Abstract
Posttranslational modification (PTM) of proteins is critical for various cellular processes. However, there are few studies examining PTMs in specific proteins using unbiased approaches. Here we report the attempt to identify the PTMs in peroxisome proliferator-activated receptor γ (PPARγ) proteins using our previously established PTM analysis system. In this study, we identified several PTMs in exogenously expressed PPARγ2 proteins from 293T cells as well as endogenous PPARγ1 proteins from a Caco-2 colon cancer cell line. The identified PTMs include phosphorylation of serine 112 and serine 81 in PPARγ2 and PPARγ1, respectively, both of which are well-known mitogen-activated protein kinase- (MAP kinase-) mediated PTMs in PPARγ proteins, thus confirming our experimental approach. Furthermore, previously unknown PTMs were also identified, demonstrating that this method can be applied to find previously unidentified PTMs in PPARγ proteins and other proteins including nuclear receptors.
1. Introduction
Peroxisome proliferator-activated receptor γ (PPARγ and NR1C3) is a ligand-dependent nuclear receptor, which was initially established as the dominant regulator of adipocyte differentiation [1, 2]. PPARγ is activated by natural ligands, such as polyunsaturated fatty acids and eicosanoids, plays a dominant role in adipose cell differentiation, modulates metabolism and inflammation in immune cells, and has strong antigrowth properties. Although PPARγ levels are the highest in adipose cells, substantial levels are also found in certain other cell types, such as the colonic epithelium and epithelial cells of the breast and prostate.
The PPARγ gene encodes two main splicing isoforms: PPARγ1 and PPARγ2. PPARγ1 is expressed in many tissues and organs including stomach and small and large intestine, whereas PPARγ2 is expressed specifically in adipocytes [1, 2]. Each PPARγ protein binds as a heterodimer with retinoid X receptor (RXR) to its recognition site called PPRE, with the sequence TGACCTxTGACCT. On binding to DNA, PPARγ recruits transcriptional coactivators such as steroid receptor coactivator 1 (SRC1) and positively regulates the expression of target genes [3, 4]. Though the underlying molecular mechanisms are unclear, PPARγ-mediated transrepression for inflammatory genes has also been described [5].
PPARγ also undergoes several PTMs in response to exogenous signals such as growth factors and adipokines, resulting in modulation of its functions [6]. For example, mitogen-activated protein kinases (MAP kinases) phosphorylate the N-terminal AF-1 domain of PPARγ (serine 84 or 112 in human PPARγ1 or PPARγ2, resp.), and this PTM inhibits ligand-dependent PPARγ transcriptional activity [7–11]. Moreover, PPARγ also undergoes SUMOylation at the C-terminal ligand-binding domain (LBD) in a ligand-dependent manner. SUMOylated PPARγ binds to NCoR corepressor complex and thereby transrepresses inflammatory genes such as iNOS genes in macrophages [12].
In the postgenomic era, it has become obvious that diversity of biological phenomena cannot be explained only by the number of different genes. Protein posttranslational modifications (PTMs) are one of the most efficient biological signals for expanding the genetic code and play key roles in diverse cellular processes such as protein transportation, DNA repair, and gene transcription [13–15]. We have previously developed a comprehensive PTM analysis system for proteins, combining biochemical and proteomic approaches [16]. In this system, we utilized nonrestrictive sequence alignment for PTM analysis and this method made it possible to identify PTMs without prior specification, enabling unbiased analysis of PTMs [17, 18].
Recent findings have revealed that the functions of epigenetic regulators, including PPARγ, are under control of PTMs in response to cellular signaling and nutrients [6, 19]. Here we applied the unrestricted comprehensive analysis to PTMs in PPARγ and identified a subset of PTMs from overexpressed PPARγ2 from 293T cells and endogenous PPARγ1 protein from Caco-2 colon cancer cells.
2. Materials and Methods
2.1. Plasmids, Antibodies, and Reagents
The expression plasmid for full-length PPARγ2 cDNA was cloned from a cDNA library of human adipocyte [20] and inserted into a pcDNA3 vector (Life Technologies, Carlsbad, CA) with a FLAG tag sequence. Anti-PPARγ antibody (#sc-7273) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Troglitazone (Sigma, St. Louis, MO) was dissolved in DMSO.
