Abstract
Toxoplasma gondii is the most common protozoan parasite of humans. Infection with T. gondii can lead to life-threatening disease as a result of repeated cycles of host cell invasion, parasite replication, and host cell lysis. Relatively little is known about the invasive mechanisms of T. gondii and related parasites within the Phylum Apicomplexa (including Plasmodium spp., the causative agents of malaria), due to difficulties associated with studying genes essential to invasion in haploid obligate intracellular organisms. To circumvent this problem, we have developed a high-throughput microscope-based assay, which we have used to screen a collection of 12,160 structurally diverse small molecules for inhibitors of T. gondii invasion. A total of 24 noncytotoxic invasion inhibitors were identified. Secondary assays demonstrated that different inhibitors perturb different aspects of invasion, including gliding motility, secretion of host cell adhesins from apical organelles (the micronemes), and extension of a unique tubulin-based structure at the anterior of the parasite (the conoid). Unexpectedly, the screen also identified six small molecules that dramatically enhance invasion, gliding motility, and microneme secretion. The small molecules identified here reveal a previously unrecognized complexity in the control of parasite motility and microneme secretion, and they constitute a set of useful probes for dissecting the invasive mechanisms of T. gondii and related parasites. Small-molecule-based approaches provide a powerful means to address experimentally challenging problems in host-pathogen interaction, while simultaneously identifying new potential targets for drug development.
Nearly one-third of all deaths in the world today are caused by infectious disease. The development of new preventative and therapeutic strategies relies on an improved understanding of the interaction between pathogens and their hosts. In many pathogenic systems, this presents a formidable experimental challenge, because standard genetic tools are either rudimentary or unavailable. Biochemical, genomic, and other approaches exist, but these lack the assumption-free power of a genetic screen.
An alternative nongenetic approach to studying mechanisms of host-pathogen interaction involves screening large structurally diverse collections of small molecules for those that disrupt the interaction. Once identified, the small molecules (or their derivatives) are used to determine the cellular components that function in the process (reviewed in refs. 1-3). The approach relies on the demonstrated ability of many small molecules to interact specifically with their targets (e.g., ref. 4). As with classical forward genetic screens, the approach is assumption-free: by sampling large unbiased collections of structurally diverse small molecules, the screen “selects” structures that perturb the process under study. Such phenotype-based high-throughput screening has recently gained momentum in the academic setting due to technological advances and the availability of small-molecule collections (2). We have applied the small-molecule approach to an experimentally challenging problem in host-pathogen interaction by seeking to identify novel inhibitors of host cell invasion by Toxoplasma gondii.
T. gondii is an important intracellular pathogen of humans and is related to Plasmodium (the causative agent of malaria), Cryptosporidium, and other parasites of medical and veterinary importance within the Phylum Apicomplexa. The pathology of toxoplasmosis is a direct result of repeated cycles of host cell invasion, parasite replication, and host cell lysis. Invasion by the T. gondii tachyzoite is a complex process involving attachment to the host cell surface, sequential secretion from three distinct secretory organelles, and movement into a parasite-induced invagination in the host cell plasma membrane (reviewed in ref. 5). Movement depends on parasite actin (6) and is powered by an unusual class of myosin motor proteins (Class XIV) found only in apicomplexan parasites (7, 8).
Despite its importance to the life cycle and pathogenicity of T. gondii, little is known about the molecular mechanisms of host cell invasion. Standard forward genetic approaches to identify parasite proteins functioning in invasion are problematic, because T. gondii tachyzoites are haploid obligate intracellular organisms; disruption of a gene essential for invasion is therefore likely to be lethal. Phenotype-based small-molecule screening offers a promising alternative approach (see also ref. 9).
We report here the use of a high-throughput microscope-based invasion assay to identify 24 noncytotoxic small-molecule inhibitors of T. gondii invasion. The inhibitors fall into discrete structural classes, and secondary assays have shown that they affect distinctly different aspects of invasion. Unexpectedly, the screen also identified six structurally independent small molecules that dramatically enhance host cell invasion. The small molecules described here represent powerful tools for studying the invasive mechanisms of T. gondii and related parasites.
Materials and Methods
High-Throughput Invasion Assay. Details of cell/parasite culture and the invasion assay can be found in Supporting Methods, which is published as supporting information on the PNAS web site. Briefly, media containing the small molecules to be tested [final concentration 8-19 μM/0.25% (vol/vol) DMSO] were added to confluent monolayers of BS-C-1 cells in 384-well plates followed immediately by the addition of parasites expressing yellow fluorescent protein (10). Plates were incubated for 15 min at 23°C, then 60-90 min at 37°C. Extracellular parasites were labeled by using either anti-T. gondii mAb 11-132 directly conjugated to Alexa546 (Molecular Probes) or mAb 11-132 followed by Alexa546-conjugated goat anti-mouse IgG. After antibody incubation, the cells were washed, fixed, and imaged with an automated image acquisition system. Captured images (four randomly selected fields per well) were analyzed by using an automated algorithm to identify the number of red and green fluorescent objects of a user-defined size and threshold value. The average number of invaded parasites per field was calculated and compared to that of control wells (buffer containing 0.25% DMSO). Invasion of <20% or >200% of the control value was considered a hit. See Supporting Methods for methods used to validate hits.
