfaces has been basically unknown in biology. IL-8 has been shown to stimulate PMNs to leave the bloodstream at a local site of mucosal inflammation, but the chemical gradient used by PMNs to move between adjacent epithelial cells and traverse the tight junction at the apical neck of these mucosal barriers has eluded identification. Our studies not only identify this factor, previously termed pathogen-elicited epithelial chemoattractant, as the eicosanoid hepoxilin A3 (hepA3) but also demonstrate that it is a key factor promoting the final step in PMN recruitment to sites of mucosal inflammation. We show that hepA3 is synthesized by epithelial cells and secreted from their apical surface in response to conditions that stimulate inflammatory events. Our data further establish that hepA3 acts to draw PMNs, via the establishment of a gradient across the epithelial tight junction complex. The functional significance of hepA3 to target PMNs to the lumen of the gut at sites of inflammation was demonstrated by the finding that disruption of the 12-lipoxygenase pathway (required for hepA3 production) could dramatically reduce PMN-mediated tissue trauma, demonstrating that hepA3 is a key regulator of mucosal inflammation.
Bacterial pathogens continually confront epithelial barriers of the body, such as those of the gastrointestinal, respiratory, and reproductive tracts. Polymorphonuclear leukocyte (PMNs) represent a class of white cells critical to defend the host from such pathogens. Previous studies have identified factors such as IL-8, secreted from the basolateral surface of epithelial barriers that establish chemical gradients essential for PMN activation and recruitment from the bloodstream (1). After this IL-8 gradient, PMNs are drawn to the lateral surfaces of epithelial cells. Migration to the actual site of bacterial infection, however, i.e., within the intestinal lumen, requires the action(s) of an additional chemical gradient established across a final barrier present at the apical neck of epithelia, the tight junction (TJ) complex. Any molecule that could function to establish a gradient across such a barrier would have unique properties: selective secretion from the apical rather than basolateral epithelial cell surface, capacity to permeate the TJ to establish a chemical gradient, and a labile nature that would prevent excessive PMN migration. Identification of such a factor has been an important unanswered question of epithelial pathobiology.
Salmonellosis, a frequent cause of diarrhea worldwide, represents one example of epithelial pathobiology where extensive PMN transmigration into the lumen is observed, in this case into small intestine crypts in response to apical infection by Salmonella typhimurium. PMN actions on the epithelium, culminating in the formation of intestinal crypt abscesses and subsequent loss of barrier function, underline the key events in mediating not only the clinical manifestations of S. typhimurium-induced enteritis (2, 3) but also idiopathic diseases associated with inflammatory bowel disease (4). To model such inflammatory events occurring at the intestinal mucosa, we have used an in vitro model of S. typhimurium infection of human intestinal epithelial cells, T84, grown as monolayers to detect the presence and obtain an initial characterization of pathogen-elicited epithelial chemoattractant (PEEC). PEEC is a small molecule (Mr < 1,500) that stimulates pertussis-toxin-sensitive G protein-coupled Ca2+ mobilization; however, in contrast to other known PMN chemoattractants, it was shown to induce no degranulation or oxidative burst even at saturating concentrations and in the presence of “primers” such as cytochalasins (5), thus distinguishing PEEC from all other factors known to affect PMN chemotaxis. Using the T84-S. typhimurium infection model (6), we have now purified and identified a molecule secreted from the apical surface of T84 cell monolayers that is stimulated by pathogenic, but not nonpathogenic, strains of S. typhimurium and demonstrated this molecule, hepoxilin A3 (hepA3), to recapitulate previously established characteristics for PEEC both in vitro and in vivo.
Experimental Procedures
Purification and Identification of PEEC. Crude preparations of PEEC were collected from the apical surface of polarized T84 cell monolayers infected with S. typhimurium SL1344 or VV341, as described (6). Samples were first passed through an Amicon ultrafiltration apparatus (Millipore) fitted with a 2,000-Da cutoff membrane. Filtrate components were bound to a Bakerbond spe octadecyl extraction column (J. T. Baker) that was subsequently eluted with water, hexane, and finally methanol. The methanol fraction was dried under vacuum and resuspended in 50:50 (vol/vol) methanol/2 mM Tris·HCl (pH 7.5) and injected onto a Vydac (Hesperia, CA) C18 (10 μm; 300 Å) semipreparative column (10 × 250 cm) equilibrated with 2 mM Tris·HCl (pH 7.5). A methanol gradient of 1-10% over 10 min, then 10-60% over 25 min, followed by 60-100% over 45 min (all at room temperature) was used to isolate active PEEC fractions. Active fractions, having no detectable absorbance at 280 nm and weak absorbance at 214 nm, were analyzed by using a Genesis C18 (4 μm, 120 Å) analytical HPLC column (4.6 × 150 mm) equilibrated with 5 mM triethylamine/acetic acid (pH 7.2). PEEC samples were chromatographed by using a linear methanol gradient of 0-100% over 60 min and analyzed by using a Finnigan LCQDeca HPLC/electrospray mass spectrometer set in the negative ion mode. Compounds were characterized for retention time, UV spectra (UV6000LP photodiode array detector), and m/z signals (Thermo Separation Products, San Jose, CA).