2.2. Cell Culture and Transfection
293T and Caco-2 cells were cultured in Dulbecco's modified Eagle medium (DMEM) plus 10% fetal bovine serum (FBS) and antibiotics. THP-1 cells were cultured in RPMI plus 10% FBS and antibiotics. For transfection of FLAG-PPARγ2 into 293T cells in the 10 cm dishes, we used Lipofectamine 2000 (Life Technologies) according to the manufacturer's instructions and incubated the cells for 24 h.
2.3. Purification of the PPARγ Proteins and Immunoprecipitation
Purification of PPARγ protein was performed as previously described [16]. Briefly, we cross-linked 2 μg of antibody with 30 μL of Protein G Dynabeads (Invitrogen) in freshly dissolved 20 mM dimethyl pimelimidate (DMP) in a 0.2 M triethanolamine buffer (pH 8.2). The cross-linking reaction was performed for 1 h at room temperature. The reaction was stopped by replacing the buffer with 50 mM Tris-HCl (pH 7.5).
Cell lysates of 293T transfected with FLAG-PPARγ2 and Caco-2 cell (from a 10 cm dish and 48 dishes, resp.) were prepared with 1% NP-40 buffer (10 mM Tris [pH 7.8], 1 mM EDTA, 150 mM NaCl, and 1% NP-40). Lysates were subjected to immunoprecipitation with Protein G Dynabeads coupled to each antibody for 3 h. The immunoprecipitates were washed by 1% NP-40 buffer and eluted with 0.1 M glycine-HCl buffer (pH 2). The eluates were boiled with Laemmli sample buffer and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then Colloidal Blue (Life Technologies), silver staining (Silver Quest, Life Technologies), or Western blotting with the indicated antibodies.
2.4. Mass Spectrometric Analysis
Mass spectrometric (MS) analysis of PPARγ protein and PTM analysis were performed as previously described [16]. Briefly, PPARγ protein was excised from the gel, reduced with 10 mM dithiothreitol solution in 0.1 M ammonium bicarbonate for 60 min at 56°C, alkylated with a 55 mM solution of iodoacetamide in 0.1 M ammonium bicarbonate in darkness for 45 min at room temperature, and in-gel digested with 25 ng/μL trypsin gold (Promega, Madison, WI) in 50 mM ammonium bicarbonate for 16 h at 37°C. Digested peptides were extracted, replaced with 0.1% formic acid in 2% acetonitrile (ACN), and subjected to analysis by electrospray ionization- (ESI-) MS/MS using an LTQ velos Orbitrap with ETD (Thermo Fisher Scientific). The nano-LC used was a DiNa system (KYA TECH Corporation, Tokyo, Japan) equipped with a C-18 ESI capillary column (100 μm × 150 mm, NIKKYO Technos, Tokyo, Japan). The gradient consisted of (A) 0.1% formic acid in 2% ACN and (B) 0.1% formic acid in 80% ACN: 0–100% B from 0 to 110 min, 100% B from 111 to 115 min, and 0% B from 116 to 120 min. The flow rate was 300 nL/min from 0 to 120 min. MS spectra were recorded from a range of m/z 350–1500 at 100,000 resolution, followed by data-dependent collision-induced dissociation (CID) MS/MS spectra and electron transfer dissociation (ETD) MS/MS spectra generated from the 20 highest intensity precursor ions. The voltage between the ion spray tip and the transfer tube was set to 1800 V. Peptides with +2 or greater charge were chosen for MS/MS experiments.
2.5. Computational Analysis for Protein Identification and PTM Analysis
Protein identification and PTM analysis of PPARγ protein were performed as previously described [16]. Briefly, for protein identification, spectra were processed using Proteome Discoverer ver. 1.3 (Thermo Fisher Scientific) against SEQUEST and subjected to a cutoff of 5% false discovery rate (FDR). The NCBI human protein database was used with a 10 ppm mass accuracy cutoff for parental MS and FT MS/MS and a 0.8 Da cutoff for ion trap MS/MS spectra. Carbamidomethylation (cysteine) was set as a fixed modification and oxidation (methionine) was set as a variable modification.
