Abstract
Inflammation is strongly associated with chronic hepatic injury and the ensuing wound healing process. Recent evidence from mouse models and human studies implicates Tolllike receptors (TLRs) as important regulators of the inflammatory response and a functional link between inflammation and fibrosis in the chronically injured liver. Here we review mechanisms by which TLR4 and TLR4 ligands from the intestinal microbiota contribute to hepatic injury, inflammation, hepatic stellate cell activation and fibrosis.
Introduction
Despite an efficient intestinal barrier, a small amount of bacteria and bacterial products continually reaches the portal circulation. The liver is the first target of translocating bacteria and their products, and usually clears the portal blood without the occurrence of significant inflammation. While the amount of bacterial products reaching the liver is minuscule under normal circumstances, increased bacterial translocation occurs in chronic liver injury and may result in hepatic inflammation. Microbial products such as lipopolysaccharide (LPS), lipopeptides, unmethylated DNA, and double-stranded RNA are potent inducers of inflammation, and even doses in the nanomolar range can induce intense inflammatory responses. Proinflammatory actions of these microbial products are mediated through a specific class of receptors, termed Toll-like receptors (TLRs). TLRs enable the host to detect signature molecules derived from pathogen, and are master regulators of innate immune responses. In the liver, TLRs are expressed in many different cell types including Kupffer cells, hepatocytes and hepatic stellate cells (HSCs). The extraordinarily powerful effects of TLRs on inflammation, their expression in the liver and the emerging concept that there is a significant hepatic exposure to TLR ligands from the intestinal microbiota, even in early stages of liver disease, suggests that TLRs act as an important link between hepatic inflammation, injury and fibrosis.
PART I: TLR receptors, TLR ligands and TLR signaling
Toll like receptors and Toll-like receptor signaling
The concept that specialized receptors allow the innate immune system to recognize invading pathogens was proposed even before the discovery of TLRs 1. One decade after the discovery of Toll and its role in drosophila development, it was recognized that Toll also exerts profound functions in the regulation of immune responses as shown first in drosophila Toll mutants 2 and later in mice expressing a mutant form of TLR4 3. TLRs are pattern recognition receptors that recognize different classes of molecular patterns specific pathogens. Upon ligand binding, TLRs allow the host to sense the presence of these pathogens and initiate subsequent immune responses. To date, ten different human TLRs as well as ligands for most of the receptors have been discovered 4. In addition to TLRs, other pattern recognition receptors exist, including the cytoplasmic proteins RIG-I-like receptors, NOD-like receptors and Dectin-1 5. TLRs contain different conserved domains responsible for ligand recognition and signaling. Leucine rich repeat (LRR) domains mediate ligand binding on the extracellular domain of TLRs, whereas Toll-IL-1R (TIR) domains, which are common to both Toll-like receptors and the IL-1 receptor 6, mediate signaling in the intracellular domain. Different TLRs not only vary in their ligand specificity but also have different cellular localizations: TLR 1, 2, 4, 5, 6, 10 are localized at the cell membrane, and TLR 3, 7, 8, 9 are localized in the endosome. The cellular localization of TLRs contributes to optimal recognition of TLR ligands as many of the pathogens that activate endosomal TLRs, such as TLR 3, 7, 8 and 9, enter through this cellular compartment. In addition, the localization of TLRs in specific cellular compartments may also reduce the risk for exposure to endogenous molecules that might falsely trigger TLR activation.
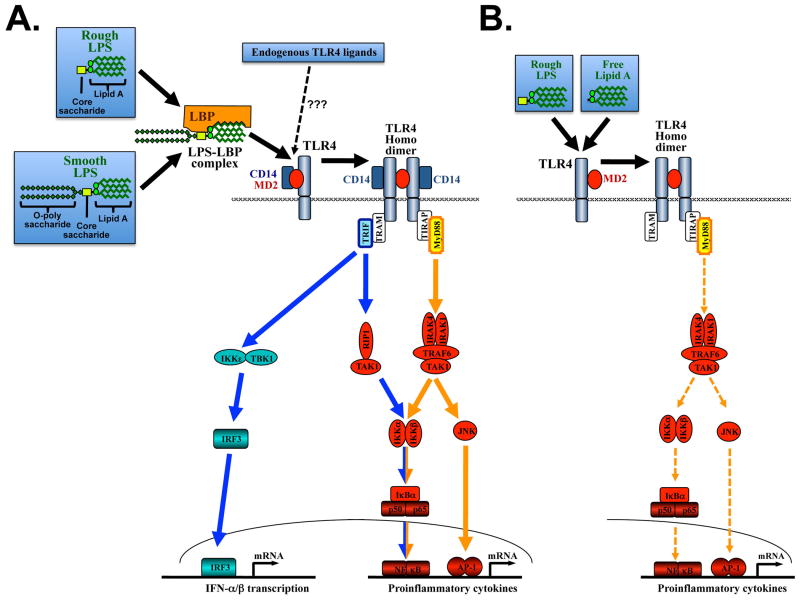
TLR4 requires several additional molecules for the induction of signaling such as LPS-binding protein (LBP), CD14 and MD-2 (see Figure 1). CD14 and MD-2 also contain LRR domains, suggesting their involvement in ligand recognition and binding. It is believed that LBP, a member of the lipid transfer protein family, helps extracting LPS from the outer membrane of Gram-negative bacteria and transferring it to CD14 in a monomeric form 7. CD14, which contains no intracellular signaling domain, then catalyzes the binding of LPS to the TLR4-MD-2 complex to initiate signaling. Binding of LPS to MD-2 induces a conformational change in MD-2 allowing the binding of a second TLR4 receptor, TLR4 homo-dimerization and signaling 8. Although each TLR detects specific ligands, key signaling molecules that mediate intracellular responses are shared by TLRs (see Figure 1). The most upstream signaling molecules are the adapter molecules MyD88 and Trif. All TLRs signal through one or, in the case of TLR4, both of these adapter molecules. The use of common signaling mediators explains why different TLR ligands often generate similar downstream signals 9,10. TLR4 requires specific adapters termed Tirap 11 12 and TRAM 13 to interact with MyD88 and Trif, respectively. Both MyD88-dependent and Trif-dependent pathways initiate the transcription of genes involved in proinflammatory pathways and antiviral interferon pathways (see Figure 1). Although MyD88 and Trif induce distinct patterns of gene expression due to preferential activation of specific pathways, there is also a fair degree of overlap 9.
Figure 1. TLR4 signaling by smooth and rough LPS.