Cell Culture. T84 monolayers with a baseline resistance ranging from 650 to 1,500 ohm·cm2 were used (6). PMNs were obtained (7) from different donors with individual experiments performed by using PMNs from single donors on individual days. PMN isolation was restricted to 10 different donors (repetitive donations) over the course of these studies. PMN transmigration results are represented as PMN cell equivalents, derived from a daily standard PMN dilution curve, which completely traversed monolayers.
PMN Transmigration Assays. The physiologically directed (basolateral-to-apical) PMN transepithelial migration assay using cell culture inserts of inverted T84 monolayers has been described (6). Human PMN were isolated from normal volunteers, as described (2, 7).
Ca2+ Mobilization and Degranulation. PMN intracellular [Ca2+] was measured in INDO-1-loaded PMN via spectrofluorimetry by using ratiometric measurements, as described (5). PMN degranulation was measured by elastase and superoxide dismutase release as previously detailed (8).
12-Lipoxygenase (LO) and 5-LO Inhibitor Treatment. For 12-LO inhibition, HBSS(+)-washed T84 cell monolayers were incubated in the presence baicalein (stock concentration at 1 mM in DMSO) for 48 h at 37°C. Subsequently, S. typhimurium SL1344 was added to the apical surface of the T84 monolayers and after an incubation of 1 h at 37°C, cells were washed free of nonadherent bacteria and then processed for the PMN transmigration assay. For 5-LO inhibition, the T84 cells were washed with HBSS(+) and then incubated for 24 h in the presence of caffeic acid (stock concentration at 22 mM in DMSO). Both inhibitors were purchased form Biomol (Plymouth Meeting, PA).
Human Intestine Xenografts. The human fetal intestinal xenograft model used in the present study has been described in detail (9). Briefly, human fetal small intestine (n = 3, gestational age 10-14 weeks) was transplanted s.c. into C.B-17 severe combined immunodeficient mice and were allowed to develop for a period between 10 and 20 weeks before use. Xenografts were infected with ≈5 × 107 wild-type S. typhimurium SL1344 in sterile HBSS(+) buffer in a 100-μl volume injected intralumenally s.c. Xenografts receiving the drug treatment were injected with 1 μM baicalein in a 100-μl volume 2 h before infection with S. typhimurium (in the continued presence of 1 μM baicalein). Consistent with previous analysis, xenograft tissue was removed 15 h after infection, extensively washed, and snap frozen in OCT compound. Histological severity of intestinal inflammation was assessed in a blinded fashion by a trained gastrointestinal pathologist and was ranked (0-3) for (1) epithelial cell damage, (2) congestion and edema, and (3) PMN infiltration (10).
Results
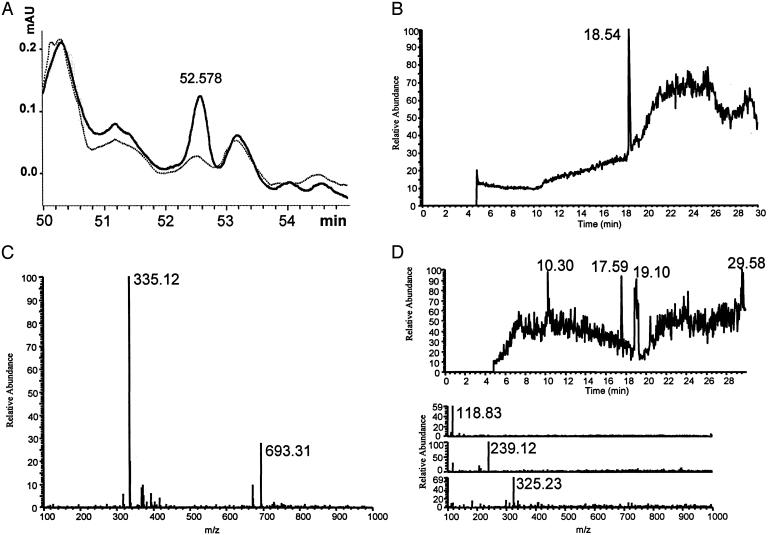
Identification of PEEC. Crude preparations of PEEC were collected from the apical surface of polarized T84 cell monolayers after infection with wild-type S. typhimurium SL1344 (5); activity-enriched preparations increased PMN cytosolic [Ca2+] and induced PMN transmigration across naïve T84 cell monolayers (5). Conditioned apical media obtained after incubation with S. typhimurium strain VV341, a nonpathogenic isogenic derivative of SL1344 rendered entry deficient by deletion of the hilA gene and failing to induce PMN transepithelial migration, was used as a negative control with similar preparations failing to show these activities. A single peak of PEEC activity was identified by using a semipreparative HPLC method (Fig. 1A). Analytical HPLC demonstrated that collected fractions contained one major component (Fig. 1B). HPLC/electrospray MS run in the negative ion mode [liquid chromatography/MS (LC/MS)] identified a prominent mass of 335 and a less prominent mass of 693 (Fig. 1C). Tandem MS analysis demonstrated the 693 mass to be consistent with a Na+ salt dimer of the lower monomer mass. A