Abstract
An elevated ferritin before allogeneic hematopoietic cell transplantation (HCT) is an adverse prognostic factor for overall survival (OS) and non-relapse mortality (NRM). Because ferritin is an imperfect surrogate of iron stores, the prognostic role of iron overload remains unclear. We conducted a patient-level meta-analysis of 4 studies that used magnetic resonance imaging to estimate pre-HCT liver iron content (LIC). An elevated LIC was not associated with a significant increase in mortality: the hazard ratio (HR) for mortality associated with LIC>7 mg/gdw (primary endpoint) was 1.4 (p=0.18). In contrast, ferritin >1000 ng/ml was a significant prognostic factor (HR for mortality 1.7, p=0.036). There was, however, no significant association between ferritin>2500 and mortality. This meta-analysis suggests that iron overload, as assessed by LIC, is not a strong prognostic factor for OS in a general adult HCT population. Our data also suggest that ferritin is an inadequate surrogate for iron overload in HCT.
INTRODUCTION
The toxicities of iron overload (IO) in patients with benign diseases overlap with some of the most severe toxicities of allogeneic hematopoietic cell transplantation (HCT) which has led to the hypothesis that IO could be common and deleterious in patients with hematologic malignancies undergoing HCT. A large body of evidence has shown that an elevated serum ferritin (SF) before HCT is associated with inferior overall survival (OS), an effect that in most studies appears mediated by an increase in non-relapse mortality (NRM)(1–15). While those studies vary in their study population and choice of SF cutoff, they almost universally agree that a high SF is associated with an adverse prognosis. A more direct estimate of total body iron burden may be obtained by measuring liver iron content (LIC), which may be assessed non-invasively by magnetic resonance imaging (MRI)(16, 17). In HCT studies evaluating both liver iron content (LIC) estimated by MRI and SF, the correlation coefficient between the two is around 0.6–0.8(12, 13, 18–21), implying that ferritin is an acceptable but imperfect surrogate of true iron burden. Alternatively, because ferritin is an acute phase reactant, its elevation in serum may betray inflammatory states including active infection or more advanced disease status, which are expected to confer an adverse prognosis in HCT independent of iron overload. There are at present 4 published studies that have directly assessed the impact of pre-HCT LIC on outcome, but differ in their conclusion despite their generally similar design4,14,15,22. We therefore undertook an individual patient data meta-analysis of all 4 studies, with the aim of clarifying whether pre-HCT LIC is, like SF, associated with worse OS or NRM.
METHODS
We obtained patient-level data from the 4 published prospective studies(3, 12, 13, 22). Survivors with < 6 months of post-HCT follow-up were excluded. We conducted a meta-analysis for OS and NRM. NRM was calculated in the competing risks framework, treating relapse as a competing risk. Within each study as a whole or for a subgroup of interest, we calculated the hazard ratio (HR) and associated p value for mortality using a univariable proportional hazards model, and for NRM using a competing risks regression model(23). The HRs were pooled with inverse variance weighting, using a random effects model(24). Heterogeneity was assessed using the Cochran’s Q statistic and the I2 calculation(24, 25).
RESULTS AND DISCUSSION
The details of the 4 cohorts and the 276 patients included in this analysis have been previously published4,14,15,22. The median age was 52 (range, 18–74) years. Half had acute myeloid leukemia, and 16% had myelodysplastic syndrome (MDS). Almost two-thirds received reduced intensity conditioning (RIC). The median pre-HCT SF was 1523 (range, 20–8878) ng/ml; 67% of patients had a ferritin over 1000 ng/ml and 23% above 2500 ng/ml. The median pre-HCT LIC was 5.0 (range, 0.3–25.4) mg/gdw, with 82% of patients having an elevated LIC (>1.8 mg/gdw), 50% an LIC>5 mg/gdw, and 28% an LIC>7 mg/gdw. The median follow-up for survivors was 22 (range, 6–44) months.