A. Strong activation of the TLR4-MD2 receptor complex is achieved when smooth and rough LPS first bind to LBP and CD14 to then trigger TLR4-MD2 activation. This activation is mediated by a conformational change of MD2 after LPS binding resulting in TLR4 dimerization and activation of two major pathways: I. MyD88-induced signals (marked in orange) predominantly activate NF-κB, IRF-7 and JNK. II Trif-dependent signals (marked in blue) predominantly activate NF-κB and IRF-3. B. Rough LPS and free Lipid A may induce TLR4 activation even in the absence of LBP and CD14. This pathway leads to TLR4 activation that is restricted to the MyD88 pathway, and results in a lower amplitude activation. Abbreviations: IRF-7, interferon regulatory factor-7; JNK, c-Jun N-terminal kinase; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa B; TLR, toll-like receptor; Trif TIR domain-containing adapter inducing IFNβ; LBP, LPS-binding protein.
TLR4 ligands
The main function of TLRs is to signal the presence of pathogens. TLRs are activated by signature molecules that are present in pathogens but not in the host, which are termed pathogen-associated molecular pattern (PAMPs). The first description of a PAMP and its proinflammatory effects can probably be attributed to Richard Pfeiffer, a collaborator of Robert Koch, who discovered in 1892 that lysates of heat-killed bacteria of the cholera-inducing infectious agent Vibrio cholerae caused toxic shock reactions in guinea pigs 14. He postulated that the heat-stable toxic principle was localized inside the bacterial cell and thus named it endotoxin to distinguish it from the already known exotoxins of Vibrio cholerae. It can be assumed that the biological effects of these bacterial preparations were probably caused to a large degree by a chemical group of substances now termed lipopolysaccharide (LPS). LPS is a component of the outer membrane of Gram-negative bacteria and the prototypical TLR4 ligand. LPS is an extremely heat-stable amphiphilic molecule composed of a predominantly lipophilic region known as lipid A, a covalently linked hydrophilic core oligosaccharide and a polysaccharide chain termed O-polysaccharide. The Lipid A portion confers the activation of TLR4, and has TLR4-agonistic effects even in the purified form. In some bacteria, LPS does not contain the O-polysaccharide and has been termed rough LPS as opposed to smooth LPS which contains the O-polysaccharide 15. Most wild-type bacteria as well as commercially available LPS contain both forms of LPS. Despite their structural differences, both smooth and rough LPS act as endotoxins since they contain lipid A, the component with biological activity towards TLR4. However, there are differences in signaling requirements between smooth and rough LPS: Smooth LPS requires the presence of LBP and CD14, whereas rough LPS (and free lipid A) may activate TLR4 in the absence of CD14 and LBP 16,17. However, CD14- and LBP-independent activation of TLR4 is not as efficient as TLR4 activation in the presence of LBP and CD14, and results in selective activation of the MyD88-dependent pathway (see Figure 1). TLR4 also recognizes proteins of viral origin, but the large majority of TLR4 biological effects in mammals seem to be linked to the recognition of LPS 18. This high promiscuity and ability to recognize chemically completely different ligands allows TLR4 to also recognize endogenous TLR4 ligands as described below.
While the main function of TLRs is the recognition of PAMPs, there is mounting evidence that TLR may be activated also by endogenous molecules. A specific class of endogenous molecules, that are associated with cellular damage or wound healing responses and may promote inflammation through TLRs and other receptor systems, has been termed damage-associated molecular patterns (DAMPs). As uncontrolled activation of TLRs by endogenous DAMPs would likely result in devastating inflammation, the activation of TLRs by DAMPs is believed to occur only under specific circumstances. This may either be a change in the environment leading to release of endogenous ligands from a cellular compartment that is usually not in contact with TLRs, or the modification of an endogenous mediator or extracellular matrix component – e.g. during inflammation or cell death - that allows binding and activation of TLRs 18,19. Among the best characterized DAMPs are HMGB1, S100 proteins, heat shock proteins, hyaluronan and fibronectin 20. Most of these ligands have been postulated to be direct agonists of TLR2 or TLR4, or both receptors, as well as other receptor systems including RAGE, CD44, scavenger receptors and galectins 20. In addition, saturated free fatty acids may have TLR2 and TLR4 agonistic activity 21,22. However, there is still ongoing controversy as to whether many DAMPs are truly bona fide TLR ligands. Many of these ligands either have been purified in bacterial systems or have a high affinity to bacterial products suggesting that bacterial products or other TLR activating substances such as lipids or DNA rather than the purified ligands themselves mediate their TLR activating effect 23. HMGB1 and hyaluronan are elevated in liver disease, and HMGB1 plays a potential role in the pathophysiology of liver disease 24,25. For these reasons, we will limit further discussion on TLR-activating DAMPs to HMGB1 and hyaluronan.
HMGB1 is a DNA-binding protein that induces bends in the helical DNA structure to facilitate multiple physical interactions of DNA with transcription factors, recombinases and steroid hormone receptors permitting transcription and other nuclear transactions to take place 26. In addition to this transcription factor-like function, HMGB1 also has cytokine-like effects that require its presence in the extracellular space 26,27. Release of HMGB1 into the extracellular space is mediated by two mechanisms: (i) Active secretion in inflammatory cells that depends on acetylation, and masking of the two nuclear-localization signals of HMGB1 thus preventing nuclear re-entry 28; (ii) passive diffusion of HMGB1 from cells that undergo necrosis 29. Importantly, HMGB1 release does not occur from apoptotic cells, presumably because HMGB1 is tightly bound to DNA within the apoptotic-cell nucleus, whereas it is only loosely bound to DNA in necrotic cells 27. Accordingly, HMGB1 has been suggested to be a signature DAMP that signals the presence of necrosis, and subsequently triggers inflammation 29. HMGB1 was initially suggested to act as a direct proinflammatory TLR 2/4 agonist; however, a direct proinflammatory activity of HMGB1 was not confirmed in recent publications 30,31. Recent data suggest instead that HMGB1 facilitates and amplifies the inflammatory response to different cytokines and TLR agonists rather than having its own proinflammatory effect 31,32. Treatment of monocytes with HMGB1 and LPS results in a higher TNF- production than LPS alone, and this proceeds via activation of TLR4, the receptor for LPS/CD14 33. The formation of a specific complex between HMGB1 and LPS can explain the empirical observation that it is difficult to produce LPS-free recombinant HMGB1.