scan of known molecules having these mass characteristics highlighted the eicosanoid class of arachidonic acid metabolites. PEEC activity had been found to be acid labile (data not shown), and this characteristic was used to further establish its identity. Several arachidonic acid metabolites having the mass identified with PEEC activity were obtained from commercial sources and analyzed for their breakdown profiles under acidic conditions. One molecule, hepA3, behaved identically with PEEC under these conditions in the formation of discrete masses with specific retention times in the LC/MS system used to identify PEEC (Fig. 1D). Similar comparison studies were performed after methylation of PEEC and authentic arachidonic acid metabolites, and only hepA3 was found to have similar characteristics as PEEC (data not shown).
Fig. 1.
Identification of PEEC. (A) Absorbance (at 214 nm) of 50- to 55-min region of a semipreparative separation of PEEC-enriched samples obtained from monolayers exposed to S. typhimurium SL1433 (solid tracing) or VV341 (lighter, dashed tracing). (B) Analytical HPLC using neutral methanol of collected PEEC fraction showing one prominent peak with a retention time of 18.5 min. (C) Negative ion mode mass profile of 18.5-min peak with prominent peak at 335 and a secondary peak at 693. (D) Analytical HPLC profile and negative ion electrospray MS analysis of PEEC material after overnight incubation in acid. (Lower) (Top) Mass of 118 with a retention time of 10.3 min. (Middle) Mass of 239 with a retention time of 17.6 min. (Bottom) Mass of 325 with a retention time of 19.1 min.
Examination of Metabolic Pathways Involving LO Activity. To substantiate hepA3 as PEEC, we examined whether synthetic hepA3 (Calbiochem) could recapitulate PEEC bioactivity. An imposed hepA3 gradient across T84 cell monolayers induced PMN transmigration and elicited an increase in PMN [Ca2+] (Table 1) in a dose-dependent manner (data not shown). Like PEEC, but in contrast to chemoattractants such as IL-8 and formyl-methionylleucyl-phenylalanine, hepA3 did not induce PMN degranulation, as assessed by elastase release (data not shown). A wide variety of bioactive lipids similar to hepA3 were analyzed for their ability to induce PMN transmigration and intracellular [Ca2+] increase in PMNs (Table 1). Arachidonic acid, a precursor of lipid metabolism that generates hepA3, also stimulated some PMN transmigration and induced an intracellular PMN [Ca2+] increase. Arachidonic acid metabolites with an hydroxyl group at the 12 carbon {12(R)-hydroxyeicosa-5Z,7E,14Z-tetraenoic acid [12(R)-HETE]} or hydroxyl groups at both the 5 and 12 carbons [5(S),12(R)-dihydroxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid (DiHETE); also known as 6-transleukotriene (LT) B4 (LTB4)] also produced measurable levels of PMN chemotactic activity (Table 1). LTB4 stimulated significant PMN transmigration and intracellular [Ca2+] increase in PMNs in our system (Table 1), but these actions appeared distinct from PEEC because this compound also induced PMN degranulation, consistent with its previously shown function in inflammatory events (11). The epoxide structure coupling carbons 11 and 12 in hepA3 is also present in hepoxilin B3 but positioned in a different chiral orientation, and this analogue was found to be inactive (Table 1). HepA3 also contains an 8-OH group, but this moiety is not sufficient for reconstituting PEEC activity [e.g., 8(S)-HETE]. Ketoeicosa-6E,8Z,11Z,14Z-tetraenoic acid has been previously shown to stimulate eosinophil migration (12) and mobilize intracellular PMN [Ca2+] (13), but this compound induced only a low level of PMN transmigration and did not stimulate mobilization of intracellular PMN [Ca2+] (Table 1). Thus, hepA3 and its precursor DiHETE were the only materials found to induce PMN transmigration and Ca2+ mobilization in the absence of a degranulation event.