Cytotoxicity Assays and Analytical Methods. Parasites and BS-C-1 cells were incubated for 60 min at 23°C in phenol red-free Hanks' buffered saline solution (HBSS) containing 4 mM Hepes, pH 7.0/0.4% (vol/vol) dialyzed FBS, and either 100 μM small molecule or 0.25% DMSO. Cell viability was determined by using the LIVE/DEAD mammalian cell viability/cytotoxicity assay (Molecular Probes) or the CellTiter-Glo luminescent cell viability assay (Promega). Small molecules were analyzed by liquid chromatography-MS or NMR as described in Supporting Methods.
Target Cell Identification. For small molecules that irreversibly inhibited invasion, the target cell was determined as follows. HBSS containing 10 mM Hepes, pH 7.0 (HH), 0.4% dialyzed FBS (dFBS) and either test molecule or 0.25% DMSO was added to confluent BS-C-1 cells in a 384-well Black/Clear Optilux plate (BD Falcon, Bedford, MA). Simultaneously, in a separate 384-well plate, freshly isolated yellow fluorescent protein-expressing parasites [resuspended to 1.5 × 107 tachyzoites/ml in HH containing 1% (vol/vol) dFBS] were mixed with test molecule or DMSO diluted in HH. After 15 min at 23°C, the BS-C-1 cells were washed once with HH, followed by the addition of 45 μl of HH and 25 μl of the pretreated tachyzoite suspension. The parasites were centrifuged onto the BS-C-1 cells (1 min, 150 × g) by using a microplate rotor. The medium was immediately removed from the wells and replaced with HH containing 0.4% dFBS and either small molecule or DMSO. The plates were incubated at 37°C for 1 h and invasion assayed as described above.
Secondary Assays. Actin sedimentation was assayed as described (11). Parasite motility, conoid extension, microneme secretion, and intracellular calcium levels were assayed as described (6, 12-14) with the modifications described in Supporting Methods.
Results
Screen Results. The high-throughput assay used to identify inhibitors of T. gondii invasion into BS-C-1 epithelial cells is summarized in Fig. 1. Each well of the 384-well plate contained either a unique small molecule or an equivalent volume of DMSO (control). Intracellular and extracellular parasites were distinguished by fluorescence microscopy: extracellular parasites were red and green; intracellular parasites were green only. Using an automated microscope, pairs of red and green fluorescence images were acquired from four randomly selected fields in each well, and the average number of intracellular parasites in each well was determined. Small molecules that reduced invasion levels to <20% compared to control wells were considered inhibitors.
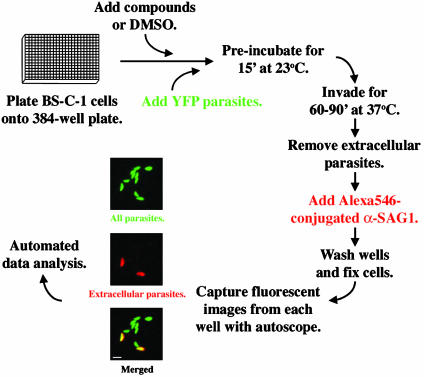
Fig. 1.
The high-throughput invasion assay. See text for details. External parasites fluoresce both green and red (yellow fluorescent protein and Alexa546), appearing yellow in merged images. Internalized parasites fluoresce green only. (Bar = 10 μm.)
Of the 12,160 small molecules screened, 65 inhibited invasion. Milligram quantities of 61 of these 65 small molecules were purchased and reassayed to identify false positives from the screen; four were no longer commercially available. Liquid chromatography-MS and/or NMR techniques were used to confirm the chemical identity and purity of the repurchased small molecules. After eliminating false positives and/or small molecules that did not meet analytical criteria, 36 inhibitors remained. Analysis of one additional inhibitor revealed that the original sample was a mixture of acetylated and deacetylated forms (Fig. 3, Inhibitors 10a and 10b). The deacetylated form (Inhibitor 10b) was purchased and determined to be >95% pure. Inhibitor 10b inhibited invasion with a potency similar to that of the original mixture and was therefore used for the remainder of the assays. Inhibitor 10a was not studied further.
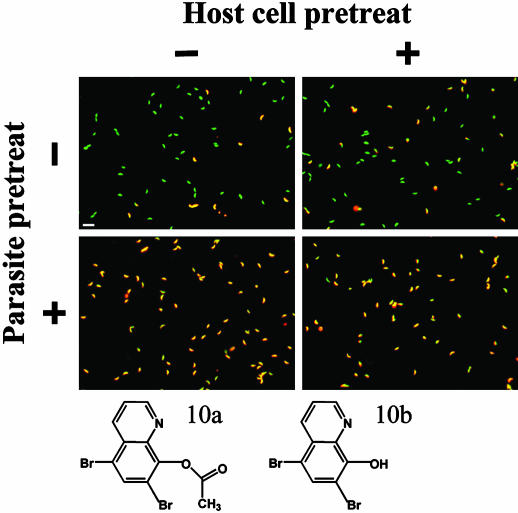
Fig. 3.