For PTM identification, spectra were processed using the MODIRO ver. 1.1 (Protagen, Bochum, Germany) software against FASTA format of human PPARγ1 and human PPARγ2 amino acid sequences. Search parameters were set as follows: two maximum missing cleavage sites, a peptide mass tolerance of 15 ppm for peptide tolerance, 1.5 Da for fragment mass tolerance (for ion trap MS/MS), 15 ppm for fragment mass tolerance (for FT MS/MS), and modification 1 of carbamidomethyl (cysteine). In all the identified PTMs, PTMs which might have occurred during sample preparations such as methylation of glutamic acid [21] were excluded from the list.
3. Results
3.1. Purification of Exogenous PPARγ2 from 293T and Endogenous PPARγ1 from Caco-2 Cells
For comprehensive analysis of PTMs in PPARγ protein, we performed the affinity purification of PPARγ proteins from 293T cells and Caco-2 colon cancer cells, which were expressing exogenous FLAG-tagged PPARγ2 and endogenous PPARγ1 protein, respectively, using the scheme shown in Figure 1(a). 1% NP-40 soluble fraction of cell lysates prepared from 293T and Caco-2 cells without PPARγ ligand was prepared and incubated with Protein G Dynabeads cross-linked with anti-PPARγ antibodies. Purified protein-antibody complexes were washed with lysis buffer and then eluted from the antibodies by adding acidic glycine-HCl buffer; eluates were then subjected to SDS-PAGE and visualized by silver staining. As shown in Figure 1(b), we successfully detected exogenous FLAG-PPARγ2 in the anti-PPARγ affinity purified eluates at the expected molecular size. We also detected endogenous PPARγ1 proteins in the anti-PPARγ affinity purified eluates from Caco-2. THP-1 human monocyte cells were used as indicators of PPARγ1 molecular size [22]. Enrichment of FLAG-PPARγ2 and endogenous PPARγ1 proteins was also confirmed by Western blotting using anti-PPARγ specific antibodies (Figure 1(c)).
Figure 1.
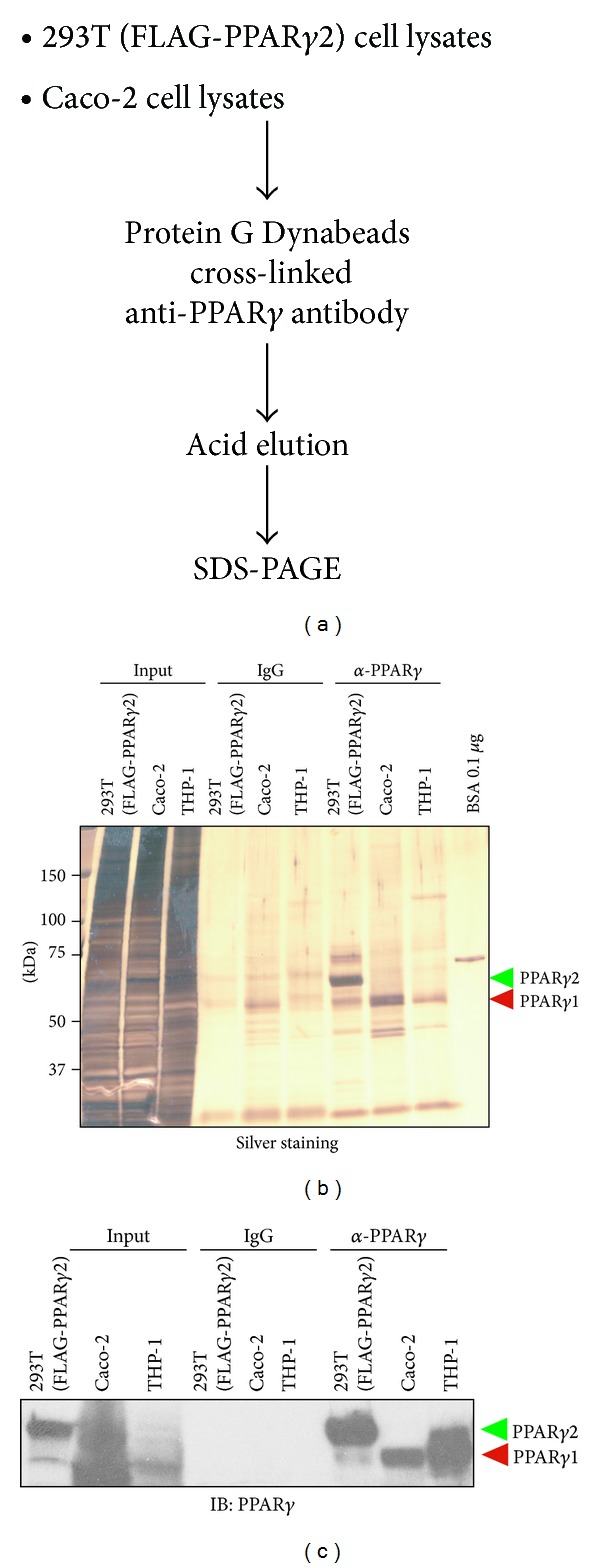