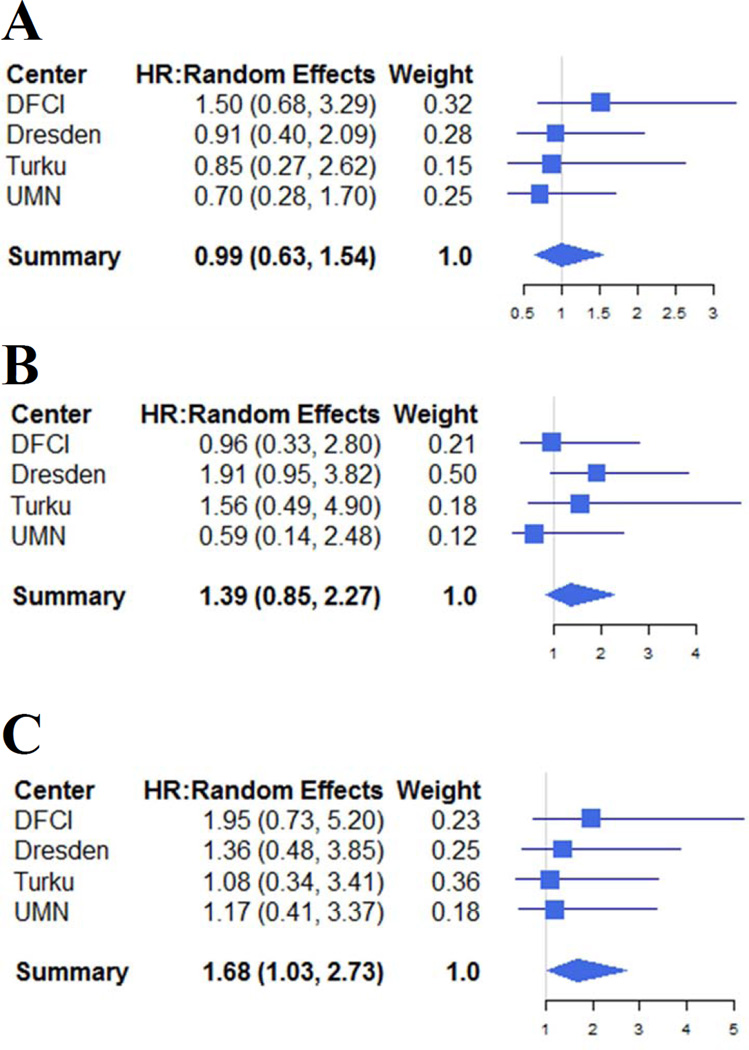
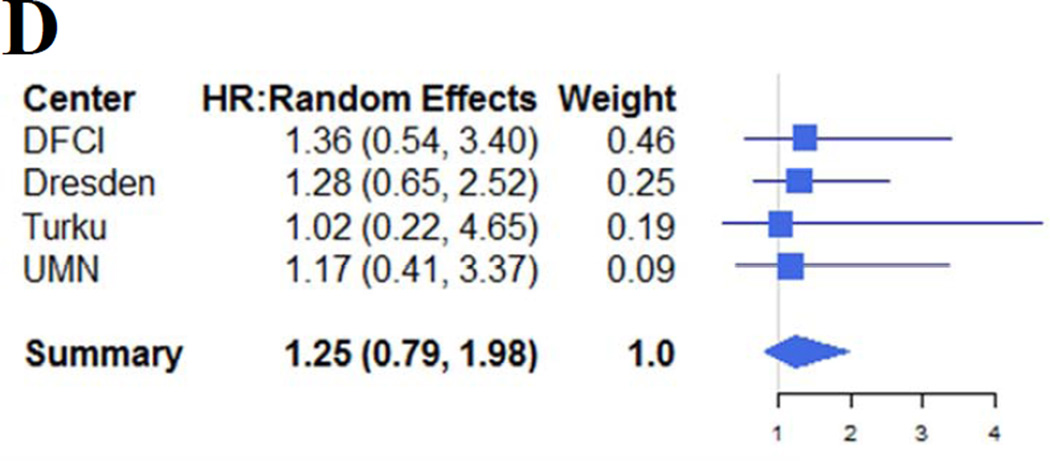
The results are summarized in Table 1. We used 2 possible pre-specified thresholds for SF (1000 and 2500 ng/ml) and for LIC (5 and 7 mg/gdw), corresponding to the ones most often used in the HCT literature and in the primary studies. For OS, the pooled HR associated with LIC>5 mg/gdw was 1.0 (p=1.0, Figure 1A); for LIC>7 mg/gdw, the HR was 1.4 (p=0.2, Figure 1B). For ferritin>1000 ng/ml, the HR was 1.7 (p=0.036, Figure 1C); however, there was no significant association between ferritin>2500 ng/ml and mortality (HR=1.3, p=0.3, Figure 1D). There was no significant heterogeneity for any of the above outcomes (Q statistic 0.1–2.7 with 3 degrees of freedom, p values 0.5–1.0, I2=0 for all). There was no significant association between any of the variables studied and NRM. We also found no significant association between LIC or SF and OS or TRM in analyses restricted to patients undergoing myeloablative conditioning (MAC), or in the subgroup of patients with MDS or acute leukemia. We also conducted an exploratory analysis restricted to patients who had undergone RIC HCT. The HR for NRM associated with LIC>7 mg/gdw was 2.2 (p=0.026), while the other HRs were not significant. One of the studies in this meta-analysis only enrolled patients undergoing MAC HCT, and was therefore not included in this subgroup analysis. As the number of patients and of events in the analysis of NRM for RIC patients was small, those results should be interpreted with caution. However, they raise the possibility that iron overload may have a particular importance among the generally older and frailer patients undergoing RIC HCT, which deserves further study.