Target cell identification. Parasites and host cells were pretreated independently with buffer containing DMSO (-) or Inhibitor 10b (+), washed, and combined in the four possible combinations for invasion assays. Intracellular parasites are green; extracellular parasites are yellow. (Bar = 20 μm.) Liquid chromatography-MS analysis of Inhibitor 10 showed that it was actually a ≈1:1 mixture of (10a) and (10b).
Although the screen successfully identified 37 inhibitors of invasion, it could not distinguish between small molecules that perturbed a specific step in invasion and those that were cytotoxic. Two assays were used to assess the cytotoxicity of each small molecule; one measured intracellular esterase activity and plasma membrane integrity and the other, intracellular ATP levels. Using these assays, 13 of the 37 invasion inhibitors were determined to be cytotoxic to the host cell and/or the parasite on the time scale of the invasion assay (60-90 min; data not shown). Thus, the screen of 12,160 small molecules yielded a total of 24 reproducible noncytotoxic inhibitors of invasion, a hit rate of 0.2% (see Fig. 7, which is published as supporting information on the PNAS web site, for structures).
Characterization of the Invasion Inhibitors. All 24 inhibitors were shown to block invasion of both BS-C-1 and human foreskin fibroblast cells. Dose-response assays (e.g., Fig. 2A) established that the lowest effective doses ranged from 3 to 100 μM (see Table 1, which is published as supporting information on the PNAS web site). None of the inhibitors disrupted BS-C-1 cell monolayers, even at the highest concentrations used (100 μM), as visualized either by transmitted light microscopy or by staining host cell actin filaments with Alexa350-phalloidin (data not shown).
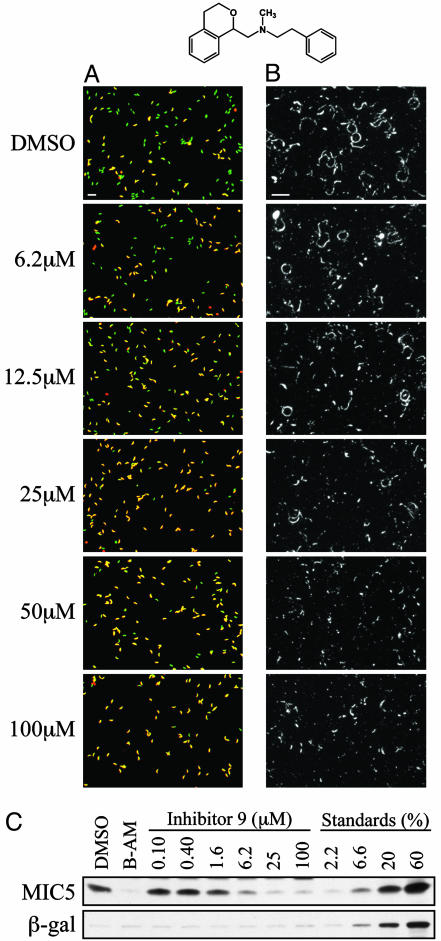
Fig. 2.
Dose-dependent inhibition of invasion, motility, and constitutive microneme secretion by Inhibitor 9 (pictured at top, racemic). (A) Invasion assay. In the merged red and green fluorescence images, intracellular parasites are green, whereas extracellular parasites appear yellow. (B) Motility assay. Trails deposited by gliding parasites are visualized by indirect immunofluorescence microscopy, using an antibody against the parasite surface protein, SAG1. (Bars = 20 μm.) (C) Microneme secretion assay. The amount of proteolytically processed (22 kDa) MIC5 constitutively secreted by the parasites, relative to untreated (DMSO) and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester [BAPTA-AM (B-AM)] pre-treated controls, was determined by Western blotting, as was the amount of β-galactosidase released from the parasites by nonspecific lysis. Only the relevant portions of the Western blots are shown. BAPTA-AM (20 μM) completely blocked the constitutive secretion of processed MIC5, as described (13). The extent of secretion and/or lysis can be estimated by a comparison to serial loadings of a total parasite extract (13): 2.2%, 6.6%, 20%, and 60% parasite equivalents are shown.
To determine whether the observed inhibitory effects were reversible, parasites and host cells were preincubated independently with each of the small molecules at 23°C, washed by centrifugation, and mixed together either in buffer containing the small molecule under test or in buffer containing DMSO. Invasion was assayed as described above. For 19 of the 24 hits, control levels of invasion were restored after small-molecule washout (data not shown); these inhibitors were classified as reversible. For the remaining five irreversible inhibitors, host cells and parasites were pretreated independently with or without the test molecule. After washing by centrifugation, inhibitor-treated and untreated parasites were added to inhibitor-treated or untreated host cells in the four possible combinations and assayed for invasion (e.g., Fig. 3). Using this assay, three of the irreversible inhibitors were found to act specifically on the parasite, whereas the other two acted on both the parasite and the host cell (Table 1).