Purification of PPARγ proteins from 293T and Caco-2 cell lysates. (a) Schematic diagram of the purification of the exogenous and endogenous PPARγ proteins. 293T and Caco-2 cell lysates were incubated with anti-PPARγ cross-linked Protein G Dynabeads as described in Section 2. Rabbit IgG cross-linked Protein G Dynabeads were used as a negative control. Bound proteins were eluted with 0.1 M glycine-HCl buffer (pH 2). (b) Isolated exogenous and endogenous PPARγ proteins. Eluted proteins were subjected to SDS-PAGE, followed by silver staining. The molecular masses of PPARγ1 and PPARγ2 are shown in the right side of the gel. (c) Enrichment of PPARγ proteins were also confirmed by Western blotting using anti-PPARγ specific antibodies.
3.2. Identification of PPARγ Proteins Using SEQUEST Algorithm
The purified FLAG-PPARγ2 from 293T cells and PPARγ1 protein from Caco-2 cells were cut from the colloidal blue-stained gel, in-gel digested with trypsin, and subjected to analysis by LC-MS/MS using a combination of collision-induced dissociation (CID) and electron transfer dissociation (ETD) activation. Precursor MS spectra were detected by Orbitrap mass analyzers (a Fourier transform (FT) mass analyzer that can measure peptide m/z with high accuracy) (ΔMS < 5 ppm), and MS/MS spectra were detected using the ion trap analyzer (ΔMS < 0.5 Da). MS spectral data were first analyzed using the SEQUEST algorithm in Proteome Discoverer ver. 1.3 and the PPARγ protein was identified with high accurate molecular weight (sequence coverage 67.92%, FDR < 5% for PPARγ2 from 293T cells, coverage 49.79%, FDR < 5% for PPARγ1 from Caco-2 cells), suggesting successful protein purification and mass measurement for the PPARγ proteins (Figures 2(a), 2(b) and 2(c)).
Figure 2.
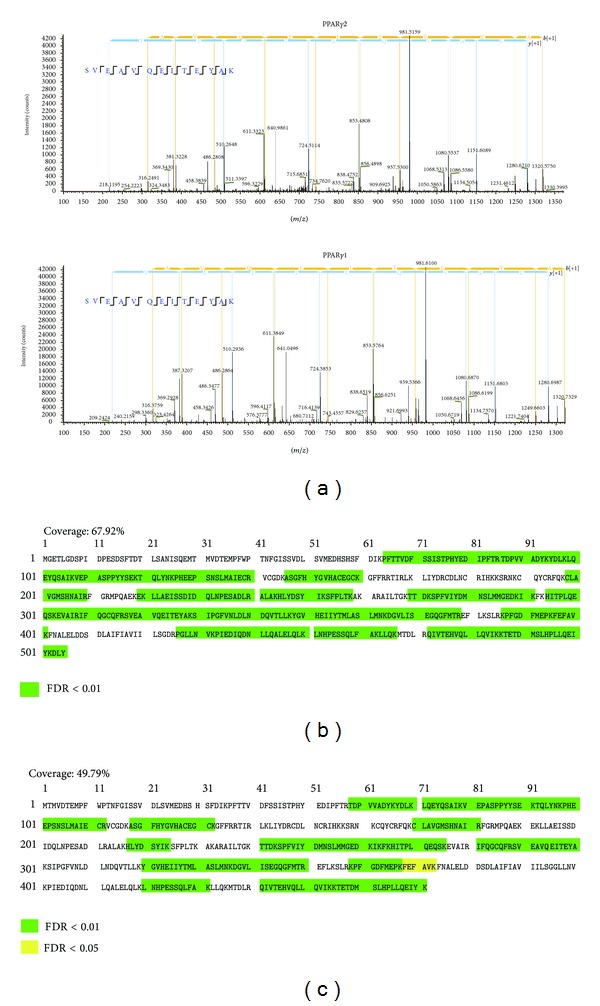
Identification of PPARγ proteins using the SEQUEST algorithm. (a) Representative MS/MS spectra of PPARγ2 proteins of the peptide [317-SVEAVQEITEYAK-329] and PPARγ1 proteins of the peptide [289-SVEAVQEITEYAK-301] assigned by SEQUEST are shown. (b) Amino acid sequence coverage of identified exogenous PPARγ2 proteins. The identified amino acid sequence is indicated. (c) Amino acid sequence coverage of identified endogenous PPARγ1 proteins. The identified amino acid sequence is indicated.