Hyaluronan is a negatively charged high molecular weight glycosaminoglycan, which is ubiquitously distributed in the extracellular matrix and a component of the basement membrane. At sites of inflammation and tissue destruction, high molecular weight hyaluronan can be broken down to lower molecular weight hyaluronan fragments via oxygen radicals and enzymatic degradation. In contrast to high molecular weight hyaluronan, low molecular weight hyaluronan has cytokine-like properties capable of inducing inflammatory gene expression in epithelial cells, endothelial cells, fibroblasts, dendritic cells (DCs), and macrophages 34. In the extracellular space, hyaluronan is bound by the CD44 receptor which mediates some of its proinflammatory effects. However, hyaluronan is able to stimulate chemokine production in peritoneal macrophages in the absence of CD44 through a TLR2 and TLR4-dependent mechanism 35. Moreover, hyaluronan stimulates maturation of dendritic cells and IL-8 production by endothelial cells, and it inhibits osteoclast differentiation in a TLR4-dependent manner 36,37. Since the disruption of basement membranes is typically associated with injury, it has been suggested that the recognition of low molecular weight hyaluronan by TLRs and other receptors is part of an injury recognition system.
PART II: TLRs and their ligands in hepatic injury and fibrogenesis
Hepatic fibrosis is the result of chronic injury and requires the concerted action of many different cell types with hepatocytes, hepatic stellate cells (HSCs) and Kupffer cells representing the key actors. Therefore, it is important to understand the role of TLRs in these hepatic cell populations as well as potential effects of TLR4 activation on hepatic injury. Current evidence suggests that TLR4, in addition to a direct effect on fibrogenesis, may also have a role in hepatic injury and thereby modulate disease progression. However, this injury-promoting effect seems occurs only in some settings: There is a reduction of injury after TLR4 inactivation in alcoholic liver disease 38-40 and nonalcoholic fatty liver disease, 41,42 but no reduction of injury in experimental fibrogenesis models such as bile duct ligation and chronic CCl4 treatment 25.
IIa. TLR expression in the liver
Kupffer cells, the resident macrophages of the liver, are among the first cells in the liver to encounter gut-derived bacteria and their products. Kupffer cells represent an important population for regulation of inflammation in response to TLR agonists as well as their clearance. In addition, Kupffer cells are also known to promote HSC activation and fibrosis 25,43,44. Kupffer cells express TLR4 and are highly responsive to LPS 45. After LPS stimulation, Kupffer cells produce TNFα, IL-1β, IL-6, IL-12, IL-18 and several chemokines 46,47. Due to the continuous exposure to low amounts of LPS, Kupffer cells may be less responsive to LPS than peripheral blood monocytes as evidenced by lower levels of CD14 48, secretion of the anti-inflammatory cytokine IL-10 and downregulated TLR4 in response to LPS 49,50. Although some studies suggest a role for Kupffer cells in the uptake and hepatic excretion of LPS 51,52, others show that Kupffer cell depletion does not reduce LPS clearance. 53. Moreover, Kupffer cells can inactivate LPS by deacetylation 54. Hepatocytes express TLR4 and are responsive to LPS, but this response is fairly weak with only two-fold elevated levels of serum amyloid A (SAA) after LPS treatment 55. The expression of TLR4 in hepatocytes is not upregulated by proinflammatory mediators 56. Hepatocytes also are believed to play a role in the uptake of endotoxin and its removal from the systemic circulation through secretion into the bile 51,53, suggesting that the uptake and removal of LPS is a potentially cooperative effect involving both hepatocytes and Kupffer cells. In the normal liver, hepatic stellate cells (HSC) are quiescent and store the majority of the body's vitamin A. In response to liver injury, quiescent HSCs activate to become the major extracellular matrix-producing cell type in the liver 57. HSCs interact with Kupffer cells and hepatocytes to promote liver fibrosis and inflammatory responses during the wound healing process. Activated mouse and human HSCs express high levels of TLR4 25,58 and CD14 58. Interestingly, even quiescent HSCs express high levels of TLR4 and are highly responsive to LPS treatment, 25 suggesting that quiescent HSCs may act as sentinels that promote inflammatory and wound healing responses after increased hepatic exposure to LPS. LPS treatment triggers inflammatory responses such as activation of IKK/NF-κ;B and JNK and the activation of NF-κ;B dependent genes in both quiescent and activated HSCs 58. LPS does not directly promote activation of HSCs, but promotes inflammatory signals and enhances HSC responses to TGFβ as discussed below. A recent study has shown that HMGB1 may directly promote HSC activation in vitro 59. However, this is in contrast to unpublished observations by our group demonstrating that HMGB1 neutralization did not reduce HSC activation in experimental fibrogenesis in vivo. Several other cell population in the liver express TLR4 and may also be involved in HSC activation and fibrogenesis such as dendritic cells and cholangiocytes 60,61.
IIb. TLR ligands in chronic liver diseases
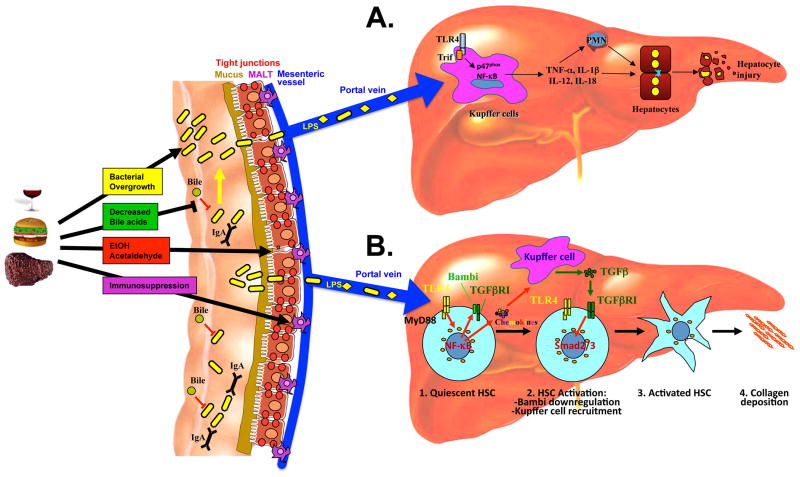
Under normal circumstances, protective mechanisms at various levels ensure that only minute amounts of bacteria and bacterial products reach the portal circulation and ultimately the liver. On the intestinal side, these include a thick layer of mucins, secretion of IgA and antimicrobial factors, a tightly sealed epithelial surface and an active mucosa-associated lymphatic tissue (MALT) (see Figure 2) 62. In addition, the presence of bile and the specific composition of the intestinal microbiota, with anaerobic bacteria outnumbering aerobic bacteria by 100:1 to 1000:1, suppress bacterial adhesion, colonization and translocation of potentially harmful and invasive microbes 63. Accordingly, selective elimination of anaerobic bacteria promotes intestinal bacterial overgrowth and translocation 63.
Figure 2. Promotion of hepatic injury and fibrogenesis through LPS-TLR4 signaling.