Secondary Assays. The process of host cell invasion involves a series of discrete steps (5), several of which can be assayed independently. Secondary assays were performed to determine whether the identified inhibitors perturbed three specific aspects of invasion: parasite motility, conoid extension, or microneme secretion.
Motility. Parasite motility is essential for invasion (6, 8). The molecular machinery that provides the power necessary to penetrate the host cell also powers gliding motility on solid substrates (6, 8). As parasites glide, they leave behind “slime trails” consisting of parasite plasma membrane proteins and lipids (15). This process can be observed using videomicroscopy (Movies 1 and 2, which are published as supporting information on the PNAS web site). We examined the effects of each inhibitor on gliding motility by treating the parasites with an inhibitor, then visualizing the trails deposited on glass coverslips using indirect immunofluoresence microscopy. Of the 24 invasion inhibitors, 21 inhibited trail formation, whereas three had no effect (Table 1). In the presence of high concentrations of the motility inhibitors, parasites left “footprints” on the coverslips, but no detectable trails; as the inhibitor concentration decreased the number of trails increased (e.g., Fig. 2B). Small molecules that inhibited trail formation did so at doses similar to those at which they inhibited invasion.
Videomicroscopy of live inhibitor-treated parasites showed that, whereas most of the inhibitors of trail deposition induced a state of rigor, a subset (Inhibitors 7, 15, and 20) caused the parasites to move quickly with frequent changes in direction (e.g., Movie 3, which is published as supporting information on the PNAS web site). This unusual nonproductive form of motility is strikingly different from that of control parasites (Movie 4, which is published as supporting information on the PNAS web site) and reminiscent of parasites treated with jasplakinolide, an actin filament stabilizer (ref. 11 and Movie 5, which is published as supporting information on the PNAS web site). However, in contrast to jasplakinolide (11), Inhibitors 7, 15, and 20 cause no detectable increase in the amount of filamentous actin in the parasite (data not shown).
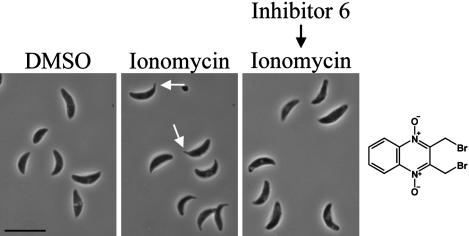
Conoid Extension. The tubulin-based conoid at the anterior end of the tachyzoite is one of the defining features of the Phylum Apicomplexa (16, 17), although its function is unknown. The conoid extends and retracts repeatedly as the parasite moves along the surface of a cell. Conoid extension can be experimentally induced by treating extracellular parasites with calcium ionophores, such as ionomycin (12). The effect of each invasion inhibitor on conoid extension was examined by adding buffer containing either ionomycin or DMSO to inhibitor-pretreated parasites, then visualizing extended conoids by phase microscopy. None of the inhibitors induced conoid extension, but one of the inhibitors, Inhibitor 6, efficiently blocked ionomycin-induced conoid extension (Fig. 4) with no effect on either parasite motility or microneme secretion (see below and Table 1).
Fig. 4.
Conoid extension assay. Phase micrographs of tachyzoites treated for 15 min with medium containing: DMSO (control); 1 μM ionomycin; or 50 μM Inhibitor 6 followed by 1 μM ionomycin. The apical end of >50% of the ionomycin-treated tachyzoites protruded (e.g., arrows). Protrusion was completely blocked by pretreatment with Inhibitor 6. (Bar = 10 μm.)
Microneme Secretion. Micronemes are small tubular secretory organelles located at the apical end of apicomplexan parasites. Many of the microneme proteins contain adhesive domains that are thought to play a role in host cell recognition and attachment (reviewed in ref. 18). The contents of the micronemes are secreted constitutively at a low basal rate, and secretion is up-regulated in response to increases in intracellular calcium (13, 19). The effect of the small molecules on constitutive microneme secretion was determined by Western blotting, using the proteolytically processed form of the soluble microneme protein, MIC5 (20), as a marker. Release of soluble β-galactosidase from the parasites was used to distinguish secretion from nonspecific parasite lysis (13). Of the 24 invasion inhibitors, 18 inhibited constitutive MIC5 secretion (e.g., Fig. 2C), four had no effect, and two increased the amount of MIC5 secreted as compared to controls (Table 1). Similar results were obtained with the processed form of the transmembrane microneme protein, MIC2, as a marker of secretion (data not shown).
Interestingly, of the 18 inhibitors of constitutive microneme secretion, 11 also inhibited calcium ionophore-induced microneme secretion, but seven had no effect on induced secretion (Fig. 5 and Table 1). None of the inhibitors of microneme secretion affected protein release from the parasite's other secretory organelles, the dense granule and rhoptries, as assayed by Western blotting with GRA8 and ROP4 specific antibodies (data not shown).