3.3. Nonrestrictive PTM Analysis for Purified PPARγ Proteins Using the MODIRO Algorithm
Next, the spectral data were searched using the MODIRO algorithm, which enables unrestricted identification of all possible PTMs in targeted proteins. As a result, various PTMs were identified in peptides derived from exogenous PPARγ2 protein from 293T cells (Figures 3(a) and 3(b)), and identified PTMs with significance score >90 were presented in Figures 3(b) and 3(c). At the top of the list, phosphorylation of serine 112 in PPARγ2 was identified with significance score = 100. This phosphorylation site is one of the well-characterized PTM sites in PPARγ2 protein, catalyzed by MAPKs and inhibiting the ligand-dependent transcription function of PPARγ [7–11]. This result confirms that our experimental approach detects the PTMs of PPARγ protein correctly. Furthermore, this PTMs list also included some PTMs such as methylation of 487 threonine residue and ubiquitination of lysine 160, which were novel PTMs for PPARγ proteins.
Figure 3.
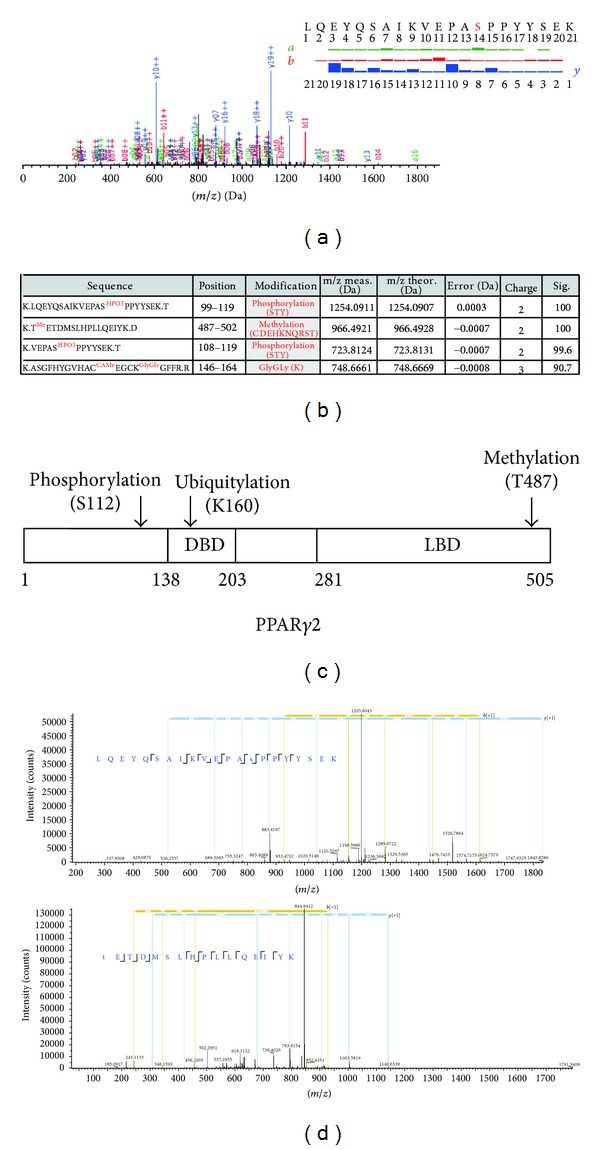
Identified PTMs in PPARγ2 proteins from 293T cells. (a) Representative MS/MS spectra of PPARγ2 proteins of the peptide [99-LQEYQSAIKVEPASHPO3PPYYSEK-119] assigned by MODIRO are shown. (b) List of identified PTMs in exogenous PPARγ2 proteins using ion trap MS/MS. Amino acid sequences, position of amino acids, identified PTMs theoretical mass, measured mass, error between measured and theoretical masses in Daltons, and significance score are listed. (c) Diagram for summary of identified PTMs in exogenous PPARγ2 proteins. (d) Identified PTMs in exogenous PPARγ2 proteins using FT MS/MS. Each responsible spectrum for S112 (top) and T487 (bottom) is shown.