Ethanol consumption, high fat diet or chronic liver injury lead to changes in the intestinal microbiota such as bacterial overgrowth and a different composition. These changes together with a reduction of bile acid, damage to tight junctions and a decreased activity of the mucosa-associated lymphatic tissue (MALT) lead to an increased translocation of bacteria and their products, and increase hepatic exposure. A. In alcoholic liver disease, LPS primarily acts on Kupffer cell to induce MyD88-independent activation of NF-κB, and activation NADPH oxidase. Release of cytokines by Kupffer cells promotes the recruitment of immune cells such as neutrophils and subsequent hepatocyte injury. B. In early stages of hepatic fibrogenesis, low amounts of LPS directly target quiescent hepatic stellate cells (HSCs) to promote their activation and fibrogenesis: (i) Upregulation of chemokines resulting in the recruitment of Kupffer cells. (ii) downregulation of the inhibitory TGFβ pseudoreceptor Bambi. These two signals complement each other and lead to an unrestricted activation of HSCs by Kupffer cell-released TGFβ. Abbreviations: HSC, hepatic stellate cell; LPS, lipopolysaccharide; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa B; Smad2/3, mothers against decapentaplegic Drosophila homologue 2/3; TGFβ, transforming growth factor β; TLR, toll-like receptor; IL, interleukin; TNF, tumour necrosis factor; Trif, TIR domain-containing adaptor inducing IFNβ; IgA, type A Immunoglobulin; MALT, mucosa-associated lymphatic tissue; PMN, polymorphonuclear leukocytes.
Whereas portal and systemic LPS levels are nearly undetectable in healthy rodents and people 64-66, LPS levels increase in chronic liver injury in a disease stage-dependent manner. Healthy subjects displayed endotoxin levels of less than 3 pg/ml, but patients with Child–Pugh classes A, B and C had endotoxin levels of 4.9 pg/ml, 7.9 pg/ml and 10.2 pg/ml, respectively 67. Chronic alcohol intake also increases endotoxin levels in peripheral blood, with alcoholic fatty liver, alcoholic hepatitis and alcoholic cirrhosis patients displaying 14 pg/ml, 16 pg/ml and 19 pg/ml endotoxin, respectively, in peripheral blood versus 2.5 pg/ml in healthy controls. 64. In addition to the elevation of LPS in chronic liver disease, acute insults such as binge drinking may also promote increased LPS translocation. Acute ingestion of alcohol in rats leads to portal endotoxin levels of 30–80 pg/ml in the portal vein 2 hours after gavage but much lower levels in peripheral blood 66,68. Thus, peripheral measurements are likely to underestimate the amount of endotoxin that reaches the liver, most likely due to the efficient clearance of endotoxin by the liver. Moreover, these data suggest that elevations of LPS are not only a feature of end-stage liver disease but may occur early in the disease course and thus actively influence disease progression. In acute and chronic liver disease, increased bacterial translocation is believed to be caused by (i) structural changes of the intestinal mucosa such as loss of tight junctions, widening of intercellular spaces and vascular congestion, (ii) defects in the mucosal immune system that promote the loss of barrier function, and (iii) changes in the composition of the intestinal microbiota such as overgrowth of bacteria in locations with normally low bacterial counts and potential overgrowth of strains that are more proficient at translocating 62. Some of the changes in the bacterial microbiota may be due to decreased intestinal levels of bile acids. Although intestinal anaerobic bacteria outnumber aerobic bacteria by far, virtually all translocating bacteria are aerobic 62. Gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, Enterococci and Streptococci not only represent the species that are most proficient at translocation but also cause the large majority of infections in patients with cirrhosis 69,70. As previously mentioned, Gram-negative bacteria contain LPS as an important component of the outer membrane of their cell wall. Thus, the aforementioned strains not only translocate but also produce LPS and probably account for a large proportion of TLR4 activation in chronic liver disease.
Currently, there is only limited data on the role of endogenous TLR4 ligands in chronic liver disease. Elevations of HMGB1 and hyaluronan have been demonstrated in experimental fibrogenesis models 25. Hyaluronan is increased in human liver fibrosis as a result of both increased production and decreased clearance 71, one result of which is use of serum hyaluronan as a marker of liver fibrosis. However, there are no data on HMGB1 in human liver disease to the best of our knowledge.
IIc. TLR4 and liver fibrosis
The development of hepatic fibrosis and cirrhosis is the result of virtually any chronic hepatic injury including viral hepatitis, alcohol, autoimmune and metabolic disease 72. The increased accumulation of extracellular matrix in the liver is directly responsible for many of the deadly complications of liver disease such as the development of portal hypertension, ascites and variceal bleeding. Whereas as substantial portion of liver fibrosis in patients is caused by alcoholic liver disease or non-alcoholic fatty liver, the relative resistance of rodents to develop fibrosis in response to alcohol and high-fat diet have resulted in a strong focus on the role of TLR4 in models of toxic and biliary liver fibrosis. We will therefore review the contribution of TLR4 to alcoholic liver disease and non-alcoholic fatty disease separately, and focus predominantly on the role of TLR4 in inflammation and injury as changes that usually precede and often promote the development of fibrosis.