Table 1. Summary results.
For each variable, outcome and subgroup of interest, the hazard ratio is given along with its associated 95% confidence interval and p value.
| Population | Outcome | Variable | HR (95CI, p value) |
|---|---|---|---|
| Overall cohort | Overall Survival | LIC>5 mg/gdw LIC>7 mg/gdw SF > 1000 ng/ml SF > 2500 ng/ml |
1.0 (0.6–1.5), p=1.0 1.4 (0.9–2.3), p=0.2 1.7 (1.0–2.7), p=0.036 1.3 (0.8–2.0), p=0.3 |
| Non-relapse mortality | LIC>5 mg/gdw LIC>7 mg/gdw SF > 1000 ng/ml SF > 2500 ng/ml |
1.0 (0.6–1.7), p=0.9 1.7 (0.9–3.3), p=0.09 1.6 (0.9–2.8), p=0.14 1.4 (0.8–2.3), p=0.3 |
|
| MAC cohort | Overall Survival | LIC>7 mg/gdw SF > 1000 ng/ml |
1.3 (0.4–4.4), p=0.6 1.5 (0.6–3.5), p=0.4 |
| Non-relapse mortality | LIC>7 mg/gdw SF > 1000 ng/ml |
Not evaluableb | |
| RIC cohorta | Overall Survival | LIC>7 mg/gdw SF > 1000 ng/ml |
1.5 (0.8–2.7), p=0.2 1.5 (0.8–2.8), p=0.2 |
| Non-relapse mortality | LIC>7 mg/gdw SF > 1000 ng/ml |
2.2 (1.1–4.6), p=0.026a 1.5 (0.7–3.3), p=0.3 |
|
| Acute leukemia + MDS | Overall Survival | LIC>7 mg/gdw SF > 1000 ng/ml |
1.2 (0.7–2.3), p=0.6 1.5 (0.7–3.1), p=0.3 |
| Non-relapse mortality | LIC>7 mg/gdw SF > 1000 ng/ml |
1.5 (0.7–3.0), p=0.3 1.1 (0.6–2.2), p=0.7 |
HR denotes hazard ratio; 95CI, 95% confidence interval; LIC, liver iron content; SF, serum ferritin; MAC, myeloablative conditioning; RIC, reduced intensity conditioning; MDS, myelodysplastic syndromes.
See text for comment
Due to the small number of events in this subgroup.
Figure 1.
Forest plot of the hazard ratios for mortality associated with LIC> 5 mg/gdw (Panel A), LIC> 7 mg/gdw (Panel B), ferritin>1000 ng/ml (Panel C) and ferritin>2500 ng/ml (Panel D). Center names are abbreviated as follows: DFCI indicates Dana-Farber Cancer Institute; Dresden, University of Dresden; Turku, Turku University Hospital; UMN, University of Minnesota.
In summary, we did not find a significant association between an elevated pre-HCT LIC and OS or NRM overall. Consistent with previous studies, we observed a significant association between SF and OS. However, this was only significant at a threshold of 1000 ng/ml, and not at a threshold of 2500 ng/ml. This could be explained if the adverse prognosis of SF is related not to iron overload but to underlying disease-related or comorbidity-related issues that elevate acute phase reactants. In that case, the degree of elevation in SF may not be relevant, only the presence of the underlying issues reflected by any significant elevation in SF. If this were true, an SF threshold of 1000 ng/ml may better capture the patient population at risk than a higher threshold. Regardless, our results suggest that the evidence to date on SF and HCT outcome may not allow us to conclude that iron overload itself is strongly detrimental after HCT.