To further confirm these identified PTMs, we analyzed the peptides by LC-MS/MS again. In this measurement, to obtain more rigorous MS/MS data and thereby more reliable PTM search results, we utilized the FT analyzer for both parental MS and MS/MS spectra. As shown in Figure 3(d), two of the three PTMs including S112 phosphorylation and T487 methylation were identified again with SEQUEST algorithm, thus confirming the initial result.
Next, PPARγ1 proteins from Caco-2 cells were analyzed in the same way. As shown in Figures 4(a), 4(b) and 4(c), phosphorylation of serine 84 was identified with significance score = 100. This serine residue corresponds to serine 112 of PPARγ2. We further tested PPARγ1 proteins from 10 μM Troglitazone-treated Caco-2 cells (24 h). However, no other PTMs besides S84 phosphorylation were identified from this analysis (data not shown).
Figure 4.
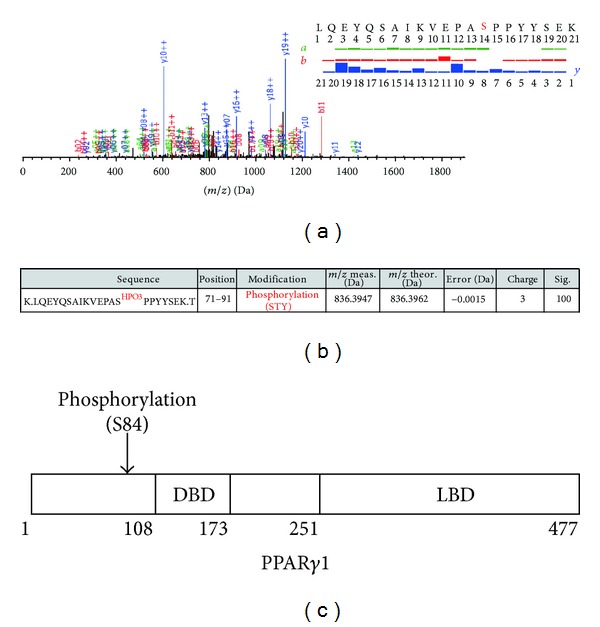
Identified PTMs in PPARγ1 proteins from Caco-2 cells. (a) Representative MS/MS spectra of PPARγ1 proteins of the peptide harboring [80-VEPASHPO3PPYYSEK-91] assigned by MODIRO are shown. (b) List of identified PTMs in endogenous PPARγ1 proteins using ion trap MS/MS. (c) Diagram for summary of identified PTM in endogenous PPARγ1 proteins.
4. Discussion
Here, we described the first report of comprehensive PTM analysis of PPARγ protein using mass spectrometry and succeeded in identifying some previously known and unknown PTMs including serine phosphorylation and threonine methylation.