As mentioned above, abundant data demonstrate that LPS is elevated in experimental models of hepatic fibrosis 25,73,74 and in patients with cirrhosis 64,67,75. Studies from the 1950s have shown that antibiotics prevent hepatic injury and fibrosis induced by CCl4 treatment or a choline-deficient diet, and that endotoxin enhances hepatic fibrosis induced by a choline-deficient diet 76,77. Recent studies using TLR4-mutant as well as gut-sterilized, CD14- and LBP-deficient mice have expanded these findings, and all emphasize the crucial role of the LPS-TLR4 pathway in hepatic fibrogenesis 25,78: (i) TLR4-mutant mice display a profound reduction in hepatic fibrogenesis in three different experimental models of biliary and toxic fibrosis 25; (ii) LBP-deficient, CD14-deficient, MyD88- and Trif-deficient mice display reduction of fibrosis after bile duct ligation 25,78; (iii) Bile-duct ligated mice, mice on a choline-deficient diet or Mdr2-deficient mice, a genetic model of liver fibrosis, have a profound decrease in fibrosis when treated with antibiotics 25,77,79. Although endogenous TLR ligands such as hyaluronan and HMGB1 are elevated in murine fibrogenesis, their contribution to fibrogenesis has not been evaluated in detail. In view of the strong anti-fibrotic effect of antibiotics, their role in hepatic fibrogenesis appears to be limited. While TLR2-deficient mice did not show a profound reduction in hepatic fibrosis following bile duct ligation 25, TLR9-deficiency decreased fibrogenesis, suggesting that some of the antifibrogenic effects of antibiotics may also be due to a reduction of TLR9 ligands from the gut microbiota 80,81. TLR4 is expressed on two key mediators of hepatic fibrogenesis, Kupffer cells and HSCs 25,45,58. Kupffer cells initiate fibrogenesis by secreting proinflammatory and profibrogenic cytokines, while HSCs are the predominant source of extracellular matrix production in the fibrotic liver 72. Although Kupffer cells express the highest levels of TLR4 in the liver and are considered a prime target of LPS, two lines of evidence suggest HSC are the crucial cell population that promotes fibrosis in a TLR4-dependent manner: (i) Both quiescent and activated HSC express high levels of TLR4 and LPS directly targets HSCs in vivo 25. (ii) TLR4-chimeric mice without functional TLR4 expression on bone marrow-derived cells still show strong fibrosis after BDL, whereas TLR4-chimeric mice that do not express functional TLR4 on resident liver cells have strongly reduced fibrosis after BDL 25. Several mechanisms likely explain the profibrogenic effects of TLR4 in hepatic stellate cells (see Figure 2B): (i) There was a strong reduction of Kupffer cell infiltration and hepatic inflammation in TLR4-deficient mice suggesting that TLR4 activation in HSCs promotes the recruitment of Kupffer cells, an important profibrogenic cell population of the liver 25. The induction of chemokines, many of which have profibrogenic effects in the liver, is likely to be an important mediator of Kupffer cell recruitment and hepatic fibrosis in response to TLR4 activation. (ii) TLR4 activation also induces a downregulation of the TGFβ pseudoreceptor Bambi and sensitizes HSCs to profibrogenic effects of TGFβ 25. In contrast to data showing that genetic or pharmacologic inactivation of Kupffer cells and chemokines that recruit Kupffer cells reduce fibrogenesis in vivo 25,43,44,82, the role of Bambi in vivo still needs to be confirmed in a knockout mouse model. Increased Kupffer cell recruitment and decreased Bambi expression probably work hand in hand to promote the activation of HSCs by Kupffer cell-released TGFβ, and subsequently hepatic fibrosis (see Figure 2B) 78.
IId. TLR4 in human liver fibrosis
Recent evidence also suggests TLR4 contributes to the development of hepatic fibrosis in patients. A recent study has identified a single nucleotide polymorphism in Tlr4 that results in a T399I substitution and confers a significantly reduced risk for fibrosis progression in patients with chronic hepatitis C virus infection 83. This polymorphism is associated with a reduced TLR4 responsiveness thus confirming the profibrogenic role of TLR4 in a clinically relevant setting. A second study, also performed in patients with chronic hepatitis C virus infection, found that multiple TLR4 variants including T399I and D299G were associated with reduced fibrogenesis 84. Although it is possible that TLR4 mutations may not only affect fibrogenesis but also the immune response towards HCV, one would expect that a non-functional TLR4 would hamper this response and thus promote viral replication and disease progression. In contrast, a third study reported no linkage between TLR4 D299G or CD14 C260T SNPs and disease progression in patients with chronic liver disease including chronic hepatitis C virus infection and alcoholic liver disease 85. A recent study has further investigated the role of TLR4 in human fibrogenesis by transfecting human HSCs with TLR4 bearing either the T399I mutation or the co-segregated D299G mutation 86. Both cell lines displayed decreased cytokine and chemokine release and Bambi downregulation in response to LPS, as well as an increase in spontaneous and drug-induced apoptosis. These results suggest that TLR4 signaling in HSCs is an important contributor to fibrogenic responses and that hyporesponsive TLR4 mutations decrease fibogenesis by reducing HSC activation and increasing HSC apoptosis.
IIe. TLR4 and alcoholic liver disease
Alcoholic liver disease is a leading cause for the development of liver fibrosis and cirrhosis in patients. As ethanol induces very little hepatic fibrosis in rodents even if applied in sublethal concentrations, e.g. by intragastric feeding, the role of TLR4 in the development of alcohol-induced liver fibrosis is somewhat difficult to study. However, there is strong evidence that LPS and TLR4 contribute to key pathophysiological aspects of alcoholic liver disease, such as inflammation and fatty liver, both of which are associated with the development of liver fibrosis in humans: (i) Alcohol consumption disrupts the intestinal epithelial barrier causing enhanced permeability 87 and subsequent elevations of endotoxin levels in the portal vein 65,88; (ii) Liver injury is strongly reduced when the intestinal Gram-negative microbiota is reduced by antibiotics or Lactobacillus, or when Kupffer cells are depleted with gadolinium chloride 38-40; (iii) Mice expressing non-functional TLR4 display strongly reduced levels of proinflammatory mediators in the liver and blunted liver injury despite elevated endotoxin levels 89; (iv) Long-term ethanol exposure sensitizes rats to the effects of LPS and strongly increases TNFα levels and liver injury 90. The injury and inflammatory-promoting effects of TLR4 appears to depend on Trif and not MyD88 as demonstrated by reduced injury in TLR4-deficient but not MyD88-deficient mice 91. NADP(H) oxidase represents another crucial downstream mediator of TLR4 in Kupffer cells during alcohol-induced liver injury 92 as mice deficient in p47phox, the main cytosolic component of NADP(H) oxidase, display reduced inflammation and liver pathology after ethanol exposure 92. Due to the lack of good animal models of alcohol-induced liver fibrosis, one has to assume that promotion of injury by TLR4 represents a mechanism that is responsible for fibrosis development in patients with alcoholic liver disease. It is likely that additional mechanisms (which cannot be studied in current animal models, e.g. TLR4-dependent activation of HSCs) contribute to disease progression in patients.
IIf. TLR4 and non-alcoholic fatty liver disease
Similar to alcoholic fatty liver, a number of studies also imply the TLR4 pathway as a major promoter of non-alcoholic fatty liver disease, and the mechanisms seem to overlap substantially: (i) Genetically obese Fa/Fa rats and ob/ob mice as well as mice on the methionine-choline-deficient exhibit increased hepatic sensitivity to endotoxin 93,94; (ii) Non-functional TLR4 and probiotics reduce hepatic injury in response to a methionine-choline-deficient diet or in ob/ob mice, respectively 41,95; (iii) TLR4 deficiency protects from high-fructose induced hepatic steatosis, injury and inflammation 42. One underlying mechanism may be the effect of high-fat diets on the intestinal microbiota and/or intestinal tight junctions, resulting in an increase in LPS translocation 96,97 which could then trigger inflammation and injury in the liver. It has also been suggested that nonalcoholic steatohepatitis is associated with an increased production of ethanol by the intestinal microbiota further emphasizing similarities between non-alcoholic fatty liver disease and alcoholic liver disease 98,99. To date, the only study assessing the role of TLR4 on hepatic fibrosis in non-alcoholic liver disease found a reduction in collagen 1α(I) expression in response to a methionine-choline-deficient diet, but there was no evidence of histological fibrosis due to the relatively short duration of the study 95. In a much earlier report published in 1957, rats receiving a methonine-diet-deficient diet were put on different antibiotic regimens 77. Whereas control rats developed cirrhosis at a rate greater than 80% and had a high mortality, rats receiving absorbable antibiotics exhibited a delayed onset of cirrhosis and reduced mortality. Non-absorbable antibiotics, e.g. a combination of neomycin and bacitracin, prevented the development of cirrhosis in most rats for as long as 750 days. However, non-absorbable and absorbable antibiotics did not have a strong impact on hepatic steatosis. In view of these data, it is very likely that TLR4 plays a role not only in the development of inflammation and steatosis but also in the fibrosis of non-alcoholic fatty liver disease.