Table 1. Evaluation of bioactive lipids for PEEC characteristics.
| Compounds tested* | PMN transmigration† | [Ca2+] increase‡ |
|---|---|---|
| 12-LO metabolites | ||
| 12(R)-HETE | 16 ± 2 | + |
| 12(S)-HETE | 12 ± 5 | − |
| 5(S), 12(R)-DiHETE | 55 ± 6 | + |
| HepA3 | 99 ± 4 | + |
| Hepoxilin B3 | 7.1 ± 1 | − |
| 5-LO metabolites | ||
| 5(S), 6(R)-DiHETE | 15 ± 1 | − |
| (±)5,6-EET | 11 ± 1 | − |
| LTA4 | 16 ± 0.2 | − |
| LTB4 | 65 ± 1 | + |
| LTC4 | 5.3 ± 1 | − |
| LTD4 | 6.2 ± 1 | − |
| 5-KETE | 19 ± 2 | − |
| 15-LO metabolites | ||
| 15(S)-HETE | 12 ± 1 | − |
| 15(S)-HETrE | 3.4 ± 0.4 | − |
| 8-LO metabolites | ||
| 8(S)-HETE | 15 ± 1 | − |
| Polyunsaturated fatty acids | ||
| Arachodonic acid | 38 ± 4 | + |
| Linoleic acid | 3.1 ± 0.3 | − |
| 13(S)-HPODE | 5.3 ± 0.7 | − |
| Sphingolipids | ||
| C2 ceramide | 10 ± 1 | − |
| C6 ceramide | 6.8 ± 0.8 | − |
| C16 ceramide | 13 ± 0.9 | − |
| C2 dihydroceramide | 7.1 ± 0.6 | − |
| C6 dihydroceramide | 6.2 ± 0.7 | − |
All lipids were tested at 500 ng/ml. DiHETE, dihydroxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid; KETE, ketoeicosa-6E-8Z,11Z,14Z-tetraenoic acid; HETrE, hydroxyeicosa-8Z,11Z,13E-trienoic acid; EET, epoxyeicosa-8Z,11Z,14Z-trienoic acid; HPODE, hydroperoxyoctadeca-9Z,11E-dienoic acid.
Inverted inserts of T84 monolayers and freshly isolated human peripheral PMNs were used to perform transmigration assays, as described (6).
Intracellular [Ca2+] was measured in INDO-1-loaded PMN via spectrofluorimetry by using ratiometric measurements, as described (5).
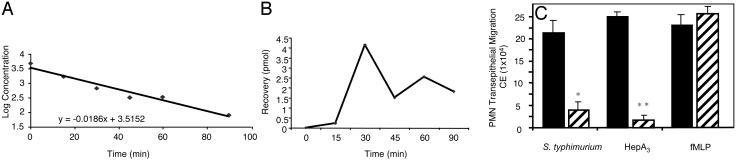
PEEC/HepA3 Establishes a Gradient in Vitro and in Vivo. Using LC/MS, we quantified the levels of intact hepA3 in the supernatants collected from the apical and basolateral surfaces of polarized epithelial cell monolayers after pathogen stimulation and observed two important findings: first, that an apical to basolateral gradient is established (210 pmol of hepA3 in the apical compartment vs. 130 pmol in the basolateral compartment after a 90-min incubation); and second, that this molecule is extremely labile. In two separate experiments, an average 50% loss of hepA3 from the apical chamber of T84 monolayers occurred in 82 min (77 and 87 min) and in only 43 min (49 and 37.5 min) in the basolateral compartment. HepA3 decay in both compartments was exponential, and the above-stated t1/2 values were determined by analyzing the slope of a best-fit line to a log-linear analysis of each data set (Fig. 2A). HepA3 levels also correlated with bioactivity assessed in the in vitro transmigration model. Both chemical and enzymatic activities (epoxide hydrolase) can rapidly act to hydrate the epoxide generated by the enzyme 12-LO to produce a completely inactive molecule trioxilin (14). Such rapid destruction is likely to be a critical aspect of normal PEEC function because a long-lived molecule might not only alter the gradient across the TJ with time but might also result in durable inflammatory events from an acute stimulus, potentially driving inflammation-related pathologies. Thus, the labile nature of hepA3 (PEEC) is an important characteristic for quelling PMN-related inflammation, and for this reason, many of our experiments have used hepA3 concentrations well above those likely required in vivo where it can be continuously synthesized and secreted in a regulated fashion.
Fig. 2.