It is unlikely that our negative results stem from our threshold choice. Based on prior reports of the correlation between SF and LIC in this patient population(18), an SF of 2500 ng/ml corresponds to an LIC around 6 mg/gdw. Therefore, the thresholds chosen for this meta-analysis should have captured the prognostic effect previously seen with SF. It is also unlikely that our results are confounded by measurement technique. While the MRI techniques used to estimate LIC differed among the 4 studies, they all used techniques that have been previously validated against liver biopsy.
It is possible that given the trends for increased overall and non-relapse mortality for elevated LIC, our negative results reflect a sample size issue, and that a larger study would have uncovered a significant effect. Our study also lacked power to definitively study the impact of iron overload among patient subgroups. Regardless, the results presented here should not be interpreted to imply that iron is irrelevant in HCT. The trends suggest a possible prognostic effect, although it does not appear as strong as suspected based on the ferritin-related literature, and may be restricted to certain subgroups. Moreover, iron may be related to disease pathology in ways that are just beginning to be understood. We suggest instead that the present study should serve as the impetus for the design of next-generation studies that do not rely primarily on SF measurement, and that explore, through careful and creative design, alternative mechanisms through which iron may influence HCT outcomes. Furthermore, several groups have already reported on the use of(26–30) or recommendation for(31) chelation therapy in the pre or post-HCT setting. Our results may weaken the premise for chelation studies in general transplant populations, and emphasize the need to design future chelation studies with a broad array of correlative endpoints that could uncover possible effects of chelation beyond simply the reduction in iron stores.
ACKNOWLEDGEMENT
P.A. was supported by an ASCO Career Development Award. This work was also supported by NIAID U19 AI 29530, NCI P01 CA142106, and the Jock and Bunny Adams Research and Education Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
P. A. designed the research, collected and analyzed the data, and wrote the paper.
H. T. K. designed the research, analyzed the data and edited the paper.
J. M. V. designed the research, collected the data, and edited the paper.
R. K. P. collected the data, and edited the paper.
M. A. I.-R. designed the research, collected the data, and edited the paper.
N. S. M. designed the research, collected the data, and edited the paper.
L. J. B. designed the research, collected the data, and edited the paper.
T. D. designed the research, and edited the paper.
B. T. designed the research, collected the data, and edited the paper.
U. P. collected the data, and edited the paper.
J. H. A. designed the research, collected the data, and edited the paper.
M. W. designed the research, collected the data, and edited the paper.
CONFLICT OF INTEREST DISCLOSURES
The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pretransplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95:476–484. doi: 10.3324/haematol.2009.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armand P, Sainvil MM, Kim HT, et al. Does iron overload really matter in stem cell transplantation? Am J Hematol. 2012;87:569–572. doi: 10.1002/ajh.23188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazuave GN, Buser A, Gerull S, Tichelli A, Stern M. Prognostic impact of iron parameters in patients undergoing allo-SCT. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.13. [DOI] [PubMed] [Google Scholar]
- 5.Kataoka K, Nannya Y, Hangaishi A, et al. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:195–204. doi: 10.1016/j.bbmt.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Kim YR, Kim JS, Cheong JW, Song JW, Min YH. Transfusion-associated iron overload as an adverse risk factor for transplantation outcome in patients undergoing reduced-intensity stem cell transplantation for myeloid malignancies. Acta Haematol. 2008;120:182–189. doi: 10.1159/000187646. [DOI] [PubMed] [Google Scholar]
- 7.Lim ZY, Fiaccadori V, Gandhi S, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2009;34:723–727. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Mahindra A, Bolwell B, Sobecks R, et al. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009;146:310–316. doi: 10.1111/j.1365-2141.2009.07774.x. [DOI] [PubMed] [Google Scholar]
- 9.Platzbecker U, Bornhauser M, Germing U, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS) Biol Blood Marrow Transplant. 2008;14:1217–1225. doi: 10.1016/j.bbmt.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42:799–805. doi: 10.1038/bmt.2008.262. [DOI] [PubMed] [Google Scholar]