Fig. 5.
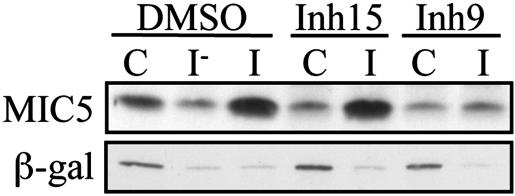
Differential effect of Inhibitors 15 and 9 on constitutive and induced microneme secretion. Tachyzoites were preincubated for 15 min at 20°C in medium containing DMSO (control), 25 μM Inhibitor 15 or 25 μM Inhibitor 9, followed by either 90 min at 37°C (constitutive secretion, lanes C) or 5 min at 37°C in the presence of 1 μM ionomycin (induced secretion, lanes I) or DMSO (lane I-). The amount of processed MIC5 or β-galactosidase released into the supernatant was assayed by Western blotting; only the relevant portions of the blots are shown. More β-galactosidase is released from the parasites after 90 min of incubation (6% of the total; data not shown) than after 5 min (2%). The structures of Inhibitors 9 and 15 are shown in Fig. 7.
Identification of Invasion Enhancing Small Molecules. Although developed to identify invasion inhibitors, the screen also yielded six enhancers of invasion (>200% invasion compared to controls; e.g., Fig. 6A) that met the same validation criteria used for the inhibitors (see Fig. 8, which is published as supporting information on the PNAS web site, for structures). The enhancers are generally more potent than the inhibitors (Table 1). None are cytotoxic to either the parasite or the host cell, and all act reversibly. Although they clearly have an effect on the parasite (see below), an additional effect on the host cell cannot be ruled out, because the target cell can only be unambiguously identified for small molecules that act irreversibly.
Fig. 6.
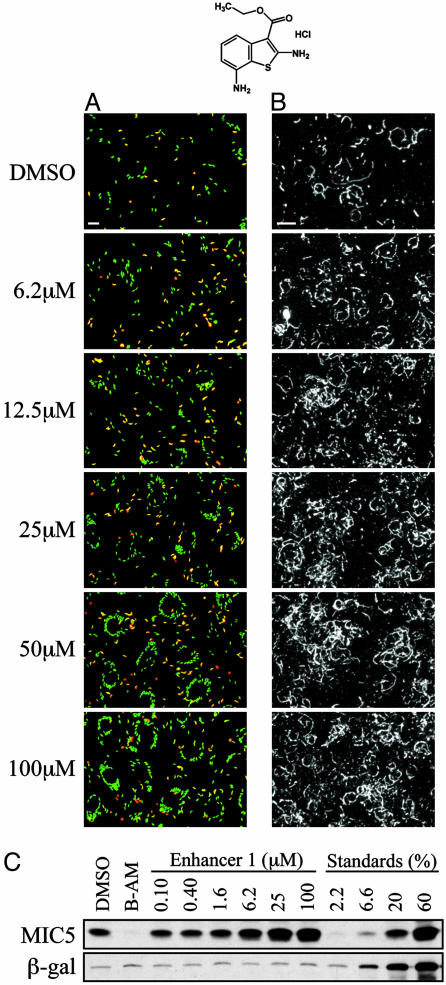
Dose-dependent enhancement of invasion, motility, and microneme secretion by Enhancer 1 (pictured at top). (A) Invasion assay. Intracellular parasites are green; extracellular parasites appear yellow. The same number of parasites was added to each well, but most of the extracellular parasites are washed away during processing for immunofluorescence. Dramatically enhanced numbers of internalized parasites are seen at increasing enhancer concentrations. (B) Motility assay. As in Fig. 2B, trails deposited by gliding parasites are visualized by indirect immunofluorescence microscopy. (Bars = 20 μm.) (C) Secretion assay. Parasites were pretreated with medium containing DMSO, 1,2-bis(2-aminophenoxy)ethane-tetraacetic acid-acetoxymethyl ester [BAPTA-AM (B-AM)] or Enhancer 1, and constitutive microneme secretion was assayed as described in Fig. 2C.
As with the inhibitors, secondary assays were performed to examine which step in invasion is affected by the enhancers. None of the enhancers induced conoid extension, nor did they block ionomycin-induced conoid extension at concentrations up to 100 μM (data not shown). In contrast, all six invasion enhancers were found to dramatically enhance parasite motility by the trail deposition assay (Fig. 6B). By live videomicroscopy, the twirling and gliding behaviors of parasites treated with enhancers were indistinguishable from those of control parasites. However, for several hours after their release from host cells, a greater proportion of the enhancer-treated parasites were actively gliding as compared to untreated controls (Movies 6 and 7, which are published as supporting information on the PNAS web site).
All of the enhancers also increased constitutive microneme secretion, by as much as 3-fold compared to controls, without a significant increase in the release of β-galactosidase (e.g., Fig. 6C). In some cases, increased secretion of both MIC2 and MIC5 could be seen at enhancer doses as low as 400 nM.