Part III: Therapeutic modulation of TLR signaling
Modulation of the intestinal microbiota by probiotics and antibiotics
Modulation of the intestinal microbiota is an emerging strategy to reduce bacterial translocation and circulating endotoxin levels. Numerous published studies have demonstrated positive effects of probiotics on parameters such as bacterial translocation, 100-102 circulating endotoxin levels 103, bacterial infection (a surrogate marker for bacterial translocation) in patients with hepatic cirrhosis. 104,105, liver injury in NASH patients 41 and animal models of NASH 106, as well as alcohol- and LPS-induced liver injury 38,101,102. However, the use of many probiotic strains and combinations of different strains make it difficult to judge the efficacy of single components and to compare studies to each other. One of the more commonly used probiotics, VSL#3, a combination of Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei and Lactobacillus bulgaricus, has shown not only a positive effect on liver injury 106 but also a significant amelioration of liver fibrosis in the methionine-choline deficient diet model of liver fibrosis 107. There are no studies that have demonstrated the efficacy of probiotic treatment in reducing hepatic fibrosis in patients, since most studies have focused on treating complications in late stages of the disease such as bacterial infection. In these stages, liver fibrosis is largely irreversible due to formation of collagen crosslinks, and an effect of probiotics on fibrosis would be very unlikely. Thus, the current data on the effect of probiotics on bacterial translocation and LPS levels, coupled with positive results from one mouse study, suggest that probiotics might exert anti-fibrogenic effects in patients. However, well-designed clinical studies are needed to determine the effect of probiotics on liver fibrosis during early disease.
A second approach to the reduction of TLR ligands is treatment with antibiotics to achieve selective intestinal decontamination of Gram-negative bacteria, the predominant source of LPS. Selective intestinal decontamination has been shown to reduce bacterial translocation in many but not all studies performed in rats. 108-110. Importantly, norfloxacin administration reduced the 1-year probability of developing spontaneous bacterial peritonitis, hepatorenal syndrome, and improved the 3-month and 1-year probability of survival compared to placebo. 111 Some of these positive effects may have been independent of the prevention of spontaneous bacterial peritonitis, e.g. related to reducing bacterial translocation and circulating levels of TLR ligands. 111,112. However, in view of the severe consequences of long-term antibiotics, these treatment regimens will be reserved for selected high-risk patients with hepatic cirrhosis, and are probably not suitable to reduce TLR agonists in patients with early stages of liver disease.
Inhibition of TLR4 activation
Several small molecule inhibitors of TLR4 have been discovered and are currently being tested in human studies. Some of these inhibitors are lipid A mimetics that bind to the TLR4-MD2 complex but lack intrinsic activity, and thus prevent binding of the lipid A portion of LPS and subsequent TLR4 activation. The TLR4 antagonist E5564 binds to a large internal pocket in MD-2 8 and dose-dependently inhibits LPS effects both in vitro and in mice and healthy human volunteers 113,114. CRX-526 is another TLR4 antagonist that mimics lipid A without activating TLR4. CRX-526 inhibited LPS-induced cytokine secretion in vitro and in vivo, and reduced colitis in two different experimental models 115. TAK-242 represents a second class of TLR4 antagonists 116 which does not target MD-2, but exerts its inhibitory effects at the intracellular domain of TLR4 as demonstrated by inhibition of a constitutively active receptor chimera in which the extracellular domain of TLR4 was replaced by CD4 117. TAK-242 prevented increases in serum levels of a wide range of cytokines in mice injected with LPS, and protected mice from LPS-induced lethality, even when administered after LPS challenge 118. A soluble TLR4-MD2 fusion protein was shown to bind LPS and to inhibit LPS-induced NF-κB and JNK activation in HSCs 119. However, the effects of this fusion protein on HSC activation were not determined in vivo.
Both E5564 and TAK-242 are currently being tested in phase III clinical trials in patients with septic shock 120. None of the TLR4/MD-2 inhibitors have been tested in chronic liver disease to the best of our knowledge. Based on the involvement of TLR4 in fibrogenesis, alcoholic liver injury and NASH, small molecule inhibitors of TLR4 might be attractive candidates for the treatment or prevention of these diseases.
Conclusion
The intestinal microbiota and TLRs represent a major link between inflammation and wound healing responses in the liver. Among the many different TLRs, TLR4 has a prominent role in (i) promoting inflammation and injury in conditions such as alcoholic liver disease and NASH and (ii) driving HSC activation and fibrogenesis. Although the role of TLR4 in fibrogenesis is well-established in animal models, there is no detailed knowledge on the changes in the gut microbiome and host that lead to the increased activation of TLRs during fibrogenesis. Current data suggest that PAMPs from the intestinal microbiota are essential contributors to TLR4-mediated inflammation and fibrosis in the liver, and that DAMPs, such as HMGB1, at best serve to amplify this response. Despite the accumulating evidence on the role of TLR4 in fibrosis in animal models and association of TLR4 SNPs with disease progression in patients with HCV, the knowledge about proinflammatory and profibrogenic effects of TLR4 in murine liver disease has not yet been transferred into therapeutic strategies. Future studies need (i) to assess the antifibrotic effects and potential side effects of TLR4 inhibitors, probiotics or other modifiers of bacterial translocation in patients with chronic liver disease, and (ii) to further study changes of the gut microbiome during fibrogenesis, to establish the basis for targeted therapies.
Acknowledgments
Grant support: This study was supported by grants U54CA126513 (PI of Project 2: RFS) and R01DK076920 (to RFS), and a grant from the Fondation de la Recherche Médicale (to JPP).