HepA3 functions in a gradient fashion. (A) Rate of hepA3 degradation (shown as log-linear plot) at the basolateral surface. Decay was exponential. t1/2 values were determined by best-fit analysis for each data set. Complex degradation events precluded calculation of accurate transport rates. (B) HepA3 recovered in the basolateral compartment of T84 monolayers after addition of 4,500 pmol to apical compartment. Data points represent means from two separate studies and indicate amount of hepA3 recovered at the basolateral surface is low. (C) Effect of 5 μg/ml basolateral addition of hepA3 (hashed bars) on the PMN transmigration normally induced (black bars) by either infection with wild-type S. typhimurium, apical addition of 5 μg/ml hepA3, or 100 mM formyl-methionylleucyl-phenylalanine (fMLP). Data (expressed as mean ± SD) represent one of at least two experiments performed in triplicate repeated at least three times. Statistical analysis (Student's t test) of the raw data reveal differences, *, P < 0.025 and **, P < 0.010, compared to their respective controls.
We next examined the ability of synthetic hepA3 to establish a chemical gradient across intact TJ barriers after apical application. Although the labile nature of hepA3 precluded attempts to calculate an accurate hepA3 transport rate across the TJ, intact hepA3 could be demonstrated in the basolateral compartment after an apical application of 4,500 pmol (Fig. 2B). HepA3 added to the basolateral surface of T84 cell monolayers, to nullify the establishment of a chemotactic gradient, significantly diminished PMN transmigration induced by either S. typhimurium or hepA3 added to the apical surfaces of these monolayers (Fig. 2C). The more rapid hepA3 degradation in the basolateral compartment (compared to the apical compartment) in the in vitro T84 infection model by using S. typhimurium likely accounts for the incomplete outcomes to negate the hepA3 gradient in these studies (Fig. 2C). Overall, our results suggest that hepA3 can establish a gradient across the epithelial TJ, an anticipated requirement for a chemoattractant that functions to draw PMNs from the lateral membrane of these cells into the lumen of mucosal tissues. Further, disruption of this hepA3 gradient paralyzed PMN transmigration.
Stimulation of PMN migration by hepA3 appears to occur via Ca2+ signaling induced by activation of an intracellular receptor (15), which leads to reorganization of Ca2+ within PMNs from the endoplasmic reticulum into mitochondria (16). HepA3 binding to an unidentified receptor in human PMNs shows clear specificity (17) in a manner similar to findings obtained in our studies (Table 1), further supporting the overall identification of hepA3 as a stimulator of PMN migration across the epithelial TJ without premature degranulation (5), previously known as PEEC.
Our hypothesis is that PMN diapedesis and entry into interepithelial sites are mediated substantially by cytokines (e.g., IL-8) secreted from the basolateral surface of epithelial cells, whereas PEEC (hepA3) secreted from the apical surface of epithelial cells would provide a concentration gradient across the TJ to draw PMNs into the mucosal lumen. To examine this concept in vivo, we used a human intestinal xenograft model (9) where an intraluminal application of 3 μg (in 100 μl of buffer) of purified bacterial flagellin was used to stimulate a basolateral IL-8 release to induce diapedesis (1, 18). Beginning 30 min after this addition (or buffer in the control set of animals), hepA3 (5 μg in 200 μl of buffer) or carrier buffer was administered over a 24-h period by using a s.c.-implanted Alzet pump that delivered at a rate of 8 μl/hr into the intestinal lumen via a catheter (continuous delivery of hepA3 was required due to its labile nature). This approach provided a separate stimulus for PMN diapedesis/interepithelial sequestration (flagellin) and a stimulus to incite PMN movement across the TJ (hepA3). PMN movement into the intestinal lumen, quantified by PMN-derived myeloperoxidase content of the intestinal tissue showed that xenografts exposed to both flagellin and hepA3 had more myeloperoxidase activity (58 ± 9 units per 100 mg of protein) than flagellin treatment alone (38 ± 6 units per 100 mg of protein). Treatment with hepA3 in the absence of flagellin (20 ± 11 units per 100 mg of protein) did not significantly affect these levels compared to animals receiving only buffer (10 ± 8 units per 100 mg of protein). These results are consistent with our hypothesis that distinct proinflammatory chemoattractants sequentially regulate PMN movement from the blood to the intestinal lumen, and that hepA3 is the factor that functions in the terminal step of this process of mucosal inflammation.