Discussion
Strengths of the Small-Molecule Approach. Small molecules that modulate the activity of specific proteins are powerful tools for studying protein function. If the mode of action of a particular small molecule is known in one system, it may be used to investigate the role of the target protein in other systems and processes. For example, cytochalasinD was critical in demonstrating that parasite actin plays a central role in host cell invasion by T. gondii (6, 21). More generally, large collections of structurally diverse small molecules can be screened to identify novel modulators of a particular biological process; the subsequent identification of the molecular targets of these small molecules implicates the targets in the process under study. This approach has recently become more accessible to the academic research community, where it has generated important new insights into several fundamental cellular processes (reviewed in refs. 1-3, 22).
Small-molecule approaches are the method of choice in systems where standard genetic tools are either unavailable or rudimentary, for dissecting the role of essential genes or recessive alleles and for examining rapid processes (2, 22, 23). All of these criteria apply to the study of host cell invasion by T. gondii. The process of invasion takes only 20-30 sec, and disruption of a gene essential for invasion by either forward or reverse genetics is problematic, because tachyzoites are haploid obligate intra-cellular organisms. Provided that the target proteins are well conserved, small molecules may also enable cross-species studies, including studies based on model organisms such as T. gondii, in a way that is not practical by using other approaches (e.g., genetics). Indeed, preliminary data suggest approximately half of the small-molecule inhibitors of T. gondii invasion reported here also inhibit the invasion of erythrocytes by the malaria parasite, Plasmodium knowlesi (data not shown). A comprehensive study with other parasites is currently underway to explore the possibility that some of the small molecules identified here target conserved components of the apicomplexan invasion machinery.
Because invasion is a complex multistep process, we predicted that several independent aspects of invasion would be vulnerable to small-molecule perturbation. Secondary assays revealed that the invasion modulators do indeed encompass a range of distinct activity profiles in terms of their effects on parasite motility, microneme secretion, and conoid extension (Table 1), suggesting that they are directed against a variety of molecular targets.
Parasite Motility Mechanisms. Actin and myosinA are central components of the machinery that powers T. gondii motility (6, 8), which is essential to parasite invasion. One goal of this study was to generate new reagents to investigate parasite motility mechanisms. Of the 24 invasion inhibitors, 21 blocked gliding motility as assayed by trail deposition. Videomicroscopy subsequently revealed that some of the inhibitors of trail deposition do not completely abolish motility. In the presence of these inhibitors, the parasites move in short back-and-forth spurts of rapid but nonproductive activity reminiscent of jasplakinolide-treated parasites (11). However, in contrast to jasplakinolide, these small molecules have no detectable effect on actin polymerization. Although the jasplakinolide results suggested that actin polymerization is the rate-limiting step in parasite motility (11), our results demonstrate that parasite motility can be enhanced by mechanisms independent of actin polymerization. The high frequency with which motility inhibitors were identified in the screen may reflect the multiple points of vulnerability presented by myosinA and its associated proteins (7, 24, 25); cytoskeletal and motor proteins may also be particularly amenable to perturbation by small molecules (26, 27).
Pharmacological Uncoupling of Microneme Secretion Pathways. The observations reported here identify microneme secretion as a potential target for drug development. Microneme secretion plays an important role in motility by releasing proteins onto the parasite surface that bind directly to ligands on the host cell (for invasion) or the substrate (for gliding motility). These secreted proteins, possibly in combination with other parasite proteins, physically couple the motor machinery of the parasite to the host cell during invasion (7, 18, 24). The micronemes secrete their contents constitutively at a low basal rate. Secretion is increased in response to agents that elevate intracellular calcium and decreased by treatment with cell-permeable calcium chelators or xestospongin (13, 14, 19). With the exception of Inhibitor 6 (see below), none of the secretion inhibitors affect conoid extension, another calcium-dependent process (12), suggesting that they are unlikely to inhibit secretion via a direct effect on parasite intracellular calcium. However, direct measurement of parasite intracellular calcium levels in response to each of the secretion inhibitors will be an important area for future investigation.
The relationship between the mechanisms underlying constitutive and calcium-induced microneme secretion is unknown, and the role of calcium-induced secretion during invasion has recently been questioned (14). We have pharmacologically un-coupled the two types of microneme secretion: of the 18 small molecules that inhibit constitutive secretion and invasion, seven had no effect on ionomycin-induced secretion. These data suggest that induced secretion is not simply an up-regulated form of constitutive secretion, but that the two processes differ mechanistically (see also ref. 28). Other data also suggest that calcium-sensitive processes within the parasite may be independently regulated (29). Alternatively, there may be two classes of micronemes: one that discharges rapidly in response to elevated intracellular calcium, and one that discharges constitutively. Other secretory cells are known to contain different pools of secretory vesicles that respond differently to applied stimuli (e.g., ref. 30).