References
- 1.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 3.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 6.Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991;351(6325):355–356. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 7.Freudenberg MA, Tchaptchet S, Keck S, et al. Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiology. 2008;213(3-4):193–203. doi: 10.1016/j.imbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130(5):906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420(6913):329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Sato S, Hemmi H, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420(6913):324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Sato S, Hemmi H, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4(11):1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 14.Pfeiffer R. Untersuchungen über das Choleragift. Z Hygiene. 1892;11:393–412. [Google Scholar]
- 15.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7(3):167–202. [PubMed] [Google Scholar]
- 16.Jiang Z, Georgel P, Du X, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6(6):565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 17.Huber M, Kalis C, Keck S, et al. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur J Immunol. 2006;36(3):701–711. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- 18.Beutler B. Neo-ligands for innate immune receptors and the etiology of sterile inflammatory disease. Immunol Rev. 2007;220:113–128. doi: 10.1111/j.1600-065X.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 19.Lotze MT, Zeh HJ, Rubartelli A, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 20.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8(4):279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JY, Zhao L, Youn HS, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279(17):16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 22.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsan MF, Baochong G. Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J Endotoxin Res. 2007;13(1):6–14. doi: 10.1177/0968051907078604. [DOI] [PubMed] [Google Scholar]
- 24.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 26.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 27.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 28.Bonaldi T, Talamo F, Scaffidi P, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22(20):5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 30.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) J Leukoc Biol. 2007;81(1):49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- 31.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180(4):2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 32.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 33.Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol. 2008;180(7):5067–5074. doi: 10.4049/jimmunol.180.7.5067. [DOI] [PubMed] [Google Scholar]
- 34.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 35.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 36.Taylor KR, Trowbridge JM, Rudisill JA, et al. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279(17):17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 37.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205(3):243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 39.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108(1):218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 40.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20(2):453–460. [PubMed] [Google Scholar]
- 41.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37(2):343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 42.Spruss A, Kanuri G, Wagnerberger S, et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50(4):1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 43.Rivera CA, Bradford BU, Hunt KJ, et al. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G200–207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- 44.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su GL, Klein RD, Aminlari A, et al. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31(4):932–936. doi: 10.1053/he.2000.5634. [DOI] [PubMed] [Google Scholar]
- 46.Seki E, Tsutsui H, Nakano H, et al. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166(4):2651–2657. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- 47.Kopydlowski KM, Salkowski CA, Cody MJ, et al. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163(3):1537–1544. [PubMed] [Google Scholar]
- 48.Lichtman SN, Wang J, Lemasters JJ. LPS receptor CD 14 participates in release of TNF-alpha in RAW 264.7 and peritoneal cells but not in kupffer cells. Am J Physiol. 1998;275(1 Pt 1):G39–46. doi: 10.1152/ajpgi.1998.275.1.G39. [DOI] [PubMed] [Google Scholar]
- 49.Knolle P, Schlaak J, Uhrig A, et al. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22(2):226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 50.Schuchmann M, Hermann F, Herkel J, et al. HSP60 and CpG-DNA-oligonucleotides differentially regulate LPS-tolerance of hepatic Kupffer cells. Immunol Lett. 2004;93(2-3):199–204. doi: 10.1016/j.imlet.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Van Bossuyt H, De Zanger RB, Wisse E. Cellular and subcellular distribution of injected lipopolysaccharide in rat liver and its inactivation by bile salts. J Hepatol. 1988;7(3):325–337. doi: 10.1016/s0168-8278(88)80005-9. [DOI] [PubMed] [Google Scholar]
- 52.Fox ES, Thomas P, Broitman SA. Clearance of gut-derived endotoxins by the liver. Release and modification of 3H, 14C-lipopolysaccharide by isolated rat Kupffer cells. Gastroenterology. 1989;96(2 Pt 1):456–461. doi: 10.1016/0016-5085(89)91571-0. [DOI] [PubMed] [Google Scholar]
- 53.Mimura Y, Sakisaka S, Harada M, Sata M, Tanikawa K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology. 1995;109(6):1969–1976. doi: 10.1016/0016-5085(95)90765-3. [DOI] [PubMed] [Google Scholar]
- 54.Shao B, Lu M, Katz SC, et al. A host lipase detoxifies bacterial lipopolysaccharides in the liver and spleen. J Biol Chem. 2007;282(18):13726–13735. doi: 10.1074/jbc.M609462200. [DOI] [PubMed] [Google Scholar]
- 55.Matsumura T, Degawa T, Takii T, et al. TRAF6-NF-kappaB pathway is essential for interleukin-1-induced TLR2 expression and its functional response to TLR2 ligand in murine hepatocytes. Immunology. 2003;109(1):127–136. doi: 10.1046/j.1365-2567.2003.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumura T, Ito A, Takii T, Hayashi H, Onozaki K. Endotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TLR4 gene expression in murine liver and hepatocytes. J Interferon Cytokine Res. 2000;20(10):915–921. doi: 10.1089/10799900050163299. [DOI] [PubMed] [Google Scholar]
- 57.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paik YH, Schwabe RF, Bataller R, et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37(5):1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 59.Kao YH, Jawan B, Goto S, et al. High-mobility group box 1 protein activates hepatic stellate cells in vitro. Transplant Proc. 2008;40(8):2704–2705. doi: 10.1016/j.transproceed.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 60.Connolly MK, Bedrosian AS, Mallen-St Clair J, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119(11):3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen XM, O'Hara SP, Nelson JB, et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J Immunol. 2005;175(11):7447–7456. doi: 10.4049/jimmunol.175.11.7447. [DOI] [PubMed] [Google Scholar]
- 62.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41(3):422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 63.Wells CL, Maddaus MA, Reynolds CM, Jechorek RP, Simmons RL. Role of anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun. 1987;55(11):2689–2694. doi: 10.1128/iai.55.11.2689-2694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12(2):162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 65.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32(5):742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 66.Mathurin P, Deng QG, Keshavarzian A, et al. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32(5):1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 67.Lin RS, Lee FY, Lee SD, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22(2):165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 68.Rivera CA, Bradford BU, Seabra V, Thurman RG. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am J Physiol. 1998;275(6 Pt 1):G1252–1258. doi: 10.1152/ajpgi.1998.275.6.G1252. [DOI] [PubMed] [Google Scholar]