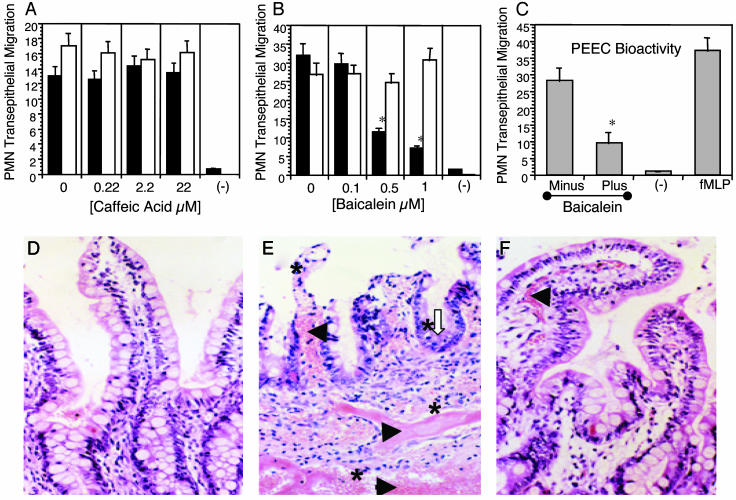
Liberation of PEEC/HepA3 Requires 12-LO Activity. There are several LO enzymes that selectively place epoxide structures from a hydroxyl residue positioned at the 5, 8, 12, or 15 carbon of arachidonic acid or its metabolites. Only arachidonic acid metabolites with modifications at either the 5 or 12 carbon demonstrated any capacity to stimulate PMN transmigration or [Ca2+] mobilization (Table 1). If indeed hepA3 functions as PEEC, disruption of 12-LO but not 5-LO activity should destroy its biological activity. Incubation of T84 cell monolayers with caffeic acid, an inhibitor of 5-LO (the enzyme required for LTB4 synthesis), failed to affect PMN transmigration induced by S. typhimurium infection (Fig. 3A). By contrast, addition of the 12-LO inhibitor, baicalein, inhibited PMN transmigration in a dose-dependent manner (Fig. 3B). Additionally, we examined the actions of 1 μM baicalein on the extent of apical hepA3 (PEEC) secretion (Fig. 3C). Apical media were collected from noninfected (-) or S. typhimurium-infected T84 monolayers in the absence (minus) or presence (+) of 1 μM baicalein and then added back to naïve T84 monolayers to assess the potential for these conditioned media to stimulate PMN transmigration. A reduced level of PEEC bioactivity assessed in this assay by the presence of 1 μM baicalein correlated with measured hepA3 levels (data not shown). Together, these results suggest that baicalein can effectively diminish the synthesis of hepA3 in T84 monolayers stimulated by S. typhimurium infection, and that this inhibition did not affect basolateral signaling events related to PMN diapedesis.
Fig. 3.
(A) PMN transepithelial migration induced by S. typhimurium (white bars) is unaffected by the specific 5-LO inhibitor caffeic acid (black bars). PMN transport across uninfected T84 monolayers (-) is also shown. (B) The 12-LO inhibitor (baicalein) induced a dose-dependent inhibition in a similar study (black bars). *, P < 0.025; Student's t test. (C) PEEC bioactivity was determined in spent apical media collected from uninfected T84 monolayers (-) or from T84 monolayers after infection with S. typhimurium SL1344 (6) that had been treated with 1 μM baicalein (Plus) or solvent control (Minus) and subsequently added to the apical compartment of naïve T84 monolayers to induce PMN transmigration. Monolayers treated with an apical application of 1 mM fMLP served as a positive control for the transmigration assay. *, P < 0.05; Student's t test. (D) Control intestinal epithelium of a xenograft injected with buffer in the absence of S. typhimurium. (E) S. typhimurium SL1344 infection of these xenografts resulted in profuse dissemination of red blood cells (black arrowheads) and a substantial PMN infiltrate (asterisk) within the mucosa and submucosa. Also shown is an early stage of crypt abscess formation (white open arrow). (F) Introduction of baicalein into the lumen of xenografts 1 hr before S. typhimurium SL1344 infection prevented the severe histopathologal events associated with S. typhimurium infection in this in vivo model. IL-8 was measured in these tissues as detailed (24). fMLP, formyl-methionyl-leucyl-phenylalanine.
Based on these in vitro findings, we next evaluated the effect of baicalein on S. typhimurium pathology by using the human intestinal xenograft model (9). Infection of these grafts with S. typhimurium results in a severe histopathology involving profuse dissemination of red blood cells in the mucosa and submucosa with a noticeable PMN infiltrate (Fig. 3 D and E). Infected xenografts also showed evidence of crypt abscesses, crypt hyperplasia, villus tip atrophy, vascular congestion, PMN margination, PMN infiltration, and edema, outcomes consistent with clinical manifestations of human salmonellosis (Fig. 3E). By contrast, mice infected with S. typhimurium subsequent to bacailein treatment showed a striking reduction in morbidity compared to control infected mice and presented xenograft tissues that were remarkably similar to those of xenografts never exposed to bacteria (Fig. 3 D and F). Blinded histopathology scores for epithelial damage, congestion, edema, and PMN infiltration showed baicalein treatment reduced pathology associated with S. typhimurium of xenografts by at least 50% [in a statistically significant manner (10)] for all assessed categories (Table 2). Due to the labile nature of hepA3 and as a method to assess inhibition of the 12-LO pathway by baicalein, we monitored the luminal secretion of a major metabolite of the 12-LO pathway, 12(S)-HETE (19) by ELISA (Assay Designs, Ann Arbor, MI). Luminal fluid collected from S. typhimurium-infected xenografts in the absence of baicalein treatment contained 114.5 ng/ml 12(S)-HETE, whereas luminal fluid collected from S. typhimurium-infected xenografts from animals treated with baicalein contained 21.5 ng/ml 12(S)-HETE (average amounts from two separate animals). Although S. typhimurium infection stimulated an increase in tissue IL-8 levels (52 ± 11 pg/mg for control xenografts vs. 225 ± 30 pg/mg after S. typhimurium infection), treatment with baicalein did not affect this increase due to S. typhimurium infection (200 ± 53 pg/mg). These data demonstrate a near complete blockade of S. typhimurium-mediated tissue damage by inhibition of the 12-LO pathway that would act to impede hepA3 apical secretion.