Conoid Extension. Inhibitor 6, which had no effect on either motility or microneme secretion, was the only identified inhibitor of conoid extension. When the two bromines in Inhibitor 6 were replaced with hydroxyl groups, the small molecule no longer blocked invasion or conoid extension (data not shown), suggesting that Inhibitor 6 may act as an alkylating agent. This is consistent with the observed irreversibility of Inhibitor 6. Even if Inhibitor 6 acts via alkylation, it does so with some specificity, because it has no effect on either microneme secretion or motility, and it acts on the parasite, not the host cell. Inhibitor 6 will be a useful tool for exploring the possible role of conoid extension in invasion.
Invasion Enhancers. An unanticipated outcome of the screen was the identification of six enhancers of invasion. The only other treatment known to enhance invasion is incubation of the parasites with spent culture supernatant containing the rhoptry protein, ROP1 (31). Parasites engineered to lack the major glycosylphosphatidylinositol-anchored surface protein, SAG1, also appear to invade fibroblasts with faster kinetics than wild-type parasites (32). The mechanism(s) underlying these effects is unknown. All six invasion enhancers significantly increase parasite motility. Intriguingly, all six also dramatically enhance microneme secretion, suggesting that secretion of adhesins from the micronemes is not only necessary but also rate-limiting in the parasite's ability to move. However, ionomycin and two of the invasion inhibitors (Inhibitors 11 and 18) inhibit motility while enhancing microneme secretion. The relationship between enhanced secretion and enhanced motility may therefore not be causal: individual small molecules that enhance both processes might affect multiple pathways within the parasite, either by acting on a single target upstream of more than one pathway or by interacting with multiple targets. Although motility and microneme secretion are both calcium-dependent processes (13, 14), none of the enhancers directly affect parasite intracellular calcium levels (data not shown). The different activity profiles of the small molecules identified here demonstrate that the relationships between induced secretion, constitutive secretion, and motility may be more complex than previously recognized.
Target Identification Strategies. A number of strategies are available for identifying the molecular targets of the invasion modulators, including biochemical purification of the target after covalently tagging it with a radiolabeled small molecule, affinity chromatography, or gel overlays with radiolabeled small molecules (reviewed in ref. 2). In addition, a potentially powerful approach to target identification in T. gondii involves generating parasites resistant to a particular small molecule by chemical mutagenesis (33) and identifying the mutated gene by high-efficiency complementation cloning (9). The recently released sequence of the T. gondii genome will facilitate target identification strategies.
In summary, small-molecule screening provides a powerful approach to studying invasion and other experimentally challenging questions in host-pathogen interaction (2, 34, 35). At the same time, the approach may generate small-molecule probes that will prove useful in other experimental systems, and it may identify protein-small molecule pairs of relevance to antimicrobial drug development.
Supplementary Material
Acknowledgments
We thank Dr. Boris Striepen (University of Georgia, Athens, GA) for providing yellow fluorescent protein-expressing parasites, Dr. Vern Carruthers (Johns Hopkins University, Baltimore) for antibodies and helpful discussions, Dr. David Sibley (Washington University, St. Louis, MO) for 2F strain parasites and anti-ACT1 antibody, and Dr. John Tallarico for assistance with liquid chromatography-MS analysis. We also thank Drs. Mary Tierney and Douglas Johnson and members of our laboratories for helpful comments on the manuscript. This work arose from a collaboration between the Institute of Chemistry and Cell Biology (Harvard Medical School) (T.J.M. and N.J.W.) and the University of Vermont (K.L.C. and G.E.W.). All technical aspects of host cell dispensing, small-molecule collections, and image acquisition/analysis were carried out through the Institute of Chemistry and Cell Biology. This work was supported by Royal Society University Research Fellowship (to N.J.W.); the Burroughs Wellcome Fund (G.E.W.); Public Health Service Grants CA22435 (to the Vermont Cancer Center), AI054961 (to G.E.W. and N.J.W.), and GM626566 (to T.J.M.); E. Merck and Merck & Co. (T.J.M.); and the Vermont Experimental Program to Stimulate Competitive Research under National Science Foundation Grant EPS-9874685 (to K.L.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: HH, Hanks' buffered saline solution containing 10 mM Hepes, pH 7.0.