Among the identified PTMs, phosphorylation of S112 and S84 in PPARγ2 and PPARγ1, respectively, was the one of the well-known PTMs in PPARγ proteins. It is reported that phosphorylation at S112 in PPARγ2 was catalyzed by a mitogen-activated protein (MAP) kinase and was involved in repression of transcriptional activity in adipocyte [7]. However, whether this modification exists in the PPARγ1 protein in colon cancer cells was elusive. Thus, in this study we could confirm the existence of S84 phosphorylation in PPARγ1 in colon cancer cells. Although the role of S84 phosphorylation in colon cancer is unclear, the phosphorylation mimic mutant of PPARγ1 (S84D) slightly derepressed PPARγ-mediated transrepression of β-catenin using the TOPflash reporter system [23], and further characterization with regard to the effect in transcriptional function is needed (Shogo Katsura, unpublished result).
In the present study, we could also identify the K160 ubiquitination and T487 methylation of PPARγ proteins, although there are few reports about these modifications. Protein ubiquitination can be targeted to a number of biological pathways such as proteasomal degradation and signal transduction depending on the numbers and linkage types of the conjugated ubiquitins [24]. Because the number and linkage types of the conjugated ubiquitins were not clear from present experiment, further detailed analyses are required to characterize the function of K160 ubiquitination in PPARγ2 protein. As for threonine methylation, it is still unclear whether this modification occurs in the sample preparation prior to MS analysis or is posttranslational [14]. We avoided methanol in each step of our experimental procedure so that proteins were not nonenzymatically methylated, although still we should be careful about this modification. However, this is a novel modification of PPARγ protein and thus our comprehensive analysis of PTMs can be applied to find previously unidentified PTMs in PPARγ proteins and other proteins including nuclear receptors.
Along with the identified phosphorylation, ubiquitination, and methylation, PPARγ protein is known to also undergo several PTMs, such as phosphorylation at other regions [25, 26], SUMOylations [12], O-GlcNAcylations [27], and ubiquitination [28, 29], although these PTMs were not identified in this study. Generally, as mass spectrometric analysis can detect relatively highly concentrated or efficiently ionized peptides [15, 17], it is possible to speculate that there are many more PTMs than what have been identified in PPARγ proteins. Because purified PPARγ proteins might be mixtures of variously modified states, we presume that, with further purification of the eluted proteins such as by isoelectric fractionation of purified PPARγ proteins or enrichment of specific modifications with PTM-recognizing antibodies, we could extend the variations of identified peptides and thus detect greater numbers of PTMs in PPARγ proteins. Those PTMs might be new candidates for drug targets to control PPARγ activities.
5. Conclusions
We have identified several modifications including phosphorylation of serines 112 and 81 in PPARγ2 and PPARγ1, respectively, and threonine methylation in PPARγ2 using our previously established PTM analysis system. This method can be applied to find previously unidentified PTMs in PPARγ proteins and other proteins including nuclear receptors.
Acknowledgments
The authors appreciate the technical assistance provided by Ms. Kei Izumikawa (University of Tokyo). This work was supported in part by priority areas from the Ministry of Education, Culture, Sports, Science and Technology (to Atsushi Yokoyama).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annual Review of Biochemistry. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 2.Voutsadakis IA. Peroxisome proliferator-activated receptor γ (PPARγ) and colorectal carcinogenesis. Journal of Cancer Research and Clinical Oncology. 2007;133(12):917–928. doi: 10.1007/s00432-007-0277-y. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Molecular Endocrinology. 2003;17(9):1681–1692. doi: 10.1210/me.2003-0116. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes and Development. 2006;20(11):1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 5.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochimica et Biophysica Acta. Molecular and Cell Biology of Lipids. 2007;1771(8):926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floyd ZE, Stephens JM. Controlling a master switch of adipocyte development and insulin sensitivity: covalent modifications of PPARγ . Biochimica et Biophysica Acta. Molecular Basis of Disease. 2012;1822(7):1090–1095. doi: 10.1016/j.bbadis.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ . Science. 1996;274(5295):2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Berger J, Zhou G, et al. Insulin- and mitogen-activated protein kinase-mediated phosphorylation and activation of peroxisome proliferator-activated receptor γ . Journal of Biological Chemistry. 1996;271(50):31771–31774. doi: 10.1074/jbc.271.50.31771. [DOI] [PubMed] [Google Scholar]