Table 2. Quantification of histopathology.
| Damage | Congestion and edema | PMN infiltration | |
|---|---|---|---|
| Buffer control | 0.1 ± 0.0 | 0.6 ± 0.2 | 0.2 ± 0.1 |
| Salmonella | 1.1 ± 0.3* | 2.3 ± 0.5* | 1.7 ± 0.4* |
| Salmonella + baicalein | 0.7 ± 0.2† | 1.3 ± 0.2†‡ | 0.8 ± 0.2†‡ |
Quantification of histopathology associated with protection from S. typhimurium-induced tissue damage by pretreatment with 1μM baicalein. Data are mean ± SEM of 6-10 grafts per group (P < 0.05).
Significant differences between buffer control and S. typhimurium
Buffer control and S. typhimurium plus baicalein (12-LO inhibitor).
S. typhimurium in the absence and presence of baicalein.
Discussion
Most likely, PMN recruitment to interepithelial sites occurs through the actions of basolateral secretion of NF-κB-regulated chemokines such as IL-8, epithelial-neutrophil-activating peptide-78, growth-related oncogene protein-α, and monocyte chemoattractant protein (1, 5, 6, 20, 21). At this site, and due to the restrictive actions of the TJ present at the neck of these adjacent epithelial cells, a distinct factor, which we have previously termed PEEC, is required for migration of PMNs across the TJ. The present studies support the identification of this factor as the eicosanoid hepA3, because it satisfies all criteria previously assigned to PEEC, inciting PMN chemotaxis across the TJ of epithelial barriers but not inducing degranulation premature to accessing the luminal site of infection. HepA3 appears to function at concentrations as low as 30-40 nM but is extremely labile and therefore is probably produced continuously during the active phase of an infection at a mucosal surface. Our studies to identify PEEC and establish its role in vivo have used a model of intestinal infection with S. typhimurium. Notably, we have identified 12-LO inhibition as a potential strategy to block inflammatory outcomes associated with these infections by blocking the production of a critical factor, hepA3, involved in the final migration of PMNs into the intestinal lumen.
The remarkable findings demonstrated by the studies herein relate to the previously unrecognized capacity of polarized epithelial cells to produce and release hepA3 in a vectoral fashion, and that, once released from the apical surface of an infected mucosal surface, hepA3 has the ability to establish chemical a gradient across the epithelial TJ, stimulating PMN transmigration without premature degranulation. It is the degranulation of PMNs that is assumed to impart much of the inflammation associated with mucosal infections (22). Orchestration of PMN movement by the intestinal epithelium likely plays a key role in both innate immune responses to food-borne pathogens and in events mediating active flares associated with inflammatory bowel disease. Although presumably the epithelium generates significant levels of hepA3 only in response to colonization by pathogens, it is possible that hepA3 generation is dysregulated under conditions such as IBD because 12-LO activity is induced at active sites of this disease (23). Although it is presently unclear whether hepA3 is secreted from the apical surface of all mucosal surfaces as a consequence of infection, initial studies have shown hepA3 clearly functions similarly in human pulmonary infections of Pseudomonas aeruginosa (B.P.H. and B.A..M, unpublished observations). Thus, it is likely that induction events by different pathogens at various mucosal surfaces of the body may use a number of stimulating determinants. Indeed, pharmacologic regulation of epithelial hepA3 generation might provide a novel and appropriate therapeutic strategy for treating epithelial inflammatory disorders.
Acknowledgments
We thank Yang Wang, Jim Bourell, and Tom Patapoff for help in LC and LC/MS protocols. Cecil Pace-Asciak is thanked for his advice on methylation of hepA3. This work was supported by National Institute of Health Grants DK-56754 (to B.A.M.), DK-47622 (to J.L.M.), and DK-2792 (to A.T.G.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PMN, polymorphonuclear leukocyte; PEEC, pathogen-elicited epithelial chemoattractant; hepA3, hepoxilin A3; TJ, tight junction; LT, leukotriene; LO, lipoxygenase; 12(R)-HETE, hydroxyeicosa-5Z,7E,11Z,14Z-tetraenoic acid; LC/MS, liquid chromatography/MS.