- 11.Sucak GT, Yegin ZA, Ozkurt ZN, Aki SZ, Yagci M. Iron overload: predictor of adverse outcome in hematopoietic stem cell transplantation. Transplant Proc. 2010;42:1841–1848. doi: 10.1016/j.transproceed.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Trottier BJ, Burns LJ, Defor TE, Cooley S, Majhail NS. Association of iron overload with allogeneic hematopoietic cell transplantation outcomes: a prospective cohort study using R2-MRI-measured liver iron content. Blood. 2013;122:1678–1684. doi: 10.1182/blood-2013-04-499772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wermke M, Schmidt A, Middeke JM, et al. MRI-based liver iron content predicts for nonrelapse mortality in MDS and AML patients undergoing allogeneic stem cell transplantation. Clin Cancer Res. 2012;18:6460–6468. doi: 10.1158/1078-0432.CCR-12-1683. [DOI] [PubMed] [Google Scholar]
- 14.Meyer SC, O'Meara A, Buser AS, Tichelli A, Passweg JR, Stern M. Prognostic impact of posttransplantation iron overload after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:440–444. doi: 10.1016/j.bbmt.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Grossekatthofer M, Guclu ED, Lawitschka A, et al. Ferritin concentrations correlate to outcome of hematopoietic stem cell transplantation but do not serve as biomarker of graftversus- host disease. Annals of hematology. 2013;92:1121–1128. doi: 10.1007/s00277-013-1737-x. [DOI] [PubMed] [Google Scholar]
- 16.Gandon Y, Olivie D, Guyader D, et al. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357–362. doi: 10.1016/S0140-6736(04)15436-6. [DOI] [PubMed] [Google Scholar]
- 17.St Pierre TG, Clark PR, Chua-anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 18.Armand P, Kim HT, Rhodes J, et al. Iron overload in patients with acute leukemia or MDS undergoing myeloablative stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:852–860. doi: 10.1016/j.bbmt.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–573. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 20.Vag T, Kentouche K, Krumbein I, et al. Noninvasive measurement of liver iron concentration at MRI in children with acute leukemia: initial results. Pediatric radiology. 2011;41:980–984. doi: 10.1007/s00247-011-2122-3. [DOI] [PubMed] [Google Scholar]
- 21.Olivieri NF, Brittenham GM, Matsui D, et al. Iron-chelation therapy with oral deferipronein patients with thalassemia major. N Engl J Med. 1995;332:918–922. doi: 10.1056/NEJM199504063321404. [DOI] [PubMed] [Google Scholar]
- 22.Virtanen JM, Itala-Remes MA, Remes KJ, et al. Prognostic impact of pretransplant iron overload measured with magnetic resonance imaging on severe infections in allogeneic stem cell transplantation. Eur J Haematol. 2013;91:85–93. doi: 10.1111/ejh.12123. [DOI] [PubMed] [Google Scholar]
- 23.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Armand P, Sainvil MM, Kim HT, et al. Pre-transplantation iron chelation in patients with MDS or acute leukemia and iron overload undergoing myeloablative allo-SCT. Bone Marrow Transplant. 2012 doi: 10.1038/bmt.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaloyannidis P, Yannaki E, Sakellari I, et al. The impact of desferrioxamine postallogeneic hematopoietic cell transplantation in relapse incidence and disease-free survival: a retrospective analysis. Transplantation. 2010;89:472–479. doi: 10.1097/TP.0b013e3181c42944. [DOI] [PubMed] [Google Scholar]
- 28.Majhail NS, Lazarus HM, Burns LJ. A prospective study of iron overload management in allogeneic hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2010;16:832–837. doi: 10.1016/j.bbmt.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Sivgin S, Baldane S, Akyol G, et al. The oral iron chelator deferasirox might improve survival in allogeneic hematopoietic cell transplant (alloHSCT) recipients with transfusional iron overload. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2013;49:295–301. doi: 10.1016/j.transci.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Sivgin S, Eser B, Bahcebasi S, et al. Efficacy and safety of oral deferasirox treatment in the posttransplant period for patients who have undergone allogeneic hematopoietic stem cell transplantation (alloHSCT) Annals of hematology. 2012;91:743–749. doi: 10.1007/s00277-011-1358-1. [DOI] [PubMed] [Google Scholar]
- 31.Alessandrino EP, Angelucci E, Cazzola M, et al. Iron overload and iron chelation therapy in patients with myelodysplastic syndrome treated by allogeneic stem-cell transplantation: report from the working conference on iron chelation of the Gruppo Italiano Trapianto di Midollo Osseo. Am J Hematol. 2011;86:897–902. doi: 10.1002/ajh.22104. [DOI] [PubMed] [Google Scholar]