References
- 1.Crews, C. M. & Splittgerber, U. (1999) Trends Biochem. Sci. 24, 317-320. [DOI] [PubMed] [Google Scholar]
- 2.Ward, G. E., Carey, K. L. & Westwood, N. J. (2002) Cell Microbiol. 4, 471-482. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber, S. L. (2003) Chem. Eng. News, 81, 51-61. [Google Scholar]
- 4.Bain, J., McLauchlan, H., Elliott, M. & Cohen, P. (2003) Biochem. J. 371, 199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black, M. W. & Boothroyd, J. C. (2000) Microbiol. Mol. Biol. Rev. 64, 607-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrowolski, J. M. & Sibley, L. D. (1996) Cell 84, 933-939. [DOI] [PubMed] [Google Scholar]
- 7.Heintzelman, M. B. (2003) Curr. Biol. 13, R57-R59. [DOI] [PubMed] [Google Scholar]
- 8.Meissner, M., Schluter, D. & Soldati, D. (2002) Science 298, 837-840. [DOI] [PubMed] [Google Scholar]
- 9.Striepen, B., White, M. W., Li, C., Guerini, M. N., Malik, S. B., Logsdon, J. M., Jr., Liu, C. & Abrahamsen, M. S. (2002) Proc. Natl. Acad. Sci. USA 99, 6304-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gubbels, M. J., Li, C. & Striepen, B. (2003) Antimicrob. Agents Chemother. 47, 309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetzel, D. M., Hakansson, S., Hu, K., Roos, D. & Sibley, L. D. (2003) Mol. Biol. Cell 14, 396-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mondragon, R. & Frixione, E. (1996) J. Eukaryotic Microbiol. 43, 120-127. [DOI] [PubMed] [Google Scholar]
- 13.Carruthers, V. B. & Sibley, L. D. (1999) Mol. Microbiol. 31, 421-428. [DOI] [PubMed] [Google Scholar]
- 14.Lovett, J. L. & Sibley, L. D. (2003) J. Cell Sci. 116, 3009-3016. [DOI] [PubMed] [Google Scholar]
- 15.Hakansson, S., Morisaki, H., Heuser, J. & Sibley, L. D. (1999) Mol. Biol. Cell 10, 3539-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, K., Roos, D. S. & Murray, J. M. (2002) J. Cell Biol. 156, 1039-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrissette, N. S. & Sibley, L. D. (2002) Microbiol. Mol. Biol. Rev. 66, 21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opitz, C. & Soldati, D. (2002) Mol. Microbiol. 45, 597-604. [DOI] [PubMed] [Google Scholar]
- 19.Lovett, J. L., Marchesini, N., Moreno, S. N. & Sibley, L. D. (2002) J. Biol. Chem. 277, 25870-25876. [DOI] [PubMed] [Google Scholar]
- 20.Brydges, S. D., Sherman, G. D., Nockemann, S., Loyens, A., Daubener, W., Dubremetz, J. F. & Carruthers, V. B. (2000) Mol. Biochem. Parasitol. 111, 51-66. [DOI] [PubMed] [Google Scholar]
- 21.Ryning, F. W. & Remington, J. S. (1978) Infect. Immun. 20, 739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer, T. U. (2003) Trends Cell Biol. 13, 270-277. [DOI] [PubMed] [Google Scholar]
- 23.Specht, K. M. & Shokat, K. M. (2002) Curr. Opin. Cell Biol. 14, 155-159. [DOI] [PubMed] [Google Scholar]
- 24.Jewett, T. J. & Sibley, L. D. (2003) Mol. Cell 11, 885-894. [DOI] [PubMed] [Google Scholar]
- 25.Herm-Gotz, A., Weiss, S., Stratmann, R., Fujita-Becker, S., Ruff, C., Meyhofer, E., Soldati, T., Manstein, D. J., Geeves, M. A. & Soldati, D. (2002) EMBO J. 21, 2149-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson, J. R. & Mitchison, T. J. (2002) Chem. Biol. 9, 1275-1285. [DOI] [PubMed] [Google Scholar]
- 27.Cheung, A., Dantzig, J. A., Hollingworth, S., Baylor, S. M., Goldman, Y. E., Mitchison, T. J. & Straight, A. F. (2002) Nat. Cell Biol. 4, 83-88. [DOI] [PubMed] [Google Scholar]
- 28.Huynh, M. H., Rabenau, K. E., Harper, J. M., Beatty, W. L., Sibley, L. D. & Carruthers, V. B. (2003) EMBO J. 22, 2082-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black, M. W., Arrizabalaga, G. & Boothroyd, J. C. (2000) Mol. Cell. Biol. 20, 9399-9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan, R. R., Greaves, J., Wiegand, U. K., Matskevich, I., Bodammer, G., Apps, D. K., Shipston, M. J. & Chow, R. H. (2003) Nature 422, 176-180. [DOI] [PubMed] [Google Scholar]
- 31.Ossorio, P. N., Schwartzman, J. D. & Boothroyd, J. C. (1992) Mol. Biochem. Parasitol. 50, 1-15. [DOI] [PubMed] [Google Scholar]
- 32.Lekutis, C., Ferguson, D. J., Grigg, M. E., Camps, M. & Boothroyd, J. C. (2001) Int. J. Parasitol. 31, 1285-1292. [DOI] [PubMed] [Google Scholar]
- 33.Pfefferkorn, E. R. & Borotz, S. E. (1994) Exp. Parasitol. 79, 374-382. [DOI] [PubMed] [Google Scholar]
- 34.Kauppi, A. M., Nordfelth, R., Uvell, H., Wolf-Watz, H. & Elofsson, M. (2003) Chem. Biol. 10, 241-249. [DOI] [PubMed] [Google Scholar]
- 35.Tsukahara, K., Hata, K., Nakamoto, K., Sagane, K., Watanabe, N. A., Kuromitsu, J., Kai, J., Tsuchiya, M., Ohba, F., Jigami, Y., et al. (2003) Mol. Microbiol. 48, 1029-1042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.