References
- 1.McCormick, B., Hofman, P., Kim, J., Carnes, D., Miller, S. I. & Madara, J. L. (1995) J. Cell. Biol. 131, 1599-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar, N. B., Nostrant, T. T. & Appelman, H. D. (1982) Am. J. Surg. Pathol. 6, 523-529. [DOI] [PubMed] [Google Scholar]
- 3.McGovern, V. J. & Slavutin, L. J. (1979) Am. J. Surg. Pathol. 3, 483-490. [DOI] [PubMed] [Google Scholar]
- 4.Yardley, J. H. & Donowitz, M. (1977) The Gastrointestinal Tract (Williams & Wilkins, Baltimore).
- 5.McCormick, B. A., Parkos, C. A., Colgan, S. P., Carnes, D. K. & Madara, J. L. (1998) J. Immunol. 160, 455-466. [PubMed] [Google Scholar]
- 6.McCormick, B. A., Colgan, S. P., Archer, C. D., Miller, S. I. & Madara, J. L. (1993) J. Cell. Biol. 123, 895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henson, P. & Oades, Z. G. (1975) J. Clin. Invest. 56, 1053-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gewirtz, A. T., Fokin, V. V., Petasis, N. A., Serhan, C. N. & Madara, J. L. (1999) Am. J. Physiol. Cell Physiol. 276, C988-C994. [DOI] [PubMed] [Google Scholar]
- 9.Savidge, T. C., Morey, A. L., Ferguson, D., Fleming, K. A., Shmakov, A. N. & Phillips, A. D. (1995) Differentiation 58, 361-371. [DOI] [PubMed] [Google Scholar]
- 10.Triadafilopoulos, G., Pothoulakis, C., O'Brien, M. & Lamont, J. T. (1987) Gastroenterology 93, 273-279. [DOI] [PubMed] [Google Scholar]
- 11.Ford-Hutchinson, A. W. (1990) Crit. Rev. Immunol. 10, 1-12. [PubMed] [Google Scholar]
- 12.Powell, W. S., Chung, D. & Gravel, S. (1995) J. Immunol. 154, 4123-4132. [PubMed] [Google Scholar]
- 13.Powell, W. S., Gravel, S. & Gravelle, F. (1995) J. Lipid Res. 36, 2590-2598. [PubMed] [Google Scholar]
- 14.Anton, R., Puig, L., Esgleyes, T., de Moragas, J. P. & Vila, L. (1998) J. Invest. Dermatol. 110, 303-310. [DOI] [PubMed] [Google Scholar]
- 15.Reynaud, D., Demin, P. M., Sutherland, M., Nigam, S. & Pace-Asciak, C. R. (1999) FEBS Lett. 446, 236-238. [DOI] [PubMed] [Google Scholar]
- 16.Mills, L., Reynaud, D. & Pace-Asciak, C. R. (1997) Exp. Cell. Res. 230, 337-341. [DOI] [PubMed] [Google Scholar]
- 17.Reynaud, D., Demin, P. & Pace-Asciak, C. R. (1996) Biochem. J. 313, 537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gewirtz, A. T., Navas, A. T., Lyons, S., Godowski, P. J. & Madara, J. L. (2001) J. Immunol. 167, 1882-1885. [DOI] [PubMed] [Google Scholar]
- 19.Sigal, E. (1991) Am. J. Physiol. 260, L13-L28. [DOI] [PubMed] [Google Scholar]
- 20.Eckmann, L., Jung, H. C., Schuer-Maly, C., Panja, A., Morzycka-Wroblewski, E. & Kagnoff, M. F. (993) Gastroenterology 105, 1689-1697. [DOI] [PubMed] [Google Scholar]
- 21.Jung, H. C., Eckmann, L., Panja, A., Frierer, J., Morzycka-Wroblewski, E. & Kagnoff, M. F. (1995) J. Clin. Invest. 95, 55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss, S. (1989) N. Engl. J. Med. 320, 365-376. [DOI] [PubMed] [Google Scholar]
- 23.Shannon, V. R., Stenson, V. F. & Holtzman, M. J. (1993) Am. J. Physiol. 64, G104-G11. [DOI] [PubMed] [Google Scholar]
- 24.Savidge, T. C., Pan, W. H., Newman, P., O'Brien, M., Anton, P. M. & Pothoulakis, C. (2003) Gastroenterology 125, 413-420. [DOI] [PubMed] [Google Scholar]