- 69.Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988;157(5):1032–1038. doi: 10.1093/infdis/157.5.1032. [DOI] [PubMed] [Google Scholar]
- 70.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28(1):26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 71.Plebani M, Burlina A. Biochemical markers of hepatic fibrosis. Clin Biochem. 1991;24(3):219–239. doi: 10.1016/0009-9120(91)80013-s. [DOI] [PubMed] [Google Scholar]
- 72.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nolan JP, Leibowitz AI. Endotoxin and the liver. III. Modification of acute carbon tetrachloride injury by polymyxin b--an antiendotoxin. Gastroenterology. 1978;75(3):445–449. [PubMed] [Google Scholar]
- 74.Grinko I, Geerts A, Wisse E. Experimental biliary fibrosis correlates with increased numbers of fat-storing and Kupffer cells, and portal endotoxemia. J Hepatol. 1995;23(4):449–458. doi: 10.1016/0168-8278(95)80204-5. [DOI] [PubMed] [Google Scholar]
- 75.Chan CC, Hwang SJ, Lee FY, et al. Prognostic value of plasma endotoxin levels in patients with cirrhosis. Scand J Gastroenterol. 1997;32(9):942–946. doi: 10.3109/00365529709011206. [DOI] [PubMed] [Google Scholar]
- 76.Luckey TD, Reyniers JA, Gyorgy P, Forbes M. Germfree animals and liver necrosis. Ann N Y Acad Sci. 1954;57(6):932–935. doi: 10.1111/j.1749-6632.1954.tb36472.x. [DOI] [PubMed] [Google Scholar]
- 77.Rutenburg AM, Sonnenblick E, Koven I, et al. The role of intestinal bacteria in the development of dietary cirrhosis in rats. J Exp Med. 1957;106(1):1–14. doi: 10.1084/jem.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Isayama F, Hines IN, Kremer M, et al. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1318–1328. doi: 10.1152/ajpgi.00405.2005. [DOI] [PubMed] [Google Scholar]
- 79.Poelstra K, Popov Y, Sverdlov DY, Sharma A, Schuppan D. Gut-derived bacterial products drive progression of liver fibrosis in mdr2-/- mice: Involvement of LPS and TLR4. Hepatology. 2009;50:819A. [Google Scholar]
- 80.Watanabe A, Hashmi A, Gomes DA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46(5):1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 81.Gabele E, Muhlbauer M, Dorn C, et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008 doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 82.Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119(7):1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang H, Shiffman ML, Friedman S, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46(2):297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Chang M, Abar O, et al. Multiple variants in toll-like receptor 4 gene modulate risk of liver fibrosis in Caucasians with chronic hepatitis C infection. J Hepatol. 2009;51(4):750–757. doi: 10.1016/j.jhep.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Von Hahn T, Halangk J, Witt H, et al. Relevance of endotoxin receptor CD14 and TLR4 gene variants in chronic liver disease. Scand J Gastroenterol. 2008;43(5):584–592. doi: 10.1080/00365520701806065. [DOI] [PubMed] [Google Scholar]
- 86.Guo J, Loke J, Zheng F, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49(3):960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1(8370):179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 88.Draper LR, Gyure LA, Hall JG, Robertson D. Effect of alcohol on the integrity of the intestinal epithelium. Gut. 1983;24(5):399–404. doi: 10.1136/gut.24.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 90.Hansen J, Cherwitz DL, Allen JI. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20(2):461–474. [PubMed] [Google Scholar]
- 91.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008 doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kono H, Rusyn I, Yin M, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106(7):867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94(6):2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szabo G, Velayudham A, Romics L, Jr, Mandrekar P. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res. 2005;29(11 Suppl):140S–145S. doi: 10.1097/01.alc.0000189287.83544.33. [DOI] [PubMed] [Google Scholar]
- 95.Rivera CA, Adegboyega P, van Rooijen N, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 97.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 98.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119(5):1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 99.Nair S, Cope K, Risby TH, Diehl AM. Obesity and female gender increase breath ethanol concentration: potential implications for the pathogenesis of nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96(4):1200–1204. doi: 10.1111/j.1572-0241.2001.03702.x. [DOI] [PubMed] [Google Scholar]
- 100.Adawi D, Kasravi FB, Molin G, Jeppsson B. Effect of Lactobacillus supplementation with and without arginine on liver damage and bacterial translocation in an acute liver injury model in the rat. Hepatology. 1997;25(3):642–647. doi: 10.1002/hep.510250325. [DOI] [PubMed] [Google Scholar]
- 101.Ewaschuk J, Endersby R, Thiel D, et al. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology. 2007;46(3):841–850. doi: 10.1002/hep.21750. [DOI] [PubMed] [Google Scholar]
- 102.Osman N, Adawi D, Ahrne S, Jeppsson B, Molin G. Endotoxin- and D-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis. 2007;39(9):849–856. doi: 10.1016/j.dld.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Liu Q, Duan ZP, Ha DK, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39(5):1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 104.Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74(1):123–127. doi: 10.1097/00007890-200207150-00021. [DOI] [PubMed] [Google Scholar]
- 105.Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. Am J Transplant. 2005;5(1):125–130. doi: 10.1111/j.1600-6143.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 106.Loguercio C, Federico A, Tuccillo C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39(6):540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 107.Velayudham A, Dolganiuc A, Ellis M, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49(3):989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Runyon BA, Borzio M, Young S, et al. Effect of selective bowel decontamination with norfloxacin on spontaneous bacterial peritonitis, translocation, and survival in an animal model of cirrhosis. Hepatology. 1995;21(6):1719–1724. [PubMed] [Google Scholar]
- 109.Llovet JM, Bartoli R, Planas R, et al. Selective intestinal decontamination with norfloxacin reduces bacterial translocation in ascitic cirrhotic rats exposed to hemorrhagic shock. Hepatology. 1996;23(4):781–787. doi: 10.1002/hep.510230419. [DOI] [PubMed] [Google Scholar]
- 110.Bauer TM, Fernandez J, Navasa M, Vila J, Rodes J. Failure of Lactobacillus spp. to prevent bacterial translocation in a rat model of experimental cirrhosis. J Hepatol. 2002;36(4):501–506. doi: 10.1016/s0168-8278(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 111.Fernandez J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133(3):818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 112.Runyon BA. A pill a day can improve survival in patients with advanced cirrhosis. Gastroenterology. 2007;133(3):1029–1031. doi: 10.1053/j.gastro.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 113.Mullarkey M, Rose JR, Bristol J, et al. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther. 2003;304(3):1093–1102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- 114.Rossignol DP, Lynn M. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J Endotoxin Res. 2002;8(6):483–488. doi: 10.1179/096805102125001127. [DOI] [PubMed] [Google Scholar]
- 115.Fort MM, Mozaffarian A, Stover AG, et al. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J Immunol. 2005;174(10):6416–6423. doi: 10.4049/jimmunol.174.10.6416. [DOI] [PubMed] [Google Scholar]
- 116.Ii M, Matsunaga N, Hazeki K, et al. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol. 2006;69(4):1288–1295. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- 117.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584(1):40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 118.Sha T, Sunamoto M, Kitazaki T, et al. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571(2-3):231–239. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 119.Schnabl B, Brandl K, Fink M, et al. A TLR4/MD2 fusion protein inhibits LPS-induced pro-inflammatory signaling in hepatic stellate cells. Biochem Biophys Res Commun. 2008;375(2):210–214. doi: 10.1016/j.bbrc.2008.07.150. [DOI] [PubMed] [Google Scholar]
- 120.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13(5):552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]