- 9.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. Journal of Biological Chemistry. 1997;272(16):10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 10.Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator- activated receptor-γ1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140(1):392–397. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 11.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. Journal of Biological Chemistry. 1997;272(8):5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 12.Pascual G, Fong AL, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ . Nature. 2005;437(7059):759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims RJ, III, Reinberg D. Is there a code embedded in proteins that is based on post-translational modifications? Nature Reviews Molecular Cell Biology. 2008;9(10):815–820. doi: 10.1038/nrm2502. [DOI] [PubMed] [Google Scholar]
- 14.Walsh CT, Garneau-Tsodikova S, Gatto GJ., Jr. Protein posttranslational modifications: the chemistry of proteome diversifications. Angewandte Chemie. 2005;44(45):7342–7372. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- 15.Seo J, Jeong J, Young MK, Hwang N, Paek E, Lee K-J. Strategy for comprehensive identification of post-translational modifications in cellular proteins, including low abundant modifications: application to glyceraldehyde-3-phosphate dehydrogenase. Journal of Proteome Research. 2008;7(2):587–602. doi: 10.1021/pr700657y. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama A, Katsura S, Ito R, et al. Multiple post-translational modifications in hepatocyte nuclear factor 4α . Biochemical and Biophysical Research Communications. 2011;410(4):749–753. doi: 10.1016/j.bbrc.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Farley AR, Link AJ. Identification and quantification of protein posttranslational modifications. Methods in Enzymology. 2009;463:725–763. doi: 10.1016/S0076-6879(09)63040-8. [DOI] [PubMed] [Google Scholar]
- 18.Ahrné E, Müller M, Lisacek F. Unrestricted identification of modified proteins using MS/MS. Proteomics. 2010;10(4):671–686. doi: 10.1002/pmic.200900502. [DOI] [PubMed] [Google Scholar]
- 19.Smith E, Shilatifard A. The chromatin signaling pathway: diverse mechanisms of recruitment of histone-modifying enzymes and varied biological outcomes. Molecular Cell. 2010;40(5):689–701. doi: 10.1016/j.molcel.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanase T, Yashiro T, Takitani K, et al. Differential expression of PPAR γ1 and γ2 isoforms in human adipose tissue. Biochemical and Biophysical Research Communications. 1997;233(2):320–324. doi: 10.1006/bbrc.1997.6446. [DOI] [PubMed] [Google Scholar]
- 21.Sprung R, Chen Y, Zhang K, et al. Identification and validation of eukaryotic aspartate and glutamate methylation in proteins. Journal of Proteome Research. 2008;7(3):1001–1006. doi: 10.1021/pr0705338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu H, Wong B, Zhou G, et al. Activation of PPARα or γ reduces secretion of matrix metalloproteinase 9 but not interleukin 8 from human monocytic THP-1 cells. Biochemical and Biophysical Research Communications. 2000;267(1):345–349. doi: 10.1006/bbrc.1999.1968. [DOI] [PubMed] [Google Scholar]
- 23.Fujisawa T, Nakajima A, Fujisawa N, et al. Peroxisome proliferator-activated receptor γ (PPARγ) suppresses colonic epithelial cell turnover and colon carcinogenesis through inhibition of the β-catenin/T cell factor (TCF) pathway. Journal of Pharmacological Sciences. 2008;106(4):627–638. doi: 10.1254/jphs.fp0071766. [DOI] [PubMed] [Google Scholar]
- 24.Komander D, Rape M. The ubiquitin code. Annual Review of Biochemistry. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 25.Choi JH, Banks AS, Estall JL, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ 3 by Cdk5. Nature. 2010;466(7305):451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi JH, Banks AS, Kamenecka TM, et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477(7365):477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji S, Park SY, Roth J, Kim HS, Cho JW. O-GlcNAc modification of PPARγ reduces its transcriptional activity. Biochemical and Biophysical Research Communications. 2012;417(4):1158–1163. doi: 10.1016/j.bbrc.2011.12.086. [DOI] [PubMed] [Google Scholar]
- 28.Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor γ is linked to ligand-dependent activation. Journal of Biological Chemistry. 2000;275(24):18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- 29.Kilroy GE, Zhang X, Floyd ZE. PPAR-γ AF-2 domain functions as a component of a ubiquitin-dependent degradation signal. Obesity. 2009;17(4):665–673. doi: 10.1038/oby.2008.616. [DOI] [PMC free article] [PubMed] [Google Scholar]


